Abstract
We previously showed that the expression of transient receptor potential canonical (TRPC)6 ion channel elevated when TRPC1 was knocked down in A7r5 cultured vascular smooth muscle cells. Therefore, the purpose of this study was to explore whether TRPC6 is also upregulated in aging rat aorta comparable to that of TRPC1 in longitudinal in vivo aging model. We further investigated a possible causal relationship between altered phenylephrine-induced contractions and the expression levels of TRPC6, a purported essential component of alpha-adrenergic receptor signaling in aging aorta. Immunoblot analysis showed that TRPC1 protein levels significantly decreased whereas TRPC6 increased drastically in aorta from 16- to 20-month-old rats compared to that from 2 to 4 months. Immunohistochemical data demonstrated spatial changes in TRPC6 expression within the smooth muscle layers along with increased detection in the adventitia of the aged rat aorta. The phenylephrine-induced contractions were potentiated in aging aorta. In conclusion, based on this aging model, TRPC6 overexpression could be related with TRPC1 downregulation and might be responsible for the increased adrenoceptor sensitivity which contributes to the development of age-related vasospastic disorders.
Keywords: Aging, Transient receptor potential, TRPC, Vascular smooth muscle
Introduction
Receptor- (ROCCs) and store-operated calcium (Ca2+) channels (SOCCs) play important roles in the regulation of intracellular Ca2+ homeostasis in vasculature. Mammalian homologs of canonical subfamily of the transient receptor potential (TRPC) genes coding for TRPC ion channel proteins are suggested to participate in ROCCs and SOCCs (Minke 2006). Among the family, TRPC1 and TRPC6 are the most predominant members expressed in vascular smooth muscle cells (VSMCs; Inoue et al. 2006). Based on the accumulated evidence, TRPC1 and TRPC6 play important roles in the regulation of vascular tone and VSMC proliferation (Albert et al. 2009; Jung et al. 2002; Sweeney et al. 2002; Yu et al. 2003, 2004; Kumar et al. 2006). In addition, adrenergic receptor (AR)-activated cation current decreases in TRPC6 antisense-treated primary cultured portal vein myocytes (Inoue et al. 2001). Furthermore, we also demonstrated recently that TRPC6 mRNA and protein levels were significantly upregulated in A7r5 aortic SMCs treated with siRNA against TRPC1 mRNA (Selli et al. 2009), suggesting that this apparent association might also be operational in intact tissue.
From a clinical point of view, prevalence of hypertension, coronary heart disease, congestive heart failure, and stroke increase with aging (Najjar et al. 2005). Although majority of age-dependent vascular disorders appear to be correlated with endothelial dysfunction (Matz et al. 2000), responses to vasoconstricting substances including noradrenaline are also potentiated (Barton et al. 1997) without any increase in density of α1D-ARs (Gurdal et al. 1995; Xu et al. 1997), a predominant AR subtype responsible for aortic contractions (Piascik et al. 1997). However, there is no evidence on age-dependent changes in expression levels of TRPC proteins that are reportedly participated in ROCCs and SOCCs. Temporal changes of TRPC expression pattern in developing embryonic brain (Strubing et al. 2003), pregnant rat myometrium (Babich et al. 2004), and gestational human placenta (Clarson et al. 2003) also suggest TRPCs’ involvement in maturation processes. Identification and validation of novel biomarkers and/or drug targets become critical in translation of preclinical research in aging model systems into clinical studies. Therefore, we investigated the effects of aging on TRPC1 and TRPC6 expression patterns in rat aorta with dysfunctional endothelium.
Materials and methods
Experimental animals
Male Sprague–Dawley rats (n = 24) housed under controlled environmental conditions (at 22°C with 12-h light/dark cycle) were longitudinally aged and tested at three age intervals (in months 2–4, 10–12, and 16–20; Csiszar et al. 2002). All animals received care according to the criteria outlined in the “Guide for Care and Use of Laboratory Animals” prepared by the National Academy of Science, which is also adopted and promulgated by Bilkent University and Ege University.
Real-time quantitative RT-PCR
Total RNA was isolated from endothelium-denuded aortic tissue samples using a guanidinium thiocyanate containing solution (TriPure®, Roche Applied Science) according to manufacturer’s instructions. RNA concentrations were calculated by measuring absorbance at 260 nm. cDNA was synthesized from total RNA using oligodT primers (RevertAid First Strand cDNA Synthesis Kit, Fermentas). To determine the relative expression levels of TRPC1 and TRPC6, real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using FastStart DNA Master SYBR Green I kit and LightCycler 2.0 (Roche Applied Science). Primer sequences are given in Table 1. Hot-start PCR was performed as follows: 10 min at 95°C for initial denaturation, followed by 50 cycles of denaturation at 95°C for 10 s, annealing at 57°C for 10 s, and elongation at 72°C for 15–19 s before the melting curve analysis. A serially diluted standard β-actin cDNA-containing plasmid with known copy number was used during each PCR to perform the linear regression for standard β-actin. By using this external calibrator, we also determined the expression levels of the housekeeping gene, β-actin, whose mRNA levels were not changed in aorta with aging (data not shown). Each PCR product was analyzed, on the basis of their individual cycle threshold, by the β-actin standard curve. All expression levels were normalized to that of internal β-actin and given as [TRPCx]/[β-actin] × 104. To confirm their integrity, PCR products were also stained with ethidium bromide after agarose (2%) gel electrophoresis.
Table 1.
Primer sequences used in PCR
| Gene | Accession no. | Primer sequence (5′–3′) | Amplicon size (bp) |
|---|---|---|---|
| TRPC1 | NM_053558 | F: TGG TAT GAA GGG TTG GAA GAC | 410 |
| R: TGC TGT TCA CAG AAG ATG CC | |||
| TRPC6 | NM_053559 | F: GAT ATC TTC AAA TTC ATG GTC ATA | 321 |
| R: ATC CGC ATC ATC CTC AAT TTC | |||
| α1D AR | NM_024483 | F: CGT GTG CTC CTT CTA CTA CC | 304 |
| R: GCA CAG GAC GAA GAC ACC CAC | |||
| β-actin | NM_031144 | F: AGT GTG ACG TTG ACA TCC GT | 244 |
| R: GAC TCA TCG TAC TCC TGC TT |
Western blot analysis
In order to see the tendency of age-related changes in TRPC1 and TRPC6 protein expression with age, the 10–12-month-old rats were also included in immunoblot analysis. Protein samples were prepared from thoracic aorta by homogenization in lysis solution (Camiolo buffer, 75 mM potassium acetate, 300 mM NaCl, 10 mM EDTA, 100 mM l-arginine basic salt, and 0.25% Triton-X 100, protease inhibitor mix). Protein concentrations were determined using Bradford assay. Proteins were separated on 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to membranes (Immobilon-P polyvinylidene difluoride, Millipore) at 25°C for 2 h at 15 V. Following 2 h blocking (with 5% skim milk in Tris-buffered saline with Tween-20), membranes were incubated with TRPC primary antibodies (1:200, Alomone Laboratories) and anti-β-actin (1:1,500, Abcam Ltd.) overnight at 4°C, then with horseradish peroxidase (HRP)-conjugated goat antirabbit secondary antibody (DakoCytomation; 1:1,500) for 1 h at room temperature. ECL Plus Detection kit (Amersham Biosciences) was used to visualize bands, and optical density of each blot was normalized to that of β-actin analyzed within the same lane and represented as relative optical density.
Immunohistochemistry
Paraffin sections (5 μm) were incubated for 30 min in 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) to quench endogenous peroxidase activity and then placed in preblocking serum of 2% bovine serum albumin in PBS for 1 h. Samples were incubated overnight at 4°C with primary polyclonal antibodies of anti-TRPC1 and anti-TRPC6 (1:100) and smooth muscle anti-α-actin (1:800, Abcam Ltd., Cambridge, Cambridgeshire, UK). Negative controls were performed by omitting the primary antibody. Following three PBS washes, each slice was incubated with biotinylated antimouse and antirabbit Ig (DakoCytomation, Denmark) and streptavidin–HRP (DakoCytomation, Denmark) for 15 min at room temperature. After 10 min PBS wash, color development was achieved by incubation with diaminobenzidine (DakoCytomation, Denmark). Finally, the slides were counterstained with hematoxylin and mounted using Faramount Aqueous Mounting Medium (DakoCytomation, Denmark).
Isometric force
Contractility experiments were performed as described previously (Tosun et al. 2007). AR-mediated contractions were induced by cumulative increases of phenylephrine (PE) concentrations (10−9–10−4 M) in endothelium-denuded vessels. In some experiments, acetylcholine (ACh)-induced endothelium-derived relaxations in PE-precontracted aortic rings were also monitored to confirm aging-related endothelial dysfunction. Force generation was normalized to cross-sectional area [Force (mN)/Area (mm2), F/CSA = (change in force × circumference)/2 × wet weight].
Statistical analysis
Data are expressed as mean ± SEM. n represents the number of animals used. Statistical significance was evaluated using Student’s t test. Values of maximal effect (Emax) and 50% effective concentration (EC50) were derived for each cumulative concentration–response curves with an iterative nonlinear least squares program (KaleidaGraph™ 3.0 by Synergy Software). Geometric means of the EC50 values (pD2) were compared. P < 0.05 was considered significant.
Results
Expression profile of TRPC mRNA
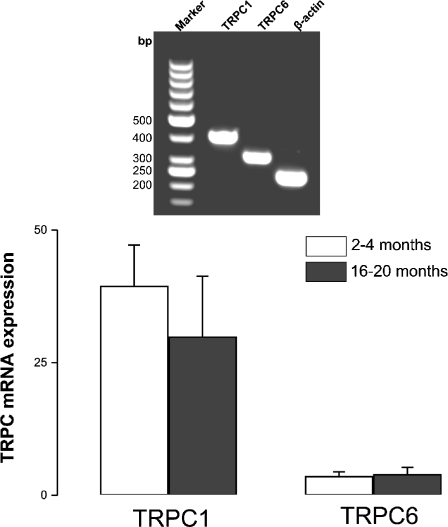
Quantitative real-time PCR was performed to determine TRPC mRNA levels in rat thoracic aorta at two different age groups. Although not statistically significant, TRPC1 expression at transcriptional level was apparently decreased with aging while no change was observed in TRPC6 (Fig. 1). Aging-dependent possible changes in AR expression pattern were also investigated. In this regard, α1D-AR mRNA levels were not altered significantly with aging (1.73 ± 0.51 and 1.33 ± 0.61, n = 3, 2–4 vs. 16–20 months old, respectively).
Fig. 1.
Real-time quantitative RT-PCR detection of TRPC1 and TRPC6 mRNAs isolated from 2- to 4- and 16- to 20-month old rat aorta. PCR products amplified with gene-specific primers (Table 1) for TRPCs and β-actin. mRNA levels were quantified based on their cycle threshold (see “Materials and methods” section for details) and normalized to internal β-actin expression. Values were given as [TRPCx]/[β-actin] × 104 (n = 4–6). Inset image confirms the specificity of the PCR products (TRPC1 410 bp, TRPC6 321 bp, and β-actin 244 bp) run on agarose gel after qRT-PCR. Marker—50-bp DNA Ladder (Fermentas)
Expression profile of TRPC proteins
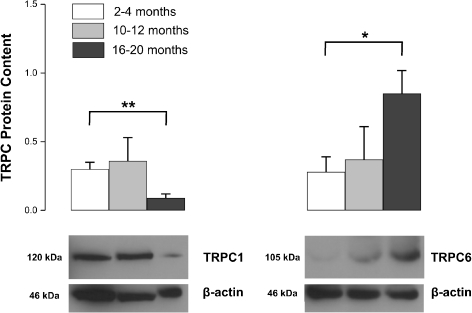
TRPC1 and TRPC6 proteins were differentially expressed during aging (Fig. 2). TRPC1 was significantly decreased at 16–20 months (P < 0.01; Fig. 2). On the other hand, TRPC6 levels were approximately threefold higher in 16–20-month-old rat aorta compared to that of 2–4 months old (P < 0.05; Fig. 2).
Fig. 2.
Western blot analysis of TRPC protein expression in 2–4-, 10–12- and 16–20-month-old rat aorta. Representative blot images for TRPC1 and TRPC6 proteins isolated from rat aorta were shown. β-actin protein was used as loading control. Protein levels were expressed as relative optical density. Shown are mean ± SEM; *P < 0.05; **P < 0.01, 2–4 months (open bars) vs. 16–20 months (closed bars), n = 5
Detection of TRPCs by immunohistochemical studies
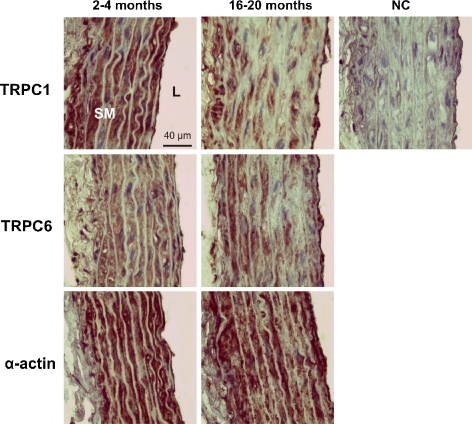
Immunohistochemical analyses of rat thoracic aorta showed drastic decreases in TRPC1 staining in old vessels (Fig. 3). Interestingly, TRPC6 protein expression was localized toward the adventitia of aortic segments from 16- to 20-month-old rats (Fig. 3) whereas staining with anti-α-actin antibody has shown a diffuse pattern throughout all VSM layers (Fig. 3). No staining was observed in negative controls (only primary antibody omitted; Fig. 3, NC).
Fig. 3.
Immunohistochemical analysis of TRPC1 and TRPC6 proteins in 2–4- and 16–20-month-old rat aorta. Positive staining in brown color is seen in smooth muscle (SM) layers. Antibody against SM α-actin used as positive control. Negative control (NC) experiments were performed in the absence of the primary antibodies. Tissue sections were counterstained with hematoxylin. L lumen
Effects of aging on vascular responsiveness
To evaluate the degree of age-dependent altered vascular reactivity pattern, organ bath experiments were performed. Although maximum contractions did not change (Table 2), PE concentration–response curves shifted to the left significantly with aging (P < 0.01, n = 6; Table 2). Endothelium-dependent vasorelaxations were also impaired significantly in aged vasculature (% maximum relaxations to ACh, 85 ± 2 vs. 62 ± 2, 2–4 and 16–20 months old, respectively; P < 0.01, n = 6).
Table 2.
Effects of aging on PE-induced α-adrenergic receptor mediated responses in rat thoracic aorta
| pD2 | Emax (F/CSA) | |
|---|---|---|
| 2–4 months | 7.45 ± 0.07 | 31.02 ± 3.12 |
| 16–20 months | 7.89 ± 0.08* | 31.28 ± 2.71 |
Data are expressed as mean ± SEM
*P < 0.01 (n = 4–6)
Discussion
TRPC ion channel proteins, especially TRPC1 and TRPC6, involved in SOCCs and ROCCs, contribute to variety of physiological functions including vasoconstriction and VSMC proliferation. The present study demonstrates a novel observation that TRPC1 protein levels significantly decreased in aging rat thoracic aorta whereas TRPC6 drastically elevated. Consistent with the previous reports, sensitivity of artery to PE was also increased with aging (Barton et al. 1997; Tabernero and Vila 1995). Thus, present data suggest that the increased vascular responsiveness during aging may be associated with the upregulation of TRPC6 protein, an essential component of the α1-AR-activated Ca2+ channels.
Significant changes observed in TRPC1 and TRPC6 protein levels rather than in transcriptional levels might have resulted from a posttranscriptional regulation of gene expression. Therefore, a slight change in mRNA level may have a great impact on protein expression due to alterations in stability/turnover of mRNA and protein. Within this context, posttranscriptional and/or posttranslational modifications could be responsible for control of gene expression, leading to an apparent discrepancy between TRPC mRNA and protein levels, as previously reported (Facemire et al. 2004; Soboloff et al. 2005; see also Anderson and Kedersha 2009 for a recent review). Posttranscriptional gene silencing appears plausible as TRPC6 protein levels was significantly upregulated in TRPC1-knocked down A7r5 VSMCs (Selli et al. 2009).
Decrease in TRPC1, reportedly a major purported component (Beech 2005) and/or a regulator of SOCC (Albert and Large 2003; Beech et al. 2003; Selli et al. 2009), suggests presence of channel disassembly in aging rat aorta. Moreover, accumulating evidence on stress-dependent differential expression profiles of TRPC1 and TRPC6 poses a novel clinical impact of these proteins in cardiovascular diseases. Among these, platelet-derived growth factor-stimulated pulmonary artery SMC proliferation has been reversed by TRPC6 antisense treatment (Yu et al. 2003). In a follow-up study, idiopathic pulmonary arterial hypertension and pulmonary arterial SMC proliferation were attenuated by TRPC6 siRNA application (Yu et al. 2004). In addition, TRPC1 and TRPC6 overexpressions have been linked to development of hypoxia-related pulmonary hypertension (Lin et al. 2004). Moreover, TRPC1 protein has been shown to be upregulated along with enhanced store-operated Ca2+ entry in cuff-injured mouse carotid artery (Kumar et al. 2006).
α1D-ARs play important roles in control of systemic blood pressure and are involved in age-related vasospasms in human subjects (Dinenno et al. 2001). On the other hand, TRPC6 has been postulated to be an essential component of the α1-AR-activated Ca2+-permeable cation channel in vascular tissues (Inoue et al. 2001; Welsh et al. 2002; Yu et al. 2004); therefore, its drastic increase (present study) may be linked to development of hypertension. Moreover, based on our recent data, TRPC6 protein expression was doubled in siTRPC1-transfected A7r5 VSMCs whose TRPC1 levels decreased 64% by gene silencing (Selli et al. 2009). Interestingly, similar interaction was also observed in cultured rat mesenteric artery in which TRPC1 mRNA expression decreased while TRPC6 mRNA expression increased after organ culture (Tai et al. 2009). However, more work is required to elucidate whether TRPC6 upregulation is a consequence of TRPC1 downregulation. Likewise, TRPC6-deficient mice studies have shown that TRPC3 mRNA expression upregulated to compensate the loss of TRPC6 (Dietrich et al. 2005). This reciprocal expression pattern may also suggest presence of a strict control mechanism for TRPC6 expression possibly to maintain AR signaling and ROCC activity.
Unaltered α1D-AR density based on the receptor binding study (Gurdal et al. 1995) and unchanged α1D-AR mRNA levels (present study) suggest that the potentiated PE responses could be due to increased coupling efficiency in AR signaling possibly carried out by elevated TRPC6 protein levels. Demonstration of TRPC6 proteins on subplasmalemmal vesicles and their rapid translocation to the cell membrane upon Gq-protein coupled receptor activation (Cayouette et al. 2004) also suggest that overexpressed ROCCs are retained available in a reservoir system rather than on the cell membrane. This may account for the increased AR sensitivity without affecting the maximum contractile force. Although not a quantitative measure, immunohistochemical data support the changes shown in immunoblot analysis for TRPC1. In contrast to Western blot analysis, TRPC6 protein staining appears to decrease. On the other hand, apparent adventitial distribution of TRPC6 protein from 16- to 20-month-old rat aorta might explain the potentiated AR-mediated responses with aging due to colocalized adrenergic innervation (Gonzalez et al. 2001). However, this speculation should be tested further by comparing slices obtained from hypertensive or aged rats concomitantly stained with TRPC6 antibody and neuronal markers. Although additional contributions of other Ca2+ signaling/entry pathways cannot be ruled out, increased TRPC6 protein expression may be responsible for enhanced AR sensitivity in aged rats. This may be confirmed by in vivo posttranscriptional gene silencing directed against TRPC6 in aging rats.
Significant loss of endothelium-dependent relaxations in aorta from aged rats (present study and Matz et al. 2000; Barton et al. 1997) suggests that potentiated AR-mediated vasoconstriction uncompensated by dysfunctional endothelium may further contribute to existing vasospasm. Consequently, TRPC1 and TRPC6 could be considered as either potential targets or biomarkers in age-related vasospastic disorders. The data presented here suggest a possible causal relationship based on longitudinal animal aging model and warrant further in vivo knockdown studies to determine the actual functional relationship.
Acknowledgments
This work was supported by The Scientific and Technological Research Council of Turkey (TUBITAK, SBAG-2735 to M.T. and graduate scholarship, BIDEB-2211 to C.S.). A partial support was also provided by the Ege University (BAP04ECZ011 and 05BIL016 to M.T.) and Bilkent University Research Grants. Authors thank Dr. R. M. Rapoport (University of Cincinnati, College of Medicine) for his critical comments on the study and I.T. Aydin (Bilkent University, Faculty of Science) for technical assistance.
References
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. doi: 10.1016/S0143-4160(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Albert AP, Saleh SN, Large WA. Identification of canonical transient receptor potential (TRPC) channel proteins in native vascular smooth muscle cells. Curr Med Chem. 2009;16:1158–1165. doi: 10.2174/092986709787581815. [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TRPC proteins in rat myometrium during pregnancy. Biol Reprod. 2004;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005;451:53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. doi: 10.1016/S0143-4160(03)00054-X. [DOI] [PubMed] [Google Scholar]
- Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem. 2004;279:7241–7246. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- Clarson LH, Roberts VH, Hamark B, Elliott AC, Powell T. Store-operated Ca2+ entry in first trimester and term human placenta. J Physiol. 2003;550:515–528. doi: 10.1113/jphysiol.2003.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol. 2004;286:F546–F551. doi: 10.1152/ajprenal.00338.2003. [DOI] [PubMed] [Google Scholar]
- Gonzalez MC, Arribas SM, Molero F, Fernandez-Alfonso MS. Effect of removal of adventitia on vascular smooth muscle contraction and relaxation. Am J Physiol Heart Circ Physiol. 2001;280:H2876–H2881. doi: 10.1152/ajpheart.2001.280.6.H2876. [DOI] [PubMed] [Google Scholar]
- Gurdal H, Cai G, Johnson MD. Alpha 1-adrenoceptor responsiveness in the aging aorta. Eur J Pharmacol. 1995;274:117–123. doi: 10.1016/0014-2999(94)00717-L. [DOI] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- Matz RL, Schott C, Stoclet JC, Andriantsitohaina R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol Res. 2000;49:11–18. [PubMed] [Google Scholar]
- Minke B. TRP channels and Ca2+ signaling. Cell Calcium. 2006;40:261–275. doi: 10.1016/j.ceca.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- Piascik MT, Hrometz SL, Edelmann SE, Guarino RD, Hadley RW, Brown RD. Immunocytochemical localization of the alpha-1B adrenergic receptor and the contribution of this and the other subtypes to vascular smooth muscle contraction: analysis with selective ligands and antisense oligonucleotides. J Pharmacol Exp Ther. 1997;283:854–868. [PubMed] [Google Scholar]
- Selli C, Erac Y, Kosova B, Tosun M. Post-transcriptional silencing of TRPC1 ion channel gene by RNA interference upregulates TRPC6 expression and store-operated Ca2+ entry in A7r5 vascular smooth muscle cells. Vascul Pharmacol. 2009;51:96–100. doi: 10.1016/j.vph.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem. 2005;280:39786–39794. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Tabernero A, Vila E. Effect of age on noradrenaline responses in rat tail artery and aorta: role of endothelium. J Auton Pharmacol. 1995;15:327–333. doi: 10.1111/j.1474-8673.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Tai K, Vandenberg G, Hamaide MC, Wibo M, Morel N. Effect of organ culture on noradrenaline-evoked contraction, calcium signalling and TRPC expression in rat mesenteric artery. J Vasc Res. 2009;46:353–364. doi: 10.1159/000189796. [DOI] [PubMed] [Google Scholar]
- Tosun M, Erac Y, Selli C, Karakaya N. Sarcoplasmic–endoplasmic reticulum Ca2+-ATPase inhibition prevents endothelin A receptor antagonism in rat aorta. Am J Physiol Heart Circ Physiol. 2007;292:H1961–H1966. doi: 10.1152/ajpheart.00298.2006. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Xu KM, Tang F, Han C. Alterations of mRNA levels of alpha1-adrenoceptor subtypes with maturation and ageing in different rat blood vessels. Clin Exp Pharmacol Physiol. 1997;24:415–417. doi: 10.1111/j.1440-1681.1997.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]