Abstract
Ca2+ entry into cells of the peripheral immune system occurs through highly Ca2+-selective channels known as CRAC (calcium release-activated calcium) channels. CRAC channels are a very well-characterized example of store-operated Ca2+ channels, so designated because they open when the endoplasmic reticulum (ER) Ca2+ store becomes depleted. Physiologically, Ca2+ is released from the ER lumen into the cytoplasm when activated receptors couple to phospholipase C and trigger production of the second messenger inositol 1,4,5-trisphosphate (IP3). IP3 binds to IP3 receptors in the ER membrane and activates Ca2+ release. The proteins STIM and ORAI were discovered through limited and genome-wide RNAi screens, respectively, performed in Drosophila cells and focused on identifying modulators of store-operated Ca2+ entry. STIM1 and STIM2 sense the depletion of ER Ca2+ stores, whereas ORAI1 is a pore subunit of the CRAC channel. In this review, we discuss selected aspects of Ca2+ signaling in cells of the immune system, focusing on the roles of STIM and ORAI proteins in store-operated Ca2+ entry.
Keywords: CRAC channels, store-operated calcium entry, T cell activation, primary immunodeficiencies
OVERVIEW
Ca2+ signaling is essential for diverse biological processes (reviewed in 1–4). Ca2+ ions are especially suited as intracellular second messengers because of the strong homeostatic mechanisms that maintain intracellular free Ca2+ concentrations ([Ca2+]i) in resting cells at 100 nM or less, in the face of extracellular Ca2+ concentrations ([Ca2+]o) that are four orders of magnitude higher (1–2 mM). Cytoplasmic Ca2+ concentrations are maintained at low levels primarily through the action of plasma membrane Ca2+-ATPases (PMCAs) that pump Ca2+ out of the cell across the plasma membrane, and the sarco-endoplasmic reticulum Ca2+-ATPases (SERCAs) that pump Ca2+ into the lumen of the endoplasmic reticulum (ER) (Figure 1). Secondary regulators of [Ca2+]i include the mitochondrial Ca2+ uniporter (MCU) that transports Ca2+ across the inner mitochondrial membrane and the electrogenic Na+-Ca2+ exchanger (NCX), which uses the entry of Na+ to power the extrusion of Ca2+ across the plasma membrane.
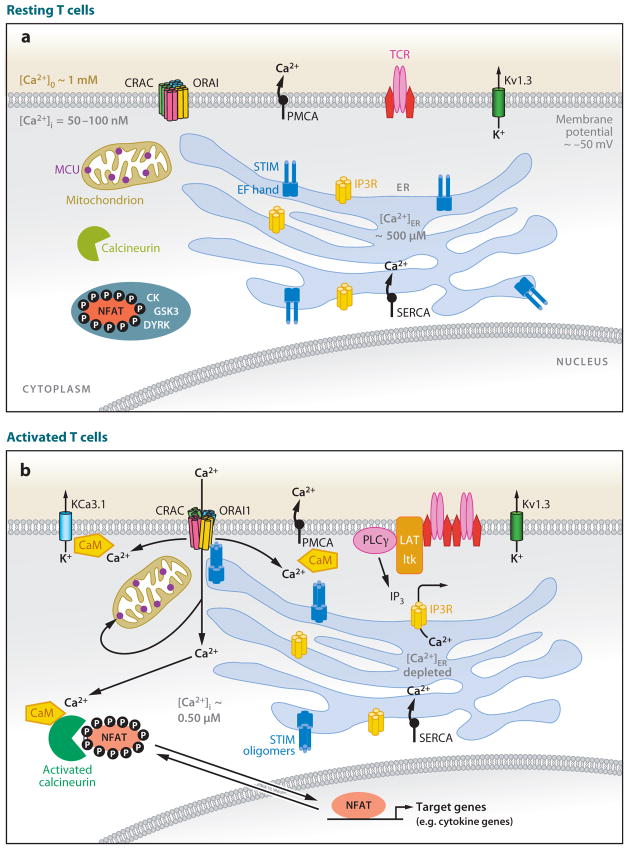
Figure 1.
Schematic diagram of the signaling pathway that connects store-operated Ca2+ entry with NFAT-dependent gene transcription in T cells. (a) Resting T cells have a membrane potential (maintained primarily by Kv1.3 K+ channels) of approximately −50 mV and intracellular free Ca2+ concentrations ([Ca2+]i) of 50–100 nM that are maintained by the plasma membrane Ca2+ ATPase (PMCA), the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) that pumps Ca2+ into the lumen of the endoplasmic reticulum (ER), and electrogenic Na+-Ca2+ exchangers (NCX, not shown). Immunoreceptors include antigen receptors on T and B cells (TCR, BCR), Fcε receptors on mast cells, or Fcγ receptors on NK cells. The concentration of free Ca2+ in the ER ([Ca2+]ER) is several hundred μM; hence the EF-hand of STIM1 is saturated with Ca2+, and STIM1 does not form higher-order oligomers (dimers are depicted, but the oligomerization state of STIM1 in resting cells is not fully defined). The transcription factor NFAT is heavily phosphorylated and localized to the cytoplasm. (b) Activated T cells. T cell receptors assemble into signaling complexes that contain scaffold proteins such as LAT and SLP-76, tyrosine kinases such as Lck, ZAP70, and Itk, and phospholipase C (PLC)γ (not all of which are shown). Inositol 1,4,5-trisphosphate (IP3) produced by PLCγbinds to IP3 receptors in the ER membrane, causing the release of Ca2+ from the ER. As a result of the depletion of ER Ca2+ stores, Ca2+ dissociates from EF-hand 1 of STIM1 and causes a conformational change (unfolding of the EF-SAM domain in the ER lumen) that leads to oligomerization (tetramers are depicted, but the oligomerization state of STIM1 in activated cells is not fully defined). The STIM oligomers move to sites of ER–plasma membrane apposition, recruit ORAI proteins to these sites, and cause CRAC channels to open. The resulting increase in [Ca2+]i causes the universal and abundant cytoplasmic Ca2+ sensor calmodulin (CaM) to bind to many channels and enzymes and modulate their activity. Among the targets of CaM are the phosphatase calcineurin, which dephosphorylates NFAT and causes its nuclear translocation, thus activating NFAT-dependent transcription; the PMCA pump whose activity is increased by CaM binding; and the KCa3.1 K+ channel that maintains membrane potential and the driving force for Ca2+ entry. Activated cells also show relocalization of mitochondria toward the plasma membrane, a process expected to maintain CRAC channel activity by diminishing Ca2+-dependent inactivation. MCU: mitochondrial Ca2+ uniporter. CK1, GSK3, DYRK: NFAT kinases.
The molecular mechanisms and consequences of Ca2+ signaling are especially well characterized in cells of the immune system, the focus of this review. In the short term (minutes), Ca2+ entry is required for mast cell degranulation and for lysis of infected or cancerous target cells by cytolytic T cells (reviewed in 5, 6). In the longer term (hours), sustained Ca2+ entry is critical for essentially all responses initiated through T cell, B cell, and Fc receptors, including proliferation and cytokine production by T cells, cytokine production by mast cells and natural killer (NK) cells, differentiation of B cells into plasma cells, and the differentiation of naive T cells into Th1, Th2, and Th17 effector subtypes. Many of these longer-term processes are regulated by the transcription factor NFAT (nuclear factor of activated T cells), which is present in a heavily phosphorylated state in the cytoplasm of resting cells, but which becomes dephosphorylated and translocates into the nucleus when [Ca2+]i elevation activates the calmodulin (CaM)-dependent phosphatase calcineurin (reviewed in 7, 8) (Figure 1).
The primary mechanism of Ca2+ influx into cells of the peripheral immune system is a process known as store-operated Ca2+ entry (reviewed in 9–13). The “store” is the ER, from which Ca2+ is released when the antigen receptors of T and B cells and the Fc receptors of mast cells and NK cells bind their appropriate ligands. Ligand binding to these receptors initiates a cascade of signaling events, among them activation of Src, Syk/ZAP70, and Tec/Btk family tyrosine kinases; kinase activation culminates in phosphorylation and activation of phospholipase C, an enzyme that hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) in the plasma membrane to produce the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. When IP3 binds to IP3 receptors (IP3R) in the ER membrane, it effects the release of ER Ca2+ stores, causing the free Ca2+ concentration in the ER to drop below its resting value of ~400–600 μM (9, 11, 13, 14) (Figure 1). However, store release by itself does not elevate [Ca2+]i sufficiently to promote long-term immune responses: Cytokine production, for instance, requires sustained calcineurin activation to support the prolonged nuclear residence of NFAT necessary for effective transcriptional activity (7, 15).
In 1986, Putney suggested that depletion of ER Ca2+ stores could evoke sustained Ca2+ influx across the plasma membrane of nonexcitable cells independently of receptor engagement, generation of second messengers, or the brief elevation of [Ca2+]i that results from ER Ca2+ release (16) (Figure 1). Parallel biophysical (patch-clamp) experiments established that lymphocytes and mast cells indeed express store-operated Ca2+ channels that can be opened in response to store depletion by various agents (reviewed in 9–12). These channels—termed CRAC (calcium release–activated calcium) channels—were eventually well characterized electrophysiologically (reviewed in 9, 10, 17), but their molecular identities, and the nature of their coupling to store depletion, remained unknown for almost 20 years.
It is now known that CRAC channels contain as their pore subunits a class of four-pass transmembrane proteins termed ORAI, gated by ER-resident single-pass transmembrane proteins known as STIM. In a notable illustration of the fact that new technologies are powerful drivers of scientific advances, STIM was discovered only after RNA interference (RNAi) had come into general use as a method for protein depletion in cultured cells (18, 19) and ORAI just as whole-genome RNAi screens were beginning to realize their potential (20–22). As described in detail in a subsequent section, we now know that ORAI1 is the pore subunit of the CRAC channel (23–25); that STIM proteins are ER Ca2+ sensors that sense ER Ca2+ concentration through an N-terminal Ca2+-binding EF-hand located in the ER lumen (13, 19, 26–28, 31); and that upon store depletion, STIM forms multimers in the ER membrane, then moves to sites of ER–plasma membrane apposition (19, 26, 29–31), where a portion of its C-terminal region gates ORAI channels directly (32–36) (Figure 1).
Here we review selected aspects of Ca2+ signaling in cells of the immune system, focusing on recent work on the molecules and mechanisms involved in Ca2+ entry through CRAC channels. Predictably, there has been an explosion of papers in the field since Drosophila Stim and mammalian STIM1 and STIM2 were identified in 2005 (18, 19) and Drosophila Orai and mammalian ORAI1, ORAI2, and ORAI3 were identified in 2006 (20–22). Several excellent reviews—indeed, volumes of reviews—summarizing each advance have been published (11–13, 17, 37–41), and the reader is referred to these for details that cannot be covered here because of space limitations. We have attempted to synthesize a large body of information for readers with an interest in immunology, and we apologize to those whose primary work has not been cited here for lack of space.
CELLULAR PATHWAYS OF CALCIUM SIGNALING IN LYMPHOCYTES
Engagement of receptors at the surface of immune cells generates intracellular messengers that create Ca2+ signals from two sources: intracellular organelles and the extracellular space. These sources are discussed below as they apply to all cells and specifically to lymphocytes.
Calcium Release from Intracellular Stores
Ca2+ signaling in response to stimulation of antigen and Fc receptors is initiated by the release of Ca2+ from intracellular stores, and several intracellular messengers have been implicated in this process. IP3 is the most extensively studied of these, dating back to 1985 when Imboden & Stobo (42) showed that anti-CD3 stimulation of Jurkat T lymphoma cells increased IP3 levels, released Ca2+ from stores, and promoted sustained Ca2+ influx. Three isoforms of the IP3R are expressed in lymphocytes, each with a characteristic sensitivity to activation by IP3 and to allosteric regulation by Ca2+ (reviewed in 43). The particular combination of isoforms and heteromultimers that are expressed can influence the dynamic patterns of Ca2+ release that occur upon antigen receptor engagement (44). Elimination of all three IP3R isoforms by homologous recombination in chicken DT40 pre-B cells completely prevents Ca2+ release in response to B cell receptor (BCR) cross-linking (45). Similarly, treatment of Jurkat T cells with IP3R1 antisense oligonucleotides or IP3R antagonists diminishes the release from Ca2+ stores in response to T cell receptor (TCR) cross-linking (46, 47), again establishing the requirement for IP3Rs in antigen receptor responses.
CRAC channels can be activated for long periods by sustained TCR engagement even though IP3 levels decline to near resting levels within 10 min (48), raising questions about whether additional second messengers may be involved in prolonging receptor-regulated Ca2+ release from the ER. One possible explanation, as yet untested, is that local IP3 generation not detectable globally may suffice to deplete Ca2+ locally in ER subregions physically involved in STIM-ORAI interaction and CRAC channel activation. On the other hand, substantial evidence suggests that cyclic ADP-ribose (cADPR) may act as a Ca2+-releasing messenger in T cells. cADPR levels rise for more than 60 min after anti-CD3 stimulation in Jurkat T cells through activation of an ADP-ribosyl cyclase; injection of cADPR releases Ca2+ from stores through type 3 ryanodine receptors, and a membrane-permeant cADPR antagonist increases the latency and decreases the duration of Ca2+ release triggered through the TCR (49). Interestingly, IP3 and cADPR appear to interact functionally: Even though they bind to distinct receptors, inhibition of IP3R signaling by IP3R antagonists also prevents Ca2+ signaling by cADPR (47). It is possible that Ca2+ released from the ER through the IP3R acts as a coactivating cofactor for the ryanodine receptor.
Nicotinic acid adenine dinucleotide phosphate (NAADP) is the most recent addition to the arsenal of Ca2+ mobilizing messengers in T cells. NAADP is the most potent Ca2+-releasing agent known, roughly 1000 times more effective than IP3. Following TCR stimulation, NAADP is produced in a biphasic fashion, reaching a transient peak approximately eightfold above baseline within 30 s, followed by a decline to baseline and a secondary smaller rise lasting more than 20 min (50). This time course is consistent with a role for NAADP in both the initiation of Ca2+ signals and their maintenance over much longer periods. Recent evidence indicates that in some cells NAADP releases Ca2+ from acidic stores such as lysosomes, endosomes, or melanosomes through twin-pore channels (TPCs) (51, 52). However, in T cells the action of NAADP is not inhibited by bafilomycin (which neutralizes acidic compartments) but is blocked by the SERCA pump inhibitor thapsigargin (which depletes ER Ca2+ stores) and is sensitive to ryanodine receptor antagonists, suggesting that NAADP instead releases Ca2+ through ryanodine receptors in the ER (53, 54).
Further work is needed to understand how the Ca2+ mobilizing actions of IP3, cADPR, and NAADP may be integrated in lymphocytes and under what conditions they are recruited. Importantly, although these messenger systems may contribute to transient release of Ca2+ under conditions of mild stimulation in vivo, activation of transcriptional pathways, most notably NFAT, requires sustained entry over tens of minutes to several hours. Because the content of the ER is finite, and recovery of released Ca2+ into the ER by SERCA pumps is incomplete owing to extrusion of a fraction of Ca2+ across the plasma membrane, Ca2+ entry from the extracellular space must occur to replenish the ER stores. The next section discusses Ca2+ channels in the plasma membrane that fulfill this sustained signaling function.
FINGERPRINTING THE CRAC CHANNEL
Patch-clamp recording techniques have been used to characterize the CRAC channel in T cells and mast cells. For whole-cell recording, a glass recording micropipette (tip diameter ~1 μm) is sealed to the cell membrane, and suction is used to break the membrane patch beneath the pipette lumen, thereby establishing electrical and physical continuity between the pipette lumen and the cytoplasm. The patch-clamp circuitry is then used to control the membrane potential and measure the total ionic current flowing through CRAC (and other) channels in the plasma membrane. During whole-cell recording, the cytoplasm of the cell slowly exchanges with the larger volume of the pipette contents. The perforated-patch technique is a less invasive mode in which the cell membrane is not broken, but a pore-forming antibiotic such as amphotericin is included in the pipette solution and inserts into the membrane under the pipette, thus providing the electrical connection to the cytoplasm that is necessary for the voltage clamp, without allowing diffusion of compounds into or out of the cell.
To measure CRAC currents specifically, K+ currents are most commonly suppressed by replacing K+ in the pipette with the impermeant cation Cs+, and TRPM7 channels are inhibited by including Mg2+ in the pipette. To make the inward Ca2+ current as large as possible, the external solution typically contains high Ca2+ (10–20 mM) and the cells are subjected to a voltage step or ramp that clamps the membrane potential to very negative values, both of which increase the driving force for Ca2+ entry.
A cluster of biophysical characteristics constitutes a unique fingerprint that distinguishes the CRAC channel from other channels. Among the most useful for discrimination are pore properties such as ion selectivity and conductance. The CRAC channel is among the most Ca2+-selective ion channels known, selecting for Ca2+ over monovalent cations such as Na+ and K+ by more than 1000:1. The Ca2+ conductance of a single CRAC channel (the unitary conductance) is estimated to be ~30 femtosiemens, which corresponds to ~104 Ca2+ ions flowing through the channel per second at a membrane potential of −100 mV. Notably, the CRAC channel resembles L-type CaV channels in its Ca2+ selectivity, but its conductance is about 100 times smaller than that of CaV, suggesting major differences in pore structure. Other distinguishing features of CRAC channels include distinct fast and slow inactivation processes driven by the elevation of intracellular Ca2+. STIM1 and CaM bind to Orai1 to favor fast inactivation, but the mechanism of slow inactivation is less well understood.
Calcium Entry Across the Plasma Membrane: The History of CRAC Channels
Since the early 1970s, from studies using 45Ca2+ to monitor Ca2+ handling it was known that mitogens like phytohemagglutinin (PHA) stimulate sustained Ca2+ uptake by human T cells and that this is essential for the stimulation of T cell proliferation (55, 56). By the 1980s, the development of vital Ca2+ indicator dyes by Tsien and colleagues (57) and patch-clamp recording by Neher, Sakmann and coworkers (58) provided the essential tools needed to begin examining this process mechanistically. Although it became known that TCR cross-linking generated IP3, which in turn released Ca2+ from the ER pool, the role of IP3 in driving Ca2+ entry was initially unclear. Early efforts to identify the entry pathway using patch-clamp recording (see sidebar, Fingerprinting the CRAC Channel) suggested a role for IP3-gated channels in the plasma membrane (59, 60). However, despite later biochemical evidence in support of plasma membrane IP3Rs (61, 62), other labs were not able to confirm the presence of IP3-gated currents in T cells or relate them directly to changes in [Ca2+]i, and their existence was questioned.
Meanwhile, by combining single-cell Ca2+ imaging with whole-cell recording from Jurkat cells, Lewis & Cahalan (63) identified a miniscule Ca2+ current that activated spontaneously during whole-cell recordings and was temporally correlated with a large increase in [Ca2+]i. The spontaneous activation was absent in perforated-patch recordings, which prevents dialysis of the cytoplasm by the pipette contents, but under these less invasive conditions the current could be activated in an oscillatory manner by PHA, a T cell mitogen. Several critical characteristics of the mitogen-regulated Ca2+ channel were established, including insensitivity to membrane potential, extremely high Ca2+ selectivity, and a very low Ca2+ conductance (as indicated by the nearly complete lack of current noise); however, its mode of activation, and in particular the role of IP3, was unclear.
During the same period, evidence was accumulating for the existence of a Ca2+ entry pathway activated by depletion of Ca2+ from the ER. In 1986, Putney formalized the concept as capacitative calcium entry, later renamed store-operated Ca2+ entry (16). Shortly thereafter, thapsigargin was introduced as a highly selective and effectively irreversible inhibitor of SERCA pumps that could deplete Ca2+ stores and activate store-operated Ca2+ entry while bypassing receptor activation and the production of IP3 (64). Thapsigargin was an extremely useful tool for demonstrating that store-operated channels exist in many if not all nonexcitable cells, including mast cells, thymocytes, and T cells (65), and in a growing number of excitable cells as well. Several other strategies were developed to reduce the free Ca2+ concentration ([Ca2+]ER) in the ER lumen, including exposure to the reversible SERCA inhibitor cyclopiazonic acid; the membrane-permeant Ca2+ buffer TPEN, which crosses the ER membrane and reduces [Ca2+]ER to low levels; and the Ca2+ ionophore ionomycin, which accumulates in ER membranes and ferries ER Ca2+ into the cytosol.
Building on these advances, Hoth & Penner (66) identified a store-operated Ca2+ current in whole-cell recordings from mast cells that was activated by intracellular Ca2+ buffers [consistent with the spontaneous activation seen earlier in Jurkat cells (63)], by ionomycin, or by IP3 in the recording pipette. They called this current the Ca2+ release-activated Ca2+, or CRAC, current. Zweifach & Lewis (67) used a different approach—thapsigargin treatment during perforated-patch recording—to identify a similar store-operated current in Jurkat T cells. Importantly, they related the properties of the CRAC current activated by thapsigargin to those of the PHA-activated current described earlier: Both had similar selectivity among Ca2+, Ba2+, and Sr2+, an extremely small apparent unitary conductance of 10–30 fS (femtosiemens), and sensitivity to inhibition by Ni2+ (67). Together, these and subsequent studies (68, 69) established the existence of store-operated Ca2+ channels in T cells and mast cells and showed that they were activated in T cells by TCR engagement and in mast cells by FcR cross-linking (70), consistent with the idea that IP3, rather than gating the channels directly, controlled them through depletion of Ca2+ from the ER.
Considerable effort was spent over the ensuing ten years to characterize the biophysical and pharmacological characteristics of the CRAC channel, thereby generating a biophysical fingerprint (see sidebar on Fingerprinting). The fingerprint, summarized in detail elsewhere (71, 72), encompasses such features as store dependency, ion selectivity, pore diameter, unitary conductance, Ca2+-dependent inactivation and potentiation, and pharmacological profile. This unique collection of properties, and in particular the extremely high Ca2+ selectivity and small unitary conductance, created a stringent benchmark for judging potential molecular candidates for the CRAC channel. For example, members of the transient receptor potential (TRP) protein family were often proposed as store-operated or CRAC channel candidates (reviewed in 73). For many of these, particularly members of the TRPC subfamily of TRP channels, their unitary conductance was much too large, and their Ca2+ selectivity far too low, to be compatible with the CRAC fingerprint. For others, such as TRPV6, many properties initially appeared to be consistent (74), and a dominant-negative TRPV6 mutant was in fact able to suppress CRAC current in Jurkat cells (75). However, subsequent studies showed discrepancies with CRAC channel pore properties (76), and the TRPV6 unitary conductance of ~40 pS for monovalent cations, initially thought to be compatible with the CRAC channel, was later found to be much too large because the CRAC channel conductance had previously been overestimated (77).
While the similar fingerprints of channels activated by thapsigargin and PHA suggested that the CRAC channel is the primary route for Ca2+ entry evoked by the TCR, subsequent genetic studies provided more definitive evidence for this conclusion. In mutant Jurkat T cells selected for a lack of store-operated Ca2+ entry, CRAC current was lost, and TCR stimulation with anti-CD3 failed to activate Ca2+ entry (78). Several human patients with hereditary severe immunodeficiency syndromes lacked thapsigargin-activated CRAC channel activity, and TCR stimulation of their T cells failed to evoke significant Ca2+ entry or Ca2+ currents detectable by electro-physiology (79–82). In one study, transfection with TRPC3 increased TCR-mediated Ca2+ influx in Jurkat mutant cells with partial defects in CRAC current (83); however, analysis of T cells from two related immunodeficient patients showed that despite normal expression of other candidate Ca2+ entry channels (TRPC1, TRPC3, TRPC4, TRPC5, TRPC7, TRPV5, and TRPV6), TCR stimulation failed to evoke Ca2+ entry (79). Although loss-of-function mutations in these potential Ca2+ entry channels were not ruled out, the Ca2+ signaling defect in these patients’ T cells was ultimately traced to a loss-of-function mutation in ORAI1, and transduction with wild-type ORAI1 was sufficient to restore normal levels of store-operated Ca2+ entry and CRAC current (20) (see following section).
RNAi SCREENS IN DROSOPHILA CELLS
The discovery of ORAI proteins awaited the emergence of a platform for genome-wide RNAi screens in Drosophila cells (85), which preceded genome-wide screens in mammalian cells by several years (85, 86). Drosophila cells have several advantages for RNAi screens (85, 87). They readily take up long double-stranded RNAs and process them into multiple overlapping 21- to 22-nucleotide small interfering RNAs that, by providing extensive coverage of the cDNA, evoke efficient knockdown of the target proteins; they lack an interferon response, and hence their use avoids the complications introduced by interferon-induced phenotypic changes in cells; the redundancy of the Drosophila genome is much lower than that of the mammalian genome, increasing the chances of observing strong loss-of-function phenotypes; and finally, off-target effects in Drosophila cells can actually be an advantage because the overlapping 21- to 22-nucleotide small interfering RNAs produced by Dicer from long double-stranded RNAs often also target mRNAs encoding several related members of a protein family.
MOLECULAR ASPECTS OF CALCIUM SIGNALING IN LYMPHOCYTES
The Discovery of STIM and ORAI
STIM and ORAI were discovered as RNAi began to be widely used as a method for the unbiased discovery of proteins in biological pathways. Meyer and colleagues (19) performed a limited RNAi screen in HeLa cells that identified human STIM1 and STIM2 as proteins whose depletion downregulated store-operated Ca2+ entry. At essentially the same time, Roos and colleagues (18) performed a limited RNAi screen in Drosophila cells, based on the demonstration by Cahalan and coworkers that Drosophila S2 cells had Ca2+-selective channels with most of the electrophysiological characteristics of mammalian CRAC channels (84). This screen identified Drosophila Stim and human STIM1 as playing key roles in store-operated Ca2+ entry. STIM was placed on the candidate list because it contained a Ca2+-binding EF-hand and a protein-protein interaction domain (a SAM domain) that led to its annotation as a signaling protein.
Drosophila Orai was discovered in the course of RNAi screens performed nearly concurrently by three separate groups (20–22) in Drosophila cells, which have several advantages for RNAi screens (85–87) (see sidebar). The Kinet and Cahalan groups used [Ca2+]i increases, imaged in a high-throughput format, as a direct readout: Cells were treated with thapsigargin in the absence of Ca2+ to evoke store depletion, and the medium was then reconstituted with Ca2+ to allow Ca2+ influx (21, 22). In both cases, knockdown of Drosophila Stim served as a positive control. Rao and colleagues (20) used an indirect screen that monitored nuclear translocation of an NFAT-GFP fusion protein ectopically expressed in Drosophila S2R+ cells. Even though Ca2+-regulated NFAT transcription factors are not represented in Drosophila (their first emergence is in vertebrates), the NFAT-GFP fusion protein translocated correctly to the nucleus of Drosophila cells in a manner that was Ca2+- and calcineurin-dependent and blocked by the calcineurin inhibitor cyclosporin A. This likely reflects the evolutionary conservation of calcineurin and NFAT kinases (CK1, DYRK, and GSK3) between Drosophila and vertebrates (88).
All three screens yielded Drosophila Orai as a robust hit. In the Ca2+ screens, which monitored [Ca2+]i for 5–10 min, depletion of hundreds or thousands of candidate proteins altered the rate or magnitude of thapsigargin-evoked calcium increase, a result that is not surprising given the tight regulation of Ca2+ homeostasis in cells (1–4). In contrast, in the NFAT translocation screen, which integrated the effect of increased [Ca2+]i over 60 min, Stim and Orai were 2 of only 16 hits that included calcineurin components and nuclear transport proteins, as expected (89).
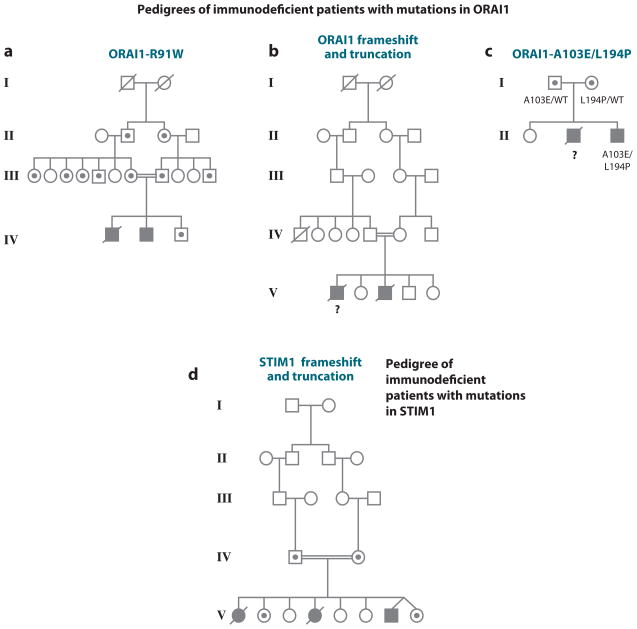
The physiological validation of Stim and Orai as players in the store-operated Ca2+ entry pathway used several approaches. The first was genetic: A familial severe immunodeficiency syndrome in two human patients was traced to a point mutation in ORAI1. The patients had presented with a rare immunodeficiency characterized by an inability to produce NFAT-dependent cytokines, whose molecular basis had progressively been traced backwards to an inability to dephosphorylate NFAT and translocate it to the nucleus and, later, to a complete absence of store-operated Ca2+ entry and CRAC current in the patients’ T cells (79, 80, 90). Notably, the patients’ parents and other heterozygous carriers of the mutant allele (who exhibited no clinical symptoms whatsoever) could be distinguished from normal individuals because their T cells showed a decrease in store-operated Ca2+ entry when [Ca2+]o was dropped to below physiological levels (0.2–0.5 mM, compared with normal [Ca2+]o levels of ~1.25 mM) (20). This permitted the unambiguous identification, through a genome-wide SNP (single nucleotide polymorphism) screen, of a 9.8 MB genomic region linked with high confidence to the mutant allele. The gene encoding ORAI1, one of the three human homologs of Drosophila Orai, fell within this interval, and it quickly became apparent that a C-to-T transition, resulting in an arginine-to-tryptophan substitution at position 91 of ORAI1, was the mutation underlying the immunodeficiency syndrome (20). More recently, investigators have shown that a nonsense mutation in STIM1 is the basis for a second hereditary immunodeficiency (91, 92), and two additional families with mutations in ORAI1 have been identified (81, 82, 93). These immunodeficiency syndromes, and mouse models of Stim and Orai deficiency, are discussed in more detail below.
A second mode of validation was reconstitution of store-operated Ca2+ entry and CRAC current in the patients’ cells by expression of wild-type ORAI1 (20); a third involved electrophysiological (whole-cell patch-clamp) studies of cells coexpressing STIM and ORAI. Soon after the first discovery of ORAI proteins, several groups reported that coexpression of Drosophila Stim and Orai in Drosophila S2 cells (22) or of human STIM1 and ORAI1 in various mammalian cell types led to a large increase in a Ca2+-selective current with the biophysical characteristics of CRAC current (94–96). The ease of studying these “monster” CRAC currents quickly led to extensive mutational, functional, and comparative analyses of the two mammalian STIM proteins, STIM1 and STIM2, and the three mammalian ORAI proteins, ORAI1, ORAI2, and ORAI3 (reviewed below). More recently, several groups have also demonstrated that the STIM1 C terminus and various fragments derived from it are capable of decorating the plasma membrane and activating constitutive CRAC currents when introduced into ORAI-expressing cells (32–36). A wealth of information has been accumulated that will inform future analyses of the structural basis for STIM-ORAI interaction. These biochemical and cell-biological studies are described in the following sections.
STIM1
STIM1 was originally assumed to be a secreted or plasma membrane protein of bone marrow stromal cells, hence the original name stromal interaction molecule (97), and it was experimentally identified as a plasma membrane protein of the chronic myeloid leukemia cell line K562 (98). In fact, STIM1 is predominantly localized in the ER (19, 29–31), and it is believed that ER-resident STIM1, not plasma membrane STIM1, controls CRAC channel opening (19, 29, 96, 99, 100). STIM1 has an ER-luminal portion of ~22 kDa after cleavage of its signal sequence, a single transmembrane segment, and a cytoplasmic portion of ~51 kDa (Figures 2, 3). ER-resident STIM1 carries out two basic functions in the CRAC channel pathway: sensing ER Ca2+ store depletion and repletion and communicating the level of Ca2+ stores to Ca2+ channels in the plasma membrane.
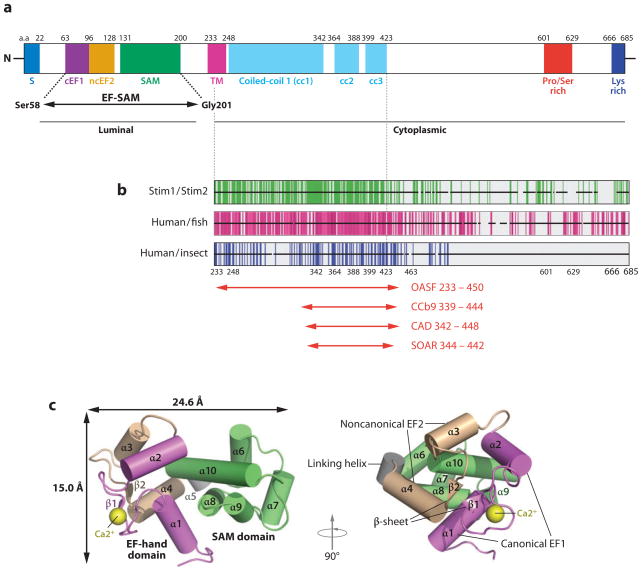
Figure 2.
Structure and properties of STIM1. (a) Domain structure of human STIM1 (adapted with permission from Reference 27). Shown are the signal peptide (S), the canonical EF-hand 1 (cEF1), the noncanonical EF-hand 2 (ncEF2), the SAM (sterile α-motif ) domain, the transmembrane domain (TM), three predicted coiled-coil regions (cc1, cc2, and cc3), the proline- and serine-rich region, and the lysine-rich (polybasic) region at the C terminus. The EF-SAM fragment whose structure was determined by NMR spectroscopy is indicated. The region to the left of the TM is located in the ER lumen, whereas the region to the right is located in the cytoplasm. Residue numbers at the approximate boundaries of the domains are indicated above the diagram. Coiled-coils cc1 and cc2 have long been recognized in STIM proteins (97, 98, 105) and are assigned high probability in STIM1 by COILS; the predicted coiled-coil cc3 is assigned a low probability by COILS in STIM1, but a relatively high probability in Aedes aegypti Stim and Anopheles gambiae Stim, and in STIM2 when core hydrophobic positions are weighted. The existence and precise boundaries of cc3 require experimental confirmation. (b) Sequence conservation in the STIM C-terminal region. Each horizontal black bar represents the human STIM1 sequence, with gaps introduced as necessary to maintain alignment with human STIM2, fish STIM1 orthologs, or insect Stim proteins, as indicated. Vertical green lines indicate identity of the human STIM1 residue with the residue at the corresponding position of human STIM2; vertical magenta lines indicate identity of the human STIM1 residue with residues at the corresponding position in at least four of five fish orthologs; vertical blue lines indicate identity with residues in at least two of three insect Stim proteins. Adapted from Reference 36. (c) Structure of the EF-SAM fragment deduced by NMR spectroscopy (adapted with permission from Reference 27). Alpha-helices are depicted as cylinders. (The canonical EF-hand 1 is magenta, the noncanonical EF-hand 2 is beige, and the SAM domain is green; the Ca2+ ion bound to EF-hand 1 is a yellow sphere.) Two views related by a 90° rotation are shown.
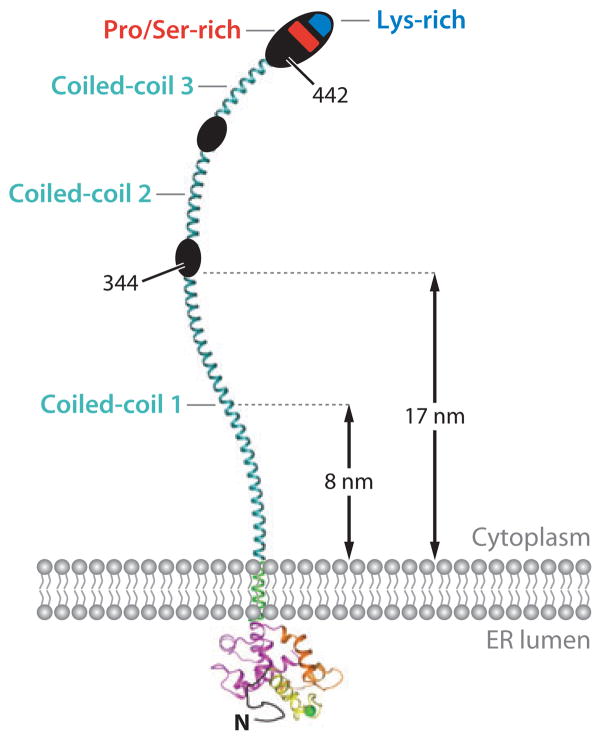
Figure 3.
Schematic representation of full-length STIM1. The cytoplasmic region contains three predicted coiled-coil regions (cyan), a serine- and proline-rich region (red ), and a polybasic tail (blue). The coiled coils can span the distance, estimated to be 8 nm (109) or ~17 nm (29), that separates the ER and the plasma membrane at the junctions where STIM and ORAI accumulate upon ER Ca2+ store depletion. For an explanation of the three coiled-coil regions, see the caption to Figure 2a.
Sensing ER Ca2+ concentration
The sensing of ER Ca2+ levels is the most thoroughly understood step in the STIM-ORAI pathway at the molecular level: Dissociation of Ca2+ from a binding site in the luminal portion of STIM1 triggers a structural change in STIM1 (27, 101). The cellular correlate of Ca2+ sensing is a relocalization of STIM1, conveniently visualized by light microscopy with GFP-fusion proteins. STIM1 is distributed throughout the ER prior to Ca2+ store depletion and collects at numerous individual spots, or puncta, upon depletion of ER Ca2+ stores (19, 29, 31, 96, 99).
The ER-luminal domain of STIM1 is responsible for Ca2+ sensing. An NMR structure of a recombinant fragment [human STIM1(58–201)] encompassing most of the STIM1 luminal domain shows a classical paired arrangement of two EF-hands followed by a sterile α motif (SAM) domain (27). Only the first EF-hand binds Ca2+, and the EF-SAM protein fragment with bound Ca2+ is monomeric (27, 101). The EF-hand pair engages an α-helix of the SAM domain in much the same way that a corresponding EF-hand pair in Ca2+-CaM engages its target peptides (13, 27) (Figure 2c). The structure of the recombinant STIM1 luminal domain in the absence of Ca2+ has not yet been determined, but biophysical measurements indicate that dissociation of Ca2+ is accompanied by substantial protein unfolding and by a transition from monomers to a mixture of dimers and larger aggregates (27, 101).
Given that the typical concentration of Ca2+ in the ER lumen is hundreds of micromolar, STIM1 must have a relatively low affinity for Ca2+ to function as a sensor. The measured Kd for Ca2+ binding to recombinant STIM1 luminal domain is ~500–600 μM at 20°C (101), and the Kd for isolated STIM1 EF-hand 1 grafted into a loop of rat CD2 domain 1 is ~500 μM at 25°C (102). Although the conditions of these in vitro determinations were not identical to conditions in cells, the values are in reasonable agreement with the sensitivity of STIM1 redistribution in cells to ER Ca2+ concentration (14, 26).
Store depletion leads to oligomerization of STIM1
Direct evidence that STIM1 in cells dimerizes or oligomerizes during Ca2+ sensing is the increased FRET between CFP-STIM1 donor and YFP-STIM1 acceptor upon store depletion (103, 104). This step precedes the appearance of STIM1 puncta subjacent to the plasma membrane (103) and thus is the earliest indicator of Ca2+ sensing by STIM1 in cells (Figure 4).
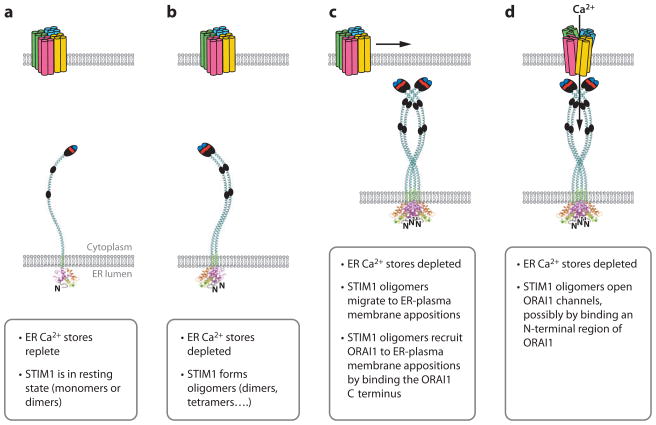
Figure 4.
Sequence of steps in store-operated Ca2+ entry. (a) Schematic diagrams of STIM1 and ORAI1 in the resting state, when ER Ca2+ stores are replete. ORAI is depicted as a tetramer for reasons discussed in the text. STIM1 is depicted as a monomer for convenience, but its oligomerization state in resting cells is not yet fully defined. (b) STIM1 oligomerization. STIM1 forms oligomers when ER stores are depleted. Oligomers are depicted here as dimers for convenience, but their stoichiometry in activated cells is unknown. (c) STIM1 redistribution. Oligomerization of STIM1 in the ER membrane is followed by migration of STIM1 to ER–plasma membrane appositions. This redistribution involves binding of the STIM1 polybasic regions to PIP2 and PIP3 in the plasma membrane. STIM1 oligomers then recruit ORAI1 to ER–plasma membrane junctions by binding a C-terminal region of ORAI1. (d ) STIM1-ORAI1 gating. STIM1 oligomers open ORAI channels, possibly by binding to an N-terminal region of ORAI1.
Ca2+ dissociation from STIM1 leads to unfolding and oligomerization of the STIM1 luminal domain. EF-hand 1 of STIM1 was recognized from its amino acid sequence (105), and disabling Ca2+ binding by point mutations in EF-hand 1 leads to constitutive localization of STIM1 in puncta (19, 31). Moreover, the same substitutions lead to constitutive activation of store-operated Ca2+ entry and CRAC current (19, 31, 106), demonstrating that disruption of Ca2+ binding to EF-hand 1 is functionally equivalent to dissociation of Ca2+ upon store depletion. Targeted, structure-based substitutions in the second EF-hand and in the SAM domain surface, intended to destabilize the close interaction between the paired EF-hands and the SAM domain, caused unfolding of the STIM1 luminal domain as predicted and also caused constitutive formation of STIM1 puncta and constitutively elevated Ca2+ entry (27).
The authors of the structural study (27) have carefully left open the question of whether the large aggregates observed with STIM1 luminal domain in solution represent the state of STIM1 luminal domain in Ca2+-depleted ER. SAM domains are in fact protein interaction domains that can assemble into large oligomeric structures (107), and one possible scenario is that the EF-hands release the SAM domain, after which the SAM domains oligomerize. However, STIM1 aggregation deviates from assembly of other characterized SAM domain multimers in two respects (27): STIM1 luminal domain in the absence of Ca2+ is seen as amorphous aggregates by electron microscopy, not in ordered polymers as with other SAM domains; and mutations in STIM1 residues that correspond to protein-protein contact sites of other SAM domains fail to impede aggregation of the STIM1 domain. In cells, oligomerization may be constrained by the tethering of STIM1 monomers to the ER membrane, by interaction with chaperones in the ER lumen, or by the protein-protein interactions of the STIM1 cytoplasmic region. Thus, it remains an open question whether the STIM1 luminal domain in cells forms large aggregates, as in vitro, or smaller ordered oligomers.
Redistribution of STIM1
The STIM1 puncta visible by light microscopy are localized at sites of close apposition between the ER and the plasma membrane, as observed by electron microscopy (29) (Figure 4). The time course of STIM1 redistribution to these sites has been followed by TIRF microscopy using fluorescently labeled STIM1 proteins. The redistribution is completed over tens of seconds (19, 29, 103), and the reverse movement of STIM1 from puncta to ER upon repletion of ER stores is comparably rapid (19, 108). The accumulation of STIM1 near the plasma membrane is further accentuated by a modest increase in the ER–plasma membrane junctional area upon store depletion in Jurkat cells (29) and by a larger increase in HeLa cells (109).
Movement of STIM1 into puncta is believed to involve local diffusion within the ER membrane, with STIM1 collecting at ER–plasma membrane contacts owing to interactions with specific proteins or lipids (103). Consistent with a diffusive mechanism, local depletion of ER stores, which may be the rule in the case of physiological stimulation, is effective in activating the CRAC current (110), and movement of STIM1 is estimated to be over a short distance, averaging approximately 2 μm, even when stores are globally depleted (103).
The interactions that retain STIM1 at ER–plasma membrane contacts are not fully defined. One key interaction maps to the short polybasic segment at the STIM1 C terminus (33, 103, 111) (see Figure 2). Involvement of the polybasic segment has led to the hypothesis that STIM is recruited by negatively charged phospholipids such as PIP2 and PIP3 (103), an established mechanism for targeting cytoplasmic and cytoskeletal proteins to the plasma membrane.
Depleting PIP2 from the plasma membrane of COS-7 cells did not prevent formation of STIM1 puncta but did lead to a modest reduction in preformed puncta (112, 113). Experience with other proteins indicates that it may be necessary to deplete both PIP2 and PIP3 to alter localization (114). Prior depletion either of PIP2 by recruitment of phosphatase to the plasma membrane or of PIP3 and PIP by inhibition of PI3K and PI4K decreased the initial migration of STIM1-EYFP to puncta in HeLa cells (115). Prior depletion of both polyphosphoinositides nearly abolished STIM1 migration to puncta (115), although a similar treatment did not dissociate preformed YFP-STIM1 puncta in COS-7 cells (113), leaving open the possibility that puncta once formed are stabilized by other interactions. Complementary in vitro data show binding of the STIM1 C terminus to liposomes containing either PIP2 or PIP3 (116). Because PIP3 is absent from the plasma membrane of naive T cells, it will be of interest to investigate whether STIM-ORAI signaling in T cells is influenced by costimulatory signals that trigger production of PIP3. Whether the net effect of costimulation would be increased CRAC channel activity is unclear, given that STIM-polyphosphoinositide interactions may have negative effects on STIM-ORAI signaling downstream of STIM1 redistribution (115).
A second factor that may contribute to STIM targeting, at least when ORAI is over-expressed, is interaction with the ORAI channel complex itself (33, 115). Neither PIP2 nor ORAI1 is preferentially localized to ER–plasma membrane contacts in resting cells, although their interaction with STIM1 is necessarily limited to these sites. Proteins known to be preferentially localized to ER–plasma membrane contacts, such as junctophilin (117, 118), have thus far not been shown to interact directly with STIM, although such interactions have not been ruled out.
A critical upstream signaling mechanism for formation of puncta is oligomerization of the STIM1 luminal domain. This has been clearly shown by introducing artificial oligomerization domains, an FRB domain and tandem FKBP12 domains, in place of the luminal domain of STIM1, and expressing the engineered proteins in cells (14). A cell-permeant ligand, rapalog, capable of bridging the inserted domains and causing assembly of multimers, triggered puncta formation and activated CRAC current in the absence of ER Ca2+ store depletion.
Why oligomerization occurring in the ER lumen would cause STIM1 to collect at ER–plasma membrane contacts is not immediately obvious. Redistribution is not directed purely by protein-protein associations within the ER lumen because removing the ~14-residue poly-basic tail at the STIM1 C terminus prevents redistribution (33, 103, 116) unless the deletion is compensated by overexpression of ORAI (33). Likewise, the inhibitory effect of depleting PIP2 and PIP3 from the plasma membrane can be compensated by overexpression of ORAI (115). Evidently, the changes in the luminal domain are conveyed in some manner to the cytoplasmic domain of STIM1. Two nonexclusive possibilities have been proposed (14, 103): (a) that oligomerization increases the avidity of STIM for plasma membrane sites in the same way that IgG or IgM avidity exceeds that of a single combining site, and (b) that oligomerization induces a conformational change in the C-terminal cytoplasmic domain of STIM1 and exposes a previously buried polybasic C-terminal segment or other sites that interact at ER–plasma membrane contacts.
STIM1 redistribution in cells shows marked cooperativity with respect to ER-luminal Ca2+ concentration (14, 26). Because each STIM1 monomer has a single Ca2+-binding site, the cooperativity indicates that oligomeric STIM1 is involved in at least one step of redistribution. The data on concentration dependence do not discriminate between whether the oligomer makes a transitory appearance or is stable. It is tempting to view the increased FRET between CFP-STIM1 and YFP-STIM1, maintained throughout redistribution to ER–plasma membrane contacts (103, 104), as evidence that a single oligomeric state is maintained. However, the solidity of this argument depends on the extent to which FRET distinguishes among dimers, small oligomers, and larger oligomers, which has not been investigated experimentally.
Importantly, physiological stimuli can elicit significant Ca2+ entry without generating large puncta. For example, puncta are not prominent in mast cells stimulated by cross-linking surface IgE with antigen (119), and STIM redistribution detected by TIRF microscopy is absent or modest in HEK293 cells stimulated with a low concentration of muscarinic agonist (120), even though these conditions elicit robust STIM-ORAI-dependent Ca2+ elevation or Ca2+ oscillations (119–121). In these examples, partial refilling of the Ca2+ stores during stimulation may limit the size of STIM-ORAI coclusters. In contrast, T cells stimulated through the TCR by treatment with superantigens or anti-CD3 antibodies exhibit clear puncta and larger clusters of STIM1 (29, 122, 123), which may reflect strong signaling and a greater degree of local store depletion, although this remains to be tested.
Additional protein-protein interactions
A portion of ER-resident GFP-STIM1 colocalizes in cells with microtubules (96, 100, 124). The prominence of the association with microtubules is variable, and it may be accentuated by GFP-STIM1 overexpression. Association with microtubules is mediated by STIM1 binding to the microtubule plus end tracking protein EB1 through a TxIP motif in STIM1 (125, 126). EB1 recruits STIM1 to sites of physical contact between growing microtubule tips and ER. Time-lapse images initially gave the impression that a fraction of cellular STIM1 is traveling along microtubules at any given moment (100). However, a later study established that individual STIM1 molecules do not move with the microtubule tip, but rather are recruited transiently from nearby in the ER and then disperse as the tip grows onward and recruits new STIM1 molecules (125).
It is not clear whether STIM1 association with extending microtubule tips has a physiological role in Ca2+ signaling. Microtubules are not required for initial CRAC channel gating in T cells and mast cells (127, 128), although microtubules contribute to the sustained Ca2+ plateau through a Ca2+-dependent movement of mitochondria toward the plasma membrane that limits slow Ca2+-dependent inactivation of the CRAC channel (128). Likewise, in HeLa cells where the STIM-microtubule interaction has been extensively studied, EB1 knockdown or treatment with taxol to suppress microtubule growth and shortening eliminated STIM-microtubule colocalization, but did not affect store-operated Ca2+ entry (125). Thus, current evidence indicates that any effect of STIM-microtubule interaction on the CRAC current is indirect, through remodeling of ER or ER–plasma membrane contacts or through regulating the availability of STIM1.
STIM1 interacts directly with TRPC proteins in biochemical and functional assays (108, 129, 130), leading to the proposal of a specific mechanism by which STIM1 gates TRPC channels (130). The relevance of this interaction to cellular Ca2+ signaling is controversial (131). Nevertheless, activation of TRPC channels offers a possible explanation for Ca2+ influx not accounted for by ORAI channels.
STIM1 is also required for activation of the ARC (arachidonate-regulated Ca2+-selective) channel, which, as indicated by the acronym, is a Ca2+ channel activated by arachidonic acid (132). The function of the ARC channel in immune cells has not been investigated, but in other cell types it does not appear to be activated by store depletion, nor is depletion required for its activation by arachidonic acid.
Lastly, activated STIM1 controls an adenylate cyclase (133). Stringent controls appear to eliminate the possibility that activation of the cyclase is through a local STIM1-dependent Ca2+ signal. The finding is very important as a first example of STIM1 integrating intracellular Ca2+ signaling with signaling in other intracellular pathways. Its biological ramifications have not been fully explored.
ORAI1
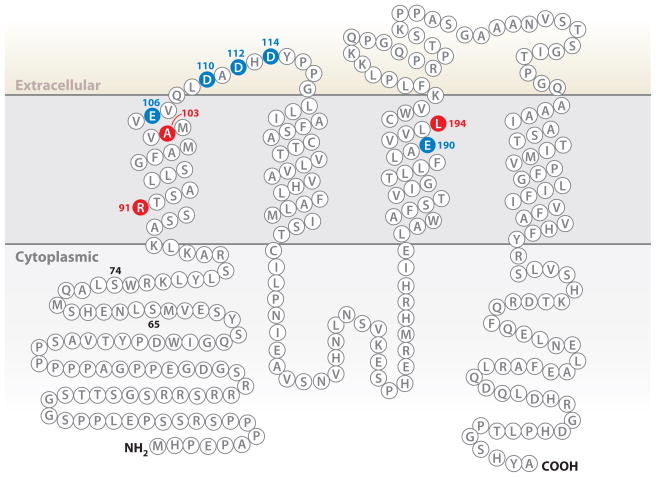
The ORAI1 monomer is a ~33-kDa plasma membrane protein with four transmembrane helices (Figure 5); glycosylation increases its apparent molecular weight on SDS gels (24, 89). There is persuasive evidence that ORAI1 assembles as a tetrameric CRAC channel (134–136). The channel opens in response to the signal conveyed by STIM1, conducts Ca2+ selectively, and, in certain cases, directs the intracellular Ca2+ signal to privileged effectors.
Figure 5.
Amino acid sequence of human ORAI1. Residues E106, D110, D112, D114, and E190, that when mutated affect channel properties, are shown in blue. Residues R91, A103, and L194, that when mutated to W, E, and P, respectively, are associated with human immunodeficiency, are shown in red (20, 93). Residues 65 and 74 are indicated; truncated ORAI1 proteins that begin at either residue are able to assemble and function as CRAC channels (33, 36, 150).
The ORAI1 channel
The transmembrane topology of the ORAI monomer (Figure 5) was established by (a) the intracellular location of N-terminal and C-terminal epitope tags and the extracellular location of an epitope tag introduced into the TM3-TM4 loop (24), (b) verification that an N-glycosylation site in the TM3-TM4 loop is glycosylated and therefore extracellular (24, 89), and (c) evidence that substitutions in the TM1-TM2 loop alter channel block by extracellular lanthanide ions (23).
The best evidence that the assembled channel is a tetramer comes from electrophysiological studies of channels made from concatenated ORAI1 monomers. CRAC currents in cells expressing a tandem tetramer of ORAI1 (unlike CRAC currents in cells expressing the monomer, tandem dimer, or tandem trimer) are insensitive to coexpression of the dominant negative E106Q monomer (134). Given that even a single E106Q substitution within the tandem tetramer compromises channel function, these results are most readily explained if the tandem tetramer forms a closed unit unable to incorporate a further monomer (134). The inability of a tandem tetramer to incorporate an ORAI monomer has been confirmed by FRET (135). Physical evidence also suggests that the channel is a tetramer because four individual bleaching steps are often resolved in single-molecule photobleaching of ORAI-GFP channels sparsely expressed in the plasma membrane (135, 136). These are technically difficult experiments in which the number of steps cannot be scored reliably for most channels (135), but two laboratories have independently concluded that open CRAC channels are tetramers (135, 136). However, one of the laboratories reports only two bleaching steps for closed Drosophila Orai channels (136). Finally, size-exclusion chromatography of purified detergent-solubilized ORAI1 complexes has supported the conclusion that the channel is a multimer (33, 137) but has not provided an accurate assessment of the number of subunits.
Redistribution of ORAI1
An outline has emerged of how the depleted-stores signal is relayed to ORAI1 channels. ORAI1 is recruited to ER–plasma membrane contacts through engagement of a C-terminal cytoplasmic segment of ORAI1 by STIM1, and a further STIM1-ORAI1 interaction gates the channel (Figure 4). The direct interactions of STIM1 with ORAI1, and their involvement in gating, are discussed below in the section on STIM-ORAI signaling.
Selectively conducting Ca2+
The biological role of the CRAC channel is to provide a permeation pathway for Ca2+ influx that effectively excludes the more abundant Na+ of physiological solutions. The negatively charged side chains of ORAI1 residues E106 in TM1 and E190 in TM3 help to create this permeation pathway, as evidenced by the markedly reduced selectivity for Ca2+ in channels with the specific replacements E106D or E190Q (23–25). More subtle changes observed on replacement of acidic residues in the TM1-TM2 loop indicate that residues immediately external to E106 also influence ion permeation (23, 25). Wild-type ORAI channels do not conduct monovalent ions in physiological solutions containing Mg2+ or Ca2+. However, in the absence of divalent ions, the currents carried by monovalent ions are sensitive probes of pore configuration. Additional experiments examining the ability of mutated channels to discriminate between Na+ and Cs+ further support the notion that residues E106 and E190 are close to the ion permeation pathway (23–25).
In an ORAI1 tetramer, four E106 side chains likely coordinate Ca2+ directly within the pore. The strict requirement for negative charges at this position in the channel is supported by the findings that ORAI1(E106A) and Drosophila Orai with the corresponding substitution fail to conduct Ca2+ (23, 24) and that a single E106Q replacement prevents Ca2+ flux when incorporated into a tandem tetramer with wild-type ORAI1 monomers (134). In contrast, the proposal that the negative charges of E190 side chains or the external acidic residues form essential Ca2+ coordination sites in the pore (25) is inconsistent with the evidence that ORAI1(E190A) and ORAI1(D110A/D112A) channels conduct Ca2+ perfectly well (24, 25). The distinctive structural contribution of these other acidic residues remains to be determined.
Other key properties that the ORAI1 channel has in common with the native CRAC channel are its very small single-channel current (138), which will contribute to tightly graded control of local and global Ca2+ concentrations, and its regulation by feedback mechanisms that will limit Ca2+ entry when local or global Ca2+ concentration is elevated (138–143) (see the sidebar above, Fingerprinting the CRAC Channel).
Communicating to effectors
The calcineurin-NFAT pathway in T cells is thought to respond primarily to global cytoplasmic Ca2+ transients. However, other effector pathways downstream of CRAC currents respond to local Ca2+ signals in microdomains near the sites of Ca2+ influx. Following the precedent of voltage-dependent Ca2+ channels and neuronal synaptic channels that conduct Ca2+, Ca2+-activated effector proteins could be localized in ORAI channel complexes or at adjacent sites within ER–plasma membrane appositions.
CRAC channel signaling is clearly restricted to microdomains in RBL (rat basophilic leukemia)-1 mast cells because loading cells with the fast Ca2+ chelator BAPTA [bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetate] diminishes Ca2+-dependent induction of the c-fos gene in these cells, whereas loading them with the slow Ca2+ chelator EGTA [ethyleneglycolbis(beta-aminoethyl ether)-N,N′-tetra-acetic acid] has no effect (144, 145). The membrane-proximal effector appears to be the Syk kinase, which couples through intermediate steps to STAT5 signaling in the cell nucleus and to phospholipase A2 and 5-lipoxygenase to elicit leukotriene production in the cytoplasm (144–147). Endothelial nitric oxide synthase (148) and Ca2+-sensitive adenylate cyclases (149) are also activated selectively by a local increase in subplasmalemmal Ca2+ occurring after store depletion, although these older studies did not show that ORAI channels, rather than TRPC or other channels, are the local source of Ca2+. The molecular tools are now available to investigate which enzymes and signaling proteins are activated in microdomains near ORAI channels in cells of the immune system.
ORAI1 is excluded from artificially close ER–plasma membrane contacts made by engineering inducible cross-links between the two membranes (112). This result suggests that the ORAI channel complex protrudes more than ~9 nm into the cytoplasm, a result that was first interpreted as indicating the presence of associated proteins. A model of the channel reconstructed from electron microscopic images of purified ORAI1 has raised the possibility that ORAI itself extends ~10 nm into the cytoplasm (137). If this conclusion is correct, the bulk of the protruding region of ORAI1 would necessarily be composed of the N-terminal section of ORAI that is dispensable for channel function. The extended surface could contribute additional interactions with STIM proteins or other regulatory proteins or serve as a scaffold for effector proteins.
STIM1-ORAI1 Signaling
Prior to store depletion, ORAI1 is distributed throughout the plasma membrane. The sequential steps in the activation of CRAC channels are that STIM1 moves to puncta, STIM1 recruits ORAI1 to puncta, and ORAI1 channels open (Figure 4). The latter two steps, which are described next, depend on direct physical interactions between STIM1 and ORAI1.
ORAI1 recruitment to puncta
Ca2+ entry through CRAC channels occurs at STIM1 puncta (30). ORAI1 colocalizes with STIM1 at puncta (30, 99, 150), and FRET between labeled STIM and labeled ORAI indicates that the proteins are closely juxtaposed (104, 119, 123, 151). STIM1 redistributes to puncta in the absence of overexpressed ORAI (19, 29, 31, 33, 99), but overexpressed ORAI does not relocalize appreciably to puncta unless it is coexpressed with STIM (33, 99), indicating that STIM is necessary to recruit ORAI to puncta.
Recruitment of ORAI1 depends on its C-terminal cytoplasmic tail, as is evident from the failure of ORAI1 lacking its C-terminal tail to colocalize with STIM1 and support CRAC current upon store depletion (104, 150). Electrophysiological recording of CRAC currents indicates that the relevant segment ends before residue 283 (141). The conclusion based on C-terminal deletion is strongly supported by studies of ORAI1 channels with the individual replacements L273S or L276D, which fail to interact with STIM1 upon store depletion (104, 151). The basis for recruitment is most likely a direct protein-protein interaction of the ORAI1 C terminus with STIM1 (33, 36, 104), with the corollary that STIM1 spans the ER–plasma membrane distance, because the short C-terminal tail of ORAI1 cannot project so far (Figures 3, 4).
ORAI1 channel gating
Studies with fully recombinant proteins expressed in bacteria or insect cells show that the STIM1 cytoplasmic region and the minimal CAD (CRAC activation domain) fragment bind directly to the C-terminal region of ORAI1 in vitro (33, 36). Circumstantial evidence suggests that this interaction is not by itself sufficient for gating. The ORAI1 ΔN truncation, missing all of the N-terminal cytoplasmic region, is expressed in the plasma membrane and accumulates at STIM1 puncta following store depletion, but it does not support the CRAC current (104, 150). In contrast, truncated ORAI1 proteins that include a short segment just N-terminal to ORAI TM1 can assemble and function as Ca2+ channels (33, 36, 150), focusing attention on the segment ORAI1(65–91).
ORAI1(R91W), the variant identified in two human patients with a hereditary immunodeficiency, interacts with STIM1 and is recruited to puncta upon store depletion (104, 151), yet it fails to conduct CRAC current (20, 79, 104, 142). The defect is intrinsic to ORAI, as shown by the fact that the corresponding R66W mutant of ORAI3 is activated neither by store depletion via STIM1 nor by 2-aminoethoxydiphenyl borate (2-APB), a compound that directly activates wild-type ORAI3 channels (152). There is no specific requirement for arginine at position 91 because ORAI1 proteins with nonconservative R91G and R91E replacements are fully functional, but replacement of R91 by residues with bulky nonpolar side chains interferes with channel function (153). These results could be explained if channel gating requires movement of this N-terminal segment of ORAI1, and substitution of bulky nonpolar residues at position 91 impairs the gating movement, but a more general effect of the N-terminal truncations or substitutions on channel structure has not been excluded (151–154).
Intriguingly, assays in vitro have detected a weak interaction of STIM1 with ORAI1 peptides corresponding to the segment ORAI1(65–91) (33, 36). Direct tests of whether this second identified STIM-ORAI interaction occurs with full-length ORAI, and whether it participates in gating, are warranted. Movement of STIM1 and ORAI1 to puncta is not obligatory for channel gating. Expression of the STIM1 cytoplasmic region alone, divorced from the luminal domain and the transmembrane tether, activates endogenous CRAC channels in Stim1−/− T cells, Jurkat T cells, and RBL cells (34, 36, 111, 140). Expression of the fragment together with ORAI1 can result in large constitutive CRAC currents in HEK293 cells (34, 104, 135, 140), without formation of visible STIM1 puncta and without visible relocalization of ORAI1 (104, 136). This finding rules out obligatory participation of specialized proteins or a distinctive lipid environment at the ER–plasma membrane contacts in channel gating. Conveniently, the finding has also provided tools to approach several questions that were not amenable to study at puncta.
The ability of the STIM1 cytoplasmic domain to activate ORAI1 channels directly has been tested in experiments with human ORAI1 channels expressed in the yeast Saccharomyces cerevisiae, which does not possess a STIM-ORAI pathway. The recombinant STIM1 C terminus elicited Ca2+ efflux from membrane vesicles isolated from yeast expressing ORAI1, but not from control vesicles containing no ORAI1 (36). As in mammalian cells, the SCID mutant ORAI1(R91W) and the pore mutant ORAI1(E106Q) did not support Ca2+ flux. The results imply that STIM1 and ORAI1 communicate directly at ER–plasma membrane contacts in mammalian cells, but they do not exclude the possibility that additional proteins in mammalian cells further modulate the efficiency of STIM-ORAI coupling.
N-terminal and C-terminal truncations of the soluble STIM1 cytoplasmic region have pinpointed a minimal fragment of STIM1 that can activate overexpressed ORAI1 and, in T cells and mast cells, native ORAI1 (32–35). The minimal region, termed SOAR (STIM-ORAI activating region), CAD, or CCb9, encompasses roughly residues 344–442 (32, 33, 35) and is contained within the functionally similar fragment termed OASF (ORAI1 activating STIM1 small C-terminal fragment) (34) (Figure 2). Notably, this region is positioned approximately 110 residues past the single transmembrane segment that tethers STIM1 to the ER. This accords with the geometry of ER–plasma membrane contacts (29, 109) and allows the part of STIM that interacts physically with ORAI proteins to be positioned near the plasma membrane by the lengthy STIM1 coiled-coil regions (Figures 3, 4).
STIM1-ORAI1 stoichiometry
Use of the STIM1 C-terminal fragment has permitted a direct approach to the stoichiometry of STIM-ORAI interaction (135). Labeled STIM C terminus and labeled ORAI, when coexpressed, do not coalesce into puncta visible by fluorescence microscopy (104, 136). Visualization of single ORAI channels by TIRF microscopy further indicates that they do not form submicroscopic aggregates, even though ORAI channels are open (135). These observations led to an experimental design in which labeled STIM1 was expressed at low levels in order to resolve its interactions with endogenous channels. Under these conditions, photobleaching of STIM1 C-terminal-EGFP bound to or near the plasma membrane proceeded in one or two steps (135). Two-step bleaching was assigned to a STIM-ORAI complex because cells expressing modestly higher levels of STIM showed increased one-step bleaching, whereas, in contrast, cells expressing exogenous ORAI1 in addition to STIM1 showed increased two-step bleaching. The interpretation that two STIM1 C-terminal domain molecules interact with ORAI in cells is in line with the finding that the recombinant C-terminal region of STIM1 exists as a dimer in vitro (32, 36), but the photobleaching result was taken to support the stronger conclusion that two STIM1 molecules activate an ORAI channel. Weaknesses in the argument are (a) the rather indirect evidence that the population with two bleaching steps corresponds to STIM-ORAI complexes, and (b) the lack of measurements to confirm that the STIM C terminus expressed at these low levels activated endogenous ORAI channels.
The picture of a STIM1 dimer, or two independent STIM1 molecules, gating an ORAI1 tetramer channel is seemingly in tension with another view: that multimer formation is necessary for CRAC current activation. The presence of a specific multimerization domain in STIM1 cytoplasmic fragments may correlate with their ability to activate the CRAC channel (34). Experimentally, the activating CAD fragment of STIM1 forms tetramers in solution, and complexes of CAD fragment and ORAI1 that have been coexpressed in insect cells, solubilized, and purified are visible as large aggregates by electron microscopy (33). However, it has not been shown that the truncated STIM1 fragment oligomerizes in the same way as the full-length protein or that the solubilized and purified ORAI channels are in an active conformation. The finding that coexpression of CAD lowers the effective diffusion coefficient of ORAI in the plasma membrane, as determined by FRAP (33), furnishes some support for ORAI1 oligomerization in cells, but it could be explained, for example, by transitory interactions of CAD with immobile cellular proteins that lower the effective concentration of freely diffusing ORAI1.
It thus remains an open question whether ORAI1 activated in situ by the STIM1 C terminus and ORAI1 activated after recruitment to puncta by full-length STIM1 are in identical complexes. The estimated average density of channels at puncta in HEK293 cells over-expressing recombinant STIM and ORAI is ~1,000 per μm2 (135), compared with a density greater than 10,000 per μm2 for the slightly larger nicotinic acetylcholine receptor in post-synaptic membranes (155). This estimate does not require that the channels be tightly packed, even in cells overexpressing ORAI and producing large currents; however, it does not rule out the ordered assembly of closely packed ORAI channels into patches with dimensions below the resolution of conventional light microscopy. The question of STIM1-ORAI1 stoichiometry will be resolved only through structural determination of the conformations and oligomerization states of full-length STIM1 and ORAI1, as well as investigations of the actual organization of STIM and ORAI at puncta in T cells and mast cells.
Additional gating mechanisms
There are continuing reports that CRAC channels can be activated by an unidentified calcium influx factor (CIF) extracted and partially purified from ER Ca2+-depleted human platelets, Jurkat T cells, and other sources (156, 157). RNAi experiments have placed CIF downstream of STIM1 and upstream of the membrane-associated phospholipase iPLA2β and ORAI1 (158). The model proposed is that CIF releases iPLA2β from inhibition by CaM, and iPLA2β in turn generates products that activate CRAC channels (159) and, in some cells, less selective Ca2+-permeable channels (160). The effectiveness of the STIM1 C terminus in activating ORAI1 channels in vitro argues strongly that CIF is not required for STIM1 to communicate with ORAI1. However, it remains possible that CIF provides a parallel pathway for ORAI activation or modulation; purification and identification of the active component of CIF will be needed to test this hypothesis.
STIM2, ORAI2, ORAI3
STIM2
STIM2 bears a marked resemblance to STIM1 in its overall structure (105). Its ER-luminal domain has paired canonical and non-canonical EF-hands and a SAM domain, and its cytoplasmic domain displays sequence similarity to STIM1 extending through a long predicted coiled-coil and beyond the C-terminal boundary of the minimal activating fragment of STIM1, as well as a polybasic C-terminal tail. The protein sequences diverge in a short segment at the N terminus and a longer segment near the C terminus. STIM2 in cells is reportedly localized exclusively in the ER (161).
STIM2 also recapitulates the basic functional properties of STIM1. Recombinant STIM2 luminal domain binds Ca2+ in vitro with an affinity suitable for sensing ER Ca2+ levels (162). Its monomeric luminal domain is somewhat more stable in the absence of Ca2+ than is the STIM1 domain, but nonetheless on loss of Ca2+ the STIM2 EF-SAM fragment undergoes a conformational change and oligomerizes (28, 162). In cells, upon depletion of Ca2+ stores, STIM2 redistributes to puncta at ER–plasma membrane contacts (26). In fact, STIM2 redistributes to puncta at higher ER Ca2+ concentrations—that is, with a smaller reduction in Ca2+ stores—than STIM1, and a fraction of STIM2 is already activated in cells with replete Ca2+ stores (26). STIM2 may also have a distinctive propensity to interact with plasma membrane lipids. In vitro data indicate that the cytoplasmic domain and the isolated polybasic tail of STIM2 bind more avidly to PIP2/PIP3 than do the corresponding fragments of STIM1 (116). Tending to counteract the partial activation in cells with replete stores and the more avid targeting to ER–plasma membrane contacts, a STIM2 EF-hand mutant that is constitutively localized at ER–plasma membrane junctions couples less effectively to ORAI1 than does the corresponding EF-hand mutant of STIM1 (120).
There has been considerable debate over the role of STIM2 in Ca2+ signaling. Over-expression of STIM2 has produced variable results, ranging from an inhibition of store-operated Ca2+ entry when STIM2 is expressed alone (161) to increases in constitutive or store-operated Ca2+ influx when STIM2 is co-expressed with ORAI1 (26, 95, 163). Over-expression of STIM2 alone partially rescues store-operated Ca2+ entry in STIM1−/− T cells (15). Collectively, these data indicate that STIM2 can engage in the same signaling pathway as STIM1, and that overexpression is not a sufficiently precise tool to tease out its biological role.
Currently, the only established role for STIM2 is a contribution to maintaining basal cytoplasmic Ca2+ levels (26), and in many cells STIM2 may have no acute signaling role. Thus, STIM1, not STIM2, is essential for agonist-driven Ca2+ oscillations in HEK293 cells (120). This does not minimize the physiological importance of STIM2. The absence of STIM2 in STIM2−/− T cells causes at most minor impairment in a short-term Ca2+ influx assay in stimulated cells, but it results in severe deficits in the sustained nuclear localization of the transcription factor NFAT and in cytokine production (15).
ORAI2 and ORAI3
ORAI2 and ORAI3 exhibit strong sequence similarity to ORAI1 in the transmembrane segments TM1–TM4. Glutamate residues corresponding to E106 and E190, which contribute to the selective Ca2+ permeability of ORAI1, are present. Overexpression of ORAI2 or ORAI3 together with STIM1 yields large Ca2+-selective currents in some expression systems (96, 139, 141, 152, 164), and the substitutions E81D and E165Q in ORAI3 have the same deleterious effect on Ca2+ selectivity as the corresponding replacements, E106D and E190Q, in ORAI1 (152). The N-terminal and C-terminal intracellular segments implicated in STIM1-ORAI1 interaction are also conserved, as is the intracellular region between TM2 and TM3 to which no function has yet been assigned. Experiments probing the interaction of STIM1 with chimeric ORAI proteins further strengthen the argument that the ORAI C terminus is essential in recruiting ORAI channels to puncta (165). On the basis of the latter work, Romanin and colleagues (165) have suggested a direct interaction between the second predicted coiled-coil of STIM1 and a predicted coiled-coil of ORAI1, although the authors note that neither a coiled-coil structure nor a direct interaction between these regions has been documented.
ORAI2 mRNA is present at high levels in murine T cells, and ORAI2 may support store-operated Ca2+ entry in ORAI1−/− T cells (166). This ORAI1-independent Ca2+ influx is especially apparent in naive T cells, but a residual store-operated Ca2+ entry and a CRAC-like current are also present in differentiated ORAI1−/− T cells (167). However, the failure of recombinant ORAI2 to reconstitute store-operated Ca2+ entry in ORAI1−/− T cells (167), despite strong recombinant protein expression at the cell surface, leaves the contribution of ORAI2 in doubt.
Several lines of evidence converge on the conclusion that ORAI3 is a subunit of the ARC channel, another highly selective Ca2+ channel that responds to arachidonic acid rather than to store depletion (168, 169). As mentioned previously, the role of the ARC channel in immune responses remains to be delineated. Another report raises the alternative possibility that ORAI1-ORAI3 heteromultimeric channels could account for store-operated currents that are less Ca2+-selective than CRAC currents (170).
MOVEMENTS OF STIM1 AND ORAI1 IN ANTIGEN-STIMULATED T CELLS
Two groups have examined the redistribution of STIM1 and ORAI1 in stimulated T cells (122, 123). Cahalan and colleagues (122) used Jurkat cells and primary human T cells transfected with GFP-ORAI1 and untagged or YFP-tagged STIM1, and stimulated them with dendritic cells (DCs) pulsed with the superantigen SEB (staphylococcal enterotoxin B). The tagged and endogenous proteins moved rapidly (within 5–10 min) to the vicinity of the T cell–DC interface [a region termed the immunological synapse, where T cell and costimulatory receptors cluster with signaling proteins (171)], and remained colocalized there for at least 30 min. A pore mutant of ORAI1 (E106A) was also able to redistribute to the T cell–DC interface, even though its expression interfered dominantly with the function of the endogenous ORAI channel and abolished Ca2+ entry through endogenous CRAC channels. These authors also showed that TCR stimulation resulted in upregulation of STIM1, ORAI1, ORAI2, and ORAI3 mRNA in activated T cells and in a corresponding increase in both thapsigargin-stimulated and TCR-stimulated Ca2+ influx.
Samelson and colleagues (123) used a different system in which Jurkat cells coexpressing ORAI1-CFP and STIM1-YFP were plated onto coverslips coated with stimulatory anti-CD3 antibodies. Under these conditions STIM1 and ORAI1 colocalized at puncta near the stimulatory surface, and at least some STIM1 and ORAI1 molecules were close enough for FRET between them to be observed. However the regions of STIM1-ORAI1 colocalization were distinct from the signaling microclusters containing TCRs and marked by phosphotyrosine, suggesting that sites of tyrosine kinase activation in the contact interface do not necessarily overlap with sites of Ca2+ influx.
Surprisingly, a large fraction of endogenous as well as fluorescently tagged STIM1 and ORAI1 proteins moved away from the stimulatory surface, eventually colocalizing in stable cap structures at the opposite pole of the cell (123). The average FRET efficiency between ORAI1-CFP and STIM1-YFP was consistently higher in the caps than in the puncta, and photobleaching experiments showed that both proteins were notably less mobile in the caps than when diffusely distributed in the ER and plasma membrane, respectively, of unstimulated cells. Formation of the caps required TCR stimulation, the activation of tyrosine kinases, and an intact cytoskeleton, but it was not dependent on Ca2+ influx. Based on their observation that the caps appeared more dynamic when Jurkat T cells were stimulated with B cells pulsed with the superantigen SEE (staphylococcal enterotoxin E) and were sometimes seen to donate STIM1 and ORAI1 to a second contact interface formed by the T cell with a newly arriving superantigen-pulsed B cell, the authors speculated that the cap may serve as a repository of STIM and ORAI proteins that could be rapidly mobilized.
OTHER PATHWAYS FOR Ca2+ ENTRY IN LYMPHOCYTES
As discussed above, the bulk of the available evidence suggests that CRAC channels form the primary route for Ca2+ entry in T cells and mast cells. Nevertheless, several studies have proposed that additional Ca2+ entry pathways contribute to Ca2+ signaling in lymphocytes. The regulation and activity of these alternative pathways are not as well understood as those of the CRAC channel. In the following sections, we discuss the current evidence for these pathways and suggest experiments that may provide more definitive tests for their roles in lymphocyte Ca2+ signaling.
Ca2+ Entry in B Cells: IP3 Receptors in the Plasma Membrane or B-SOC?
The high sensitivity of CRAC channels to inhibition by trivalent cations has been exploited to reveal other routes of Ca2+ entry into lymphocytes. In two studies, treatment of chicken DT40 pre-B cells with 300 nM Gd3+ or La3+ (sufficient to fully block CRAC current) failed to fully suppress BCR-triggered Ca2+ entry (172, 173). What is the source of this residual BCR-dependent Ca2+ entry? Taylor and colleagues (173) propose that it occurs through plasma membrane IP3Rs, based on recordings of single-channel currents in whole-cell recordings from DT40 and mouse B cells. Several lines of evidence support the argument that the currents reflect IP3Rs in the plasma membrane: Channel activity was absent in IP3R–knockout DT40 cells, the single-channel conductance varied with expression of known IP3R pore mutants, and the currents could be blocked by extracellular bungarotoxin when IP3Rs with bungarotoxin-binding sites were expressed. Surprisingly, however, regardless of the amount of IP3R cDNA used to transfect, only two to three IP3-gated channels were detected in the plasma membrane. This constancy of expression is apparently not regulated by feedback from channel activity or Ca2+ entry, as it also applies to mutant IP3Rs with reduced IP3 binding or low conductance (174). Together, the findings of Taylor and colleagues (173, 174) suggest that just two to three high-conductance IP3Rs account for about half of the Ca2+ entry triggered by BCR stimulation, whereas the remainder is conducted by thousands of low-conductance CRAC channels. If true, this could exert a powerful influence on the specificity of Ca2+ signaling, particularly if specific effector proteins are localized within the Ca2+ microdomains of the IP3R. One essential test of these results would be to identify the individual sites of influx through the IP3Rs using Ca2+ imaging techniques; knowing the location of these Ca2+ hotspots not only would confirm the electrophysiological results, but also would likely offer helpful clues regarding downstream pathways or mechanisms of channel regulation.
A second group recently confirmed that BCR stimulation evoked trivalent-insensitive, non-CRAC-mediated Ca2+ entry into DT40 cells but concluded that it was not conducted through IP3Rs (175). Morita and colleagues (175) observed that BCR stimulation could elicit La3+-insensitive Ca2+ entry into IP3R–deficient DT40 cells, but only if stores had first been fully depleted with thapsigargin. The response in DT40 cells required STIM1 expression but was not affected by knocking down Orai1 or Orai2. These and other findings suggest a pathway, referred to as B-SOC, that is STIM1-dependent and regulated by the combination of store depletion and a signal from the BCR. The channel underlying this process has not been identified. To validate this B-SOC pathway, it will be necessary to distinguish it from the CRAC channel by characterizing its biophysical and pharmacological properties using patch-clamp techniques; because biochemical factors and membrane potential can also influence Ca2+ signals, Ca2+ imaging experiments alone cannot describe the underlying pathway directly or in enough detail to allow a meaningful comparison. Further work will be needed to clarify the relation between B-SOC and the IP3Rs described by Taylor et al.
CaV Channels in T Cells
Voltage-gated Ca2+ (CaV) channels execute diverse roles in electrically excitable cells: CaV channels open upon membrane depolarization, and the resulting Ca2+ entry initiates muscle contraction and vesicle exocytosis and shapes electrical activity, to name but a few functions. The roles of CaV channels in electrically inexcitable cells such as lymphocytes have been more controversial. The inhibitory effects of l-type CaV antagonists (nifedipine, diltiazem, and verapamil, among others) on TCR-mediated Ca2+ responses originally prompted the notion that human T cells express functional CaV channels (176, 177). However, the lack of specificity of these drugs for CaV channels creates complications: At the high doses that were used they also block K+ channels, which can indirectly inhibit Ca2+ signaling via CRAC channels by depolarizing the membrane and diminishing the driving force for Ca2+ entry (178–180). Moreover, one CaV antagonist, nifedipine, may affect the store-operated Ca2+ entry process itself (181). Finally, depolarization of T cells fails to elicit a [Ca2+]i rise or to evoke inward Ca2+ currents (122, 182, 183). Thus, if a CaV channel were to conduct Ca2+ in T cells, it would have to be activated through a novel biochemical pathway rather than through membrane depolarization.
Molecular studies have provided additional evidence for CaV channel expression in T cells. Cav1.1, 1.2, and 1.4 transcripts and proteins as well as auxiliary β subunits have been detected in murine T cells, and their levels of expression increased after stimulation with anti-CD3 and anti-CD28 in vitro (183). Alternatively spliced transcripts of Cav1.4 channels have been detected in human T, B, and Jurkat cells; these splicing events are predicted to create deletions that might account for the observed lack of voltage-dependent gating (182, 184). [Ca2+]i elevations evoked by anti-CD3 were partially suppressed in CD4+ T cells from mice lacking either β3 or β4 subunits (183), possibly explaining the cytokine secretion defects in these mice. Similarly, TCR-mediated Ca2+ signaling was partially inhibited in CD4+ T cells from mice genetically disrupted for expression of the scaffold protein AHNAK1 (185). AHNAK1 is a very large scaffold protein (~700 kDa) that binds several proteins, including PLC, S100b, the annexin 2 complex, and the CaV β2 subunit (185). Cav1.1 and Cav1.2 protein levels were diminished in AHNAK1−/− cells, and Cav1.1 in the membrane fraction was reduced, correlating with the smaller Ca2+ responses (185). Interestingly, AHNAK1 is expressed in CD8+ T cells only after they differentiate to CTLs, and expression of Cav1.1 is upregulated in parallel. Like AHNAK1−/− CD4+ T cells, CTLs from AHNAK1−/− mice show reduced Ca2+ elevation in response to TCR stimulation, as well as reduced granzyme B production and cytolytic activity (186).
Can these studies be reconciled with the evidence that Orai Ca2+ channels are responsible for Ca2+ influx in peripheral mouse and human T cells? In humans, loss of expression or function of ORAI1 results in a complete lack of TCR-mediated Ca2+ influx as well as in profound immunodeficiency (79–82, 93). Murine T cells deficient in β4, β3, or AHNAK1 fail to exhibit substantial Ca2+ entry following TCR stimulation, but are not impaired for Ca2+ entry evoked by store depletion with thapsigargin, implying that CRAC channel activation by full store depletion is intact. One possibility is that CaV β subunits and the AHNAK1 scaffold influence Ca2+ influx and T cell effector functions indirectly, perhaps by influencing the local generation of IP3 [bulk IP3 generation was unaffected in AHNAK1−/− T cells (185)], or some additional mechanism that does not directly involve Ca2+ permeation through CaV channels.
An essential step in validating CaV channels as a Ca2+ permeation pathway in peripheral T cells is to demonstrate that they conduct a measurable Ca2+ current after activation by the TCR. The most straightforward way to do this is to confirm that mutations of the critical ion selectivity–producing glutamate residues in the CaV pore alter the ion selectivity of the TCR-induced current. This type of experiment was done originally with Cav1 channels (187), and an analogous mutagenesis of the Orai1 subunit of the CRAC channel supplied the definitive proof that Orai1 forms the pore of the CRAC channel (23–25). A direct demonstration of currents carried by CaV channels in T cells will help settle the debate regarding their role as a parallel pathway for TCR-triggered Ca2+ influx.
In conclusion, further studies will be needed to determine the contributions of CaV channels, IP3Rs, or other modes of non-store-operated Ca2+ entry to Ca2+ influx in lymphocytes, particularly in defined subsets of immune cells and under a range of stimulation conditions. Notably, murine thymocytes that completely lack store-operated Ca2+ entry as a result of targeted disruption of the Stim1 and Stim2 genes show no developmental impairment (discussed below), suggesting that STIM-independent modes of Ca2+ entry may operate during T cell development. These possibilities deserve further investigation.
Modulation of Calcium Signals
The rate at which Ca2+ enters the cell through open CRAC channels is determined by the Ca2+ concentration gradient and the voltage difference across the plasma membrane (membrane potential). Thus, even though they do not conduct Ca2+ directly, various channels can modulate calcium signals in T cells through their ability to alter the T cell membrane potential (normally ~−50 mV, with the cell interior at negative potential relative to the outside) (188). The most thoroughly understood are the K+-selective channels. These have been extensively reviewed elsewhere (41, 180); here, we give a brief overview of their functions.
K+ channels
Kv1.3 is expressed in human and mouse T cells. It is a voltage-gated channel (hence Kv channel), which opens upon depolarization beyond ~−50 mV (i.e., when the difference in potential between the inside and outside of the cell is reduced, as would occur upon opening of nonselective cation channels or Ca2+-selective CRAC channels). By allowing K+ to leave the cells, opening of the Kv1.3 channel sets the resting membrane potential of the T cell near this value (i.e., ~−50 mV), thus resisting the depolarizing influence of Ca2+ influx through CRAC channels. Blockade of Kv1.3 with highly specific blockers inhibits T cell activation in vitro in a dose-dependent manner, which has been linked mechanistically to depolarization and reduced Ca2+ entry (41, 189).
In addition, human T and B cells also express KCa3.1, a class of intermediate-conductance K+ channels. This channel is voltage-independent but is activated by Ca2+ (hence KCa channel). The KCa3.1 channel opens when Ca2+ binds to CaM bound to the channel C terminus (half-maximal activation by ~0.5 μM Ca2+) and hyperpolarizes the cell, thus increasing the driving force for Ca2+ influx and causing a greater overall increase of [Ca2+]i. Interestingly, KCa3.1 is negatively regulated by the histidine phosphatase PHPT-1, and knockdown of PHPT-1 has been associated with increased Ca2+ signals and proliferation (190).
Recent studies have revealed important changes in Kv1.3 and KCa3.1 expression during T cell activation and differentiation in vivo. Kv1.3 is more abundant than KCa3.1 in naive resting T cells and central and effector memory T cells, enabling this K+ channel to play the dominant role in maintaining membrane potential and Ca2+ influx. However, upon activation, central memory cells upregulate KCa3.1, whereas effector memory cells upregulate Kv1.3 (180). This creates a difference in their dependency on K+ channel subtypes that may be exploited for therapeutic purposes. Kv1.3 levels are high in T cells from human patients with a variety of autoimmune diseases (191), and clofazimine, a Kv1.3 channel inhibitor, has been used successfully in treatment of lupus, psoriasis, and chronic graft-versus-host disease in human patients (192), while other selective Kv1.3 inhibitors have been effective in treating diverse autoimmune disease models in animals (191, 193).
Recently, members of the twin-pore class of K+ channels (K2P) have been reported in T cells. In one study, a voltage-independent K+ current developed during whole-cell recording from Jurkat cells and resembled in some ways TRESK, a K2P family member that contributes to the resting potential in diverse cells (194). In human T cells, there is immunological evidence for the acid-sensitive TWIK-related channels TASK1 and TASK3, and an anandamide-sensitive current in these cells has been attributed to TASK channels (195). Anandamide suppressed experimental allergic encephalomyelitis in rats, raising the possibility that these channels could provide an additional therapeutic target in T cells (195), but the general lack of specificity of anandamide and other TASK inhibitors complicates efforts to assess TASK channel functions in vivo. Given reports that K+ currents in human and rat T cells are completely inhibited after treatment with highly specific blockers of Kv1.3 and KCa3.1 (ShK-Dap22 and TRAM-34, respectively) (41, 196), further work will be needed to establish roles for the K2P family of K+ channels in lymphocytes.
TRPM4
The nonselective cation channel TRPM4 (197) may also play an important role in regulating membrane potential and Ca2+ signaling in T cells. TRPM4 is activated by Ca2+, and once open it would be expected to drive the lymphocyte membrane potential toward 0 mV, just the opposite of the KCa channel. Launay et al. (198) show evidence that TRPM4 protein is present in thymocytes, the D10 T cell line (Th2), and Jurkat T cells and that increased [Ca2+]i activated a nonselective current in Jurkat that was inhibited by transfection with a dominant-negative TRPM4 mutant. The same inhibitory mutant suppressed PHA-induced [Ca2+]i oscillations and IL-2 production, leading to the suggestion that TRPM4 causes periodic depolarization that provides negative feedback to help terminate each oscillation and prevent [Ca2+]i from climbing to a high plateau. These results differ from earlier studies on Jurkat T cells in several respects. First, Launay et al. did not observe the prominent KCa current that was activated by μM Ca2+i in prior studies (199). Also, PHA had been shown previously to evoke oscillations of CRAC current in Jurkat cells without any apparent nonselective current (63). The reasons for these discrepancies are not clear but could reflect clonal variation in Jurkat cell lines. However, previous studies of primary human T cells also failed to reveal a Ca2+-activated nonselective cation channel like TRPM4, and intracellular dialysis of resting or activated human T cells with up to 10 μM Ca2+i evoked large KCa currents without any detectable nonselective current (200). Characterization of TRPM4 currents in normal human T cells will be an important step toward establishing their functional roles.
Mitochondria
The activity of CRAC channels can be modulated by mitochondria, and recent studies suggest that this may serve to sustain the activity of CRAC channels during interactions of T cells with antigen-presenting cells (APCs). Shortly after Ca2+ enters the cell through CRAC channels, energized mitochondria take up a portion of it through the MCU, accumulate it, and release it back into the cytosol through a Na+-Ca2+ exchange mechanism (201). In human T cells and Jurkat cells, sustained elevation of [Ca2+]i in response to store depletion requires uptake and release of Ca2+ by the mitochondria (201). One mechanism by which energized mitochondria may sustain CRAC channel activity is by preventing slow Ca2+-dependent inactivation of CRAC channels (202). Mitochondria are thought to inhibit inactivation by taking up Ca2+ and thereby reducing the free Ca2+ near the inactivation site or by locally generating ATP, a Ca2+ buffer (203–205). Interestingly, in Jurkat T cells Ca2+ influx through CRAC channels causes mitochondria to move toward the plasma membrane through a microtubule- and kinesin-dependent process, and sustained [Ca2+]i elevation is reduced when translocation is prevented, e.g., by treatment with nocodazole or by loading with antikinesin mAb (206). The accumulation of mitochondria near sources of Ca2+ has also been observed in other cells and occurs by a mechanism in which local [Ca2+]i elevation uncouples mitochondria from kinesin-driven movement along microtubules (207, 208).
Directed mitochondrial movement also results from polarized signaling through the TCR. Mitochondria accumulate at contacts between T cells and anti-CD3 beads, and this is dependent on Ca2+ entry through CRAC channels (209). The movement in this case is not nocodazole-sensitive, however; instead, it is inhibited by actin filament depolymerization with latrunculin B. Preventing the movement with latrunculin B did reduce the sustained component of Ca2+ signaling, but the actin dependency of immune synapse formation and strength of TCR signaling makes it difficult to draw a firm conclusion about the specific role of mitochondrial movement in this case. However, it is intriguing that CRAC channels also localize to the synapse (122, 123), raising the possibility that mitochondria function as part of a self-regulating signaling complex that promotes sustained Ca2+ entry at the synapse.
The dynamics of Ca2+ signaling in T cells are also modulated by time-dependent changes in Ca2+ pumping across the plasma membrane (210). In Jurkat T cells, Ca2+ extrusion occurs via PMCA4b. At resting [Ca2+]i, the PMCA is autoinhibited through an interaction between its C terminus and a region near the Ca2+ transport site (211). After [Ca2+]i rises, pump activity is initially low, but over tens of seconds Ca2+-CaM binds to the C terminus, displacing the inhibitory domain and allowing pump activity to increase. The effect of this PMCA modulation is to act as a high-pass filter, allowing transients to pass relatively unimpeded but effectively reducing sustained signals. This behavior contributes to the generation of biphasic spike and plateau responses commonly seen with strong TCR stimulation (210).
Mechanisms and Functions of Complex Calcium Signaling Patterns
The diversity of mechanisms that create and modulate Ca2+ signals in lymphocytes creates the potential for complex signaling dynamics, which is likely to have important consequences for the strength and specificity of signaling through downstream pathways. At high levels of stimulation using strong antigens, anti-TCR cross-linking, or artificial store depletion, a biphasic response is typical. This consists of an early rise to a peak, followed by a gradual decline over tens of seconds to an elevated plateau. Under these strong stimulation conditions, one would expect Ca2+ store depletion to remain relatively constant. Quantitative modeling has shown how this dynamic signature can arise from PMCA modulation (210).
At lower levels of stimulation, either with antigen or anti-TCR cross-linking or partial depletion of stores, [Ca2+]i oscillations are the more common response. A particularly dramatic example is seen with human T cells stimulated with a variety of agents (SERCA inhibitors, ionomycin, or anti-CD3) that only partially deplete the intracellular stores (212). Under these conditions, [Ca2+]i oscillates with a regular period of 100–200 s. The complete mechanism of this response is not known; however, evidence suggests that the oscillations are derived from the repetitive and coordinated opening and closing of CRAC channels, rather than from repetitive Ca2+ release from the ER. In support of this idea, PHA-evoked [Ca2+]i oscillations are terminated immediately by block of CRAC Ca2+ fluxes, and, in perforated-patch recordings from Jurkat cells, PHA evokes oscillations of CRAC current even under voltage-clamp conditions (63). Single-cell measurements from human T cells show that store content and the Ca2+ influx rate oscillate out of phase with each other (212). One model proposed to explain this oscillatory behavior is that intrinsic delays in the feedback between changes in store content and CRAC channel activity cause each to overshoot and undershoot its equilibrium value. Given our current understanding of the mechanism for CRAC channel activation, these delays may arise from the time it takes STIM1 to accumulate at ER–plasma membrane junctions following store depletion and to dissipate after stores refill. Other processes may also contribute to the oscillations, such as activation of Ca2+-activated K+ channels and TRPM4 channels, which could provide positive or negative feedback, respectively, during the rise and fall of each oscillation through their influence on membrane potential (198). However, the ability of CRAC current to oscillate at a constant membrane potential (63) shows that the basic oscillatory machinery does not require these other channels. A fuller understanding of the oscillation mechanism will entail quantitative modeling that incorporates an accurate characterization of each conductance and their interactions through changes in membrane potential and [Ca2+]i. Given the current pace of progress in understanding the CRAC channel, this may soon become possible.
[Ca2+]i oscillations may serve important functions in lymphocytes by enhancing the efficiency and the specificity of Ca2+ signaling to the nucleus. In Jurkat cells and RBL cells, artificially generated oscillations enhance the efficiency of signaling through NFAT by a limited amount of Ca2+ or IP3 (213–215). In addition, the activation of different transcriptional pathways shows a distinct dependency on oscillation frequency that enhances the specificity with which pathways are recruited; NFAT requires frequent spikes (period <200 s) for significant activity, whereas NF-κB is activated by spike periods as long as 30 min (213). The mechanism underlying these differences is likely to derive from the kinetics of transcription factor activation and deactivation. For example, NFAT translocation to the nucleus is regulated by a relatively rapid dephosphorylation/phosphorylation cycle; in this case, oscillations enhance the nuclear accumulation of NFAT provided that their period is shorter than the lifetime of the dephosphorylated state, so that each pulse of Ca2+ causes an incremental increase in nuclear NFAT (215). In contrast, NF-κB is activated through the proteolysis of the inhibitory IκB subunit; because resynthesis of IκB is a slow process, deactivation is also slow, and even infrequent oscillations are able to drive NF-κB accumulation in the nucleus (216).
These studies highlight the possibility that lymphocytes may receive specific information encoded in the amplitude and pattern of Ca2+ signals. To assess whether such a decoding system operates in vivo, naturally occurring Ca2+ signals must be characterized in cells exposed to the physiological stimuli that control their behavior and developmental fate. Several recent studies have used two-photon microscopy to measure [Ca2+]i signals as T cells navigate within three-dimensional immune tissues. In thymic slices, thymocytes undergoing positive selection display irregular oscillations that are associated strongly with immotility, and increased [Ca2+]i appears to be necessary and sufficient to stop cell migration (217). Thus, during development Ca2+ signals act in a positive feedback loop that stops the cells from migrating once they encounter and recognize an antigen-MHC complex; this “stop signal” then may help prolong the signaling that drives transcriptional programs leading to positive selection. Mature T cells in lymph node explants also display [Ca2+]i fluctuations that increase in frequency and amplitude on contact with antigen-primed DCs (218). As in the thymus, Ca2+ spikes in naive lymph node T cells were associated with decreased velocity, consistent with the ability of Ca2+ to slow T cell motility. [Ca2+]i elevation and oscillations have also been seen in B cells contacting antigen-bearing DCs in the lymph node (219). A third study showed that only high-potency peptides can cause a [Ca2+]i rise in lymph node T cells in vivo, and again, the Ca2+ signal was necessary for motility arrest (220). It remains to be seen whether Ca2+ is a universal motility stop signal in the immune system, what other signals participate in immobilizing cells at sites of productive contacts with APCs, and how the balance between these influences may change during development.
BIOLOGICAL CONSEQUENCES OF STIM AND ORAI DEFICIENCIES
Hereditary Immunodeficiencies in Humans Resulting from Mutations in STIM1 and ORAI1
In human patients, defects in CRAC channel function lead primarily to severe immunodeficiency. A handful of patients with rare hereditary immunodeficiencies have been identified who display a concomitant loss of T cell cytokine expression, store-operated Ca2+ entry, and CRAC current (20, 79–82, 90–93). Three sets of siblings from three families have mutations in ORAI1, and three others from a single family have mutations in STIM1 (Figure 6). The mutations are recessive because the heterozygous relatives are not immunodeficient. Because other family members (STIM2, ORAI2, ORAI3) do not compensate for mutations in STIM1 and ORAI1, the data suggest strongly that ORAI1 and STIM1 are the predominant family members responsible for store-operated Ca2+ entry in human T cells. Patients with mutations in STIM2, ORAI2, or ORAI3 have not yet been identified.
Figure 6.
Pedigrees of immunodeficient patients with mutations in (a–c) ORAI1 and (d ) STIM1. (Filled symbols, patients; strike-through, deceased; ?, DNA unavailable for sequencing; dot within symbol, individual is heterozygous for the mutant allele.) Adapted with permission from References 91, 93.
R91W, a loss-of-function mutation in ORAI1
T cells from the two patients who have been most completely characterized were unable to produce some NFAT-dependent cytokines or to show changes in the expression of diverse Ca2+-regulated genes, upon stimulation (80, 90). This was traced to an almost complete loss of store-operated Ca2+ entry and CRAC channel current, resulting in very minor, transient dephosphorylation and nuclear translocation of NFAT in stimulated T cells (79, 80, 90). Relatives who were heterozygous carriers of the mutant allele were identified by the fact that they showed decreased store-operated Ca2+ entry, but only at subphysiological [Ca2+]o (20). Genome-wide SNP analyses, together with the Drosophila RNAi screen, established the genetic basis for the immunodeficiency as an R91W mutation in ORAI1, near the beginning of the first transmembrane domain (20) (Figure 6). Why this mutation is nonfunctional is not yet understood at a biochemical level (see previous section).
The defect in store-operated Ca2+ entry in the T cells and fibroblasts of these patients could be rescued by expression of wild-type ORAI1, but not by overexpression of ORAI2 above its endogenous levels in cells. There was a minor degree of rescue by overexpressed ORAI3. The biophysical differences in the channel formed by ORAI1, ORAI2, and ORAI3 were discussed above; in particular, channels formed by ORAI2 may not be gated by store depletion in a physiological context, although overexpression of ORAI2 and STIM1 does result in increased Ca2+ entry evoked by store depletion.
ORAI1 mutations leading to loss of ORAI1 protein expression
ORAI1 mutations are also responsible for two other familial severe immunodeficiency syndromes, originally described in the mid-1990s (81, 82). In each case, the underlying mutations result in an essentially null phenotype for ORAI1 (93) (Figure 6). In one of the families, where the parents were second cousins and their two affected children were homozygous for the mutant allele, insertion of an adenine in exon 1 of ORAI1 gave rise to a frameshift mutation that altered the sequence of the first transmembrane domain and introduced a stop codon that terminated the protein at residue 112 (81, 93). There was no detectable expression of the mutant protein (or mRNA, possibly due to nonsense-mediated decay) and no detectable Ca2+ entry into the patient’s fibroblasts upon store depletion with thapsigargin. Store-operated Ca2+ entry was restored upon reconstitution of the mutant fibroblasts with wild-type ORAI1, but not with STIM1.
In the second family, where the parents were unrelated, the affected patient appears to have inherited a different mutant ORAI1 allele from each of his heterozygous (and clinically normal) parents (82, 93) (Figure 6). This patient’s two independent missense mutations, A103E and L194P, are located in the first and third transmembrane regions of ORAI1, respectively, in relative proximity to two essential glutamate residues, E106 and E190, in the Ca2+-conducting pore. Nevertheless, both mutations result in loss of protein expression rather than in loss of function with unimpaired expression. Endogenous ORAI1 protein levels appeared to be diminished in the patient’s fibroblasts, and ORAI1 cDNAs bearing the individual mutations were not expressed at detectable levels even in HEK293 cells. As with T cells and fibroblasts bearing the R91W mutant in ORAI1 discussed above, the defect in store-operated Ca2+ entry in this patient’s fibroblasts was rescued by expression of wild-type ORAI1 and to some degree by overexpression of ORAI3 above its endogenous levels in these cells. Again, as with the R91W mutant T cells and fibroblasts, there was no rescue upon overexpression of ORAI2.
A STIM1 mutation leading to loss of STIM1 protein expression
A fourth hereditary immunodeficiency was traced to a nonsense mutation in STIM1 (91) (Figure 6). In two patients from this family, insertion of an adenine in the third exon of STIM1 resulted in a frameshift that introduced a stop codon shortly thereafter, at residue 136. The predicted short N-terminal fragment of STIM1 was not detected in the patient’s fibroblasts. Store-operated Ca2+ entry was almost undetectable but could be restored by reintroduction of wild-type STIM1. There was also considerable rescue by overexpressed STIM2, indicating that both STIM1 and STIM2 can couple to ORAI1 and suggesting that endogenous STIM2 is not expressed at high enough levels to contribute to CRAC channel function or does not couple as effectively as STIM1 to ORAI1 in fibroblasts. These possibilities may be distinguished by measuring the relative levels of endogenous and ectopically expressed STIM1 and STIM2 and by measuring the ability of purified recombinant STIM1 and STIM2 to open the ORAI1 channel in cell-free systems.
Clinical manifestations
The clinical manifestations of patients with homozygous STIM1 and ORAI1 mutations have been described in detail (90–93) and are summarized very briefly here. The predominant clinical phenotype is a severe T cell immunodeficiency, marked in the affected infants by recurrent viral, fungal, and bacterial infections. Lymphocyte development is unaffected, with total lymphocyte counts, numbers of T, B, and NK cells, and serum immunoglobulin levels generally falling in the normal range, but T cell activation and proliferation are strongly impaired. Another consistent symptom is a congenital, nonprogressive myopathy that manifests in infants as muscular hypotonia and persists in surviving patients whose immune deficiency has been rescued by transfer of hematopoietic precursor cells. Histological examination of one of the patients showed atrophy of type II muscle fibers (93). Clinically, the myopathy eventually results in severe chronic pulmonary disease with increased mucus retention in the airways. The myopathy is consistent with the well-established requirement for ORAI1 and STIM1 in store-operated Ca2+ entry in skeletal muscle and for STIM1 in myoblast differentiation (reviewed in 92, 221, 222). A less consistent manifestation, observed in some but not all surviving patients, is ectodermal dysplasia, a syndrome that encompasses defects in formation of dental enamel as well as anhydrosis with impaired sweat production and consequent heat intolerance (90–93).
These data suggest that the major clinical consequences of loss-of-function mutations in ORAI1 and STIM1 are limited to the immune system, skeletal muscle, and certain ectodermally derived tissues. Such selective impairment is surprising given that ORAI1 and STIM1 are both widely expressed and that store-operated Ca2+ channels with the characteristics of CRAC channels have been described in diverse nonimmune cell types (reviewed in 92). The most likely explanation is that in most nonimmune cell types deficiencies in ORAI1 and STIM1 are compensated for by expression of other STIM and ORAI family members. The therapeutic implications are obvious—compounds that selectively inhibit the channel function of ORAI1 or coupling between ORAI1 and STIM1 have the potential to suppress immune function selectively, without having deleterious effects on other organ systems. As such they might lack the toxicity of current immunosuppressive agents, such as cyclosporin A and FK506.
Patients with STIM1 mutations also showed evidence of lymphoproliferation and autoimmune disease, presenting with thrombocytopenia and (in some cases) autoimmune hemolytic anemia (91, 92). In the one patient in whom this determination could be made, the autoimmunity correlated with decreased numbers of regulatory T cells (Tregs) (91). This phenotype is reminiscent of that observed in mice lacking Stim1 and Stim2 selectively in T cells (15), as discussed below.
Functional Consequences of Stim1, Stim2, and Orai1 Mutations in Mice
The immunological and extraimmunological phenotypes of mice with targeted disruptions of the Stim1, Stim2, and Orai1 genes are only briefly described here, given that they have been exhaustively covered in several recent reviews (39, 92, 221–225). All three genes are required for survival: On the C57BL/6 background, mice with a targeted global deletion of Orai1 or Stim1 (or an R93W knock-in mutation introduced into the Orai1 gene) die at late embryonic ages in utero or perinatally within a day of birth, and Stim2-deficient mice survive for only ~5 weeks (15, 166, 167, 226–228). Crossing to outbred mouse strains prolongs the survival of some Stim1-deficient mice to approximately 7 days and rescues the neonatal lethality of a fraction of Orai1-deficient mice (15, 166, 167). Surviving Orai1-deficient mice show a strong phenotype of thin skin, sporadic hair loss, and cyclical alopecia (167), resembling that described in mice with a keratinocyte-specific deletion of the Cnb1 gene (which encodes a regulatory subunit of calcineurin) (229).
Here, we focus on T cells and mast cells, the cells of the immune system whose phenotypes have been best characterized in these mice. Briefly, the effect of Orai1 and Stim1 deficiency varies depending on cell type. To illustrate, mast cell degranulation and leukotriene production are almost completely abolished with individual deletions of either Stim1 or Orai1 (166, 227); the function of Tregs is more resistant because they are present and functional in the absence of Stim1 but are severely compromised in numbers and suppressive function upon deletion of both Stim1 and Stim2 (15); and, finally, thymocyte development is surprisingly unaffected even when all Stim function is lost, as in fetal liver chimeras generated with precursors doubly deficient in Stim1 and Stim2 (M. Oh-hora, personal communication). A possible scenario is that Ca2+ influx in developing thymocytes occurs through a STIM-independent, non-store-operated pathway, for instance one of those discussed in the context of peripheral T cells in the previous section.
Orai1 deficiency
Mice with a gene-trap mutation in the Orai1 gene were used to investigate the role of Orai1 in mast cell and T cell function (166). Mast cells developed normally in the absence of Orai1 but showed essentially no degranulation or synthesis of leukotriene C4 in response to stimulation with antigen IgE, even though store-operated Ca2+ entry was not completely abolished. Thymic T cell development was unaffected in these mice, as in mice with a targeted deletion of the Orai1 gene (167). In both strains of mice, store-operated Ca2+ entry and cytokine production were moderately impaired in naive Orai1-deficient T cells compared with wild-type T cells, possibly because of compensation by other Orai family members (166, 167) (discussed above). However, previously activated or differentiated T cells showed considerable downregulation of Orai2 and Orai3 mRNA and displayed a much more striking decrease in cytokine production (167). Store-operated Ca2+ entry in response to anti-IgM stimulation was also diminished, albeit not completely lost, in Orai1-deficient B cells relative to wild-type B cells (167). It remains to be tested whether the T and B cell impairments translate into immune deficiency in vivo.
Stim1 and Stim2 deficiencies
As observed for mast cells from mice with a gene trap mutation in Orai1 (166), mast cells from mice with a targeted disruption of the Stim1 gene were poorly functional in vitro and in vivo (227). In T cells, Stim1 deficiency resulted in a profound loss of store-operated Ca2+ entry, whereas the lack of Stim2 had only a minor effect in these short-term (10–60 min) assays, possibly because Stim2 constitutes less than 5% of total Stim protein in T cells (15). However, T cells lacking Stim2 were unable to sustain NFAT nuclear translocation and showed a disproportionate decrease in cytokine production (15), consistent with the hypothesis that, as ER stores refill, STIM1 is more rapidly inactivated, whereas STIM2 may remain active and continue to gate ORAI channels for a prolonged time (26).
Perhaps the most unexpected immune phenotypes were observed when both the Stim1 and Stim2 genes were conditionally and selectively deleted in T cells using CD4Cre. Over the course of approximately four months, these doubly deficient mice developed a notable lymphoproliferative phenotype characterized by splenomegaly, lymphadenopathy, infiltration of leukocytes into many organs, and signs of autoimmunity in the form of blepharitis and dermatitis (15). The lymphoproliferative phenotype was associated with a striking decrease in the number and function of Tregs. The Treg loss of function was at least partly cell intrinsic because mixed bone marrow chimera experiments showed that the Treg developmental and functional defects persisted even in the presence of circulating wild-type T cells in the recipient chimeric mice. However, the development of splenomegaly and lymphadenopathy was mostly prevented in the chimeras, as well as in Stim1fl/fl, Stim2fl/fl, CD4Cre mice that received wild-type CD4+ CD25+ Tregs at a young age. Together, these findings suggest that store-operated Ca2+ entry through the STIM-Orai1 pathway is essential for Treg development and function (15), a finding confirmed in Stim1-null bone marrow chimeras generated independently by another group (228).
CONCLUSIONS AND PERSPECTIVES
To summarize, our view of Ca2+ signaling in immune cells, and of the field of store-operated Ca2+ entry more generally, was revolutionized 3–4 years ago with the discovery of STIM and ORAI. These discoveries were followed quickly by a dramatic increase in our understanding of how these proteins work together in lymphocytes and other nonexcitable cells to effect store-operated Ca2+ entry. Many challenges remain. From a structural and biophysical point of view, we are far from a precise understanding of how ORAI channels are gated by STIM1. From a signaling and cell biological point of view, we have almost no information on the many factors that are likely to modulate STIM-ORAI coupling in cells. Much remains to be learned about how STIM-ORAI signaling contributes to the development and function of different subsets of immune cells; the possible contributions of other channels to processes such as thymocyte development need to be explored; and the physiological functions of ORAI2 and ORAI3 remain to be elucidated. We encourage the community to participate in these efforts and hope that the coming years bring many important advances.
Acknowledgments
We thank Dr. Yubin Zhou for help with the illustrations, Jake Banfield-Weir for administrative and secretarial assistance, and the National Institutes of Health for support.
- Nuclear factor of activated T cells (NFAT)
a family of four Ca2+-regulated transcription factors whose translocation from the cytoplasm of resting cells to the nucleus of activated cells requires dephosphorylation of a regulatory domain by the Ca2+-dependent serine/threonine phosphatase calcineurin
- CRAC channel
a store-operated Ca2+ channel composed of ORAI proteins and gated by STIM proteins
- Thapsigargin
a highly selective and irreversible inhibitor of SERCA Ca2+ ATPases, which pump Ca2+ from the cytoplasm back into the ER. Treatment with thapsigargin reduces the free Ca2+ concentration in the ER lumen ([Ca2+]ER) because the Ca2+ that leaves the ER through unspecified leak pathways is not effectively restored
- TRP channels
transient receptor potential channels
- Punctum (pl. puncta)
bright spots observed by fluorescence microscopy where fluorescently labeled STIM proteins accumulate after depletion of ER Ca2+ stores
- Ca2+ microdomain
a local region near Ca2+ channels where free Ca2+ concentration is elevated. Microdomains are often too small to be visualized by Ca2+ imaging but their presence can be inferred by showing that a fast Ca2+ chelator (BAPTA) blocks a downstream effect, whereas a slow Ca2+ chelator (EGTA) does not
- CaV channels
voltage-gated Ca2+ channels that open when the membrane potential becomes depolarized
- Kv channel
voltage-gated K+ channel that maintains the negative membrane potential that drives Ca2+ influx
- KCa channel
K+ channel that is activated by increased intracellular Ca2+
Footnotes
DISCLOSURE STATEMENT
P.H. and A.R. are founders and scientific advisors to Calcimedica, Inc. The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Patrick G. Hogan, Email: hogan@idi.harvard.edu.
Richard S. Lewis, Email: rslewis@stanford.edu.
Anjana Rao, Email: arao@idi.harvard.edu.
LITERATURE CITED
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–22. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Di Capite J, Parekh AB. CRAC channels and Ca2+ signaling in mast cells. Immunol Rev. 2009;231:45–58. doi: 10.1111/j.1600-065X.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 6.Pores-Fernando AT, Zweifach A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol Rev. 2009;231:160–73. doi: 10.1111/j.1600-065X.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- 7.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 8.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 9.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 10.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 11.Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32:235–45. doi: 10.1016/j.tibs.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Feske S. Calcium signaling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 13.Stathopulos PB, Ikura M. Structurally delineating stromal interaction molecules as the endoplasmic reticulum calcium sensors and regulators of calcium release-activated calcium entry. Immunol Rev. 2009;231:113–31. doi: 10.1111/j.1600-065X.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 14.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–42. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–43. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Prakriya M. The molecular physiology of CRAC channels. Immunol Rev. 2009;231:88–98. doi: 10.1111/j.1600-065X.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 21.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–23. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–29. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–33. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 25.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–79. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–39. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Stathopulos PB, Zheng L, Ikura M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem. 2009;284:728–32. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 29.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–25. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, et al. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–26. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, et al. Minimal requirement for store-operated calcium entry: STIM1 gates ORAI1 channels in vitro. Nat Struct Mol Biol. 2010;17:112–16. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–87. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 38.Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–77. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao A, Hogan PG. Calcium signaling in cells of the immune and hematopoietic systems. Immunol Rev. 2009;231:5–9. doi: 10.1111/j.1600-065X.2009.00823.x. [DOI] [PubMed] [Google Scholar]
- 41.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imboden JB, Stobo JD. Transmembrane signaling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985;161:446–56. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor CW, Rahman T, Tovey SC, Dedos SG, Taylor EJ, Velamakanni S. IP3 receptors: some lessons from DT40 cells. Immunol Rev. 2009;231:23–44. doi: 10.1111/j.1600-065X.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 44.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–8. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–88. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayaraman T, Ondriasová E, Ondrias K, Harnick DJ, Marks AR. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc Natl Acad Sci USA. 1995;92:6007–11. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guse AH, da Silva CP, Berg I, Skapenko AL, Weber K, et al. Regulation of calcium signaling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 48.Guse AH, Roth E, Emmrich F. Intracellular Ca2+ pools in Jurkat T-lymphocytes. Biochem J. 1993;291:447–51. doi: 10.1042/bj2910447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunerth S, Langhorst MF, Schwarzmann N, Gu X, Huang L, et al. Amplification and propagation of pacemaker Ca2+ signals by cyclic ADP-ribose and the type 3 ryanodine receptor in T cells. J Cell Sci. 2004;117:2141–49. doi: 10.1242/jcs.01063. [DOI] [PubMed] [Google Scholar]
- 50.Gasser A, Bruhn S, Guse AH. Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J Biol Chem. 2006;281:16906–13. doi: 10.1074/jbc.M601347200. [DOI] [PubMed] [Google Scholar]
- 51.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–9. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steen M, Kirchberger T, Guse AH. NAADP mobilizes calcium from the endoplasmic reticular Ca2+ store in T-lymphocytes. J Biol Chem. 2007;282:18864–71. doi: 10.1074/jbc.M610925200. [DOI] [PubMed] [Google Scholar]
- 54.Dammermann W, Guse AH. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J Biol Chem. 2005;280:21394–99. doi: 10.1074/jbc.M413085200. [DOI] [PubMed] [Google Scholar]
- 55.Whitney RB, Sutherland RM. Characteristics of calcium accumulation by lymphocytes and alterations in the process induced by phytohemagglutinin. J Cell Physiol. 1973;82:9–20. doi: 10.1002/jcp.1040820103. [DOI] [PubMed] [Google Scholar]
- 56.Whitney RB, Sutherland RM. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J Cell Physiol. 1972;80:329–37. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]
- 57.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 58.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 59.Kuno M, Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987;326:301–4. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- 60.Kuno M, Goronzy J, Weyand CM, Gardner P. Single-channel and whole-cell recordings of mitogen-regulated inward currents in human cloned helper T lymphocytes. Nature. 1986;323:269–73. doi: 10.1038/323269a0. [DOI] [PubMed] [Google Scholar]
- 61.Khan AA, Steiner JP, Klein MG, Schneider MF, Snyder SH. IP3 receptor: localization to plasma membrane of T cells and cocapping with the T cell receptor. Science. 1992;257:815–18. doi: 10.1126/science.1323146. [DOI] [PubMed] [Google Scholar]
- 62.Khan AA, Steiner JP, Snyder SH. Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc Natl Acad Sci USA. 1992;89:2849–53. doi: 10.1073/pnas.89.7.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, et al. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 65.Putney JW, Bird GS. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993;14:610–31. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- 66.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–56. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 67.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–99. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Premack BA, McDonald TV, Gardner P. Activation of Ca2+ current in Jurkat T cells following the depletion of Ca2+ stores by microsomal Ca2+-ATPase inhibitors. J Immunol. 1994;152:5226–40. [PubMed] [Google Scholar]
- 69.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–86. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, McCloskey MA. Immunoglobulin E receptor-activated calcium conductance in rat mast cells. J Physiol. 1995;483:59–66. doi: 10.1113/jphysiol.1995.sp020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–21. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 72.Cahalan M, Zhang S, Yeromin A, Ohlsen K, Roos J, Stauderman K. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–44. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 74.Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 2001;410:705–9. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- 75.Cui J, Bian J-S, Kagan A, McDonald TV. CaT1 contributes to the stores-operated calcium current in Jurkat T-lymphocytes. J Biol Chem. 2002;277:47175–83. doi: 10.1074/jbc.M205870200. [DOI] [PubMed] [Google Scholar]
- 76.Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, et al. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J Biol Chem. 2001;276:47767–70. doi: 10.1074/jbc.C100607200. [DOI] [PubMed] [Google Scholar]
- 77.Clapham DE. Sorting out MIC, TRP, and CRAC ion channels. J Gen Physiol. 2002;120:217–20. doi: 10.1085/jgp.20028618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fanger CM, Hoth M, Crabtree GR, Lewis RS. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J Cell Biol. 1995;131:655–67. doi: 10.1083/jcb.131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feske S, Prakriya M, Rao A, Lewis R. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med. 2005;202:651–62. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–24. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 81.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–35. [PubMed] [Google Scholar]
- 82.Le Deist F, Hivroz C, Partiseti M, Thomas C, Buc HA, et al. A primary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood. 1995;85:1053–62. [PubMed] [Google Scholar]
- 83.Philipp S, Strauss B, Hirnet D, Wissenbach U, Mery L, et al. TRPC3 mediates T-cell receptor-dependent calcium entry in human T-lymphocytes. J Biol Chem. 2003;278:26629–38. doi: 10.1074/jbc.M304044200. [DOI] [PubMed] [Google Scholar]
- 84.Yeromin AV, Roos J, Stauderman KA, Cahalan MD. A store-operated calcium channel in Drosophila S2 cells. J Gen Physiol. 2004;123:167–82. doi: 10.1085/jgp.200308982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Echeverri CJ, Perrimon N. High-throughput RNAi screening in cultured cells: a user’s guide. Nat Rev Genet. 2006;7:373–84. doi: 10.1038/nrg1836. [DOI] [PubMed] [Google Scholar]
- 86.Moffat J, Sabatini DM. Building mammalian signaling pathways with RNAi screens. Nat Rev Mol Cell Biol. 2006;7:177–87. doi: 10.1038/nrm1860. [DOI] [PubMed] [Google Scholar]
- 87.Sharma S, Rao A. RNAi screening: tips and techniques. Nat Immunol. 2009;10:799–804. doi: 10.1038/ni0809-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–50. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 89.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, et al. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–43. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 90.Feske S, Dräger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- 91.Picard C, McCarl CA, Papolos A, Khalil S, Lüthy K, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–80. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCarl C, Picard C, Khalil S, Kawasaki T, Rother J, et al. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 2009;124:1311–18. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJS, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–73. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–65. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 96.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, et al. Large store-operated calcium selective currents due to coexpression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–90. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J Cell Biol. 1996;134:771–82. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, et al. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–55. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 99.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–76. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 100.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–9. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 102.Huang Y, Zhou Y, Wong HC, Chen Y, Chen Y, et al. A single EF-hand isolated from STIM1 forms dimer in the absence and presence of Ca2+ FEBS Lett. 2009;276:5589–97. doi: 10.1111/j.1742-4658.2009.07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–22. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 105.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–85. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–45. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiao F, Bowie JU. The many faces of SAM. Sci STKE 2005. 2005:re7. doi: 10.1126/stke.2862005re7. [DOI] [PubMed] [Google Scholar]
- 108.Smyth JT, Dehaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–72. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orci L, Ravazzola M, Le Coadic M, Shen WW, Demaurex N, Cosson P. STIM1-induced pre-cortical and cortical subdomains of the endoplasmic reticulum. Proc Natl Acad Sci USA. 2009;106:19358–62. doi: 10.1073/pnas.0911280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ong HL, Liu X, Tsaneva-Atanasova K, Singh BB, Bandyopadhyay BC, et al. Relocalization of STIM1 for activation of store-operated Ca2+ entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca2+ store. J Biol Chem. 2007;282:12176–85. doi: 10.1074/jbc.M609435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, et al. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 112.Várnai P, Tóth B, Tóth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J Biol Chem. 2007;282:29678–90. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 113.Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T. Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J Biol Chem. 2009;284:21027–35. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heo WD, Inoue T, Park WS, Kim ML, Park BO, et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–61. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J. 2009;425:159–68. doi: 10.1042/BJ20090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ercan E, Momburg F, Engel U, Temmerman K, Nickel W, Seedorf M. A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER. Traffic. 2009;10:1802–18. doi: 10.1111/j.1600-0854.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- 117.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 118.Cuttell L, Vaughan A, Silva E, Escaron CJ, Lavine M, et al. Undertaker, a Drosophila Junctophilin, links Draper-mediated phagocytosis and calcium homeostasis. Cell. 2008;135:524–34. doi: 10.1016/j.cell.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 119.Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20:389–99. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–29. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wedel B, Boyles RR, Putney JW, Jr, Bird GS. Role of the store-operated calcium entry proteins Stim1 and Orai1 in muscarinic cholinergic receptor-stimulated calcium oscillations in human embryonic kidney cells. J Physiol. 2007;579:679–89. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci USA. 2008;105:2011–16. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barr VA, Bernot KM, Srikanth S, Gwack Y, Balagopalan L, et al. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: puncta and distal caps. Mol Biol Cell. 2008;19:2802–17. doi: 10.1091/mbc.E08-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smyth JT, DeHaven WI, Bird GS, Putney JW., Jr Role of the microtubule cytoskeleton in the function of the store-operated Ca2+ channel activator STIM1. J Cell Sci. 2007;120:3762–71. doi: 10.1242/jcs.015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grigoriev I, Gouveia SM, Van Der Vaart B, Demmers J, Smyth JT, et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–82. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–76. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 127.Bakowski D, Glitsch MD, Parekh AB. An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current ICRAC in RBL-1 cells. J Physiol. 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–9. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 129.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–45. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, et al. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–48. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, et al. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009;587:2275–98. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–15. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signaling mediated by STIM1. Nat Cell Biol. 2009;11:433–42. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- 134.Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586:419–25. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ji W, Xu P, Li Z, Lu J, Liu L, et al. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc Natl Acad Sci USA. 2008;105:13668–73. doi: 10.1073/pnas.0806499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–20. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maruyama Y, Ogura T, Mio K, Kato K, Kaneko T, et al. Tetrameric Orai1 is a teardrop-shaped molecule with a long, tapered cytoplasmic domain. J Biol Chem. 2009;284:13676–85. doi: 10.1074/jbc.M900812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamashita M, Navarro-Borelly L, McNally BA, Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol. 2007;130:525–40. doi: 10.1085/jgp.200709872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, et al. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang SL, Kozak JA, Jiang W, Yeromin AV, Chen J, et al. Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. J Biol Chem. 2008;283:17662–71. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci USA. 2009;106:14687–92. doi: 10.1073/pnas.0904664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Derler I, Fahrner M, Muik M, Lackner B, Schindl R, et al. A Ca2+ release-activated Ca2+ (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2+-dependent inactivation of ORAI1 channels. J Biol Chem. 2009;284:24933–38. doi: 10.1074/jbc.C109.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci USA. 2009;106:15495–500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ng SW, Nelson C, Parekh AB. Coupling of Ca2+ microdomains to spatially and temporally distinct cellular responses by the tyrosine kinase Syk. J Biol Chem. 2009;284:24767–72. doi: 10.1074/jbc.M109.011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Parekh AB. Local Ca2+ influx through CRAC channels activates temporally and spatially distinct cellular responses. Acta Physiol (Oxf) 2009;195:29–35. doi: 10.1111/j.1748-1716.2008.01919.x. [DOI] [PubMed] [Google Scholar]
- 146.Chang WC, Di Capite J, Singaravelu K, Nelson C, Halse V, Parekh AB. Local Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels stimulates production of an intracellular messenger and an intercellular proinflammatory signal. J Biol Chem. 2008;283:4622–31. doi: 10.1074/jbc.M705002200. [DOI] [PubMed] [Google Scholar]
- 147.Ng SW, di Capite J, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283:31348–55. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 148.Lin S, Fagan KA, Li KX, Shaul PW, Cooper DM, Rodman DM. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J Biol Chem. 2000;275:17979–85. doi: 10.1074/jbc.275.24.17979. [DOI] [PubMed] [Google Scholar]
- 149.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–29. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–56. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 151.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol. 2008;586:5383–401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schindl R, Bergsmann J, Frischauf I, Derler I, Fahrner M, et al. 2-aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size. J Biol Chem. 2008;283:20261–67. doi: 10.1074/jbc.M803101200. [DOI] [PubMed] [Google Scholar]
- 153.Derler I, Fahrner M, Carugo O, Muik M, Bergsmann J, et al. Increased hydrophobicity at the N terminus/membrane interface impairs gating of the severe combined immunodeficiency-related ORAI1 mutant. J Biol Chem. 2009;284:15903–15. doi: 10.1074/jbc.M808312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thompson JL, Mignen O, Shuttleworth TJ. The Orai1 severe combined immune deficiency mutation and calcium release-activated Ca2+ channel function in the heterozygous condition. J Biol Chem. 2009;284:6620–26. doi: 10.1074/jbc.M808346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heuser JE, Salpeter SR. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979;82:150–73. doi: 10.1083/jcb.82.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bolotina VM, Csutora P. CIF and other mysteries of the store-operated Ca2+-entry pathway. Trends Biochem Sci. 2005;30:378–87. doi: 10.1016/j.tibs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 157.Bolotina VM. Orai, STIM1 and iPLA2β: a view from a different perspective. J Physiol. 2008;586:3035–42. doi: 10.1113/jphysiol.2008.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Csutora P, Peter K, Kilic H, Park KM, Zarayskiy V, et al. Novel role for STIM1 as a trigger for calcium influx factor production. J Biol Chem. 2008;283:14524–31. doi: 10.1074/jbc.M709575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Csutora P, Zarayskiy V, Peter K, Monje F, Smani T, et al. Activation mechanism for CRAC current and store-operated Ca2+ entry: calcium influx factor and Ca2+-independent phospholipase A2β-mediated pathway. J Biol Chem. 2006;281:34926–35. doi: 10.1074/jbc.M606504200. [DOI] [PubMed] [Google Scholar]
- 160.Trepakova ES, Csutora P, Hunton DL, Marchase RB, Cohen RA, Bolotina VM. Calcium influx factor directly activates store-operated cation channels in vascular smooth muscle cells. J Biol Chem. 2000;275:26158–63. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]
- 161.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, et al. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16:1465–70. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 162.Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ region of STIM1 and STIM2. Biochem Biophys Res Commun. 2008;369:240–46. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 163.Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, et al. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 2008;22:752–61. doi: 10.1096/fj.07-9449com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–56. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 165.Frischauf I, Muik M, Derler I, Bergsmann J, Fahrner M, et al. Molecular determinants of the coupling between STIM1 and Orai channels: differential activation of Orai1–3 channels by a STIM1 coiled-coil mutant. J Biol Chem. 2009;284:21696–706. doi: 10.1074/jbc.M109.018408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gwack Y, Srikanth S, Oh-hora M, Hogan PG, Lamperti ED, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–22. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol. 2008;586:185–95. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mignen O, Thompson JL, Shuttleworth TJ. The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. J Physiol. 2009;587:4181–97. doi: 10.1113/jphysiol.2009.174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schindl R, Frischauf I, Bergsmann J, Muik M, Derler I, et al. Plasticity in Ca2+ selectivity of Orai1/Orai3 heteromeric channel. Proc Natl Acad Sci USA. 2009;106:19623–28. doi: 10.1073/pnas.0907714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dustin ML. The cellular context of T cell signaling. Immunity. 2009;30:482–92. doi: 10.1016/j.immuni.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morita T, Tanimura A, Nezu A, Kurosaki T, Tojyo Y. Functional analysis of the green fluorescent protein-tagged inositol 1,4,5-trisphosphate receptor type 3 in Ca2+ release and entry in DT40 B lymphocytes. Biochem J. 2004;382:793–801. doi: 10.1042/BJ20031970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dellis O, Dedos SG, Tovey SC, Taufiq-Ur-Rahman, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–33. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 174.Dellis O, Rossi AM, Dedos SG, Taylor CW. Counting functional inositol 1,4,5-trisphosphate receptors into the plasma membrane. J Biol Chem. 2008;283:751–55. doi: 10.1074/jbc.M706960200. [DOI] [PubMed] [Google Scholar]
- 175.Morita T, Tanimura A, Baba Y, Kurosaki T, Tojyo Y. A Stim1-dependent, noncapacitative Ca2+-entry pathway is activated by B-cell-receptor stimulation and depletion of Ca2+ J Cell Sci. 2009;122:1220–28. doi: 10.1242/jcs.041640. [DOI] [PubMed] [Google Scholar]
- 176.Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J Biol Chem. 2004;279:19566–73. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- 177.Kotturi MF, Hunt SV, Jefferies WA. Roles of CRAC and Cav-like channels in T cells: more than one gatekeeper? Trends Pharmacol Sci. 2006;27:360–67. doi: 10.1016/j.tips.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 178.Randriamampita C, Bismuth G, Debré P, Trautmann A. Nitrendipine-induced inhibition of calcium influx in a human T-cell clone: role of cell depolarization. Cell Calcium. 1991;12:313–23. doi: 10.1016/0143-4160(91)90005-y. [DOI] [PubMed] [Google Scholar]
- 179.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-dependent ion channels in T-lymphocytes. J Neuroimmunol. 1985;10:71–95. doi: 10.1016/0165-5728(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 180.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–89. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Colucci A, Giunti R, Senesi S, Bygrave FL, Benedetti A, Gamberucci A. Effect of nifedipine on capacitive calcium entry in Jurkat T lymphocytes. Arch Biochem Biophys. 2009;481:80–85. doi: 10.1016/j.abb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 182.Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J Biol Chem. 2004;279:19566–73. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- 183.Badou A, Jha MK, Matza D, Mehal WZ, Freichel M, et al. Critical role for the beta regulatory subunits of Cav channels in T lymphocyte function. Proc Natl Acad Sci USA. 2006;103:15529–34. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kotturi MF, Jefferies WA. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol. 2005;42:1461–74. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 185.Matza D, Badou A, Kobayashi KS, Goldsmith-Pestana K, Masuda Y, et al. A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation. Immunity. 2008;28:64–74. doi: 10.1016/j.immuni.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Matza D, Badou A, Jha MK, Willinger T, Antov A, et al. Requirement for AHNAK1-mediated calcium signaling during T lymphocyte cytolysis. Proc Natl Acad Sci USA. 2009;106:9785–90. doi: 10.1073/pnas.0902844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev Physiol. 2003;65:133–59. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- 188.Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Annu Rev Immunol. 1995;13:623–53. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- 189.Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Dev. 2003;6:640–47. [PubMed] [Google Scholar]
- 190.Srivastava S, Zhdanova O, Di L, Li Z, Albaqumi M, et al. Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the K+ channel KCa3.1. Proc Natl Acad Sci USA. 2008;105:14442–46. doi: 10.1073/pnas.0803678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103:17414–19. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ren YR, Pan F, Parvez S, Fleig A, Chong CR, et al. Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PLoS One. 2008;3:e4009. doi: 10.1371/journal.pone.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, et al. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 2005;67:1369–81. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pottosin II, Bonales-Alatorre E, Valencia-Cruz G, Mendoza-Magaña ML, Dobrovinskaya OR. TRESK-like potassium channels in leukemic T cells. Pflugers Arch. 2008;456:1037–48. doi: 10.1007/s00424-008-0481-x. [DOI] [PubMed] [Google Scholar]
- 195.Meuth SG, Bittner S, Meuth P, Simon OJ, Budde T, Wiendl H. TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 critically influence T lymphocyte effector functions. J Biol Chem. 2008;283:14559–70. doi: 10.1074/jbc.M800637200. [DOI] [PubMed] [Google Scholar]
- 196.Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, et al. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA. 2001;98:13942–47. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 198.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–77. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 199.Grissmer S, Lewis RS, Cahalan MD. Ca2+-activated K+ channels in human leukemic T cells. J Gen Physiol. 1992;99:63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Grissmer S, Nguyen AN, Cahalan MD. Calcium-activated potassium channels in resting and activated human T lymphocytes. Expression levels, calcium dependence, ion selectivity, and pharmacology. J Gen Physiol. 1993;102:601–30. doi: 10.1085/jgp.102.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–48. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zweifach A, Lewis RS. Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J Biol Chem. 1995;270:14445–51. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 203.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci USA. 2000;97:10607–12. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gilabert JA, Parekh AB. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J. 2000;19:6401–7. doi: 10.1093/emboj/19.23.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Parekh AB. Mitochondrial regulation of store-operated CRAC channels. Cell Calcium. 2008;44:61–63. doi: 10.1016/j.ceca.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 206.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–9. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 207.Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–72. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–74. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci USA. 2007;104:14418–23. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bautista DM, Hoth M, Lewis RS. Enhancement of calcium signaling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J Gen Physiol. 2002;541:877–94. doi: 10.1113/jphysiol.2001.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Caride AJ, Elwess NL, Verma AK, Filoteo AG, Enyedi A, et al. The rate of activation by calmodulin of isoform 4 of the plasma membrane Ca2+ pump is slow and is changed by alternative splicing. J Biol Chem. 1999;274:35227–32. doi: 10.1074/jbc.274.49.35227. [DOI] [PubMed] [Google Scholar]
- 212.Dolmetsch RE, Lewis RS. Signaling between intracellular Ca2+ stores and depletion-activated Ca2+ channels generates [Ca2+]i oscillations in T lymphocytes. J Gen Physiol. 1994;103:365–88. doi: 10.1085/jgp.103.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–36. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 214.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–41. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 215.Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–32. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lewis RS. Calcium oscillations in T-cells: mechanisms and consequences for gene expression. Biochem Soc Trans. 2003;31:925–29. doi: 10.1042/bst0310925. [DOI] [PubMed] [Google Scholar]
- 217.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–51. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 218.Wei SH, Safrina O, Yu Y, Garrod KR, Cahalan MD, Parker I. Ca2+ signals in CD4+ T cells during early contacts with antigen-bearing dendritic cells in lymph node. J Immunol. 2007;179:1586–94. doi: 10.4049/jimmunol.179.3.1586. [DOI] [PubMed] [Google Scholar]
- 219.Qi H, Egen JG, Huang AYC, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–76. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 220.Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–44. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 221.Rosenberg PB. Calcium entry in skeletal muscle. J Physiol. 2009;13:3149–51. doi: 10.1113/jphysiol.2009.172585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dirksen RT. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol. 2009;13:3139–47. doi: 10.1113/jphysiol.2009.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–66. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 224.Baba Y, Kurosaki T. Physiological function and molecular basis of STIM1-mediated calcium entry in immune cells. Immunol Rev. 2009;231:174–88. doi: 10.1111/j.1600-065X.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 225.Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009;231:210–24. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 226.Bergmeier W, Oh-Hora M, McCarl CA, Roden RC, Bray PF, Feske S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood. 2009;113:675–78. doi: 10.1182/blood-2008-08-174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 228.Beyersdorf N, Braun A, Vögtle T, Varga-Szabo D, Galdos RR, et al. STIM1-independent T cell development and effector function in vivo. J Immunol. 2009;182:3390–97. doi: 10.4049/jimmunol.0802888. [DOI] [PubMed] [Google Scholar]
- 229.Mammucari C, di Vignano AT, Sharov AA, Neilson J, Havdra MC, et al. Integration of Notch1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2006;8:666–76. doi: 10.1016/j.devcel.2005.02.016. [DOI] [PubMed] [Google Scholar]