Abstract
Advanced glycation endproducts (AGE) are oxidative products formed from the reaction between carbohydrates and a free amino group of proteins that are provoked by reactive species (RS). It is also known that AGE enhance the generation of RS and that the binding of AGE to a specific AGE receptor (RAGE) induces the activation of the redox-sensitive, pro-inflammatory transcription factor, nuclear factor-kappa B (NF-ĸB). In this current study, we investigated the anti-oxidative effects of short-term kaempferol supplementation on the age-related formation of AGE and the binding activity of RAGE in aged rat kidney. We further investigated the suppressive action of kaempferol against AGE's ability to stimulate activation of pro-inflammatory NF-ĸB and its molecular mechanisms. For this study, we utilized young (6 months old), old (24 months old), and kaempferol-fed (2 and 4 mg/kg/day for 10 days) old rats. In addition, for the molecular work, the rat endothelial cell line, YPEN-1 was used. The results show that AGE and RAGE were increased during aging and that these increases were blunted by kaempferol. In addition, dietary kaempferol reduced age-related increases in NF-κB activity and NF-ĸB-dependant pro-inflammatory gene activity. The most significant new finding from this study is that kaempferol supplementation prevented age-related NF-κB activation by suppressing AGE-induced nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase). Taken together, our results demonstrated that dietary kaempferol exerts its anti-oxidative and anti-inflammatory actions by modulating the age-related NF-κB signaling cascade and its pro-inflammatory genes by suppressing AGE-induced NADPH oxidase activation. Based on these data, dietary kaempferol is proposed as a possible anti-AGE agent that may have the potential for use in anti-inflammation therapies.
Keywords: Kaempferol, Aging, NF-κB, AGE, NADPH oxidase, Anti-inflammation
Introduction
Advanced glycation endproducts (AGE) result from the reaction between carbohydrates and free amino group of proteins. The formation of AGE adducts involves a complex cascade of reactions including condensations, rearrangements, fragmentations, and oxidative modifications, which are found in various tissues and are known to progress with age and alter cellular structure and function. Age-related hyperglycemia and diabetes are common characteristics of aging process in which glucose serum levels elevate and facilitate the formation of AGE (Wautier and Guillausseau 2003).
AGE, rather than oxidative stress, is one of the culprits of vascular dysfunction (Schmidt et al. 1999). Several lines of evidence have demonstrated a pivotal role for AGE in the vascular endothelial dysfunction associated with normal aging and age-related chronic diseases (Bucala and Cerami 1992; Brownlee 1995a; Vlassa et al. 1994; Thornally 1998). The intricate involvement of AGE in cellular oxidative damage comes from their ability to disturb the redox status through their interaction with the specific receptor for AGE (RAGE). It was shown that the incubation of human endothelial cells with AGE prompts hydrogen peroxide generation and is accompanied by decreased anti-oxidant enzymes, diminished glutathione and the activation of protein kinase C (PKC) (Jiang et al. 2004). It is also known that the interaction of RAGE with its ligands generates RS via the activation of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), nitric oxide synthase (NOS) and cyclooxygenase (COX) (Wautier et al. 2001; Sumi and Ignarro 2004; Shi and Vanhoutte 2008).
RAGE is a member of the immunoglobulin superfamily of cell surface molecules (Hofmann et al. 1996; Bierhause et al. 1988). RAGE is expressed on endothelial cells and has been shown to initiate cellular signaling leading to the generation of oxidative stress through the activation of the redox-sensitive transcription factor, nuclear factor-κB (NF-κB) (Yan et al. 1996).
Pertinent to the current study is that a key target of RAGE signaling is nuclear NF-κB because the promoter region of RAGE contains functional binding elements for NF-κB (Li and Schmidt 1997). Translocation of cytosolic NF-κB into the nucleus is the key step for the initiation of transcription for a number of proteins, including intercellular adhesion molecules, growth factors, cytokines, and RAGE itself (Bierhause et al. 1988; Kislinger et al. 1999; Ramana et al. 2004).
Recent studies of AGE and RAGE structures have highlighted their important roles in aging and age-dependent disease processes. Because the interaction between AGE and RAGE are so closely linked to age-related endothelial dysfunction and NF-κB dependant chronic vascular disease (Lu et al. 2004), it seems reasonable to expect that the attenuation of AGE and RAGE could have an immediate impact on the NF-κB signaling pathway.
Kaempferol is a polyphenol compound and a known anti-oxidant compound that also possesses anti-inflammatory properties resulting from its ability to diminish the formation of RS (Bronska et al. 2003). Kaempferol causes the inhibition of inducible nitric oxide synthase and cyclooxygenase-2 and the down-regulation of the NF-κB pathway (García-Mediavilla et al. 2007). It is well documented that various anti-oxidants can inhibit the oxidative glycation of reducing sugars with tissue proteins (Yamaguchi et al. 2000). For example, flavonoids, such as rutin and quercetin are shown to have inhibitory effects on insulin, hemoglobin and albumin glycosylation (Asgary et al. 2002). To date, however, the effects of dietary kaempferol as an age-related anti-inflammatory and anti-glycation agent, particularly related to AGE, are little reported.
In a recent study, we focused on the effect AGE have on the activation of pro-inflammatory NF-κB and the possible underlying the mechanism by which kaempferol inhibits NF-κB activation (Kim et al. 2007). In this study, we attempt to document evidence showing the inhibitory effect of short-term kaempferol feeding on age-related NF-κB signaling and NF-κB-dependant gene activities through its suppression of AGE-induced NADPH oxidase activation.
Materials and methods
Animals
Specific pathogen-free male Fischer 344 rats were obtained from Samtako (Osan, Korea) and were fed a diet of the following composition: 21% soybean protein, 15% sucrose, 43.65% dextrin, 10% corn oil, 0.15% a-methionine, 0.2% choline chloride, 5% salt mix, 2% vitamin mix, and 3% Solka-Floc. Rats at 6 and 24 months of age were used as young and old rats, respectively. Kaempferol (0.01% and 0.02%) was mixed with the rat chow and was fed to the two dosages of 24-month-old rats for 10 days. Rats in two dosages consumed an average of 2 mg/kg or 4 mg/kg of the kaempferol per day, respectively. This experiment designed with a short-term feeding is base on our previous study, where anti-oxidant or anti-inflammatory effect of several flavonoid in aged rat were fed for 10 days. We published the effect of these agents in aged rats by Kim et al. 2006, Go et al. 2005 and Jung et al. 2009. It is our reason and intent to show the efficacy of dietary supplementation with a short period, rather than long-term feeding. Rats were sacrificed by decapitation and the kidneys quickly removed. The tissue was immediately frozen in liquid nitrogen and stored at −80°C. For our current study, we choose to use the kidney because of its vulnerability to age-related oxidative stress and inflammatory responsiveness.
Cell culture system
Rat prostate endothelial cell lines, YPEN-1 was obtained from American Type Culture Collection (Rockville, ND, USA). YPEN-1, which is responsive to oxidative stress, its well molecularly characterized redox-sensitive NF-κB modulation by anti-oxidants and our lab's long experience and familiarity with cell samples for the molecular work on age- and redox-related changes. The cells were grown in DMEM (Dulbecco's Modified Eagle Medium, Nissui, Tokyo, Japan) containing 2 mM l-glutamine, 100 mg/mL streptomycin, 2.5 mg/L amphotericin B and 5% heat-inactivated fetal bovine serum. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2/95% air. Cells were discarded after 3 months, at which time, new cells were obtained from frozen stocks. Cells at the exponential phase were used for all experiments.
Plasmids
NF-κB activity was examined using a luciferase plasmid DNA, pTAL-NF-κB that contains a specific binding sequence for NF-κB (Becton Dickinson (BD) Biosciences, Franklin Lakes, NJ, USA).
Reagents
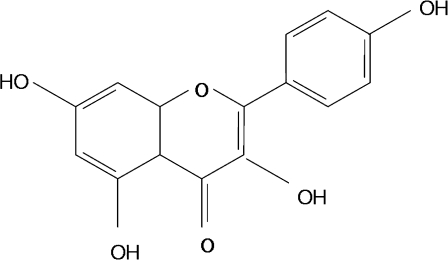
Kaempferol was obtained from Shaanxi Huike Botanical Development Coperation (Beijing, China, Fig. 1). Bovine serum albumin, rutin, quecertin, AA861, DUP697, DPI, and PAO were obtained from Sigma Chemical Corporation (St. Louis, MO, USA). Sodium azide, d(+)-glucose and fructose obtained from Junsei Chemical Corporation (Tokyo, Japan). 2,7-dichlorodihydrofluorescein diacetate and l-tc were obtained from Molecular Probes, Inc. (Eugene, OR, USA) AGE were obtained from Circulex™ (Tokyo, Japan). Immobilon-P transfer membrane was obtained from Millipore Corp. (Bedford, MA, USA). Antibodies to RAGE, phospho IκBα, IκBα, p65, p50, MMP-9, MCP-1, Rantes, VCAM-1, ICAM-1, β-actin, and Histone H1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody to ATPase was obtained Abcam PLC (Abcam plc, Cambridge, UK), and antibody to phospho p65 was obtained from Cell Signaling (New England Bio Labs, Hurtsfoldshire, UK). Enhanced chemiluminescence (ECL) Western blotting detection reagents were from Amersham Life Science Inc. (Arlington Heights, IL, USA).
Fig. 1.
Chemical structure of kaempferol. Levels of AGE were determined by fluorescence in the kidney homogenate taken from rats
Kidney homogenate preparation
All solutions, tubes, and centrifuges were maintained at 0–4°C. The preparation of nuclear extract was based on previous methods (Corsini et al., 1997). Kidney (300 mg) was homogenized with 2 ml of homogenate buffer A (10 mM HEPES, pH 7.8, 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, 0.1 mM PMSF, 1 μM pepstatin, and 1 mM p-aminobenzamidine) with a tissue homogenizer for 20 s. Homogenates were kept on ice for 15 min, 125 μl of 10% Nonidet p40 (NP40) solution was added, and mixed for 15 s, and the mixture was then centrifuged at 12,000 rpm for 2 min, then the supernatant containing cytosol proteins was obtained. The pelleted nuclei were washed once with 400 μl of buffer A plus 25 μl of 10% NP40, centrifuged, suspended in 50 μl of buffer C (50 mM HEPES, pH 7.8, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF and 10% (v/v) glycerol), mixed for 20 min, and centrifuged at 12,000 rpm for 10 min. The supernatant containing nuclear proteins was stored at −80°C.
Western blot analysis
The prepared samples in gel loading buffer, pH 6.8 (12.5 mM Tris, 4% sodium dodecyl sulfate (SDS), 20% glycerol, 10% 2-mercaptoethanol, and 0.2% bromophenol blue) in a ratio of 1:1 were boiled for 5 min. Equal amounts of proteins (30 ug) for each sample were separated on 8–17% SDS-polyacryamide minigel at 100 V and transferred to a polyvinylidiene fluoride membrane at 100 V for 90 min in a wet transfer system (Bio-Rad, Hercules, CA). The membrane was immediately placed into a blocking solution (5% w/v skim milk powder in TBS-Tween buffer containing 10 mM Tris, 100 mM NaCl, and 0.1 mM Tween-20, pH 7.4) at room temperature for 1 h. The membrane was washed in TBS-Tween buffer for 30 min and then incubated with a primary antibody (diluted 1:500 in TBS-Tween buffer) at room temperature for 3 h. After three 10-min washings in TBS-Tween buffer, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody from sheep (diluted 1:10,000 in TBS-Tween buffer) at room temperature for 2 h. After three 10-min washings in TBS-Tween buffer, antibody labeling was detected using ECL and exposed to radiographic film. Pre-stained blue protein marker (Bio-Rad, Hercules, CA) was used for molecular-weight determination.
EMSA
Electrophoretic mobility shift assay (EMSA) method was used to characterize the binding activities of NF-κB transcription factors in nuclear extracts (Kerr 1995). NF-κB oligonucleotide sequence was 5′-GAGAGGCAAGGGGATTCCCTTAGTTAGGA-3′. The protein-DNA-binding mixture containing 20 μg of nuclear protein extract was incubated for 20 min at 4°C in binding medium containing 5% glycerol, 1 mM MgCl2, 50 mM NaCl, 0.5 mM EDTA, 2 mM DTT, 1% NP-40, 10 mM Tris (pH 7.5), unspecific binding was blocked with 1 μg of poly(dI-dC) poly(dI-dC). Radiolabeled transcription factor consensus oligonucleotide (20,000 cpm of 32P) was added, and the complete mixture was incubated for additional 20 min at room temperature. DNA-binding complexes were resolved by 7% native polyacrylamide gel electrophoresis with 0.5 × TBE (50 mM Tris, 45 mM boric acid, and 0.5 mM EDTA) for 90 min at 200 V. The gel was dried and exposed to Fuji X-ray film 1 ∼ 2 days at −80°C.
Measurement of AGE contents
AGE contents were determined by the method of Nakayama et al. (1993). Briefly, minced renal tissue was delipidated by shaking gently with chloroform and methanol (2:1 v/v) overnight. After washing, the tissue was homogenized in 0.1 N NaOH, followed by centrifugation at 8,000 g for 15 min at 4°C. The amounts of AGE in these alkali-soluble samples were measured at an emission wavelength of 350 nm and excitation wavelength at 450 nm against a blank of 0.1N NaOH solution using spectrofluorometric detector (Bio-Tek Instrument, Inc., FL, ×800 microplate fluorescence reader).
Measurement of AGE-induced RS generation of YPEN cells
To determine intracellular RS generation activity, YPEN-1 cells were seeded in a 96-well plates. After 1 day, the medium was changed to a fresh serum-free medium. The cells were treated with or without kaempferol pre-incubated for 1 h. After treatment with AGE (100 μg/ml) for 30 min, the medium was replaced with a fresh serum-free medium, and DCFDA (2.5 μM) was added. The fluorescence intensity of DCF was measured every 5 min for 1 h using the microplate fluorescence reader TECAN (Salzburg, Austria) with excitation and emission wavelengths of 485 and 535 nm, respectively.
Transfection and luciferase reporter assay for NF-κB activity
The activity of NF-κB was examined using a luciferase plasmid DNA, pTAL-NF-κB that contains a specific binding sequence for NF-κB (BD Biosciences, Francisco, CA). Transfection was carried out using the FuGENE 6 reagent (Roche, Indianapolis, IN, USA). Briefly, 2 × 104 cells per each well were seeded in 48-well plates. When cultured cells reached about 50% confluence, cells were treated with 0.1 μg DNA/0.1 μl FuGENE 6 complexes in a total volume of 500 μl normal media (5% serum contained) for 24 h. Subsequently, pre-treated various RS source inhibitors were treated after the plate was changed with serum-free media and incubated for overnight (16 h). AGE were treated and incubated for 6 h. Cells were washed with PBS and transfered to the Steady-Glo Luciferase Assay System (Promega Corporate Headquarters, Madison, WI, USA), 48-well plates. Luciferase activity was measured by a luminometer GENios (Tecan Instruments, GmbH, Austria). The obtained raw luciferase activities were normalized by protein concentrations.
Statistical analysis
Analysis of variance (ANOVA) was conducted to analyze significant differences among all groups. Differences among the means of individual groups were assessed by the Fischer's protected LSD post hoc test. The statistical significance of the difference between the groups (age and dietary supplemented) was determined by one-factor ANOVA followed by the Fischer's protected LSD post hoc test. Values of p < 0.05 were considered statistically significant.
Results
Inhibition of AGE and RAGE expression by kaempferol
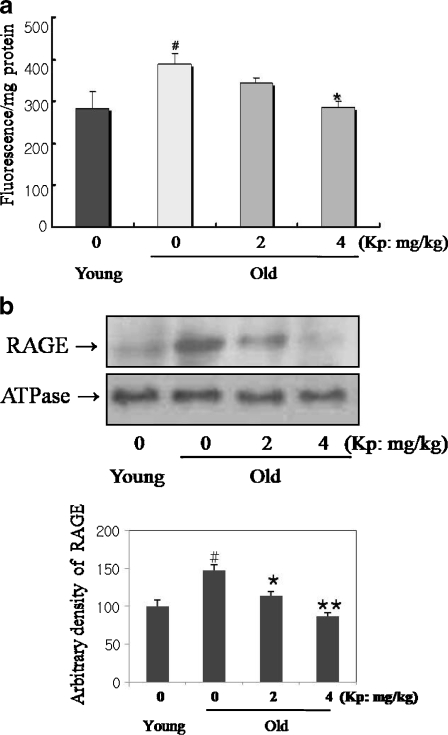
AGE contents in kidney homogenate were determined by fluorometry, and the results are shown in Fig. 2a. AGE levels increased in the old rats compared with the young. However, the kaempferol-fed old groups showed decreased AGE accumulation compared to their control counterpart. We determined RAGE protein levels by Western blot. RAGE protein expression, as shown in Fig. 2b, was increased in aged kidneys, but was significantly suppressed in the 4 mg/kg kaempferol-fed old rats. In these experiments, we used ATPase for the membrane fraction marker. These results indicate that kaempferol inhibited age-related AGE accumulation and RAGE expression in aged rat.
Fig. 2.
Inhibition of AGE and RAGE expression by kaempferol in aged rat. a Effect of aging and kaempferol on AGE-modified protein formation were analyzed by fluorescence in rat kidney. b Inhibition of age-related RAGE expression by kaempferol. The old groups showed increased levels of AGE fluorescence, but kaempferol reduced these increases. Western blot analysis was performed to detect RAGE protein level in kidney. RAGE, the receptor for AGE, protein expression was increased in aged kidneys. However, kaempferol suppressed RAGE expression in the old rats. ATPase was membrane marker. #p < 0.05 compared with young group; *p < 0.05, **p < 0.001 compared with old group, fed the normal diet
Inhibition of age-related NF-κB activation by kaempferol
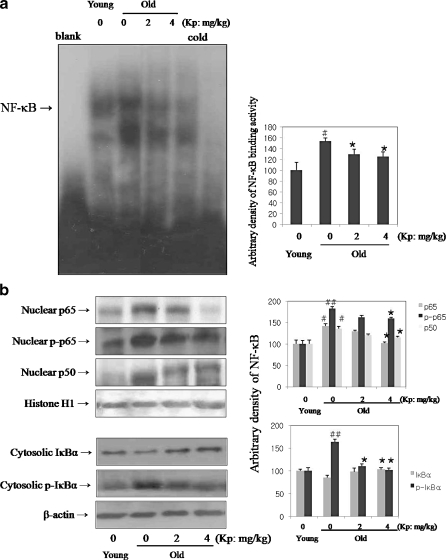
We investigated DNA-binding activity by kaempferol in aged rat. For verification of DNA-binding of NF-κB, EMSA was carried out with nuclear proteins in young, old, and kaempferol-fed old rats. The results in Fig. 3a, lanes 2 and 3, show that the DNA-binding activity of NF-κB increased with age, while kaempferol suppressed NF-κB expression compared to control. The displacement pattern of NF-κB band as a result of incubation with specific antibodies thus supports the specificity of NF-κB binding to its consensus motif. Furthermore, the binding specificity of NF-κB was demonstrated using 100-fold excess of an unlabeled oligolnulcleotide, which competed for binding.
Fig. 3.
Inhibition of age-related NF-κB activation. a Inhibition of age-related NF-κB binding activity by kaempferol in aged rat. b Inhibition of age-related NF-κB translocation through IκBα degradation by kaempferol in aged rat. To verify the DNA-binding of NF-κB, electroohoretic mobility shift assay (EMSA) was carried out with nuclear proteins in young, old, and kaempferol-fed old rats. DNA-binding activity of NF-κB with age was increased, while kaempferol suppressed NF-κB expression in (a). As shown in (b), increased degradation of the IκBα with aging was decreased by kaempferol. Nuclear translocation of NF-κB increased with age, but kaempferol inhibited this change. Furthermore, increased phosphorylation of p65 subunit with age was suppressed by kaempferol. EMSA and Western blot as described in “Materials and methods”. #p < 0.05 compared with young group; *p < 0.05, **p < 0.001 compared with old group, fed the normal diet
We further investigated inhibition of age-related NF-κB translocation through IκBα degradation by kaempferol in aged rat. As shown in Fig. 3b, increased phosphorylation of the IκBα with aging was decreased by kaempferol. Subsequently, IκBα deteriorated with aging, and kaempferol suppressed IκBα degradation in the cytosol fraction. The nuclear translocation of NF-κB subunit, p65 and p50 also increased with age, but kaempferol inhibited this change. Furthermore, increased phosphorylation of p65 subunit with age was suppressed by kaempferol. The phosphorylation of p65 up-regulated NF-κB transcriptional activity. Therefore, these results show that kaempferol inhibited age-related NF-κB activation in aged rat.
Inhibition of NF-κB-dependant gene expressions by kaempferol
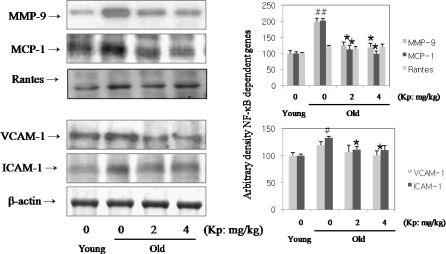
To determine the activation of NF-κB-dependent genes and adhesion molecule expressions with age, expressions of MMP-9, MCP-1, RANTES, VCAM-1 and ICAM-1 were examined. These genes have been known to be associated with inflammation and to have an NF-κB binding site in their promoter regions and to be controlled by NF- κB activation (Umezawa and Chaicharoenpong 2002). Therefore, to elucidate the changes in NF-κB DNA-binding activity that correlates with NF-κB-dependent gene expression, we examined the gene expression of these genes. As shown in Fig. 4, MMP-9, MCP-1, RANTES, VCAM-1, and ICAM-1 levels increased with age, but kaempferol decreased these levels. These results suggest that kaempferol modulates NF-κB activation and NF-κB-dependent gene expressions.
Fig. 4.
Inhibition of NF-κB-dependant gene expression by kaempferol in aged rat. To determine NF-κB-dependent genes and adhesion molecule expressions with age, expressions of MMP-9, MCP-1, RANTES, VCAM-1, and ICAM-1 were examined. As shown in this data, MMP-9, MCP-1, RANTES, VCAM-1, and ICAM-1 levels increased with age, but kaempferol reduced these levels with age. #p < 0.05 compared with young group; *p < 0.05, **p < 0.001, ***p < 0.001 compared with old group, fed the normal diet
Inhibition of AGE-induced oxidative stress by kaempferol in endothelial YPEN-1 cells
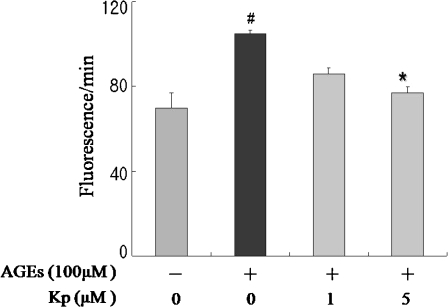
To assess the inhibitory effect of kaempferol on AGE-generated oxidative status, total RS was measured in endothelial YPEN-1 cells. As shown in Fig. 5, the results revealed that pre-incubation of kaempferol at concentrations of 1 and 5 μM decreased intracellular AGE-induced RS generation in a dose-dependent manner. These results show that short-term dietary kaempferol supplementation effectively exerted an anti-oxidative effect on AGE-induced oxidative stress.
Fig. 5.
Inhibition of AGE-induced oxidative stress by kaempferol in YPEN-1 cells. The cells were incubated in serum-free media in order to avoid RS scavenging activity. The results revealed that pre-incubation with kaempferol at concentrations of 1 and 5 μM decreased intracellular RS generation induced by AGE in a dose-dependent manner. #p < 0.05 compared with AGE exposure; *p < 0.05 compared with pre-treatment of kaempferol
Inhibition of AGE-induced NF-κB activation by kaempferol in YPEN-1 cells
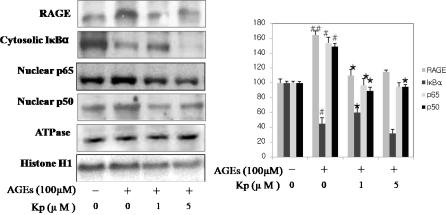
We wished to determine whether kaempferol treatment modulates NF-κB activation by AGE-induced RS in YPEN-1 cell. As shown in Fig. 6, kampferol significantly inhibited AGE-induced RAGE expression. We also investigated whether kaempferol suppresses NF-κB activation by AGE-induced RS through nuclear translocation of p65 and p50 subunits and IκB phosphorylation. YPEN-1 cells were incubated with kaempferol for 1 h before treatment with AGE. Western blot analysis was used to study the results, which suggest that RAGE and NF-κB activation levels increased by AGE and that kaempferol suppressed these increases.
Fig. 6.
Inhibition of NF-κB activation by AGE-induced RS in YPEN-1 cells. In order to determine NF-κB activation by AGE-induced RS and its ability to be modulated by kaempferol. Kaempferol suppressed NF-κB activation by AGE-induced RS via RAGE expression, which was significantly inhibited by kaempferol. Using Western blot analysis, we also investigated the ability of kaempferol to suppress NF-κB activation by AGE-induced RS through the nuclear translocation of p65 and p50 subunits and IκB phosphorylation. YPEN-1 cells were incubated with kaempferol for 1 h before treated AGE. RAGE and NF-κB activation levels increased by AGE but kaempferol was able to stem these increases
Inhibition of NF-κB activity through AGE-induced RS in YPEN-1 cells
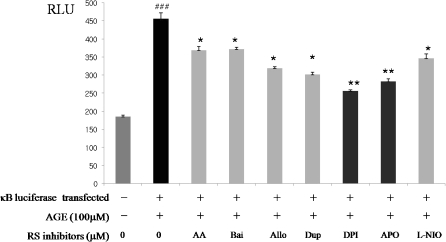
In this experimentation, effort was put forth to determine the intracellular generation source of RS that is responsible for AGE-induced oxidative stress. To do that, several inhibitors known to suppress RS-producing enzymes were utilized: AA861 (11.91 μM) and baicalein (10.93 μM) for LOX inhibitor, XOD inhibitor allopurinol (11.51 μM), COX inhibitors DuP-697 (5.95 μM), NADPH oxidase inhibitors DPI(7.15 μM) and APO(13.33 μM) and nitric oxide synthase inhibitor L-NIO (14.32 μM). We measured the IC50 of each inhibitor base on AGE-induced RS scavenging activity (data not shown). Luciferase activity was detected by AGE treatment in the absence or presence of a RS-generating source after transient transfection of a plasmid containing NF-κB consensus and reporter. NF-κB luciferase activity increased by more than twofold in cells exposed to 100 μM AGE for 6 h. For the NF-κB activation, the potential sources of several RS-generating enzymes were examined using specific enzyme inhibitors for them. The interesting and significant finding was that among all inhibitors tested; only the NADPH oxidase inhibitors, DPI (dibenziodolium chloride; 43.84%) and APO (apocynin; 37.98%) significantly blocked AGE-induced NF-κB activation as shown in Fig. 7. Thus, these results indicate NF-κB activation by AGE-induced RS generation through NADPH oxidase activation.
Fig. 7.
Inhibition of NF-κB activation from AGE-induced NADPH oxidase activation in YPEN-1 cells. To find the intracellular RS source responsible for AGE-induced oxidative stress, inhibitors of several intracellular RS-producing enzymes were applied in the experiments. We used several inhibitors known to suppress RS-producing enzymes: AA: AA861 (11.91 μM) and Bai: baicalein (10.93 μM) for LOX inhibitor, XOD inhibitor Allo: allopurinol (11.51 μM), COX inhibitors Dup: DuP-697 (5.95 μM), NADPH oxidase inhibitors DPI: dibenziodolium chloride (7.15 μM ) and APO: apocynin (13.33 μM) and nitric oxide synthase inhibitor L-NIO (14.32 μM). NF-κB activation induced by RS from the formation of AGE was monitored by the NF-κB luciferase assay. ##p < 0.001 compared with exposure to AGE; *p < 0.05, **p < 0.001 compared with pre-treatment with the RS source inhibitor
Discussion
AGE are deleterious oxidative products that are generated from glucose and other reducing sugars through a series of non-enzymatical reactions forming Schiff base and Amadori compounds (Labuza and Baisier 1992). These glycated products undergo further complex reactions to become cross-linked, fluorescent oxidative products, AGE (Slatter et al. 1998; Winlove et al. 1996). One major deleterious effect is that they can generate RS intermediates, which can induce cellular activation and inflammation (Bucala and Cerami 1992; Brownlee 1995b; Vlassa et al. 1994; Thornally 1998).
Several lines of evidence suggest a pivotal role for AGE-induced oxidative stress in normal aging and in the pathogenesis of many age-related chronic conditions, like endothelial dysfunction. The close interaction between AGE and RAGE is further exhibited in the pathogenesis of numerous age-related diseases such as renal, cardiovascular and neurodegenerative diseases (Yan et al. 1996; Ramasamy et al. 2005). The wide spread distribution of AGE has been detected in various tissues including kidney, lung, brain, and heart from aged and diabetic patients (Schleicher et al. 1997; Liang et al. 2003).
There are many reports regarding the inhibitory effects of flavonoids on AGE. Recently, studies showed that flavonoids suppress AGE-mediated oxidative stress to attenuate the pro-inflammatory response via MAPK signaling pathway (Huang et al. 2006). Furthermore, kaempferol, a flavonoids derived from Nelumbo nucifera is shown to inhibit oxidative stress and the formation of AGE (Jung et al. 2008).
The purpose of our current study is to determine whether treatment with kaempferol supplements can inhibit AGE accumulation and RAGE-induced NF-κB activation and to identify the major culprit responsible for the NF-κB activation in aged rats.
Our results demonstrate that the short-term feeding of kaempferol to aged rats modulated both AGE accumulation and RAGE expression. RAGE expression is characteristically dependent on NF-κB transcriptional activity. Our data revealed further that kaempferol suppressed age-related NF-κB activation and its pro-inflammatory genes through the suppression of AGE-induced NADPH oxidase activation.
In our study, kaempferol down-regulated AGE-induced NF-κB signaling and the kaempferol-fed, old rats showed suppressed NF-κB activation through the inhibition of IκBα phosphorylation and translocation of the p65, p50 heterodimer. Cytoplasm NF-κB binds to a member of the IκBα inhibitor protein family in an inactive state (Hatada et al. 2000), and following stimulation, IκBα is phosphorylated and then degraded. Unbound NF-κB translocates into the nucleus and transactivates various downstream genes (Umezawa et al. 2000). In our experiments, kaempferol-fed, old rats also suppressed p65 phosphorylation. P65 phosphorylation elevates NF-κB transcription activity. Accordingly, kaempferol down-regulated NF-κB dependant pro-inflammatory genes such as MMP-9, MCP-1, RANTES and adhesion molecules such as VCAM-1 and ICAM-1, genes correlated with vascular inflammation.
To delineate further the anti-oxidative role of kaempferol, we focused on the source of RS generation, more specifically AGE-induced RS generation and kaempferol’s effects. Results show that kaempferol likely suppressed intracellular RS generation induced by AGE. This finding is in agreement with a previous report that kaempferol inhibited intracellular RS generation (Lee et al. 2008) in which NF-κB activation by AGE-induced RS was down-regulated by kaempferol through the inhibition of phosphorylation of IκBα and the translocation of p65 and p50 in rat endothelial cell. We confirmed those findings in our current endothelial cell system, where kaempherol showed similar effects to inhibit the accumulation of age-related AGE and AGE-induced activation of NF-κB in aged rat. Thus, our data clearly showed that short-term dietary kaempherol has anti-oxidative effect and modulates NF-κB activation.
Although kaempherol's anti-oxidative role is well-recognized, the intracellular site of its action has not been investigated to date. Our attempt utilizing specific inhibitors to narrow down the possible site revealed new insights. Data in Fig. 7 that were generated from the NF-κB luciferase assay using the two NADPH oxidase inhibitors, APO and DPI showed AGE-induced NF-κB activation to be significantly blocked. The results indicate that kaempferol's attenuation of AGE-induced NF-κB activation is likely due to the inhibition of RS generation by NADPH oxidase.
It is worth mentioning the significance of NADPH oxidase inhibition. NADPH oxidase is a plasma membrane-bound enzyme complex that has been implicated as a major source of intracelluar RS, particularly in response to AGE exposure, which may be excerbated during aging. Sustained NADPH oxidase activation compromises several anti-oxidant systems by depleting NADPH (Hamilton et al. 2001; Csiszar et al. 2002). It has been clearly demonstrated that RS generated by NADPH oxidase are involved in signal transduction in response to several cytokines, Ang II, PDGF, and TNF-α that rapidly activate NADPH oxidase, followed by a rise in intracellular O-2 and H2O2 levels and activation of signaling molecules. (Griendling et al. 2000; Li and Shah 2001). More recently, it was reported that AGE-induced RS generation by NADPH oxidase is causally related to cellular dysfunction and vascular inflammation (Cai et al. 2004). However, age-related NF-κB activation through NADPH oxidase activation by AGE-induced RS generation has not been not fully explored. In this light, our current findings on keampherol’s preferential inhibition of NADPH oxidase are therefore interesting and noteworthy. This innate suppressive action of kaempferol at the plasma site may be the important contributing property that makes this flavanoid such an effective anti-oxidant with diverse actions.
In conclusion, kaempferol suppressed the activation of NF-κB and NF-κB dependant genes and adhesion molecules by inhibiting NADPH oxidase caused by increased AGE during the aging process. We further propose that because of its anti-inflammatory and anti-oxidative properties, kaempferol may be a potential therapeutic used in the treatment and prevention of age-related inflammatory processes and age-related diseases.
Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (20090093226). We thank Aging Tissue Bank for providing research samples.
References
- Asgary S, Naderi GA, Zadegan NS, Vakili R. The inhibitory effects of pure flavonoids on in vitro protein glycosylation. J Herb Pharmacother. 2002;2:47–55. doi: 10.1300/J157v02n02_05. [DOI] [PubMed] [Google Scholar]
- Bierhause A, Hofmann MA, Ziegler R, Nawroth PP. The AGE/RAGE pathway in vascular disease and diabetes mellitus. Part І. The AGE-concept. Cadiovasc. Res. 37:586-600. Biogerontology. 1988;4:399–408. doi: 10.1016/s0008-6363(97)00233-2. [DOI] [PubMed] [Google Scholar]
- Bronska M, Czuba ZP, Krol W. Effect of flavone derivatives on interleukin 1β mRNA expression and IL-1β protein synthesis in stimulated RAW 264.7 macrophages. Scand J Immunol. 2003;57:162–166. doi: 10.1046/j.1365-3083.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 1995;6865:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Bucala R, Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/S1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- Cai W, He JC, Zhu L, Peppa M, Lu C, Uribarri J, Vlassara H. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. 2004;3:285–291. doi: 10.1161/01.CIR.0000135587.92455.0D. [DOI] [PubMed] [Google Scholar]
- Corsini E, Terzoli A, Bruccoleri A, Marinovich M, Galli CL. Induction of tumor necrosis factor-alpha in vivo by a skin irritant, tributyltin, through activation of transcription factors: its pharmacological modulation by anti-inflammatory drugs. J Invest Dermatol. 1997;108(6):892–896. doi: 10.1111/1523-1747.ep12292696. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;11:1159–1166. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, González-Gallego J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 2007;2- 3:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Go EK, Jung KJ, Kim JY, Yu BP, Chung HY. Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci. 2005;60(10):1252–1264. doi: 10.1093/gerona/60.10.1252. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;2 Part 2:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- Hatada EN, Krappmann D, Scheidereit C. NF-kappaB and the innate immune response. Curr Opin Immunol. 2000;12(1):52–58. doi: 10.1016/S0952-7915(99)00050-3. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguch A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neutath MF, Slattery T, Beach D, McClary J, Nagashimura M, Moreser J, Stern D, Schmidt AM. RAGE mediates a novel pro inflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1996;97:889–901. doi: 10.1016/S0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Huang SM, Wu CH, Yen GC. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGE. Mol Nutr Food Res. 2006;12:1129–1139. doi: 10.1002/mnfr.200600075. [DOI] [PubMed] [Google Scholar]
- Jiang XH, Tu SP, Cui JT, Lin MC, Xia HH, Wong WM, Chan AO, Yuen MF, Jiang SH, Lam SK, Kung HF, Soh JW, Weinstein IB, Wong BC. Antisense targeting protein kinase C alpha and beta1 inhibits gastric carcinogenesis. Cancer Res. 2004;16:5787–5794. doi: 10.1158/0008-5472.CAN-03-1172. [DOI] [PubMed] [Google Scholar]
- Jung HA, Jung YJ, Yoon NY, Jeong da M, Bae HJ, Kim DW, Na DH, Choi JS. Inhibitory effects of Nelumbo nucifera leaves on rat lens aldose reductase, advanced glycation endproducts formation, and oxidative stress. Food Chem Toxicol. 2008;12:3818–3826. doi: 10.1016/j.fct.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, Chung HY. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009;58(3):143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- Kerr LD. Electrophoretic mobility shift assay. Methods Enzymol. 1995;254:619–632. doi: 10.1016/0076-6879(95)54044-X. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park HR, Lee JS, Chung TS, Chung HY, Chung J. Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology. 2007;8(4):399–408. doi: 10.1007/s10522-007-9083-9. [DOI] [PubMed] [Google Scholar]
- Kim JY, Jung KJ, Choi JS, Chung HY. Modulation of the age-related nuclear factor-kappaB (NF kappaB) pathway by hesperetin. Aging Cell. 2006;5(5):401–411. doi: 10.1111/j.1474-9726.2006.00233.x. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Fu C, Huber B, Ou W, Taguchi A, Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern DM, Schmidt AM. N(epsilon)-(carboxymethyl) lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Bio Chem. 1999;44:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- Labuza TP, Baisier W. The role of the Federal Government in food safety. Crit Rev Food Sci Nutr. 1992;31:165–176. doi: 10.1080/10408399209527566. [DOI] [PubMed] [Google Scholar]
- Lee VS, Dou J, Chen RJ, Lin RS, Lee MR, Tzen JT. Massive accumulation of gallic acid and unique occurrence of myricetin, quercetin, and kaempferol in preparing old oolong tea. J Agric Food Chem. 2008;17:7950–7956. doi: 10.1021/jf801688b. [DOI] [PubMed] [Google Scholar]
- Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;26:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- Li J-M, Shah AM. Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc Res. 2001;52:477–486. doi: 10.1016/S0008-6363(01)00407-2. [DOI] [PubMed] [Google Scholar]
- Liang YX, Wang Z, Li DD, Jiang JM, Shao RG. Effects of aging and advanced glycation on gene expression in cerebrum and spleen of mice. Biomed Environ Sci. 2003;16:323–332. [PubMed] [Google Scholar]
- Lu C, He JC, Cai W, Liu H, Zhu L, Vlassara H. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci USA. 2004;32:11767–11772. doi: 10.1073/pnas.0401588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Mitsuhashi T, Kuwajima S, Aoki S, Kuroda Y, Itoh T, Nakagawa S. Immunochemical detection of advanced glycation end products in lens crystallins from streptozocin-induced diabetic rat. Diabetes. 1993;42(2):345–350. doi: 10.2337/diabetes.42.2.345. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;11:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Yan SF, Schmidt AM. The RAGE axis and endothelial dysfunction: maladaptive roles in the diabetic vasculature and beyond. Trends Cardiovasc Med. 2005;15(7):237–43. doi: 10.1016/j.tcm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;3:457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Cir Res. 1999;84:489–497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vanhoutte PM. Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br J Pharmacol. 2008;3:639–651. doi: 10.1038/bjp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatter DA, Murray M, Bailey AJ. Formation of a dihydropyridine derivative as a potential cross-link derived from malondialdehyde in physiological systems. FEBS Lett. 1998;421(3):180–184. doi: 10.1016/S0014-5793(97)01554-8. [DOI] [PubMed] [Google Scholar]
- Sumi D, Ignarro LJ. Regulation of inducible nitric oxide synthase expression in advanced glycation end product-stimulated raw 264.7 cells: the role of heme oxygenase-1 and endogenous nitric oxide. Diabetes. 2004;53(7):1841–1850. doi: 10.2337/diabetes.53.7.1841. [DOI] [PubMed] [Google Scholar]
- Thornally PJ. Cell activation by glycated proteins: AGE receptoer recognition factors and functional classification of AGE. Cell Mol Biol. 1998;44:1013–1023. [PubMed] [Google Scholar]
- Umezawa K, Chaicharoenpong C. Molecular design and biological activities of NF-kappaB inhibitors. Mol Cells. 2002;4(2):163–167. [PubMed] [Google Scholar]
- Umezawa K, Ariga A, Matsumoto N. Naturally occurring and synthetic inhibitors of NF-kappaB functions. Anticancer Drug Des. 2000;5(4):239–244. [PubMed] [Google Scholar]
- Vlassa H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- Wautier J, Guillausseau P. Advanced glycation end products, their receptors and diabetic angiopathy. Diabets Metab. 2003;29(1):86–87. doi: 10.1016/S1262-3636(07)70013-7. [DOI] [PubMed] [Google Scholar]
- Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280(5):E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- Winlove CP, Parker KH, Avery NC, Bailey AJ. Interactions of elastin and aorta with sugars in vitro and their effects on biochemical and physical properties. Diabetologia. 1996;39(10):1131–1139. doi: 10.1007/BF02658498. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48(2):180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao J, Magashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]