Abstract
In assisted reproductive technology (ART), the pregnancy and birth rates following in vitro fertilization (IVF) attempts are still low. Recently, apoptotic markers have been suggested as new criteria for oocyte and embryo quality selection. Many studies have provided evidence that poor oocyte and embryo quality can be associated with apoptosis. The aim of this review is to summarize our current knowledge on the apoptotic process in oocytes and embryos, and focus on the possibility for using apoptotic markers as a reliable and predictive marker to select competent oocytes and embryos during IVF. Moreover, it is currently accepted that IVF failures, linked to poor embryo quality, are, in part, associated with suboptimal in vitro culture conditions. Here, we also review the current state of knowledge concerning how the genetic control of apoptosis during folliculogenesis and pre- implantation embryonic development is affected by in vitro culture conditions during IVF. In the future, identification of apoptotic markers in ART for oocyte and embryo selection should result in the development of new agonistic or antagonistic molecules of apoptosis by medicinal chemistry.
Keywords: Apoptosis, Biological Markers, Embryonic Development, Female, Fertilization in Vitro, Humans, Male, Oocytes, cytology, Signal Transduction
Keywords: IVF, Apoptosis markers, Genetic control, Oocyte, Embryo, Microenvironment, Diagnosis
I. Introduction
Infertility is a public health issue affecting some 60 000 reproductive-age couples every year in France. Female infertility represents 60 % of these cases. In vitro fertilization (IVF) is a possible solution for many of these couples. Despite the enormous progress made in IVF, many couples, however, are subject to repeated IVF failure and birth rates following IVF are still little more than 15 % per initiated cycle [1]. Though the reasons for this are both subtle and complex, poor quality of the embryos, that is itself conditioned by poor quality of the gametes, is obviously an important criterion. Many studies have provided evidence that poor embryo quality and bad grade (III or IV) can be associated with apoptosis [2, 3]. Although apoptosis might be induced, or facilitated, on account of the sub-optimal in vitro culture condition of the embryo, its primary cause could also derive from apoptotic signalling already triggered in the oocyte. Therefore, the possibility of using apoptotic markers as reliable markers to select competent oocytes and embryos, which were hitherto selected by “operator- dependent” evaluation of morphological criteria, opens new perspectives in ART centers.
Here, we review the current state of knowledge concerning the genetic control of apoptosis in the oocyte and embryo as well as how this process is affected by in vitro culture conditions.
II. Apoptosis
Apoptosis is a form of programmed cell death that is indispensable to embryonic development, homeostasis and in the surveillance of aggressions such as infection, uncontrolled cell division or severe cellular damage [4]. During development, cells are produced in excess. Structures specific to each organ are produced by the selective ablation of certain cells by apoptosis. The number of cells in an organ is therefore finely controlled by an equilibrium between cell proliferation and cell death. The classical example is the morphogenesis of the fingers that involves the apoptotic demise of cells in the inter-digital spaces during embryogenesis. Similarly, the developing nervous system comprises a large excess of neurons, over half of which will be eliminated by apoptosis in order to establish correctly connected neural networks. Development of the reproductive system is no exception; for example the Müllerian ducts that develop into the uterus, the Fallopian tubes and the upper vagina are initially present in both the male and the female foetus, but are lost in the male through apoptosis. Dysregulation of apoptosis is associated with a number of human pathologies, such as Huntington’s disease, Alzheimer’s disease and AIDS as well as playing a preponderant role in carcinogenesis.
II-1. The morphological features of apoptosis
When cells die by apoptosis, they progressively acquire a characteristic morphology arising from profound changes in structure and function. These changes, that are very different to those observed in necrosis, allow the dying cell to be detached from its neighbours and to be eliminated by phagocytosis. The structural modifications include nuclear and cytoplasmic condensation, the alteration of organelles such as mitochondria and lysosomes and the invagination of the plasma membrane and nuclear envelope [5–7]. Membrane blebbing gives the cell the appearance of a bunch of grapes that can be easily visualised by real-time video microscopy [8]. The sum of these structural modifications leads to the fragmentation of the cell into membrane-bound apoptotic bodies that will be phagocytosed by surrounding cells [5, 6]. Modification of membrane asymmetry, accompanied by the externalisation of phosphatidylserine (PS), allows the recognition of these apoptotic bodies by phagocytic cells.
It is possible to use a specific ligand of PS, annexin V, to detect and quantify apoptotic cells by immunofluorescence and flow cytometry (Fig. 1) [9]. The apoptotic process also involves the fragmentation of DNA by endonucleases. Genomic DNA is preferentially cleaved in the internucleosomal regions leading to fragments of DNA with sizes that are multiples of 200 base pairs. These fragments can be separated by agarose gel electrophoresis giving a characteristic ladder pattern. The fragmented DNA can also be detected by the histochemical labelling of the free 3′OH ends created by the endonucleases using a technique called TUNEL (Terminal deoxynucleotidyl Transferase-mediated dUTP-biotin Nick End Labelling) [10–12].
Fig. 1.
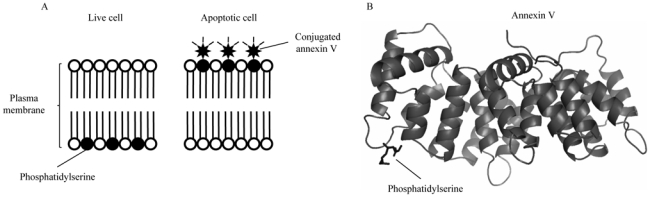
Principle of the annexin V apoptosis detection. (A) After initiating apoptosis, cells translocate the membrane phosphatidylserine from the inner face of the plasma membrane to the cell surface. Once on the cell surface, phosphatidylserine can be easily detected by staining with a fluorescent conjugate of Annexin V, a protein that has a high affinity for phosphatidylserine. (B) Structure of phosphatidylserine-bindig annexin V. Annexin A5 core (PDB code 1A8A) domains are intracellular phosphatidylserine-binding domains.
II-2. Apoptotic Genes
Two complementary approaches, biochemical analysis and genetical analysis of simple model organisms (notably C. elegans and D. melanogaster), have been particularly fruitful in the description of apoptosis, which is the most abundant and best studied of the cell death mechanisms [13, 4]. The core enzymatic machinery of apoptosis is composed of a family of cysteine proteases called caspases. To date, 14 caspases have been identified in man; though it is probable that this list is not exhaustive [14–18]. Caspases are omnipresent in the form of zymogens, the activation of which is subject to a number of regulatory mechanisms [14–18]. Members of the Bcl2 and IAP (Inhibitor of Apoptosis) families of proteins are fundamental elements in the pathways that control apoptosis [19–21]. Two major pathways regulate apoptosis (Fig. 2). The extrinsic pathway is activated by the binding of ligands to membrane- bound death receptors of the TNFR family [22]. The intrinsic pathway involves a plethora of signals that converge on the mitochondria via the pro- and the anti- apoptotic members of the Bcl2 family [19–23]. The extrinsic pathway links into the intrinsic pathway via a pro- apoptotic Bcl2-family protein, Bid [24].
Fig. 2.
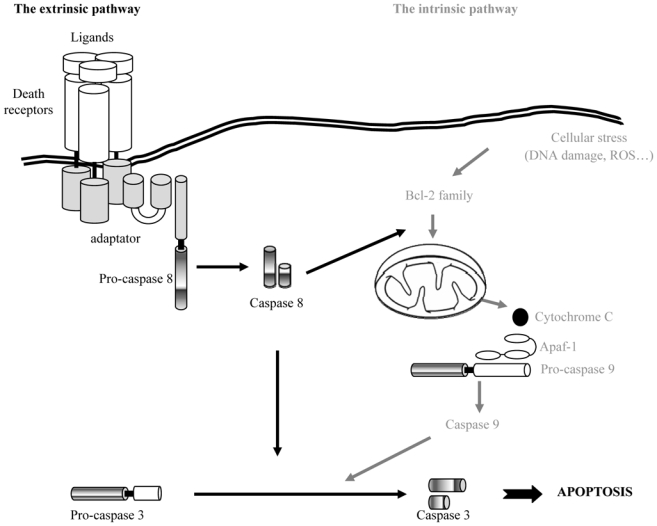
Schematic representation of the two major transduction pathways of apoptotic signalling. The first one, called the extrinsic pathway, is activated by the binding of ligands to membrane-bound death receptors of the TNFR family. The second pathway, called the intrinsic pathway, involves a plethora of signals that converge on the mitochondria via the pro- and the anti- apoptotic members of the Bcl2 family. The extrinsic pathway links into the intrinsic pathway via a pro- apoptotic Bcl2-family protein, Bid.
Apoptotic phenomena have been described in the blastocyst and, more recently, throughout the development of the pre-implantation embryo [25, 26]. The development of the embryos could well depend on an equilibrium between cellular proliferation and apoptosis. Accumulating data indicate that sub-optimal in vitro culture conditions severely affect the survival of the embryos. However, it is also highly probable that the poor quality of the fertilized oocytes, that are already apoptotically compromised, is a major factor in embryonic survival. This underlines the considerable importance of studying the genetic control of apoptosis in both the gametes and the pre-implantation embryos.
III. Oocyte apoptosis
III-1. During the foetal period
The definitive stock of primordial follicles, that will ultimately enter into the follicular growth phase, during adult life, is established during foetal oogenesis. More than 90 % of germinal cells present in the foetus at mid-gestation will have disappeared by birth. Experimental results from animal models confirm that this lost of germinal cells occurs through apoptosis during gonad mitosis, prophase of meiosis I and primordial follicle formation. Apoptotic cells have been identified, by in situ labelling, both in man [27, 28] and in the mouse [29]. Owing to the limits imposed on the exploitation of human tissue samples, most available data derive from experiments with other animal species. The invalidation of genes in the mouse, especially members of the Bcl2 family and the caspases, have been invaluable in establishing their key roles in oocyte and follicular apoptosis [30, 31]. The implication of these pathways in the regulation of germ-cell number in the human foetal ovary has largely been established using immunolocalisation techniques. Thus, certain members of the Bcl2 family of proteins, such as the anti-apoptotic Bcl2 and Mcl1 and the pro-apoptotic Bax [28, 32] in addition to caspases 2, 3,7,8 and 9 have all been detected [33, 34]. The presence of cleaved, active, forms of the initiator caspases 8 and 9 and executioner caspases 2 and 7 have been confirmed by western blotting; although this is not the case for caspase 3, one of the best characterised executioner caspases [33]. The presence of activated caspase 3 has been observed, however, by immunohistochemistry in all species tested. The number of germinal cells expressing active caspase 3 increases between the 14th and 19th weeks of gestation, but then falls to zero in primordial follicles in both human [33] and mouse [34]. The invalidation of the caspase 3 gene in the mouse suggests that this protease is primordial in the apoptosis of granulosa cells, but not in germinal cells, during ovarian development [35, 36]. In contrast, the inactivation of the caspase 2 or Bax genes, in the mouse, leads to a dramatic increase in the number of primordial follicles in the post-natal ovary [37, 38]. The study of the expression profiles of the actors and regulators of apoptosis, therefore, appears to be crucial in order to define the mechanisms and signalling pathways implicated in this physiological process.
A number of trophic factors, identified in the developing ovary, have been shown to reduce the loss of both human [39] and rodent [40] germinal cells in vitro. Although the causes, and the regulation, of this apoptosis are not yet fully understood, recent studies have implicated the tyrosine kinase receptor TrkB whose principal ligands are the neurotrophins BDNF (Bone-Derived Neurotrophic Factor) and NT4 (neurotrophin 4) [41]. TrkB is a so-called dependence receptor that can transmit two opposing signals: a survival signal in the presence of the principal ligands, and an apoptotic signal in their absence. The implication of TrkB has been confirmed by the invalidation of its catalytic activity in the mouse, the use of specific inhibitors in in vitro culture of human and mouse ovaries and the localisation of neurotrophin 4 mRNA [41].
III-2. In the Adult
In the adult human ovary, the individual fate of a follicle (growth/ovulation vs atresia) is tightly regulated by a dialogue of death and survival signalling, including both endocrine factors (gonadotropins) and paracrine factors (e.g. GDF9, BPM15). Only a small proportion of the primordial follicles present at birth progress to ovulation, the others die by apoptosis during the regulated process of follicular atresia.
Two types of follicular atresia can be distinguished. Basal atresia principally affects reserve follicles as they start to grow. This type of atresia, that is observed throughout reproductive life, and that is independent of the menstrual cycle, is characterised by the premature involution of the oocyte and by mild nuclear fragmentation in the granulosa cells [36]. Preantral follicles, and follicles at the start of antrum, show high levels of atresia (≈ 30 %), whereas follicles between the sizes of 0.5 and 2 mm show lower levels (≈ 15 %). In addition to this basal atresia, larger follicles (> 2 mm) are subjected to cyclic atresia. This type of atresia starts with high levels of nuclear fragmentation in granulosa cells, followed by a rapid degeneration of the oocyte. The highest levels of atresia are observed in the mid-luteal phase (73 % for 2 – 5 mm follicles and 100 % for 6 – 10 mm follicles). A number of studies of human and mouse oocyte quality have reported high levels of DNA fragmentation, as analysed by TUNEL, associated with low fecundity [42–44]. The expression of caspase 3 is greatly increased in pre-ovulatory follicles entering into atresia [45] and activated caspase 3 has been detected not only in the oocyte [34], but also in the granulosa cells of atretic follicles in the mouse ovary [46]. Apoptosis could, therefore, be a means of eliminating defective or fertilization-incompetent oocytes. The description of the apoptotic cascade, as well as its induction and regulation, have, and continue to be, the object of a very large number of studies (Table I). It is probable that the death receptor Fas expressed in the oocytes of primordial and primary follicles in the post-natal ovary, is involved in apoptotic signalling [43]. Fas ligand (Fas-L) is expressed in oocytes of primary, secondary and tertiary follicles, adding weight to the argument that the couple Fas/Fas-L is involved in the regulation of oocyte apoptosis [47]. Moreover, the activation of the Fas pathway during oocyte maturation in vitro increases the incidence of apoptosis of cells of the cumulus oophorus [48] and granulosum [49]. In addition, soluble Fas, a truncated form of Fas that acts as an inhibitor of Fas-induced apoptosis, is found at high concentration in follicular fluid and in the medium bathing the cumulus oophorus/mature oocyte complex [50]. Other factors that induce apoptosis have also been incriminated, including cytokines such as TNF-α and l’IL-6 [51–53]. These latter may act either by inducing the uncontrolled production of free radicals, or by increasing intracellular Ca2+, that in turn leads to endonuclease activation, either by activating pro-apoptotic genes of the Bcl-2 family (Bax, BclXS), or by generating second messengers such as ceramide that lead to caspase activation [54, 55]. Indeed, the knockout of either Bax or caspase 2 leads to a spectacular increase in the number of primordial follicles [57, 37]. Nevertheless, these apoptotic signals are not present in the developing follicle in normal conditions. One theory that could explain this is that cell proliferation activates a default apoptotic programme that, unless countered by survival signalling, leads to the inexorable demise of the cell. Here, the principal survival signal is Follicle-Stimulating Hormone (FSH) that is prerequisite to the survival of post-antrum stage follicles. FSH both inhibits the expression of pro-apoptotic genes of the Bcl2 family (Bax and BclXS) and stimulates the production of growth factors, such as EGF, TGFα, bFGF and the IGFs, that have a positive effect on the oxidative stress response. In addition, when small antral follicles from rat are cultured in the presence of FSH, they show increased follicular growth, reduced apoptosis and an increased expression of XIAP (X-linked Inhibitor of Apoptosis) [58]. Originally identified in baculovirus, the IAPs are a family of survival proteins that regulate cell fate by modulating post-mitochondrial apoptotic signalling. Several members have been identified in man, including XIAP [59]. A causal relationship between XIAP and apoptosis has been confirmed by the fact that inhibition of its expression induces apoptosis and represses follicular development, even in the presence of FSH. XIAP would, therefore, seem to play a key role in FSH-stimulated follicular development. We have recently demonstrated, by transcriptome analysis, that oocytes also strongly express two other anti-apoptotic factors, Bcl2L10/DIVA and survivin (another member of the IAP family), as well as a pro-apoptotic factor (BNIP1) [60]. The equilibrium between these pro- and anti- apoptotic factors may contribute to the regulation of oocyte apoptosis in the adult.
Table I.
mRNA expression of the Bcl-2 family and caspases during human pre-implantation embryo development.
| Ovary |
Oocyte |
Early embryo development stage |
|||||
|---|---|---|---|---|---|---|---|
| zygote | 2 cells | 8 cells | Blastocyst | ||||
| Bcl-2 family | |||||||
| Pro-apoptotic members: | Bak | +/− | n.d | + | + | + | + |
| Bax | +/− | +/− | + | + | + | + | |
| Bid | n.d | n.d | − | + | + | − | |
| Bad | n.d | n.d | + | + | + | + | |
| Anti-apoptotic members: | Bcl-2 | +/− | + | + | + | + | + |
| Bcl-xL | +/− | +/− | +/− | − | + | − | |
| Bcl-w | n.d | n.d | + | + | − | − | |
| Caspases | |||||||
| Caspase 2 | + | + | + | + | + | + | |
| Caspase 3 | +/− | + | + | +/− | + | + | |
This table shows the data of several publications and the contradictory results. +: detected, −: not detected, +/−: detected and not detected, n.d: not determined.
It is interesting to note that, contrary to the well established anti-apoptotic role of gonadotropins during early antral follicle development, Luteinizing Hormone (LH) and FSH stimulate caspase 3 and caspase 7 activities in in vitro cultures of rat pre-ovulatory follicles, potentialising apoptosis in theca cells but not granulosa cells [61]. This strongly suggests that apoptosis of theca cells is a physiological process that is necessary for gonadotropin-induced ovulation [62].
Although the growing body of literature that has been generated over the past few years has permitted the identification of the major actors regulating apoptotic signalling in oocytes, the reason that it should occur at all remains a mystery. A number of hypotheses might be suggested, such as the selection of oocytes prior to ovulation or the surveillance of their genomic integrity.
IV. Apoptosis in early stage of embryos development
The term early embryo stages refers to the developing from the zygote (resulting from the fertilization of the oocyte) to the moment that it implants in the uterus, at the blastocyst stages, and establishes an intense molecular dialogue with the maternal tissues. Indeed, during this period, zygote migrates along the genital tract to the uterus and the embryo undergoes important developmental stages and cellular differentiation. The apoptotic phenomena, observed throughout the development of the pre-implantation embryo, are necessary for the elimination of genetically abnormal or mutated cells [3]. The degree of apoptosis, that is particularly elevated at the blastocyst stage, however, cannot exceed a certain threshold without leading to the collapse of embryonic homeostasis and developmental arrest. Despite this “natural selection” during the pre-implantation stages, there is still a substantial loss (estimated at 20 to 25 % in vivo) on account of defective implantation [63].
The critical phase of implantation process, occurring between the sixth and the fifteenth day post fertilization, has been little studied because of difficulties encountered in the culture of human embryos from these stages of development. Despite efforts to improve the culture conditions (reduced glucose concentration, amino-acid supplements, growth factors in the culture medium, controlled hypoxia) of early stages of in vitro embryo culture, only 20 – 40% of embryos reach the blastocyst stage after 5 – 6 days post fertilization, and very few of these have the morphological characteristics of implantation-stage embryos.
Embryos are selected essentially on the basis of morphological criteria: number and regularity of blastomeres, rate of development, and embryonic fragmentation percentage [64, 65] (Fig. 3). The low implantation rate of embryos aged between 2 and 3 days (10 – 12 %) might be explained by uterine hyper-motricity and the inappropriate timing of transfer. Indeed, these embryos are placed in an unsuitable environment because, in vivo, they would still be in the Fallopian tubes, and would not reach the uterus until the blastocyst stage i.e. 5 to 6 days post fertilisation. This is one of the reasons that have led to the in vitro culture of embryos up to the blastocyst stage. In addition to synchronising the embryo to the endometrium, the prolonged culture in vitro also allows the selection of the most competent embryos, with the highest developmental potential, and the elimination of those that are blocked at a stage corresponding to genomic activation (6 – 8 cell stage, D3 post-insemination). Thus, most IVF teams transfer 1 or 2 embryos at the blastocyst stage and achieved better pregnancy success rates per blastocyst compared to embryo transfer on day 2 or 3 [66]. In many embryos, the embryonic genome is not activated and they remain blocked at the 4 – 8 cell stage. Arrested development at this stage is possibly due to genetic factors, apoptosis, maternal age or paternal genome.
Fig. 3.
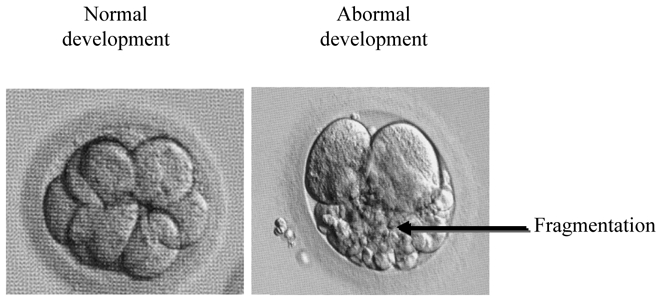
Morphological aspect of early embryo developement. Left, normal embryonic development caracterised by few or no fragmentation; Right, abnormal embryonic development with fragmentation.
In addition, around two-thirds of the embryos obtained show cellular fragmentation > 15%. This fragmentation is observed in both in vivo and in vitro fertilized embryos [63, 2]. The origin, the role and the mechanism of this fragmentation remain to be clarified. However, there is now little doubt that it does affect embryonic development. Indeed, a number of studies have highlighted a negative correlation between the degree of fragmentation and development to the blastocyst stage; excessive fragmentation being associated with diminished embryonic viability and a low implantation rate [67]. Several hypotheses have been proposed to explain the negative effect of fragmentation on pre-implantation embryonic development. First, the fragments could physically disrupt cell-cell interactions, interfering with embryonic development, especially during compaction, cavitation and blastocyst formation [68, 69]. Secondly, the fragments could reduce the cytoplasmic volume, depleting essential organelles or polarised domains that are indispensable for correct development [70]. Thirdly, ultrastuctural analysis reveals that blastomeres that are adjacent to fragments show signs of degeneration; probably due to the liberation of toxic substances by the fragments [71, 2].
On account of the resemblance of these fragments to apoptotic bodies, a number of groups have taken an interest in the role of apoptosis during the development of pre-implantation embryos cultured in vitro [72]. It is probable that the activation of apoptosis in human pre-implantation embryos prevents the transmission of damaged DNA. This apoptosis is preponderant after compaction, mainly at the morula and blastocyst stages, initially amongst the embryonic stem cells (cells of the inner mass) and later throughout the foetus [70]. In embryos with low, or no, fragmentation, 7–8% of the cells of the inner mass undergo apoptosis. Two hypotheses have been proposed. In the first hypothesis, the number of cells in the inner mass of the developing blastocyst is maintained at equilibrium by regulated proliferation and cell death [73]. The fact that embryos with a low morphological quality, and a reduced number of cells in the inner mass, have a lower rate of apoptosis than embryos that are morphologically perfect argues in favour of a role for apoptosis in the maintenance of cellular homeostasis. The second hypothesis proposes that, in the developing foetus, apoptosis eliminates cells with an altered genome. Indeed, aneuploid cells can appear in the inner mass. Until recently, these fragments were thought to be structures that are independent of the blastomeres, and simply derived from the mass of embryonic cells. This notion, however, is currently under debate. Several studies have reported a fragmentation of blastomeres as well as trophoectodermal cells [74, 75]. Importantly, a recent study has shown that severe fragmentation, equivalent to the loss of at least one blastomere, at two days post fertilization, is associated with a drastic decrease in the rate of formation of blastocysts at six days post fertilization (12% vs 60 % in non-fragmented embryos) [76].
Human early embryos that are marked by an excessive fragmentation on day 6 post fertilization, or that have an arrested development, display a high proportion of cells undergoing apoptosis (over 15 % of the cells), characterized by cytoplasmic and nuclear fragmentation, condensed chromatin, fragmented DNA and phagocytosis. These characteristics have been observed for the most part by classical microscopy techniques and have not been confirmed by biochemical markers such as TUNEL or annexin V binding [70, 77, 78]. Whether embryonic fragmentation is associated with the activation of an apoptotic cascade is, therefore, a valid question. The ratio of the expression of transcripts of pro-apoptotic (Bak, Bax, Bid, Bik, Bad) and anti-apoptotic (Bcl2, BclXL, Mcl1, Bclw) members of the Bcl2 family is invariable throughout normal embryonic development in both man and mouse (Table I) [79, 80]. At the protein level, only Bax is constitutively present, the other members of the family show variable expression levels throughout development, with some data in the literature being contradictory [80, 81]. Bcl2 expression, however, appears to be restricted to specific cells of the inner mass, whereas Bax is localised in the cytoplasm of the blastomeres, the cells of the inner mass and the trophectodermal cells. Similarly, an increased expression of Bax has been described in human blastocysts with poor quality morphology [72, 75], whilst Bcl2 has been reported to be over-expressed in mouse blastocysts with good morphology as compared to blastocysts with a fragmented morphology [79]. It would appear, therefore, that blastocysts with a high degree of fragmentation are characterised by disequilibrium between pro- and anti-apoptotic members of the Bcl2 family of proteins [79, 72, 82]. Caspase 2 and 3 transcripts are detectable at all developmental stages of the human pre-implantation embryo [82, 76]. Increased caspase 3 activity, as well as increased expression of Harakiri, a pro-apoptotic protein of the Bcl2 family, has been reported in fragmented embryos as of the 4-cell stage [76].
Finally, survivin, a member of the IAP family, plays a key role in embryonic development. Survivin is highly expressed throughout embryonic life and is absent from differentiated tissues. It is re-expressed, however, in most common cancers and in tumour-derived cell lines [83]. The anti-apoptotic function of this protein is associated with its capacity to bind certain caspases (especially caspases 3, 7 and 9). Recently, alternative transcripts have been shown to give rise to proteins with different (and sometimes opposing) apoptotic or anti-apoptotic effects [84]. Survivin appears to act during mitosis. It associates with the mitotic spindle and appears to be regulated by the absence of tension at the kinetochores. Transgenic embryos, lacking survivin expression, die in utero from massive apoptosis [85]. Similarly, down regulation of survivin expression, by antisense RNA, provokes metaphase arrest and various anomalies, depending on the model studied. In the early mouse embryo this is translated by developmental arrest at the morula or blastocyst stage, followed by apoptosis [86]. In addition this apoptosis can be inhibited by inhibitors of caspases 3 and 9. Survivin is, therefore, an essential anti-apoptotic gene, that is expressed throughout all stages of development of the human pre-implantation embryo and that can protect embryos from apoptosis by inhibiting apoptotic pathways implicating caspases.
Despite some controversy over the role of apoptosis in inducing fragmentation, the altered expression of apoptotic regulatory proteins does seem to be linked to embryonic viability.
V. Micro-environment and apoptotic signalling
We have described above the state of our knowledge concerning the genetic control of apoptosis in the oocyte and the embryo, and will now examine the consequences of culture conditions on folliculogenesis and the development of the early embryo development.
It is currently accepted that IVF failures, linked to poor embryo quality, are, in part, associated with suboptimal in vitro culture conditions [74, 78]. Apoptosis in early embryos might be provoked by the lack of maternally derived factors, such as essential growth factors and cytokines [87–89]. However, the mechanisms that trigger apoptosis in suboptimal in vitro culture conditions have not yet been clearly identified. Ideally, an experimental model should take into account the three-dimensional (3D) structure of the tissue. Classical cell culture, however, obliges cells to adapt to physical and environmental constraints that are very different to those encountered in vivo. Indeed, cell behaviour has to adapt to the unnatural rigidity of the support and to the lack of other cells above and below. Within the organism, cells are organised into tissues and bound to the extracellular matrix that is composed of molecules such as collagen, fibronectin and laminin. The spatial constraints of 3D cell culture aim to recreate these conditions, such that the cells correctly perceive and interpret the biochemical and biophysical cues of the microenvironment; especially those related to their binding to the extracellular matrix and neighbouring cells and their response to growth factors [90–93]. In the context of 3D culture, cells conserve the characteristics of a tissue, whilst remaining amenable to experimentation. The importance of 3D culture is especially crucial in the study of complex cellular systems, such as epithelia. Indeed the composition of the extracellular matrix, as well as its topographical organisation are essential elements contributing to the survival, migration and differentiation of epithelial cells that are otherwise unable to polarize correctly and differentiate [94]. Epithelial cell polarisation, that depends on binding to the extracellular matrix, via integrins, and to neighbouring cells, via adherent and tight junctions, both affects, and is affected by, intracellular signalling pathways including the PI(3)K pathway [95, 96]. One of the effectors of this pathway is the serine/threonines kinase, Akt that constitutes a signalling node that regulates cell survival [97]. The substrates of Akt include several components of the core apoptotic machinery.
In conclusion, an understanding of the molecular mechanisms involved in cell death decisions necessitates the use of experimental models that recapitulate major aspects of physiological conditions, such as the extracellular matrix and growth factor composition.
V-1. Impact of the microenvironment on folliculogenesis
V-1-1. Culture medium and microenvironment
A number of studies have highlighted the importance of the culture media employed in in vitro maturation, as they affect not only the maturation of the oocytes, but also the subsequent development of the embryo. For instance, the use of basal media (bicarbonate buffered physiological saline containing pyruvate, glucose and lactate) with low concentrations of non-essential amino acids and vitamins, but lacking purine precursors (glutamine, glycine, aspartic acid) and hypoxanthine (responsible for meiotic arrest), and supplemented with human serum seems to significantly increase the survival and maturation of oocytes [98].
For over a decade now embryologists have recognized the importance of preserving the microenvironment [99]. Follicle culture, for instance, has given way to the culture of fragments of ovarian cortex as numerous studies have reported that the growth and development of the oocytes and follicles are better when the integrity of the oocyte/granulosa cell/follicular component interactions is preserved. Indeed, follicle survival is significantly increased when the ovarian tissues are only partially dispersed [100]. In these conditions it is possible to maintain inter-follicular interactions, as well as the action of various factors present in the follicular liquid. However, it is difficult to maintain the three dimensional nature of the tissue in vitro and the follicles degenerate after a few days in culture. The culture of follicles or ovarian tissues on a monolayer of extracellular matrix does allow the maintenance of follicular structure over a longer period of time [100], but the culture systems suffer from the lack of extracellular matrix contact on the upper side of the biological sample.
V-1-2. Hormones and Growth Factors
Primordial follicles present in the ovary represent the stock of follicles for the reproductive lifetime. As the follicle is characterised by rapid growth, intense interaction between follicle cells and growth factors, hormones and steroids is necessary in order to transmit elements indispensable for the maturation of the oocyte. Notably, cumulus oophorus cells secrete, in an FSH dependent manner, glycolytic intermediates (lactate, pyruvate …) as nutrients for the germline. Indeed, the oocytes, themselves, have little, or no, glycolytic activity [101] and increased glycolytic activity in cells of the cumulo-oocyte complex is associated with the complete maturation of human oocytes in vitro [102] (Fig. 4). The hormone FSH is already used to supplement most culture media used for the maturation of oocytes in vitro [102]. Protocols, however, remain to be standardised. Indeed, the concentrations of FSH vary between laboratories. In certain animal species, treatment with high doses of exogenous gonadotropins affects the pre-implantation period as well as post implantation development. Studies of cumulus cells-oocyte complexes in vitro have shown that the metabolism of pyruvate, but not that of glucose, is affected by high doses of FSH. This example highlights the urgent need to standardise protocols for the in vitro maturation of oocytes [102].
Fig. 4.
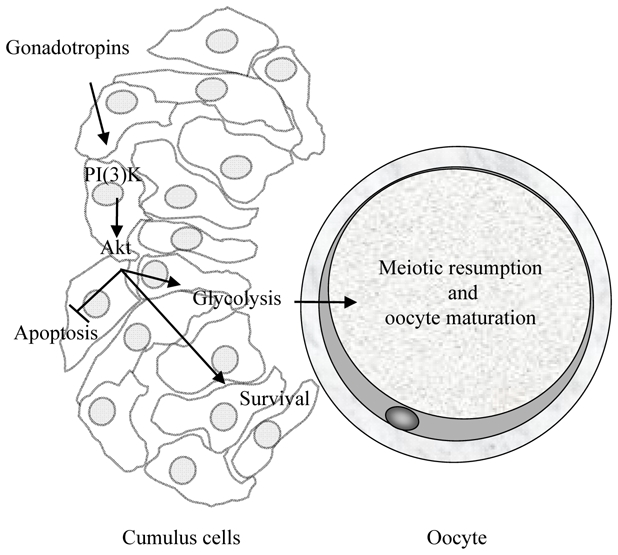
Key role of the PI(3)K/Akt signalling in the cumulo-oocyte complexe survival. The PI(3)K/Akt signalling pathway in cumulus cells plays a determining role in the suppression of both spontaneous and gonadotropin-stimulated meiotic re-entry in oocyte as well as in oocyte survival.
The mechanisms permitting the consumption of glucose by cumulus oophorus cells, following gonadotropic stimulation, is currently well understood and involves the activation of the PI(3)K/AKT (phosphoinositide-3-kinase/V-akt murine thyoma viral oncogene) [102, 103]. This signalling pathway plays a determining role in the suppression of both spontaneous and gonadotropin-stimulated meiotic re-entry in oocytes [102]. Indeed, the low levels of Akt activity found in the cumulus, as well as those observed immediately after the collection of the oocte in its follicle, are necessary for meiotic arrest. Increased Akt activity favours the production of progesterone by the cumulus cells, which in turn favours meiotic resumption and survival signalling (Fig. 4). The pharmacological inhibition of the PI(3)K pathway, blocking the activation of Akt, increases caspase-3 activity and the proportion of apoptotic cells in the cumulus oophorus, and affects meiotic resumption stimulated by porcine oocytes [104]. The use of basal media for the culture of cumulo-oocyte complexes, however, does not allow the maintenance of Akt activity, which decreases drastically within a few hours of culture [104]. It is probable that the loss of survival signalling in the cumulus cells-oocyte complexes provokes the apoptosis of cells in the cumulus oophorus. As interactions between these cells and the oocytes are necessary for embryonic development, decreases in their numbers have a negative effect on development rate [105].
A number of factors are involved in follicular progression and growth; notably the tyrosine kinase receptor cKit, that is synthesized by the oocyte, and its ligand, KITL that is present at the surface of the follicle cells. Mice with naturally occurring KITL mutations form primordial follicles that are incapable of progressing to the primary follicle stage. All of the stages from the formation of follicles through their progression up to the secondary follicle stage are independent of hypophyseal gonadotropins, and are regulated instead by intra-ovarian paracrine factors. Of these, three are members of the TGFβ (Transforming Growth Factor) super family: AMH (Anti-Mullerien Hormone), that appears to drive primordial follicles into the following stages, GDF9 (Growth Differentiation factor) that is implicated in stages posterior to the primary follicle and BMP15 (Bone Morphogenetic Protein) that affects the proliferation of follicle cells [106–110]. GDF9 is expressed very early during follicular development, and is secreted by oocytes within growing follicles [111]. The addition of GDF9 to the culture medium enhances survival and follicular progression up to the secondary follicle stage in cultured ovarian slices [112]. In this same model, other growth factors, such as IGF-I, IGF-II and insulin have been shown to be oocyte survival factors [113–115].
Transcriptome studies, along with the completed genome sequences of several species (man, mouse, cow, pig etc.), have led to the identification of novel transcripts expressed in the developing ovary [116, 117]. This should allow the improvement of ovary and early embryo culture conditions in the near future.
V-2. The impact of the microenvironment on the early embryo development
Studies with mice have demonstrated that in vitro culture increases cell death in the embryos to a degree that is dependent upon the culture medium [78, 118]. There is now a good deal of evidence that the cellular microenvironment influences the degree of fragmentation in early embryo stages. For example, embryos cultured on a monolayer of feeder cells (e.g. fibroblasts) have a lower degree of fragmentation than those cultured alone [119]. Likewise, embryos cultured in small volumes, or those expressing growth factors and their receptors, have a lower incidence of fragmentation [120, 121]. Thus, in order to take into account nutritional (modification of glucose metabolism) and environmental (passage from the Fallopian tubes to the uterus) changes of the developing embryo in vivo, two different culture media are used sequentially in most IVF centers today: one for culture up to the 4-cell stage, the other for culture up to the activation of the genome and compaction. Most mammalian cells cultivated in the absence of serum (or other extracellular signalling molecules), however, undergo apoptosis. In most cell types, the proteins necessary for the execution of the death program are constitutively expressed, but are maintained in an inactive state by extracellular survival signals. Although few data are available concerning the exact composition of the fluid surrounding the embryo in vivo, evidence is now accumulating as to the role of growth factors, of both maternal and embryonic origin, in the development of the pre-implantation embryo. Notably, the addition of growth factors, such as TGF-α, IGF-I, or insulin, to the culture medium is beneficial to the development of the embryo, increasing the formation and number of blastocyst cells (60% of embryos at the blastocyst stage in the presence of IGF-I vs 35% without IGF-I). In addition, the presence of IGF-I or TGF-α strongly represses the number of apoptotic nuclei in the human blastocyst [72, 98, 118, 89]. Other growth factors, such as LIF (leukaemia inhibitory factor) and HB-EGF (Heparin Binding EGF) increase blastocyst formation by 24 and 30 % respectively [122, 123]. Further studies will be necessary, however, before these results can be extrapolated in terms of implantation and clinical pregnancy rate.
VI. Apoptotic markers and pre-implantation potential
Taken together, the above review of the literature suggest that apoptotic markers might be of predictive value in the selection of competent oocytes and early embryos of high quality. With this in mind, the results of Malamitsi-Puchner et al [49] are of particular interest as they underline the correlation between the level of soluble Fas in the follicular fluid and/or the cumulus cells-oocyte complex with both the morphological quality of the pre-implantation embryos and the clinical pregnancy success rate. Indeed, these authors demonstrate that high soluble Fas levels, in the follicular fluids, are a reflection of oocyte maturity. In addition, low levels of soluble Fas in the cumulus cells-oocyte complex are only observed following fertilization of the oocyte, when the morphology of the pre-implantation embryo is perfect or in the case of a successful clinical pregnancy [49]. However, this study, albeit promising, involved only a small number of patients and needs to be verified on a larger scale in order to confirm the predictive potential, for IVF, of soluble Fas levels in the follicular fluid and/or the cumulus cells-oocyte complex.
Other studies suggest that telomere length in the oocyte is a predictive factor of cytoplasmic fragmentation in the embryo [124]. Telomeres that are too short are known to lead to genomic instability, with unrepairable damage to DNA that can lead to apoptosis, suggesting that shortened telomeres in the oocyte might be responsible for increased apoptosis in the human pre-implantation embryo. However, the inverse correlation between telomere length, as measured by Fluorescent In Situ Hybridization (FISH), and cytoplasmic fragmentation has only been observed as of the 3rd day after fertilization, the moment at which the pre-implantation embryos are transferred into the uterus [124]. Taking into account the technical difficulties and complexity (24 to 48 h) of molecular cytogenetic techniques, such as FISH, it is difficult to envisage that it can be a pertinent diagnostic tool in the evaluation of oocyte and embryo quality. In addition, recent studies indicate that up to 26 % of FISH analyses involving oocytes are artefactual [125, 126], introducing an element of doubt as to the reliability of the technique.
Finally, classical apoptotic markers (nuclear fragmentation, annexin 5 labelling, caspase activation), as described above, do not currently appear to be very promising. Data in the literature do not yet give a clear indication as to whether embryonic fragmentation is indeed associated with activation of the apoptotic cascade. Although alterations in the expression of protein regulators of apoptosis (Bcl-2 family members and IAPs) do appear to be associated with the viability of embryos, the evidence in the literature remains contradictory [70, 77, 78, 80, 81, 88]. Thus, there is still a need to identify novel markers, especially those involved in early events within the apoptotic signalling pathways. The identification of a genetic signature as a reliable way to predict the fertilisability of the oocyte and the quality of its pre-implantation embryonic development remains a crucial goal. Identifying gene expression profiles is the first indispensable step in the identification of potential molecular markers of the expression of abnormal genes in the oocytes and the pre-implantation embryos.
Although this type of strategy has already been applied to cumulus cells, oocytes and human supernumerary embryos [116, 117, 127, 128], the samples were in each case biologically stimulated or super-ovulated and, therefore, far from being in a physiologically normal environment. Using a recent technology, laser dissection microscopy, Arrazota et al [128] isolated primordial oocytes from sections of rhesus monkey ovary and uncovered 84 novel genes implicated in the survival and maturation of oocytes. It is also difficult to draw specific conclusions from such global data and further work will be required to validate the predictive value of the candidate genes in order to develop novel diagnostic genetic tests for oocyte and embryo quality.
VII. Conclusion and perspectives
Despite much progress in IVF over the past decades, more than 50% of human embryos cultured in vitro do not reach the blastocyst stage on account of developmental defaults. This is due, in part, to defective oocyte maturation, for which suboptimal culture conditions are probably a contributing factor. Little is known of the molecular events driving early embryonic development and transcriptome and proteome studies should lead not only to major advances in our understanding of the first week of human life, but also to the identification of pertinent and reliable markers of oocyte and embryo quality.
Acknowledgments
We thank the direction of the University-Hospital of Montpellier for support and the ART team for their assistance during this study. Partial financial support for this study was provided by the Ferring pharmaceutical company.
References
- 1.Hamamah S. Oocyte and embryo quality: is their morphology a good criterion? J Gynecol Obstet Biol Reprod. 2005;34(7 Pt 2):5S38–5S41. [PubMed] [Google Scholar]
- 2.Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71(5):836–842. doi: 10.1016/s0015-0282(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 3.Delimitreva SM, Zhivkova RS, Vatev IT, Toncheva DI. Chromosomal disorders and nuclear and cell destruction in cleaving human embryos. Int J Dev Biol. 2005;49(4):409–416. doi: 10.1387/ijdb.041909sd. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88(3):347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 5.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann G, Haouzi D, Moreau A, Durand-Schneider AM, Bringuier A, Berson A, Mansouri A, Fau D, Pessayre D. Opening of the mitochondrial permeability transition pore causes matrix expansion and outer membrane rupture in Fas-mediated hepatic apoptosis in mice. Hepatology. 2000;31(3):674–683. doi: 10.1002/hep.510310318. [DOI] [PubMed] [Google Scholar]
- 8.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 9.Van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31(1):1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Gold R, Schmied M, Rothe G, Zischler H, Breitschopf H, Wekerle H, Laussmann H. Detection of DNA fragmentation in apoptosis: application of in situ nick translation to cell culture systems and tissue sections. J Histochem Cytochem. 1993;41:1023–1030. doi: 10.1177/41.7.8515045. [DOI] [PubMed] [Google Scholar]
- 11.Migheli A, Attanasio A, Schiffer D. Ultrastructural detection of DNA strand breaks in apoptotic neural cells by in situ end-labelling techniques. J Pathol. 1995;176:27–35. doi: 10.1002/path.1711760106. [DOI] [PubMed] [Google Scholar]
- 12.Mundle SD, Gao XZ, Khan S, Gregory SA, Preisler HD, Raza A. Two in situ end labeling techniques reveal different patterns of DNA fragmentation during spontaneous apoptosis in vivo and induced apoptosis in vitro. Anticancer Res. 1995;15:1895–1904. [PubMed] [Google Scholar]
- 13.Hengartner MO, Horvitz HRC. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76(4):665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 14.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;8:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 15.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326 (Pt1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornberry NA. The caspase family of cysteine proteases. Br Med Bull. 1997;53(3):478–490. doi: 10.1093/oxfordjournals.bmb.a011625. [DOI] [PubMed] [Google Scholar]
- 17.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14(1):32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 19.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 20.Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998;252(1):1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 21.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 22.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 23.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 25.Levy R. Genetic regulation of preimplantation embryo survival. Int Rev Cytol. 2001;210:1–37. doi: 10.1016/s0074-7696(01)10002-1. [DOI] [PubMed] [Google Scholar]
- 26.Hardy K, Spanos S, Becker D, Iannelli P, Winston RM, Stark J. From cell death to embryo arrest: mathematical models of human preimplantation embryo development. Proc Natl Acad Sci U S A. 2001;98(4):1655–1660. doi: 10.1073/pnas.98.4.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Pol A, Vaccina F, Foraboscu A, Cavazzuti E, Marzoni L. Apoptosis of germ cells during human prenatal oogenesis. Hum Reprod. 1997;12:2235–2241. doi: 10.1093/humrep/12.10.2235. [DOI] [PubMed] [Google Scholar]
- 28.Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, te Velde ER, Stenbäck F, Heikinheimo M, Tapanainen JS. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab. 2001;86(7):3421–3429. doi: 10.1210/jcem.86.7.7679. [DOI] [PubMed] [Google Scholar]
- 29.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 30.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136(8):3665–3668. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- 31.Pru JK, Tilly JL. Programmed cell death in the ovary: insights and future prospects using genetic technologies. Mol Endocrinol. 2001;15:845–853. doi: 10.1210/mend.15.6.0646. [DOI] [PubMed] [Google Scholar]
- 32.Quenby SM, Gazvani MR, Brazeau C, Neilson J, Lewis-Jones DI, Vince G. Oncogenes and tumour suppressor genes in first trimester human fetal development. Mol Hum Reprod. 1999;5:737–741. doi: 10.1093/molehr/5.8.737. [DOI] [PubMed] [Google Scholar]
- 33.Albamonte MS, Willis MA, Albamonte MI, Jensen F, Espinosa MB, Vitullo AD. The developing human ovary: immunohistochemical analysis of germ-cell- specific VASA protein, BCL-2/Bax expression balance and apoptosis. Hum Reprod. 2008;23(8):1895–1901. doi: 10.1093/humrep/den197. [DOI] [PubMed] [Google Scholar]
- 34.Fenwick MA, Hurst PR. Immunohistochemical localization of active caspase-3 in the mouse ovary: growth and atresia of small follicles. Reproduction. 2002;124:659–665. doi: 10.1530/rep.0.1240659. [DOI] [PubMed] [Google Scholar]
- 35.Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, Tilly JL. Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology. 2001;142:2468–2480. doi: 10.1210/endo.142.6.8078. [DOI] [PubMed] [Google Scholar]
- 36.Feldmann G, Benifla JL, Madelenat P. Apoptosis of granulosa cells as a predictive marker of in vitro fertilization success ? Gynecol Obstet Fertil. 2006;34 (7–8):547–582. doi: 10.1016/j.gyobfe.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL, Yuan J. Defects in regulation of apoptosis in caspase-2- deficient mice. Genes Dev. 1998;12(9):1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morita Y, Perez GI, Maravei DV, Tilly KI, Tilly JL. Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol Endocrinol. 1999;13:841–850. doi: 10.1210/mend.13.6.0306. [DOI] [PubMed] [Google Scholar]
- 39.Martins da Silva SJ, Bayne RA, Cambray N, Hartley PS, McNeilly AS, Anderson RA. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation prior to primordial follicle formation. Dev Biol. 2004;266:334–345. doi: 10.1016/j.ydbio.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Dolci S, Pesce M, De Felici M. Combined action of stem cell factor, leukemia inhibitory factor, and cAMP on in vitro proliferation of mouse primordial germ cells. Mol Reprod Dev. 1993;35:134–139. doi: 10.1002/mrd.1080350206. [DOI] [PubMed] [Google Scholar]
- 41.Spears N, Molinek MD, Robinson LL, Fulton N, Cameron H, Shimoda K, Telfer EE, Anderson RA, Price DJ. The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development. 2003;130(22):5481–5491. doi: 10.1242/dev.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez GI, Tilly JL. Cumulus cells are required for the increased apoptotic potential in oocytes of aged mice. Hum Reprod. 1997;12(12):2781–2783. doi: 10.1093/humrep/12.12.2781. [DOI] [PubMed] [Google Scholar]
- 43.Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2(11):838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Zhang L, Wang X. Maturation and apoptosis of human oocytes in vitro are age-related. Fertil Steril. 2000;74(6):1137–1141. doi: 10.1016/s0015-0282(00)01597-1. [DOI] [PubMed] [Google Scholar]
- 45.Boone DL, Carnegie JA, Rippstein PU, Tsang BK. Induction of apoptosis in equine chorionic gonadotropin (eCG)-primed rat ovaries by anti-eCG antibody. Biol Reprod. 1997;57:20–27. doi: 10.1095/biolreprod57.2.420. [DOI] [PubMed] [Google Scholar]
- 46.Hurst PR, Mora JM, Fenwick MA. Caspase-3, TUNEL and ultrastructural studies of small follicles in adult human ovarian biopsies. Hum Reprod. 2006;21(8):1974–1980. doi: 10.1093/humrep/del109. [DOI] [PubMed] [Google Scholar]
- 47.Kim JM, Boone DL, Auyeung A, Tsang BK. Granulosa cell apoptosis induced at the penultimate stage of follicular development is associated with increased levels of Fas and Fas ligand in the rat ovary. Biol Reprod. 1998;58:1170–1176. doi: 10.1095/biolreprod58.5.1170. [DOI] [PubMed] [Google Scholar]
- 48.Rubio Pomar FJ, Roelen BA, Slot KA, van Tol HT, Colenbrander B, Teerds KJ. Role of Fas-mediated apoptosis and follicle-stimulating hormone on the developmental capacity of bovine cumulus oocyte complexes in vitro. Biol Reprod. 2004;71(3):790–796. doi: 10.1095/biolreprod.104.028613. [DOI] [PubMed] [Google Scholar]
- 49.Chen Q, Yano T, Matsumi H, Osuga Y, Yano N, Xu J, Wada O, Koga K, Fujiwara T, Kugu K, Taketani Y. Cross-Talk between Fas/Fas ligand system and nitric oxide in the pathway subserving granulosa cell apoptosis: a possible regulatory mechanism for ovarian follicle atresia. Endocrinology. 2005;146(2):808–815. doi: 10.1210/en.2004-0579. [DOI] [PubMed] [Google Scholar]
- 50.Malamitsi-Puchner A, Sarandakou A, Baka S, Vrachnis N, Kouskouni E, Hassiakos D. Soluble Fas concentrations in the follicular fluid and oocyte-cumulus complex culture medium from women undergoing in vitro fertilization: association with oocyte maturity, fertilization, and embryo quality. J Soc Gynecol Investig. 2004;11(8):566–569. doi: 10.1016/j.jsgi.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Relationships between concentrations of tumor necrosis factor-alpha and nitric oxide in follicular fluid and oocyte quality. J Assist Reprod Genet. 2000;17(4):222–228. doi: 10.1023/A:1009495913119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zolti M, Ben-Rafael Z, Meirom R, Shemesh M, Bider D, Mashiach S, Apte RN. Cytokine involvement in oocytes and early embryos. Fertil Steril. 1991;6(2):265–272. doi: 10.1016/s0015-0282(16)54483-5. [DOI] [PubMed] [Google Scholar]
- 53.Maeda A, Inoue N, Matsuda-Minehata F, Goto Y, Cheng Y, Manabe N. The role of interleukin-6 in the regulation of granulosa cell apoptosis during follicular atresia in pig ovaries. J Reprod Dev. 2007;53(3):481–490. doi: 10.1262/jrd.18149. [DOI] [PubMed] [Google Scholar]
- 54.Kim MR, Tilly JL. Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochim Biophys Acta. 2004;1644(2–3):205–210. doi: 10.1016/j.bbamcr.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Lin CF, Chen CL, Lin YS. Ceramide in apoptotic signaling and anticancer therapy. Curr Med Chem. 2006;13(14):1609–1616. doi: 10.2174/092986706777441986. [DOI] [PubMed] [Google Scholar]
- 56.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 57.Tilly JL, Tilly KI, Perez GI. The genes of cell death and cellular susceptibility to apoptosis in the ovary: a hypothesis. Cell Death Differ. 1997;4(3):180–187. doi: 10.1038/sj.cdd.4400238. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Rippstein PU, Tsang BK. Role and gonadotrophic regulation of X- linked inhibitor of apoptosis protein expression during rat ovarian follicular development in vitro. Biol Reprod. 2003;68:610–619. doi: 10.1095/biolreprod.102.007807. [DOI] [PubMed] [Google Scholar]
- 59.Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- 60.Assou S, Anahory T, Pantesco V, Le Carrour T, Pellestor F, Klein B, Reyftmann L, Dechaud H, De Vos J, Hamamah S. The human cumulus--oocyte complex gene-expression profile. Hum Reprod. 2006;21(7):1705–1719. doi: 10.1093/humrep/del065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yacobi K, Wojtowicz A, Tsafriri A, Gross A. Gonadotropins enhance caspase-3 and -7 activity and apoptosis in the theca-interstitial cells of rat preovulatory follicles in culture. Endocrinology. 2004;145:1943–1951. doi: 10.1210/en.2003-1395. [DOI] [PubMed] [Google Scholar]
- 62.Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo- luteal transformation. Connect Tissue Res. 2003;44:50–57. [PubMed] [Google Scholar]
- 63.Pereda J, Cheviakoff S, Croxatto HB. Ultrastructure of a 4-cell human embryo developed in vivo. Hum Reprod. 1989;4(6):680–688. doi: 10.1093/oxfordjournals.humrep.a136967. [DOI] [PubMed] [Google Scholar]
- 64.Holte J, Berglund L, Milton K, Garello C, Gennarelli G, Revelli A, Bergh T. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007;22(2):548–557. doi: 10.1093/humrep/del403. [DOI] [PubMed] [Google Scholar]
- 65.Scott L, Finn A, O’Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Reprod. 2007;22(1):230–40. doi: 10.1093/humrep/del358. [DOI] [PubMed] [Google Scholar]
- 66.della Ragione T, Verheyen G, Papanikolaou EG, Van Landuyt L, Devroey P, Van Steirteghem A. Developmental stage on day-5 and fragmentation rate on day-3 can influence the implantation potential of top- quality blastocysts in IVF cycles with single embryo transfer. Reprod Bio l Endocrinol. 2007;5:2. doi: 10.1186/1477-7827-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebner T, Yaman C, Moser M, Sommergruber M, Polz W, Tews G. Embryo fragmentation in vitro and its impact on treatment and pregnancy outcome. Fertil Steril. 2001;76(2):281–285. doi: 10.1016/s0015-0282(01)01904-5. [DOI] [PubMed] [Google Scholar]
- 68.Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum Reprod. 2000;15(12):2634–2643. doi: 10.1093/humrep/15.12.2634. [DOI] [PubMed] [Google Scholar]
- 69.Van Blerkom J, Davis P, Alexander S. A microscopic and biochemical study of fragmentation phenotypes in stage-appropriate human embryos. Hum Reprod. 2001;16(4):719–729. doi: 10.1093/humrep/16.4.719. [DOI] [PubMed] [Google Scholar]
- 70.Antczak M, Van Blerkom J. Temporal and spatial aspects of fragmentation in early human embryos: possible effects on developmental competence and association with the differential elimination of regulatory proteins from polarized domains. Hum Reprod. 1999;14(2):429–447. doi: 10.1093/humrep/14.2.429. [DOI] [PubMed] [Google Scholar]
- 71.Trounson A, Sathananthan AH. The application of electron microscopy in the evaluation of two- to four-cell human embryos cultured in vitro for embryo transfer. J In Vitro Fert Embryo Transf. 1984;1(3):153–165. doi: 10.1007/BF01139208. [DOI] [PubMed] [Google Scholar]
- 72.Hardy K. Appoptosis in the human embryo. J Reprod Fert. 1999;4:125–134. doi: 10.1530/ror.0.0040125. [DOI] [PubMed] [Google Scholar]
- 73.Jurisicova A, Varmuza S, Casper RF. Programmed cell death and human embryo fragmentation. Mol Hum Reprod. 1995;2(2):93–98. doi: 10.1093/molehr/2.2.93. [DOI] [PubMed] [Google Scholar]
- 74.Hardy K. Cell death in the mammalian blastocyst. Mol Hum Reprod. 1997;3(10):919–925. doi: 10.1093/molehr/3.10.919. [DOI] [PubMed] [Google Scholar]
- 75.Hardy K, Stark J, Winston RM. Maintenance of the inner cell mass in human blastocysts from fragmented embryos. Biol Reprod. 2003;68(4):1165–1169. doi: 10.1095/biolreprod.102.010090. [DOI] [PubMed] [Google Scholar]
- 76.Jurisicova A, Antenos M, Varmuza S, Tilly JL, Casper RF. Expression of apoptosis-related genes during human preimplantation embryo development: potential roles for the Harakiri gene product and Caspase-3 in blastomere fragmentation. Mol Hum Reprod. 2003;9(3):133–141. doi: 10.1093/molehr/gag016. [DOI] [PubMed] [Google Scholar]
- 77.Levy R, Benchaib M, Cordonier H, Guerin JF. Apoptosis in the pre-implantation embryo. Contracept Fertil Sex. 1998;26(7–8):536–541. [PubMed] [Google Scholar]
- 78.Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med. 1998;4(12):1421–1424. doi: 10.1038/4013. [DOI] [PubMed] [Google Scholar]
- 79.Exley GE, Tang C, McElhinny AS, Warner CM. Expression of caspase and BCL-2 apoptotic family members in mouse preimplantation embryos. Bio l Reprod. 1999;61(1):231–239. doi: 10.1095/biolreprod61.1.231. [DOI] [PubMed] [Google Scholar]
- 80.Metcalfe AD, Hunter HR, Bloor DJ, Lieberman BA, Picton HM, Leese HJ, Kimber SJ, Brison DR. Expression of 11 members of the BCL-2 family of apoptosis regulatory molecules during human preimplantation embryo development and fragmentation. Mol Reprod Dev. 2004;68(1):35–50. doi: 10.1002/mrd.20055. [DOI] [PubMed] [Google Scholar]
- 81.Warner CM, Cao W, Exley GE, McElhinny AS, Alikani M, Cohen J, Scott RT, Brenner CA. Genetic regulation of egg and embryo survival. Hum Reprod. 1998;13(Suppl3):178–190. doi: 10.1093/humrep/13.suppl_3.178. [DOI] [PubMed] [Google Scholar]
- 82.Spanos S, Rice S, Karagiannis P, Taylor D, Becker DL, Winston RM, Hardy K. Caspase activity and expression of cell death genes during development of human preimplantation embryos. Reproduction. 2002;124(3):353–363. doi: 10.1530/rep.0.1240353. [DOI] [PubMed] [Google Scholar]
- 83.Li F, Brattain MG. Role of the Survivin gene in pathophysiology. Am J Pathol. 2006;169(1):1–11. doi: 10.2353/ajpath.2006.060121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson ME, Howerth EW. Survivin: a bifunctional inhibitor of apoptosis protein. Vet Pathol. 2004;41(6):599–607. doi: 10.1354/vp.41-6-599. [DOI] [PubMed] [Google Scholar]
- 85.Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KHA. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 86.Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, Shimizu Y, Tanaka T. Survivin acts as an antiapoptotic factor during the development of mouse preimplantation embryos. Dev Biol. 2003;256(2):331–341. doi: 10.1016/s0012-1606(02)00135-5. [DOI] [PubMed] [Google Scholar]
- 87.O’Neill C. The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update. 2008;14(3):275–288. doi: 10.1093/humupd/dmn002. [DOI] [PubMed] [Google Scholar]
- 88.Bedaiwy M, Shahin AY, AbulHassan AM, Goldberg JM, Sharma RK, Agarwal A, Falcone T. Differential expression of follicular fluid cytokines: relationship to subsequent pregnancy in IVF cycles. Reprod Biomed Online. 2007;15(3):321–325. doi: 10.1016/s1472-6483(10)60346-x. [DOI] [PubMed] [Google Scholar]
- 89.Spanos S, Becker DL, Winston RM, Hardy K. Anti-apoptotic action of insulin- like growth factor-I during human preimplantation embryo development. Biol Reprod. 2000;63(5):1413–1420. doi: 10.1095/biolreprod63.5.1413. [DOI] [PubMed] [Google Scholar]
- 90.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 91.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70(9–10):537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 93.Radisky D, Muschler J, Bissell MJ. Order and disorder: the role of extracellular matrix in epithelial cancer. Cancer Invest. 2002;20:139–153. doi: 10.1081/cnv-120000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walpita D, Hay E. Studying actin-dependent processes in tissue culture. Nat Rev Mol Cell Biol. 2002;3:137–141. doi: 10.1038/nrm727. [DOI] [PubMed] [Google Scholar]
- 95.Weaver VM, Peterson OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three- dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;172(2):221–236. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- 99.Roberts R, Franks S, Hardy K. Culture environment modulates maturation and metabolism of human oocytes. Hum Reprod. 2002;17(11):2950–2956. doi: 10.1093/humrep/17.11.2950. [DOI] [PubMed] [Google Scholar]
- 100.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12(5):1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 101.Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod. 1999;60(6):1446–1452. doi: 10.1095/biolreprod60.6.1446. [DOI] [PubMed] [Google Scholar]
- 102.Roberts R, Stark J, Iatropoulou A, Becker DL, Franks S, Hardy K. Energy substrate metabolism of mouse cumulus-oocyte complexes: response to follicle- stimulating hormone is mediated by the phosphatidylinositol 3-kinase pathway and is associated with oocyte maturation. Biol Reprod. 2004;71(1):199–209. doi: 10.1095/biolreprod.103.023549. [DOI] [PubMed] [Google Scholar]
- 103.Hoshino Y, Yokoo M, Yoshida N, Sasada H, Matsumoto H, Sato E. Phosphatidylinositol 3-kinase and Akt participate in the FSH-induced meiotic maturation of mouse oocytes. Mol Reprod Dev. 2004;69(1):77–86. doi: 10.1002/mrd.20150. [DOI] [PubMed] [Google Scholar]
- 104.Shimada M, Ito J, Yamashita Y, Okazaki T, Isobe N. Phosphatidylinositol 3- kinase in cumulus cells is responsible for both suppression of spontaneous maturation and induction of gonadotropin-stimulated maturation of porcine oocytes. J Endocrinol. 2003;179(1):25–34. doi: 10.1677/joe.0.1790025. [DOI] [PubMed] [Google Scholar]
- 105.Blondin P, Sirard MA. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev. 1995;41(1):54–62. doi: 10.1002/mrd.1080410109. [DOI] [PubMed] [Google Scholar]
- 106.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 107.Hayashi M, McGee EA, Min G, Klein C, Rose UM, van Duin M, Hsueh AJ. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology. 1999;140(3):1236–1244. doi: 10.1210/endo.140.3.6548. [DOI] [PubMed] [Google Scholar]
- 108.Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod. 2002;67(3):1018–1024. doi: 10.1095/biolreprod.101.002527. [DOI] [PubMed] [Google Scholar]
- 109.Gueripel X, Brun V, Gougeon A. Oocyte bone morphogenetic protein 15, but not growth differentiation factor 9, is increased during gonadotropin-induced follicular development in the immature mouse and is associated with cumulus oophorus expansion. Biol Reprod. 2006;75(6):836–843. doi: 10.1095/biolreprod.106.055574. [DOI] [PubMed] [Google Scholar]
- 110.McNatty KP, Reader K, Smith P, Heath DA, Juengel JL. Control of ovarian follicular development to the gonadotrophin-dependent phase: a 2006 perspective. Soc Reprod Fertil. 2007;64:55–68. doi: 10.5661/rdr-vi-55. [DOI] [PubMed] [Google Scholar]
- 111.Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, Louhio H, Tuuri T, Sjöberg J, Bützow R, Hovata O, Dale L, Ritvos O. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;(8):2744–2750. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- 112.Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;7(1):316–321. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- 113.Lackey BR, Gray SL, Henricks DM. The insulin-like growth factor (IGF) system and gonadotropin regulation: actions and interactions. Cytokine Growth Factor Rev. 1999;10(3–4):201–217. doi: 10.1016/s1359-6101(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 114.Woodward TL, Xie J, Fendrick JL, Haslam SZ. Proliferation of mouse mammary epithelial cells in vitro: interactions among epidermal growth factor, insulin-like growth factor I, ovarian hormones, and extracellular matrix proteins. Endocrinology. 2000;141(10):3578–3586. doi: 10.1210/endo.141.10.7701. [DOI] [PubMed] [Google Scholar]
- 115.Weber GM, Moore AB, Sullivan C. In vitro actions of insulin-like growth factor-I on ovarian follicle maturation in white perch (Morone americana) Gen Comp Endocrinol. 2007;151(2):180–187. doi: 10.1016/j.ygcen.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 116.Assou S, Le Carrour T, Tondeur S, Strom S, Gabelle A, Marty S, Nadal L, Pantesco V, Réme T, Hugnot JP, Gasca S, Hovatta O, Hamamah S, Klein B, De Vos J. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells. 2007;25(4):961–973. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gasca S, Pellestor F, Assou S, Loup V, Anahory T, Dechaud H, De Vos J, Hamamah S. Identifying new human oocyte marker genes: a microarray approach. Reprod Biomed Online. 2007;4(2):175–183. doi: 10.1016/s1472-6483(10)60785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brison DR, Schultz RM. Increased incidence of apoptosis in transforming growth factor alpha-deficient mouse blastocysts. Biol Reprod. 1998;59(1):136–144. doi: 10.1095/biolreprod59.1.136. [DOI] [PubMed] [Google Scholar]
- 119.Morgan K, Wiemer K, Steuerwald N, Hoffman D, Maxson W, Godke R. Use of video cinematography to assess morphological qualities of conventionally cultured and cocultured embryos. Hum Reprod. 1995;10(9):2371–2376. doi: 10.1093/oxfordjournals.humrep.a136301. [DOI] [PubMed] [Google Scholar]
- 120.Kane MT, Morgan PM, Coonan C. Peptide growth factors and preimplantation development. Hum Reprod Update. 1997;3(2):137–157. doi: 10.1093/humupd/3.2.137. [DOI] [PubMed] [Google Scholar]
- 121.Teruel M, Smith R. Effect of embryo density and growth factors on in vitro preimplantation development of mouse embryos. Acta Physiol Pharmacol Ther Latinoam. 1997;47(2):87–96. [PubMed] [Google Scholar]
- 122.Dunglison GF, Barlow DH, Sargent IL. Leukaemia inhibitory factor significantly enhances the blastocyst formation rates of human embryos cultured in serum-free medium. Hum Reprod. 1996;11(1):191–196. doi: 10.1093/oxfordjournals.humrep.a019016. [DOI] [PubMed] [Google Scholar]
- 123.Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13(6):1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- 124.Keefe DL, Franco S, Liu L, Trimarchi J, Cao B, Weitzen S, Agarwal S, Blasco MA. Telomere length predicts embryo fragmentation after in vitro fertilization in women-toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005;192(4):1256–1260. doi: 10.1016/j.ajog.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 125.Cupisti S, Conn CM, Fragouli E, Whalley K, Mills JA, Faed MJ, Delhanty JD. Sequential FISH analysis of oocytes and polar bodies reveals aneuploidy mechanisms. Prenat Diagn. 2003;23:663–668. doi: 10.1002/pd.665. [DOI] [PubMed] [Google Scholar]
- 126.Pellestor F, Andréo B, Anahory T, Déchaud H, Hédon B, Hamamah S. The cytogenetics of human oocytes: 40 years of progress. Gynecol Obstet Fertil. 2005;33(5):283–292. doi: 10.1016/j.gyobfe.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 127.Bermúdez MG, Wells D, Malter H, Munné S, Cohen J, Steuerwald NM. Expression profiles of individual human oocytes using microarray technology. Reprod Biomed Online. 2004;8(3):325–337. doi: 10.1016/s1472-6483(10)60913-3. [DOI] [PubMed] [Google Scholar]
- 128.Arraztoa JA, Zhou J, Marcu D, Cheng C, Bonner R, Chen M, Xiang C, Brownstein M, Maisey K, Imarai M, Bondy C. Identification of genes expressed in primate primordial oocytes. Hum Reprod. 2005;20(2):476–483. doi: 10.1093/humrep/deh498. [DOI] [PubMed] [Google Scholar]


