Abstract
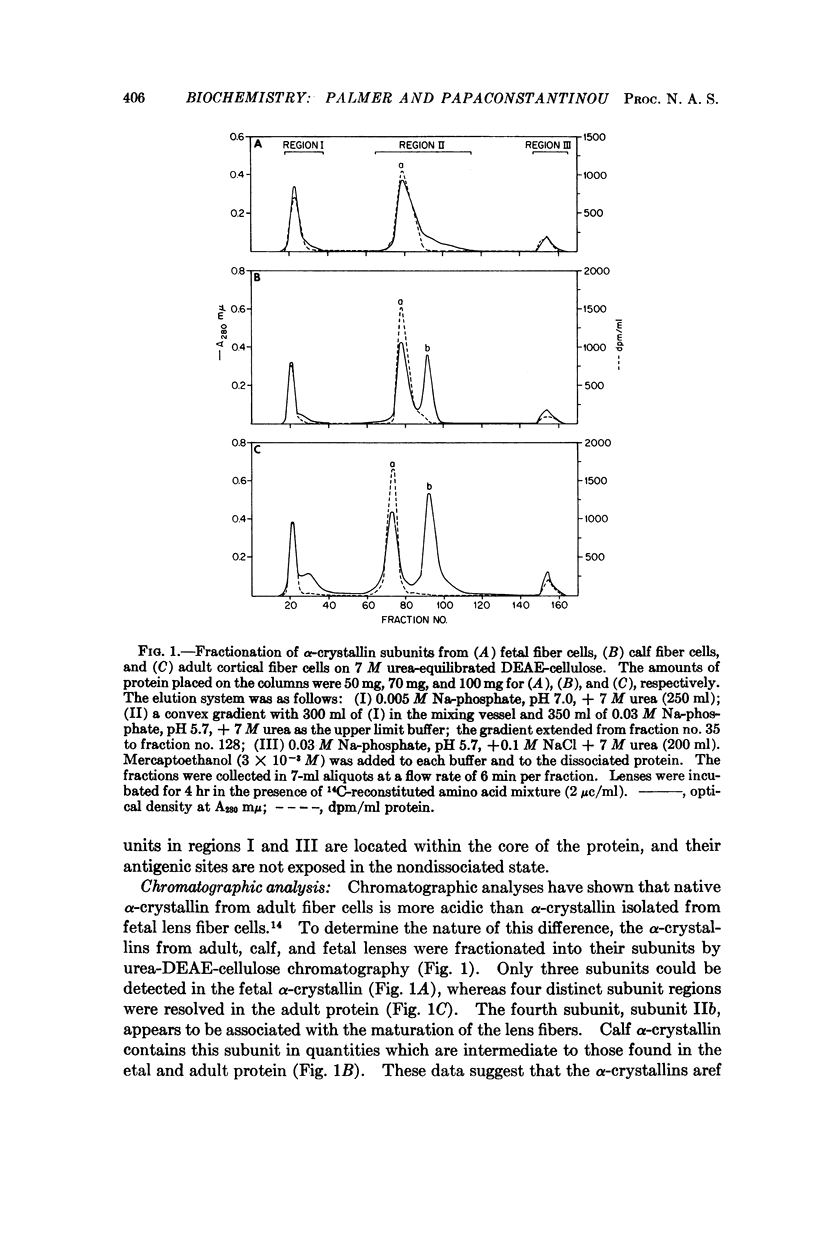
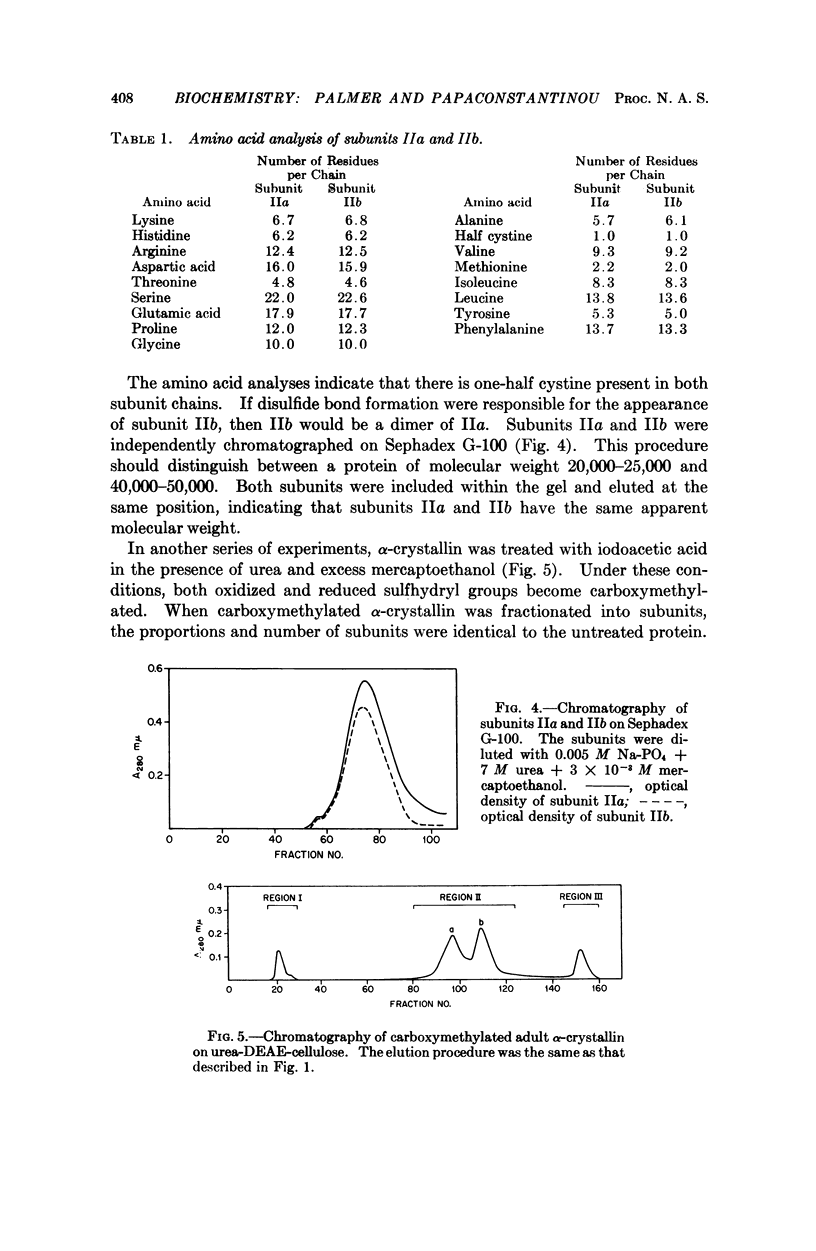
Fetal α-crystallin from the bovine lens can be resolved into three major subunit fractions by ion exchange chromatography. A fourth subunit gradually arises during the development of the lens. Incorporation studies indicate that this subunit is not formed by de novo synthesis but by the chemical conversion of one subunit into another. Possible mechanisms for this conversion are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOEMENDAL H., BONT W. S., JONGKIND J. F., WISSE J. H. Splitting and recombination of alpha-crystallin. Exp Eye Res. 1962 Jun;1:300–305. doi: 10.1016/s0014-4835(62)80015-3. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Klippenstein G. L., Holleman J. W., Klotz I. M. The primary structure of Golfingia gouldii hemerythrin. Oder of peptides in fragments produced by tryptic digestion of succinylated hemerythrin. Complete amino acid sequence. Biochemistry. 1968 Nov;7(11):3868–3878. doi: 10.1021/bi00851a012. [DOI] [PubMed] [Google Scholar]
- Moss B., Ingram V. M. Hemoglobin synthesis during amphibian metamorphosis. I. Chemical studies on the hemoglobins from the larval and adult stages of Rana catesbeiana. J Mol Biol. 1968 Mar 28;32(3):481–492. doi: 10.1016/0022-2836(68)90336-7. [DOI] [PubMed] [Google Scholar]
- Palmer W. G., Papaconstantinou J. Biochemistry of bovine lens proteins. 3. Chemical and physical properties of alpha-crystallin subunits. Biochemistry. 1968 Jan;7(1):243–253. doi: 10.1021/bi00841a029. [DOI] [PubMed] [Google Scholar]
- Palmer W. G., Papaconstantinou J. Increase in the complexity of alpha-crystallins during differentiation of lens cells. Nature. 1968 Jul 13;219(5150):170–172. doi: 10.1038/219170a0. [DOI] [PubMed] [Google Scholar]
- Stewart J. A., Papaconstantinou J. Lactate dehydrogenase isozymes and their relationship to lens cell differentiation. Biochim Biophys Acta. 1966 May 26;121(1):69–78. doi: 10.1016/0304-4165(66)90349-7. [DOI] [PubMed] [Google Scholar]
- WILT F. H. The ontogeny of chick embryo hemoglobin. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1582–1590. doi: 10.1073/pnas.48.9.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]




