Abstract
Public health agencies utilize aerial insecticides to interrupt an active West Nile virus (WNV) transmission cycle, which may expose WNV-infected birds to these agents. Although resmethrin has been considered benign to birds, no studies have evaluated whether the environmentally employed form of resmethrin with PBO synergist (synergized resmethrin (SR)) can suppress avian immunity to WNV infection and enhance a bird's host competence. Recognizing that wild birds confront toxicological stressors in the context of various physiological states, we exposed four groups (n = 9–11) of 9-week old chickens (Gallus domesticus) to drinking water with either SR (three alternate days at 50 µg/l resmethrin + 150 µg/l piperonyl butoxide), CORT (10 days at 20 mg/l to induce subacute stress), the combination of SR and CORT, or 0.10% ethanol vehicle coincident with WNV infection. Compared to controls, SR treatment did not magnify but extended viremia by one day, and depressed IgG; CORT treatment elevated (mean, 4.26 log10 PFU/ml) and extended viremia by two days, enhanced IgM and IgG, and increased oral virus. The combination of SR and CORT increased the number of chickens that shed oral virus compared to those treated with CORT alone. None of the chickens developed a readily infectious viremia to mosquitoes (none ≥ 5 log10 PFU/ml), but viremia in a CORT-exposed chicken was up to 4.95 log10 PFU/ml. Given that SR is utilized during WNV outbreaks, continued work toward a complete risk assessment of the potential immunotoxic effects of SR is warranted. This would include parameterization of SR exposures with immunological consequences in wild birds using both replicating (in the laboratory) and non-replicating (in the field) antigens. As a start, this study indicates that SR can alter some immunological parameters, but with limited consequences to primary WNV infection outcome, and that elevated CORT mildly enhances SR's immunotoxicity in chickens.
Keywords: resmethrin, corticosterone, west nile virus, avian, immunotoxicology, insecticide
1. Introduction
Debate over arbovirus control strategies remains contentious because concern regarding the relative risk of viral infection and environmental toxicant exposure is high, but inadequately characterized. Taking this into account, mosquito control agencies employ aerial insecticides only after arbovirus surveillance data indicate high local mosquito-infection-rates. Successfully mitigating the risk of adult mosquito-control insecticides (“adulticides”) to non-target species such as humans, beneficial insects, domestic animals, aquatic and terrestrial wildlife, while increasing their efficacy to reduce arbovirus outbreak intensity requires targeted scientific data from animal toxicity studies and environmental modeling and monitoring activities. For example, pyrethroid spraying activities were recently shown to reduce the number of flying mosquitoes (Elnaiem et al. 2008), while a related study indicated, for the first time, fewer human West Nile virus (WNV) cases in sprayed compared to unsprayed areas (Carney et al. 2008). Although these studies demonstrated short-term insecticide efficacy during specific outbreaks, spatiotemporally varied conditions such as vegetation density and mosquito resistance to a particular insecticide can affect mosquito-killing efficacy. Moreover, these studies did not and very few others have attempted to monitor environmental concentrations of adulticides or potential adulticide-exposure in non-target organisms in conjunction with mosquito control activities. Those that have investigated environmental concentrations of pyrethroids after adulticiding have detected resmethrin at 0–0.293 ppb (Abbene et al. 2005), permethrin at 0–9.40 ppb (Pierce et al. 2005) and piperonyl butoxide synergist at 0–60 ppb (Abbene et al. 2005; Schleier et al. 2008). Permethrin in sediments near agricultural activities have been found up to 459 ppb (Weston et al. 2004). Higher sediment than water pyrethroid concentrations are not surprising given the hydrophobicity of pyrethroids, suggesting oral exposures of non-target terrestrial species could be of a pulsed (water) or chronic (food) nature.
Evaluating the risk of insecticides to non-target species prior to governmental registration involves a consideration of the chemical's environmental fate and transport and any potential effects to those species given predicted environmental concentrations. However, risk assessment procedures often mandate only an evaluation of the active ingredient in a commercial formulation of an insecticide and do not require techniques that evaluate avian immunotoxicity (EPA 2008). This is an important issue for insecticides used to control WNV or other arboviruses because an immunosuppressed avian host may be more infectious to mosquitoes. Furthermore, a comprehensive evaluation of an adulticide's risk to wild birds should take into account the effect of natural variation in host health status due to exposures to multiple stressors on the immunotoxicity of a commercial formulation (i.e., not solely the active ingredient) of an insecticide.
During arbovirus control efforts, the formulation of resmethrin that is disseminated (Scourge® or synergized resmethrin (SR)) includes the synergist piperonyl butoxide (PBO, a p450 inhibitor (Casida 1980) and petroleum distillates). Resmethrin is highly toxic to terrestrial invertebrates, such as honey bees (Apis mellifera) (Murray 1985), and many aquatic taxa {Demoute, 1989 #88} including lobsters (Zulkosky et al. 2005) and fish (Paul et al. 2005), but has been considered relatively benign to mammals and birds (Neuschl et al. 1995). However, various investigators have found altered immune responses in mice, (Blaylock et al. 1995) chickens (McCorkle et al. 1980) and lobsters (De Guise et al. 2005) exposed to pyrethroids while others have shown that permethrin activates the hypothalamic pituitary adrenal axis in mice (de Boer et al. 1988). These studies are insufficient for an assessment of the human (Peterson et al. 2006) or ecological (Davis et al. 2007) risks of adulticides because they used high (parts per million) levels of only the active ingredients of a commercial insecticide and exposed only healthy subjects, not recognizing that physiological status can vary greatly in nature (McEwen and Wingfield 2003). Thus, to determine whether SR may be immunotoxic to birds at ppb levels, we assessed whether the domestic chicken (Gallus domesticus) might experience a higher WNV pathogenicity when exposed to three alternate days of waterborne SR, and if subacute elevations of corticosterone influences its immunotoxicity.
2. Methods
2.1 Experimental Design
The chickens utilized for this study were treated humanely with due consideration to the alleviation of their distress and discomfort, and according to University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC) protocol #A01059 and US Geological Survey National Wildlife Health Center IACUC protocol #EP040811. These protocols followed guidelines established in “The Guide for the Care and Use of Laboratory Animals” (1996)
The chicken was selected because it is readily available, can be selected for defined biological factors and is accustomed to captivity, and so provides ample sample sizes. Moreover, it constitutes a good initial organism to determine how significantly the environmental stressors used in this study might cause a bird to produce an elevated viremia without risking mortality. Species that naturally experience a higher viremia (e.g. American robin (Turdus migratorius), house sparrow (Passer domesticus) or American crow (Corvus brachyrhynchos) (Komar et al. 2003)) might succumb to an immunosuppression that results in a lethal viremia, effectively reducing the sample size and therefore the statistical power to detect subtle changes in infection outcome. The chicken was also chosen because factors such as age and sex (and thus reproductive hormones), previous toxicant exposures and infection status can all be easily controlled through the purchase of specific pathogen free chickens. And lastly, given that disease-related bird losses can be costly to growers, many commercially available reagents are available for detailed immunological studies of the chicken; however, these reagents do not predictably cross-react with the analogous biological molecules of other avian species.
Two experiments were performed for this study: (1) a pilot study to determine whether corticosterone (CORT)-exposed chickens (exposed to CORT but not SR, as described in the Supplementary Material) were immunosuppressed as demonstrated by reduced antibody response to sheep red blood cell (SRBC) inoculation; and, (2) an experiment to determine whether SR and CORT independently or interactively altered immunity to WNV. The pilot experiment is specifically described in Supplementary Material-Methods and only differs from below in that WNV and SR were not used, and 2 rather than 4 groups of 9–11 chickens were used. The specifics of the WNV study follow.
Forty-six SPF chicken eggs were obtained from Charles River Laboratories (Chicago, IL). The eggs were hatched, and chicks were raised without handling for 6 weeks at the University of Wisconsin-Madison Poultry Research Laboratory (UW PRL), Madison, WI, and then moved to a BSL-3 facility (USGS NWHC, Madison, WI) for the remainder of the experiment. We randomly distributed the chickens to 4 groups of 9–11 individuals. These birds were maintained at a 12:12 light:dark cycle and fed ad lib with UW PRL ration.
Four groups of 9-week old chickens (n = 9–11, see Table) were given drinking water that was mixed with one of four possible combinations of SR and corticosterone (CORT) in 0.10% ethanol vehicle: vehicle controls; CORT; SR; CORT+SR. CORT (Sigma #C2505, St. Louis, MO) was given for 10 continuous days from −6 days post inoculation (DPI) to 3 DPI at 20 mg/L drinking water; SR was given for 3 alternate days on −3, −1, & 1 DPI as 50 µg resmethrin + 150 µg PBO + petroleum distillates/L drinking water (diluted from Scourge4+12®, Bayer Environmental Science, Research Triangle Park, NC); CORT+SR: CORT was given as above and SR was added only on days as SR was above; 0.10% ethanol vehicle controls. CORT was given to simulate subacutely (not acutely or chronically) elevated adrenal activity, as the duration of elevated CORT is inextricably linked to immunological effect (Martin 2009) and chronic elevations (i.e., multiple weeks) are not likely in nature given an elevated risk of mortality for afflicted animals. We based the CORT concentration on a pilot study performed by the current authors (see Supplementary Material) in which antibody to SRBCs and corticosterone were measured, and on a study performed by Post et al. (Post et al. 2003). The SR water concentrations used in this study were determined by extrapolating from the manufacturer's label instructions (3 parts resmethrin: 1 part PBO at a rate of 3.18 g resmethrin/acre) if 1 acre of wetland was exposed and the upper 6 inches of water was sampled (Terracciano SA, personal communication). Chickens were challenged subcutaneously with either 100 µl bovine-albumin viral media (BA-1) containing 105 PFUs of American crow isolate 16399-3 WNV or with 100 µl BA-1 (n = 6, data not shown).
Table.
West Nile viremia profiles (PFU/ml [%positive]) and mean ± SEM viremia-days (VD = (mean log10 PFU/ml) × days viremic). Virus detection limit was 1.70 log10 PFU/ml
| Treatment Effect | 1 DPI | 2 DPI | 3 DPI | 4 DPI | VDa |
|---|---|---|---|---|---|
| Vehicle (n = 10) | 1.70–4.05 [100] | 1.70–2.70 [80] | < 1.7 [0] | < 1.7 [0] | 3.72±0.47 |
| SR (n = 10/11)b | 2.30–3.56 [91] | 1.70–2.90 [91] | 1.85–2.60 [50] | < 1.7 [0] | 4.81±0.45 |
| CORT (n = 9)c | 2.60–4.83 [89] | 3.30–4.95 [100] | 1.70–3.18 [44] | 1.70 [22] | 6.83±0.50* |
| CORT+SR (n = 10) | 3.78–4.86 [100] | 3.20–4.32 [100] | 1.70–2.18 [40] | < 1.7 [0] | 6.38±0.47 |
Notes:
Whole model results: F = 15.5252, R2 = 0.64, P < 0.0001
The SR group began with 11 subjects, but 1 subject was sacrificed on 3 DPI for a study that could not be completed. Thus, n = 11 to 2 DPI and n = 10 until the termination of the experiment (i.e., on 14 DPI).
One CORT subject was not properly inoculated and was thus removed from the analysis. Thus n = 9 for group CORT.
P < 0.0001
2.2. Sampling Protocol
After delivery from the UW PRL brooder facility, the chickens were not handled for 7 days. On the 8th day of housing (−16 DPI), mock fecal sampling was performed daily for 7 days. Each bird was captured from its pen and placed in a plastic poultry crate (0.142 m3) within the larger pen until defecation occurred (~10 minutes).
Starting on −9 DPI, fecal samples were collected daily to establish a baseline CORT level prior chemical and WNV treatments (Figure S1). Fecal samples were held on ice for up to 1 hour prior to freezing at −20°C. Blood samples, and oral and cloacal swabs were collected on −9 DPI for baseline virology and serology, and on DPI 1 – 5, 7, 10, and 14 to track the immune response to WNV. A blood smear was made, and blood samples were allowed to clot at room temperature for 30 minutes, chilled on wet ice, then centrifuged at 5000 × g for 15 minutes; serum was removed and frozen at −80°C until analysis. All oral and cloacal swabs were chilled on wet ice after collection, and then frozen at −80°C within 1 hour of sampling. Birds were weighed every blood-sampling day. All birds were euthanized via CO2 asphyxiation on 14 DPI.
2.3. Analysis of Fecal Metabolites of Corticosterone
We measured fecal glucocorticoid metabolites (FGM) but not CORT (Mostl et al. 2002) to quantitatively relate a non-invasive measure of “stress” to viremia and to verify that CORT given in drinking water was biologically available. Briefly, samples were thawed from −20°C to 95°C for 30 minutes, suspended in 60% methanol-water, vortexed on a multitube vortexer for 30 minutes, followed by 20 minutes of centrifugation at 2000 × g to clarify the metabolite suspension. One ml of this suspension was evaporated at 60°C for 24 hours then frozen at −20°C until analyzed for chicken CORT metabolites with a 3-α-11-oxo structure by EIA (Mostl et al. 2002; Mostl et al. 2005).
2.3. White Blood Cell Differential Counts
Blood smears were stained with Wrights-Giemsa. The heterophil:lymphocyte (H:L) ratio was calculated after identifying a total of 100 of these cells per smear at 1000×. This was performed as another measure of “stress” (Gross and Siegel 1983).
2.4. Serum Antibody (IgG and IgM) to WNVE
Serum anti-WNV envelope protein (WNVE)-IgG antibodies were detected by using a sandwich ELISA developed in our laboratory. We detected IgM using procedures based on Johnson et al. (Johnson et al. 2003). Pseudotiters are reported rather than titers because antibody levels were determined by comparison to a standard serum dilution curve rather than by a dilution of all samples. To estimate a standard curve from which we derived the reported psuedotiters, high positive controls (pooled chicken sera from 14 DPI for IgG and 10 DPI for IgM) were serially diluted two-fold from 1:100 to 1:25,600. Serum samples were considered WNVE-antibody positive if the ratio between its average optical density (OD), and the average OD of the negative control (pooled chicken sera from −9 DPI) was greater than 2.0. IgG pseudotiters for positive samples were then calculated from the standard curve's optical density versus dilution slope equation. See Supplementary Material for complete assay protocol details.
2.5. Virus Detection
Vero cells were used to detect the presence of virus in serum, oral and cloacal swab media by plaque formation. Viremia (plaque forming units (PFU)/ml serum) was calculated from the serum dilution that produced between 5 and 30 plaques per well. Oral and cloacal swab samples were deemed virus positive when 1 or more plaques were visible at a 1/5 dilution.
2.6. Statistical Analysis
All data were analyzed for main (SR and CORT) and interactive (SR*CORT) effects using a 2 × 2 model. Cell culture time-course data were analyzed with a Poisson-linked generalized linear mixed model in which treatment was the fixed effect and subject was the random effect ('lmer' function, R 2.8.1, the R Foundation for Statistical Computing). Antibody pseudotiters were analyzed with a general linear mixed model (Gaussian) with effects modeled as in cell culture time-course data ('lme' function, R 2.8.1). Percent anti-WNVE-IgG/IgM and oral swab positive data were tested by Fisher's exact test. The fraction of days a bird was oral swab positive was tested by 2-way ANCOVA. These tests were performed with SAS JMP IN 5.1.2 (Cary, NC).
3. Results
3.1. Stress Response
As anticipated, chickens exposed to CORT excreted more FGM (Figure S1) and exhibited higher heterophil:lymphocyte ratios (Figure S2) than vehicle-controls (P < 0.0001 for both endpoints), but SR exposure did not impact these measurements. The addition of CORT and SR did not interactively alter FGM or heterophil:lymphocyte ratio. None of the chickens exhibited mortality or clinical signs indicative of morbidity in response to the treatments used in this study.
3.2. Viremia and Oral Virus Shedding
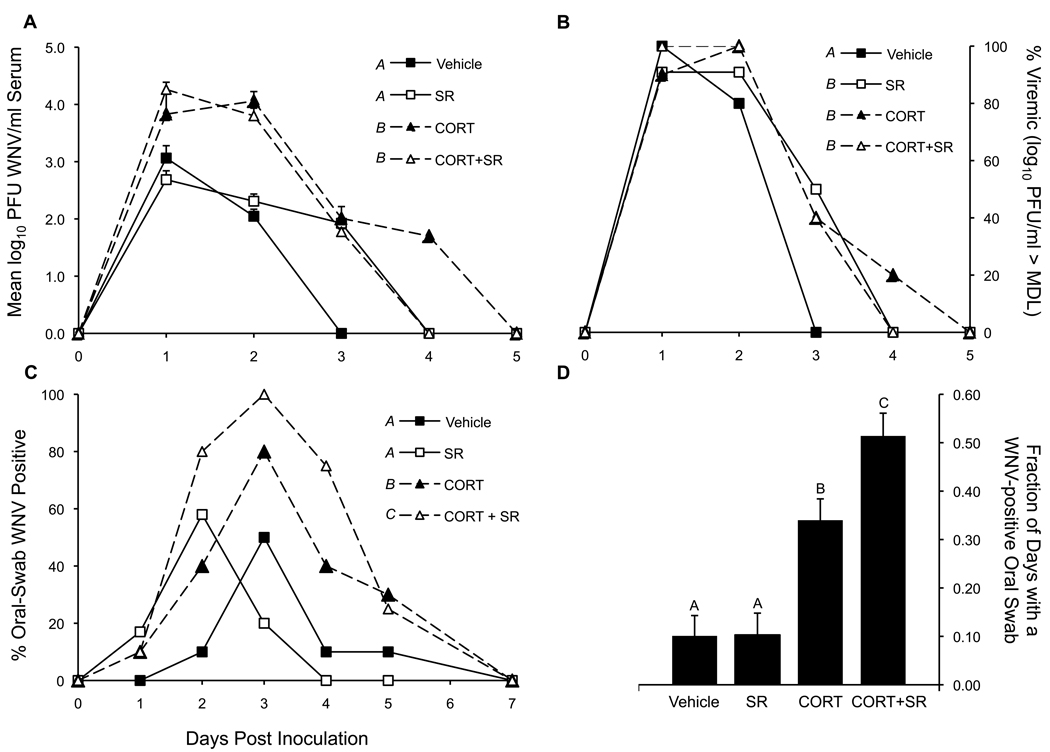
CORT was the only statistically significant factor that affected differences in total viremia across the experimental period (P < 0.0001). Neither SR nor CORT+SR treatment altered total viremia response curves compared to controls and CORT treatment, respectively (Figure 1a). However, 50% of the birds treated with SR alone were viremic one day longer than controls (3 DPI vs. 2 DPI, respectively, P = 0.0163) (Figure 1b), although SR-exposed birds on 3 DPI were just above the virus detection threshold of 1.70 log10 PFU/ml (Table). Corticosterone treatment elevated viremia on 2 DPI by a mean of 102 and a median of 91.8 fold over controls (Figure 1a), respectively, and extended it by 2 days (44% and 22% WN viremia positive on DPI 3 and 4, respectively) compared to controls (Figure 1b and Table). Mean viremia was nominally highest among the CORT+SR-treated birds on 1 DPI (4.26 log10 PFU/ml serum) compared to vehicle controls' mean viremia of 3.06 log10 PFU/ml on the same day. CORT-treated birds experienced a peak viremia of 4.95 log10 PFU/ml on 2 DPI and vehicle-controls reached a peak viremia of 4.05 log10 PFU/ml on 1 DPI. No virus was detected in any birds on or after 5 DPI.
Figure 1. Viremia and oral shedding profiles.
(a) Mean ± SEM viremia for all subjects. Means presented as on the Y-axis in log10 PFU/ml when > minimum detection limit (MDL); or, as 0.00 log10 PFU/ml when < MDL (MDL for Vero cell plaque forming assay = 1.70 log10 PFU/ml). Viremia statistics summarize total viremia response curves. P < 0.0001. (b) Percent of chickens within a treatment group with a viremia greater than the MDL for the Vero cell plaque-forming assay (i.e., % positive for WN viremia within a treatment group). P < 0.05 between different letter superscripts for 3 DPI (c) Percent of chickens within a treatment group that were shedding oral virus on a given DPI. (d) Mean ± SEM fraction of days a chicken shed virus while it was alive from 1–5 DPI. P < 0.05 between different letter superscripts for panel c and d.
The viremia-day value (VD = mean log10 PFU/ml of a bird for all days that the bird was viremic multiplied by the number of days that the bird was viremic) for vehicle-treated birds was lower (mean VD 3.72) than all other treatment-groups: CORT most significantly boosted VD (mean VD 6.83, P < 0.0001), CORT and SR did not interactively alter VD (CORT+SR group mean VD 6.38, P = 0.1118), and SR did not impact VD (mean VD 4.81, P = 0.5023) (Table).
West Nile virus was detectable on oral swabs for up to 5 DPI (Figure 1c). Cumulatively, vehicle, SR, CORT and CORT+SR treated-birds shed live WNV in 16.0, 20.4, 40.0 and 58.7% of oral swabs taken, respectively. Corticosterone-treated birds shed virus orally for more days (DPI 1 to 5) than vehicle (DPI 2 to 5) or SR (DPI 1 to 3) treated birds. When comparing the level of oral shedding between groups from DPI 2 – 4 (days of largest group-wise differences), we found that more CORT+SR-treated birds shed oral virus than CORT-treated birds and than all others (P = 0.0079 and P < 0.0001, respectively) but vehicle controls and SR treated birds did not statistically differ by this measure (P = 0.4449) (Figure 1c). We assessed overall (1–5 DPI) treatment effects on the fraction of days a bird was oral-swab-positive and adjusted this to the number of days a bird was living because 1 SR-treated bird was sacrificed for a study that could not be completed. We found that SR did not, but CORT (P < 0.0001) and the interaction of SR and CORT (P = 0.0347) significantly augmented the fraction of days a bird was alive with oral virus (Figure 1d). Cloacal swab data are not presented because shedding from the cloaca was minimal, sporadic, and not related to treatment.
3.3. Antibody to West Nile Virus
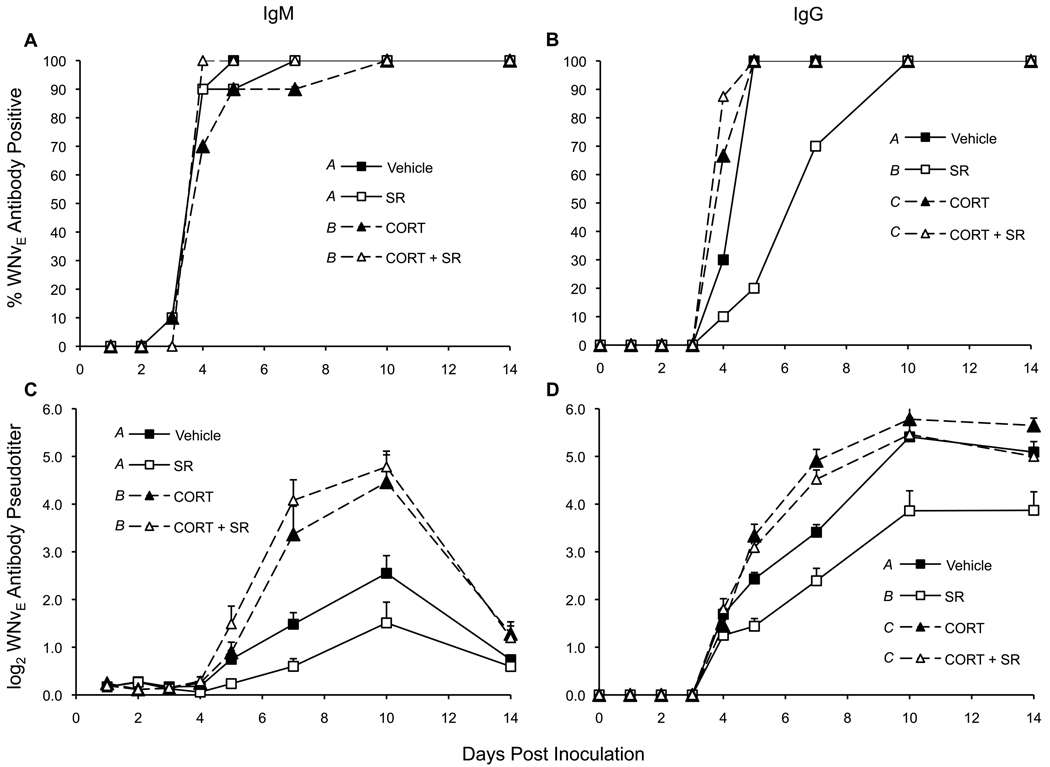
WNVEIgM (IgM) activity was first detected on 3 DPI (Figure 2a) whereas WNVEIgG (IgG) was not detected until 4 DPI (Figure 2b). Both IgM (Figure 2c) and IgG (Figure 2d) did not increase in quantity until 4 DPI. IgM peaked at 10 DPI followed by a consistent drop by 14 DPI; no change was seen in IgG pseudotiters between 10 and 14 DPI. We are not able to assess when IgG might have peaked because all birds were sacrificed on 14 DPI.
Figure 2. WNVEIgM and WNVEIgG profiles.
(a) IgM and (b) IgG were considered WNVE-antibody positive by ELISA when the OD of sample wells were > 2.0 times the OD of negative control wells. The mean ± SEM log2 pseudotiters of (c) IgM and (d) IgG were calculated by inserting a sample's OD into a dilution curve of WNVE-antibody positive chicken sera. P < 0.0001 between different letter superscripts.
Calculating the percent of birds positive for IgM and IgG reactive to WNV E protein in ELISA, we found that there were no treatment-related patterns in IgM, but treatment did impact this measure of IgG. Low levels of IgM activity were first detected on 3 DPI in 10% of the birds of each treatment group except CORT+SR treated birds; most birds were IgM-positive by 4 DPI. Corticosterone accelerated IgG seroconversion compared to vehicle-controls (77.5% compared to 30% becoming IgG-positive on 4 DPI, respectively) (CORT > vehicle, P = 0.0142). Thereafter, all CORT-treated birds and vehicle-controls were IgG-positive. SR treatment attenuated IgG production (Figure 2b). On 4 DPI, 10% were IgG positive to WNVE compared to 30% of controls; on 5 DPI, 20% were IgG-positive compared to 100% of controls; 70% were positive on 7 DPI, and on 10 DPI, 100% seroconverted. However, these differences were only statistically significant on 5 DPI (SR < vehicle, P = 0.0004) and for the total number of IgG2-positive birds from 4–14 DPI (SR < vehicle, P = 0.0031).
During the days of greatest treatment-wise differences (IgM, 3–10 DPI; IgG, 4–14 DPI), IgG pseudotiters varied more strongly with treatment than IgM. CORT enhanced both IgM (Figure 2c) and IgG (Figure 2d) quantity (P < 0.0001) whereas SR did not affect IgM levels (P = 0.4522) but suppressed IgG (P < 0.0001). There was no interaction detected between CORT and SR the production of IgM or IgG. At 14 DPI, IgM levels were the same between all treatment groups, whereas IgG levels in CORT-treated birds' statistically matched vehicle controls, and SR-treated birds exhibited lower IgG levels compared to all others (P < 0.05).
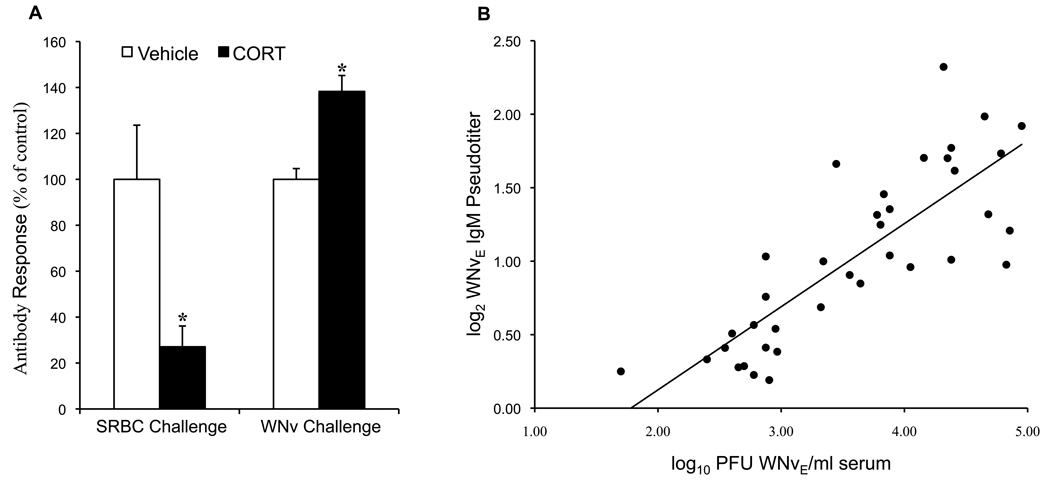
The finding that CORT treatment was associated with advanced seroconversion compared to controls, prompted us to compare the present data to a pilot study in which sheep RBCs (SRBCs) rather than WNV were used as the test antigen. We found that although antibody to WNV was higher (on 7 DPI), antibody to SRBCs was significantly depressed (on 6 DPI) (Figure 3a) in CORT-treated chickens compared to controls, and that bursa weights responded to CORT treatment equally with both antigens (data not shown). We hypothesized that antibody (IgM or IgG) production was directly related to the magnitude of viremia and found a positive correlation; the amount of virus present correlated with IgM more strongly than with IgG (R2 = 0.67 vs. 0.38, respectively) (Figure 3b shows IgM data).
Figure 3. Effect of antigen on antibody production.
(a) In a pilot experiment, CORT-exposed chickens were inoculated (i.p.) with 10% sheep red blood cells (SRBC) in sterile PBS and tested for antibody production on day 6 post inoculation (by hemagglutination inhibition assay) and this was compared to CORT-exposed chickens' production of anti-WNVE IgM (by ELISA) on day 7 post inoculation (* indicates statistical significance at P < 0.0005). (b) Correlation between viremia and anti-WNVE IgM production (F=69.6, R2=0.67, P < 0.0001). (WNVEIgG correlation not shown, F=21.3, R2=0.38, P < 0.0001.)
3.4. Relationship between viremia and fecal corticosterone
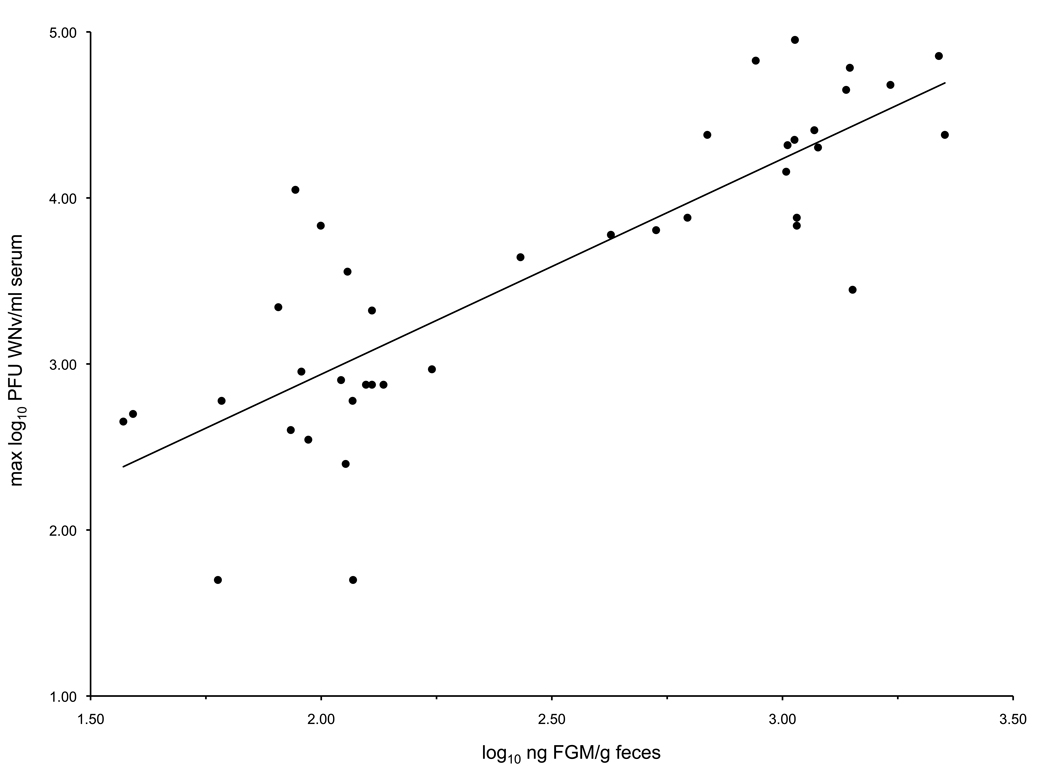
Fecal glucocorticoid metabolites measured on the day of maximum viremia correlated positively with that day's viremia (F=83.8, R2=0.69, P < 0.0001) (Figure 4).
Figure 4.
Correlation between FGM and maximum WN viremia (F=83.8, R2=0.69, P < 0.0001).
4. Discussion
Our findings demonstrate that corticosterone elevation and SR exposure in chickens led to an altered immunological response to WNV infection, with each treatment and treatment interaction yielding different results. Compared to controls, birds exposed to corticosterone exhibited a higher and longer viremia, produced higher IgM and IgG pseudotiters, and more birds shed oral virus; whereas, SR-exposed birds had similar levels of viremia, but were viremic one day longer, produced lower IgG psuedotiters, and shed similar levels of virus. We observed an interaction between CORT and SR exposure in just one endpoint: more birds treated with CORT+SR shed oral WNV than birds treated with CORT or SR alone.
Given the choice of the domestic chicken as a model organism for these studies and its associated advantages as noted above, future studies are poised to pursue two distinct research avenues: (1) detailed mechanistic studies of how the nature of these stressors impacts immunity to WNV in the chicken; and, (2) investigations of the significance of these immunoaltering stressors on the ecology of WNV transmission dynamics in more host competent species such as the American robin or house sparrow. The following discussion points explore these two directions in the context of our data.
4.1. Immunotoxicity of Synergized Resmethrin
We exposed the chickens to a scenario with potential environmental relevance in which adulticides are sprayed every other day for three days, leading to transient SR residues in water. With the SR concentrations used in this study (50 ppb resmethrin and 150 ppb PBO) and the estimated low SR dose (≅0.03 µg/kg; or, 4×10−7 X resmethrin's LD50 red-winged blackbird (Agelaius phoeniceus) of 75 mg/kg, and 6×10−9 X resmethrin's LC50 (bobwhite quail, Colinus virginianus) of >5000 ppm (2006)), we predicted interactive immunosuppressive effects with CORT, but no specific immunotoxic effects. However, as indicated above, we observed both types of effects. Compared to controls, we found that SR did not magnify viremia but extended it by 1 day, enhanced oral shedding of WNV in CORT-treated birds, and suppressed WNVEIgG (the antibody isotype that is relevant to subsequent infections) production. Synergized resmethrin did not affect total viremia over the course of the experiment but did cause IgG suppression, indicating that IgG was related to SR treatment but not viremia. This finding also suggests that the level of IgG suppression in SR-treated birds did not impact the immune defense against first WNV infection in these chickens. This is perhaps not surprising, as IgM (antibody isotype relevant to first infections) levels were not affected by SR. The importance of SR-related IgG suppression in the outcome of a subsequent WNV infection should be tested.
We are not aware of any other published studies that have tested the impact of the commercial formulation of pyrethroids on avian immunity. Immunosuppression upon permethrin exposure has been demonstrated in chickens, but the chickens were orally (feed) exposed for 6 weeks to only the active ingredient of mg/kg (ppm) permethrin (McCorkle et al. 1980) (compared to the 3 alternate days of exposure to ppb levels of the commercial formulation of resmethrin (SR) used in this study). In a variety of other studies, pyrethroids have been found to produce no effects on immunity (rats exposed to mg/kg permethrin levels for 28 days (Institoris et al. 1999)), immunosuppression (lowered lymphocyte activity in mice orally exposed to 40 µg/kg (0.1% mouse LD50) (Blaylock et al. 1995)), or immunostimulation (higher antibody forming cells in rats exposed to mg/kg deltamethrin (Madsen et al. 1996)). These discrepancies might be due to concentration differences or species differences in the gastro-intestinal absorption or metabolism of pyrethroids. We suggest that the combined effects of species differences in pyrethroid metabolism and chemical exposure regimes account for these contrasting results because pyrethroids are orally bioavailable to mammals (Miyamoto 1976) and birds (Christopher et al. 1985). Considering that published studies have shown immunostimulation after mg/kg pyrethroid exposures and that the current study resulted in mild IgG suppression at µg/kg pyrethroid exposures, we hypothesize that low pyrethroid levels (µg/kg) are antibody suppressive and high levels (mg/kg) are immunostimulating. Immunomodulation may occur through the alteration of cytokine profiles, as Diel et al. (Diel et al. 1999) reported pyrethroid-induced IL-4/INF-© ratio shifts in human lymphocyte culture depending on the subject's immune status and culture duration. Perhaps different pyrethroid concentrations [or formulations] differently affect this ratio leading to either antibody suppression (reduced ratio) or antibody stimulation (increased ratio). The former could account for the current observations (Figure 2d), while the later would provide a basis for the reported allergenic effects of pyrethroids (Diel et al. 1998; Hoellinger et al. 1987). Based on our findings in chickens, further avian immunotoxicologal work should determine whether wild birds (that host WNV) are affected by SR exposure similarly or differently from chickens, and if IgG suppression would have any affect on the limitation of viremia upon second WNV infection.
4.2. Corticosterone’s impact on the immune response to WNV
This is the first study to quantitatively relate the immune response of any bird to a non-invasive measurement of corticosterone (i.e., FGM), and thus provides a convenient means to predict a chicken's potential susceptibility to WNV. Further, mechanistic insights on a chicken's immunity to WNV are possible given the extensive (yet incomplete) literature on CORT's effects on immunity.
Our observations that the heterophil:lymphocyte ratio (Figure S2), oral shedding, viremia (Figure 1) and antibody (Figure 2) were all higher than controls in CORT-exposed birds may be explained by understanding that, in general, CORT causes a shift away from immunity focused on intracellular pathogens to extracellular agents through a polarization from TH1 to TH2 cytokines (Daynes and Araneo 1989). Specifically, IFN-γ (a key TH1 and WNV cytokine (Shrestha et al. 2006)) falls, whereas IL-4 (a major TH2 cytokine) rises upon CORT exposure (Daynes and Araneo 1989). CORT also causes heterophils (avian phagocytic cells) to exit and lymphocytes to enter subcutaneous tissues, perhaps leading to a reduced ability to phagocytize extravascular antigens.
It is plausible that the higher viremia and thus antigenic stimulus caused by CORT's presumed immunosuppressive effects on innate immunity, led to enhanced IgM, and this, coupled with a shift towards TH2 type responses explain the enhanced level of IgG. Perhaps the signal provided by a replicating antigen to the immune system overrides the negative impact of CORT on antibody production because CORT significantly depressed antibody levels in chickens exposed to non-replicating SRBC antigens (Figure 3). The finding that CORT-treated birds exhibited higher viremia and higher antibody levels suggests the relative importance of innate immunity over antibody upon primary WNV infection in birds, or that the antibody generated or measured was not specifically neutralizing to WNV.
4.3. Mild interactive effects of SR and corticosterone
An interaction between CORT and SR was observed only in elevated oral shedding profiles (Figure 1c,d), an endpoint indicative of a toxicological effect, but not as important to WNV transmission dynamics as an altered viremia. Corticosterone increased oral shedding of WNV (69% of CORT-treated birds were oral swab positive vs. 26% of control birds on 2–4 DPI). Comparing CORT- to CORT+SR-treated birds, there was a consistent increase (53% vs. 86% positive for DPI 2 – 4, respectively) in oral shedding by CORT+SR-exposed birds. This might be due to a physically destructive effect of SR on infected cells lining the oral mucosa leading to leakage of intracellular contents into the oral cavity although we have no direct evidence for this speculation. Perhaps in conjunction with this effect, the higher viral load in CORT-exposed birds (compared to vehicle or SR-treated birds), and given this study's result that SR lowered IgG production, SR may have reduced the amount of mucosal antibody (e.g., IgA), and led to a reduced sequestration of WNV in the mouths of CORT+SR treated birds. However, the relationship between secretory IgA levels and the magnitude of WNV oral shedding in birds is unknown.
Mechanism aside, this finding is more interesting immunotoxicologically than epidemiologically because oral virus shedding is not considered to be a major factor in WNV transmission. Additionally, the inconsistent relationship between the effects of CORT+SR treatment on the endpoints evaluated leads us to conclude that CORT did not greatly alter SR's immunotoxicity.
4.4. An Immunological Perspective
Based on the above, we suggest that CORT does but SR does not have an effect on innate immunity and that this is the primary determinant of an effective immune response to first infection with WNV in chickens. Specifically, given that (1) viremia magnitude and IgM and IgG levels were higher in CORT-exposed birds than vehicle-control birds; and, (2) total viremia was the same in SR-exposed and vehicle-control birds (even though SR extended it by 1 day), but IgG was lower in the former than the latter group, we suggest that innate immunity is more important in viremia limitation than antibody production to first infection. However, this is a somewhat tenuous conclusion because IgM production was not suppressed by SR, and we did not evaluate the neutralizing capacity of the serum using the plaque reduction neutralization test. To more conclusively assess the relevance of ELISA-measured IgG suppression (SR exposure) or magnification (CORT exposure), a second WNV challenge is required to monitor viremia levels along with antibody measurements by ELISA and the plaque reduction neutralization test.
4.5 Evaluating Immunosuppression in Multiply Stressed Subjects
This study evaluated whether physiological stress (i.e., subacute CORT elevation as can periodically occur during an animal's life cycle) enhances the immunotoxicity of SR in the chicken. Although our findings were mostly negative for CORT*SR interactions, this study illustrates the importance of a consideration of other immunoaltering physiological factors such as CORT elevation (i.e., fecal glucocorticoid metabolite (FGM) levels) when interpreting the impact of a toxicant's role in detected immunoalteration (Pruett et al. 2009). This is because although FGM levels were the same in vehicle- and SR-exposed birds, IgG was reduced only after SR exposure. In studying the immunotoxicological impacts of SR exposure in wild birds, simultaneously measuring FGM levels and SR exposure (e.g., presence of SR metabolites bird excreta or less convincingly, SR residues in their food or water) would help to determine whether SR or general physiological stress caused any observed immunosuppression.
4.5. An Environmental Perspective
With pyrethroid-resistant mosquitoes continuing to emerge (Brogdon and McAllister 1998; N'Guessan et al. 2007), the capacity for pyrethroids to interrupt WNV outbreaks (Carney et al. 2008; Reddy et al. 2006), and the currently demonstrated antibody suppressive impacts in chickens, further work should aim to place these findings into an environmental and epidemiological context. For example, the insecticide levels used in this study were 171 (resmethrin) and 2.5 (piperonyl butoxide) times more than what has been found in water after adulticiding campaigns for WNV (Abbene et al. 2005; Schleier et al. 2008) but 10% of what has been found in sediments near agriculture (Weston et al. 2004). Even so, this study suggests that physiological stress (i.e., subacutely elevated CORT) presents a higher potential of augmenting WN viremia in birds than SR exposure, and that CORT would not appreciably enhance SR's immunotoxicity. Similarly impacted chickens would likely not significantly augment local WNV transmission dynamics. This is because host viremia levels below 5 log10 PFU/ml are not thought to infect an epidemiologically significant number of mosquitoes (Chamberlain et al. 1954; Komar et al. 2003; Lord et al. 2006; Reisen et al. 2008)and neither CORT- (maximum mean viremia, 4.26 log10 PFU/ml on 1 DPI) nor SR- (maximum mean viremia, 2.68 log10 PFU/ml on 1 DPI) exposed birds achieved this level. However, understanding the balance between reduced vector abundance and host immunocompetence on local WNV transmission dynamics upon SR aduliticing campaigns remains to be studied. Given our results which suggest an immunotoxicity of SR in chickens, more WNV host-competent species (i.e., species that naturally support a higher viremia [than chickens]) like the American robin (Hamer et al. 2009) or house sparrow should be utilized to investigate this question through both laboratory and field studies. Species like the American crow may not be appropriate for these types of studies due to its high susceptibility and potential for death by WNV infection.
Our results highlight the advantages (i.e., real-life consequences of toxicant-induced immunomodulation) as well as the difficulties (i.e., elevated anti-WNV antibody but suppressed anti-SRBC antibody upon CORT exposure) of studying immunotoxicity using live virus infections rather than non-replicating antigens. But given that we have found immunotoxicity at ppb levels of SR, existing ecological risk assessments of pyrethroids should be updated with further field and laboratory based immunotoxicological studies. For example, these may involve an evaluation of the type (air, water, soil or sediment) and magnitude of SR exposure on WNV infection outcome in wild-caught birds in the laboratory, and on immunocompetence in free-ranging birds using non-replicating antigens in the field. Additionally, the impacts of PBO synergism on resmethrin's immunotoxicity should be specifically investigated in order to further understand what exposure factors account for our data. Together, this would help to determine the larger significance of this study's results that SR altered some immunological parameters, but with limited consequences to primary WNV infection outcome, and that elevated CORT mildly enhanced SR's immunotoxicity in chickens.
Supplementary Material
Acknowledgements
We thank L. Karwal, M. Lund, D. Berndt of U.S. Geological Survey, and J. Neimuth, C. Fassbinder-Orth, J. Berkowitz, B. Basler, K. Montenero of UW-Madison for their animal handling and assay assistance. We are also grateful to S. Terracciano (U.S. Geological Survey) and E. Paul (N.Y. Department of Environmental Conservation) for their advice concerning pyrethroid concentrations used in this study. Finally, we thank M. Cook (UW-Madison) and J. Fair (Los Alamos National Laboratory) for their constructive comments on an earlier version of this manuscript. Financial support for this work was provided by the USGS Biological Resources Discipline through the Great Lakes–Northern Forest Cooperative Ecosystems Study Unit, University of Wisconsin–Madison award 02HQAG0112. We also acknowledge the National Research Service Award Predoctoral Traineeship awarded to M.D.J., National Institutes of Environmental Health Sciences Training Grant T32 ES07015, Molecular and Environmental Toxicology Center, University of Wisconsin–Madison.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Elnaiem D-EA, Kelley K, Wright S, Laffey R, Yoshimura G, Reed M, Goodman G, Thiemann T, Reimer L, Reisen WK, Brown D. Impact of Aerial Spraying of Pyrethrin Insecticide on Culex pipiens and Culex tarsalis (Diptera: Culicidae) Abundance and West Nile Virus Infection Rates in an Urban/Suburban Area of Sacramento County, California. Journal of Medical Entomology. 2008;45:751–757. doi: 10.1603/0022-2585(2008)45[751:ioasop]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Carney R, Husted S, Jean C, Glaser C, Kramer V. Efficacy of Aerial Spraying of Mosquito Adulticide in Reducing Incidence of West Nile Virus, California, 2005. Emerging Infectious Diseases. 2008;14:747–754. doi: 10.3201/eid1405.071347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbene IJ, Fisher SC, Terracciano SA USGS. Concentrations of Insecticides in Selected Surface Water Bodies in Suffolk County, New York, Before and After Mosquito Spraying, 2002–04. Reston, VA, U.S.A: 2005. [Google Scholar]
- Pierce RH, Henry MS, Blum TC, Mueller EM. Aerial and tidal transport of mosquito control pesticides into the Florida Keys National Marine Sanctuary. Rev Biol Trop. 2005;53 Suppl 1:117–125. [PubMed] [Google Scholar]
- Schleier JJ, Peterson RKD, Macedo PA, Brown DA. Environmental concentrations, fate, and risk assessment of pyrethrins and piperonyl butoxide after aerial ultralow-volume applications for adult mosquito management. Environmental Toxicology and Chemistry. 2008;27:1063–1068. doi: 10.1897/07-532.1. [DOI] [PubMed] [Google Scholar]
- Weston DP, You J, Lydy MJ. Distribution and toxicity of sediment-associated pesticides in agriculture-dominated water bodies of California's Central Valley. Environmental Science & Technology. 2004;38:2752–2759. doi: 10.1021/es0352193. [DOI] [PubMed] [Google Scholar]
- E.P.A. U.S., editor. Final rule. Washington D.C., U.S.A: 2008. Protection of environment. Data requirements for pesticides. [Google Scholar]
- Casida JE. Pyrethrum flowers and pyrethroid insecticides. Environ Health Perspect. 1980;34:189–202. doi: 10.1289/ehp.8034189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. Acute and residual toxicity of a new pyrethroid insecticide, WL85871, to honey-bees. Bull Environ Contam Toxicol. 1985;34:560–564. doi: 10.1007/BF01609776. [DOI] [PubMed] [Google Scholar]
- Jean-Pierre D. A brief review of the environmental fate and metabolism of pyrethroids. Pesticide Science. 1989;27:375–385. [Google Scholar]
- Zulkosky AM, Ruggieri JP, Terracciano SA, Brownawell BJ, McElroy AE. Acute toxicity of resmethrin, malathion and methoprene to larval and juvenile American lobsters (Homarus Americanus) and analysis of pesticide levels in surface waters after Scourge, Anvil, and Altosid application. Journal of Shellfish Research. 2005;24:795–804. [Google Scholar]
- Paul EA, Simonin HA, Tomajer TM. A comparison of the toxicity of synergized and technical formulations of permethrin, sumithrin, and resmethrin to trout. Arch Environ Contam Toxicol. 2005;48:251–259. doi: 10.1007/s00244-003-0110-9. [DOI] [PubMed] [Google Scholar]
- Neuschl J, Kacmar P, Poracova Toxicologic evaluation of supermethrin, a pyrethroid insecticide, in rabbits and pheasants. Vet Med (Praha) 1995;40:383–386. [PubMed] [Google Scholar]
- Blaylock BL, Abdel-Nasser M, McCarty SM, Knesel JA, Tolson KM, Ferguson PW, Mehendale HM. Suppression of cellular immune responses in BALB/c mice following oral exposure to permethrin. Bull Environ Contam Toxicol. 1995;54:768–774. doi: 10.1007/BF00206111. [DOI] [PubMed] [Google Scholar]
- McCorkle F, Taylor R, Martin D, Glick B. The effect of permethrin on the immune-response of chickens. Poultry Science. 1980;59:1568–1568. doi: 10.3382/ps.0591324. [DOI] [PubMed] [Google Scholar]
- De Guise S, Maratea J, Chang ES, Perkins C. Resmethrin immunotoxicity and endocrine disrupting effects in the American lobster (Homarus americanus) upon experimental exposure. Journal of Shellfish Research. 2005;24:781–786. [Google Scholar]
- de Boer SF, van der Gugten J, Slangen JL, Hijzen TH. Changes in plasma corticosterone and catecholamine contents induced by low doses of deltamethrin in rats. Toxicology. 1988;49:263–270. doi: 10.1016/0300-483x(88)90007-8. [DOI] [PubMed] [Google Scholar]
- Peterson R, Macedo P, Davis R. A human-health risk assessment for West Nile virus and insecticides used in mosquito management. Environmental Health Perspectives. 2006;114:366–372. doi: 10.1289/ehp.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RS, Peterson RK, Macedo PA. An ecological risk assessment for insecticides used in adult mosquito management. Integr Environ Assess Manag. 2007;3:373–382. [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Grossblatt N, editor. The Guide for the Care and Use of Laboratory Animals. Washington D.C., U.S.A.: National Academies Press; 1996. [PubMed] [Google Scholar]
- Martin LB. Stress and immunity in wild vertebrates: Timing is everything. Gen Comp Endocrinol. 2009 doi: 10.1016/j.ygcen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Post J, Rebel JM, ter Huurne AA. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult Sci. 2003;82:1313–1318. doi: 10.1093/ps/82.8.1313. [DOI] [PubMed] [Google Scholar]
- Mostl E, Maggs JL, Schrotter G, Besenfelder U, Palme R. Measurement of cortisol metabolites in faeces of ruminants. Vet Res Commun. 2002;26:127–139. doi: 10.1023/a:1014095618125. [DOI] [PubMed] [Google Scholar]
- Mostl E, Rettenbacher S, Palme R. Measurement of corticosterone metabolites in birds' droppings: an analytical approach. Ann N Y Acad Sci. 2005;1046:17–34. doi: 10.1196/annals.1343.004. [DOI] [PubMed] [Google Scholar]
- Gross WB, Siegel HS. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 1983;27:972–979. [PubMed] [Google Scholar]
- Johnson AJ, Langevin S, Wolff KL, Komar N. Detection of anti-West Nile virus immunoglobulin M in chicken serum by an enzyme-linked immunosorbent assay. J Clin Microbiol. 2003;41:2002–2007. doi: 10.1128/JCM.41.5.2002-2007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E.P.A. U.S., editor. Reregistration eligibility decision for resmethrin. Washington D.C., U.S.A.: 2006. [Google Scholar]
- Institoris L, Undeger U, Siroki O, Nehez M, Desi I. Comparison of detection sensitivity of immuno- and genotoxicological effects of subacute cypermethrin and permethrin exposure in rats. Toxicology. 1999;137:47–55. doi: 10.1016/s0300-483x(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Madsen C, Claesson MH, Ropke C. Immunotoxicity of the pyrethroid insecticides deltametrin and alpha-cypermetrin. Toxicology. 1996;107:219–227. doi: 10.1016/0300-483x(95)03244-a. [DOI] [PubMed] [Google Scholar]
- Miyamoto J. Degradation, Metabolism and toxicity of synthetic pyrethroids. Environmental Health Perspectives. 1976;14:15–28. doi: 10.1289/ehp.761415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher RJ, Munger CE, Ivie GW. Distribution and depletion of [C-14] resmethrin isomers administered orally to laying hens. Pesticide Science. 1985;16:378–382. [Google Scholar]
- Diel F, Horr B, Borck H, Savtchenko H, Mitsche T, Diel E. Pyrethroids and piperonyl-butoxide affect human T-lymphocytes in vitro. Toxicol Lett. 1999;107:65–74. doi: 10.1016/s0378-4274(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Diel F, Detscher M, Schock B, Ennis M. In vitro effects of the pyrethroid S-bioallethrin on lymphocytes and basophils from atopic and nonatopic subjects. Allergy. 1998;53:1052–1059. doi: 10.1111/j.1398-9995.1998.tb03814.x. [DOI] [PubMed] [Google Scholar]
- Hoellinger H, Lecorsier A, Sonnier M, Leger C, Do Cao T, Nguyen Hoang N. Cytotoxicity, cytogenotoxicity and allergenicity tests on certain pyrethroids. Drug Chem Toxicol. 1987;10:291–310. doi: 10.3109/01480548709042988. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Araneo BA. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989;19:2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, Diamond MS. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Schwab C. Patterns of Immunotoxicity Associated with Chronic as Compared with Acute Exposure to Chemical or Physical Stressors and their Relevance with Regard to the Role of Stress and with Regard to Immunotoxicity Testing. Toxicol. Sci. 2009;109:265–275. doi: 10.1093/toxsci/kfp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerg Infect Dis. 1998;4:605–613. doi: 10.3201/eid0404.980410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MR, Spielman A, Lepore TJ, Henley D, Kiszewski AE, Reiter P. Efficacy of resmethrin aerosols applied from the road for suppressing Culex vectors of West Nile virus. Vector Borne and Zoonotic Diseases. 2006;6:117–127. doi: 10.1089/vbz.2006.6.117. [DOI] [PubMed] [Google Scholar]
- Chamberlain RW, Sikes RK, Nelson DB, Sudia WD. Studies on the North American arthropod-borne encephalitides. VI. Quantitative determinations of virus-vector relationships. Am J Hyg. 1954;60:278–285. doi: 10.1093/oxfordjournals.aje.a119721. [DOI] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerging Infectious Diseases. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC, Rutledge CR, Tabachnick WJ. Relationships Between Host Viremia and Vector Susceptibility for Arboviruses. Journal of Medical Entomology. 2006;43:623–630. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Fang Y, Martinez VM. Does Variation in Culex (Diptera: Culicidae) Vector Competence Enable Outbreaks of West Nile Virus in California? Journal of Medical Entomology. 2008;45:1126–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host Selection by Culex pipiens Mosquitoes and West Nile Virus Amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.