Abstract
Dissociation process of glutathione-gold(I) polymers in aqueous solution resulted in the formation of a class of ~2 nm gold nanoparticles. Different from the same sized but NaBH4 reduced gold nanoparticles, these nanoparticles exhibit strong luminescence but no surface plasmon absorption. Luminescence lifetimes of the nanoparticles were found strongly dependent on excitation wavelengths, and singlet and triplet excited states involving the emission were found degenerate in energy. X-ray photoelectron spectroscopic studies showed that nearly 40~50% gold atoms in the luminescent nanoparticles were in gold(I) state, which are responsible for the unique optical properties of the luminescent gold nanoparticles. These luminescent nanoparticles can be considered an intermediate state between luminescent gold(I) complexes and reduced nonluminescent gold nanoparticles.
Introduction
Polymeric gold (I) thiolates, with a linear RS-Au-SR motif, are well-known intermediates during the synthesis of monolayer thiol protected gold nanoparticles (NPs).1–10 After addition of strong reducing agents such as NaBH4, gold (I)-sulfur bonds of the polymers are often broken, and reduced gold atoms aggregate to form nanometersized particles through aurophilic interactions.1–7 As demonstrated in Brust’s studies, the majority of gold atoms in the nanoparticles were in Au(0) state under such strong reducing conditions.1 Since material properties of gold NPs are strongly dependent on the number of free electrons in the nanoparticles,11 valence states of gold atoms are expected to have significant influence on material properties of gold nanoparticles, which, however, is still far from full understanding.
Glutathione (GSH) coated gold NPs(GS-AuNPs) have been widely used to probe structure-property relationships of noble metals on the nano scale.2,7,12–20 Size-dependent absorption and fluorescence were observed from different sized GS coated gold nanoparticles.2,7,12–20 Similar to other thiolated ligands, polymeric GS-Au (I) motif was also formed during the synthesis of GS-AuNPs. More recently, it was found that the size of GS-AuNPs can be accurately controlled by simply tuning the sizes of polymeric GS-Au (I) nanoparticles.7 While strong reducing agents were often introduced to reduce GS-Au (I) polymer into the nanoparticles, GSH itself is also a weak reducing agent and can reduce Au (3+) to Au (0) with a stoichiometric process: 3GSH + Au (3+) → Au (0) + 3/2GSSG + 3H+.12 However, how this reaction influences the stability of polymeric GS-Au (I) thiolates and the formation of gold NPs is still not clear.
Herein, we report our recent discoveries on polymeric GS-Au (I) thiolates. First, while large polymeric GS-Au (I) NPs with size around 100–150 nm were quickly formed after mixing GS with Au3+ ions, these polymeric NPs were actually not stable in aqueous solution. Some of them continued growing into bigger particles and others slowly dissociated into smaller ~20 nm polymeric NPs, which eventually resulted in the formation of ~2 nm luminescent gold NPs. Second, time-resolved spectroscopic studies on these luminescent gold NPs showed that luminescence lifetimes of the NPs changed from microseconds to nanoseconds when the excitation wavelength was shifted from 420 nm to 530 nm, implying that singlet and triplet excited states are degenerate in the luminescent NPs. Third, X-ray photoelectron spectroscopic (XPS) studies on the valence states of gold atoms in these luminescent gold NPs indicated that nearly 40~50% gold atoms of the NPs are in the gold(I) state, different from the previously reported GS coated gold nanoparticles/clusters created using strong reducing agents.12–13,16 Once these luminescent gold NPs were further reduced by NaBH4, the luminescence vanished, further suggesting that the oxidation states of gold atoms in the NPs play a vital role in their optical properties.
Experimental
Chemicals
Tetrachloroauric acid trihydrate (HAuCl4·3H2O) and sodium borohydride (NaBH4) were purchased from Fisher Sci. Glutathione in the reduced form (GSH) and other chemicals were purchased from Sigma Aldrich. All the chemicals were commercially available and used as received.
Synthesis of polymeric GS-Au (I) Nanoparticles
GSH aqueous solution (500 µL, 25 mM) was mixed with 500 µL HAuCl4 aqueous solution at 1: 1 or 1:2 molar ratios, and polymeric GS-Au(I) was formed immediately at room temperature.
Synthesis of GS-coated luminescent gold NPs (GS-AuNPs)
The polymeric GS-Au(I) NPs were found not stable and some of them slowly dissociated at room temperature. The color of solution changed from colorless to pale yellowish after two weeks. The solution was first centrifuged at 21,000 g for 5 min. to remove the large NPs or polymers. The supernatant was further purified by adding a small amount of ethanol into the aqueous solution (the ratio between water and ethanol is 2:1). Under this condition, the luminescent gold NPs were precipitated out of the solution while the free GS and gold ions were still in the solution. The precipitates were then re-suspended in aqueous solution and further purified using a size exclusive column to ensure that all free gold ions or GS were removed. The final product was then precipitated out of the solution by adding ethanol and dried under vacuum for 3 hours.
Equipment
The size distributions of polymeric GS-Au (I) and luminescent nanoparticles in the aqueous solution were determined using Brookhaven 90Plus Dynamic Light Scattering Particle Size Analyzer (DLS). To prepare a TEM sample, GS-AuNP solution was drop casted onto a carbon-coated Formvar copper grid, and high resolution transmission electron microscopes (TEM) images of GS-AuNPs were taken using a 200kV Jeol 2100F TEM at 120 kV. The fluorescence spectra were obtained using a Hitachi F-7000 fluorescence spectrophotometer. The UV/Vis absorption spectra of GS-AuNPs were measured using a Varian Cary 50 UV-Vis spectrophotometer. The valence states of GS-Au(I) polymer and GS-AuNPs were characterized using a Perkin Elmer X-ray photoelectron spectrometer (XPS) PHI 5600 ESCA system. Binding energies (BE) of Au 4f2/7 electrons were used as a signature to characterize Au oxidation states with alkyl chain C 1s BE (284.6 eV) as an internal reference. Lifetimes of GS-AuNPs were measured using a PTI time-resolved Fluorescence Lifetime Spectrometer.
Results and Discussion
Dissociation of polymeric GS-Au(I) NPs
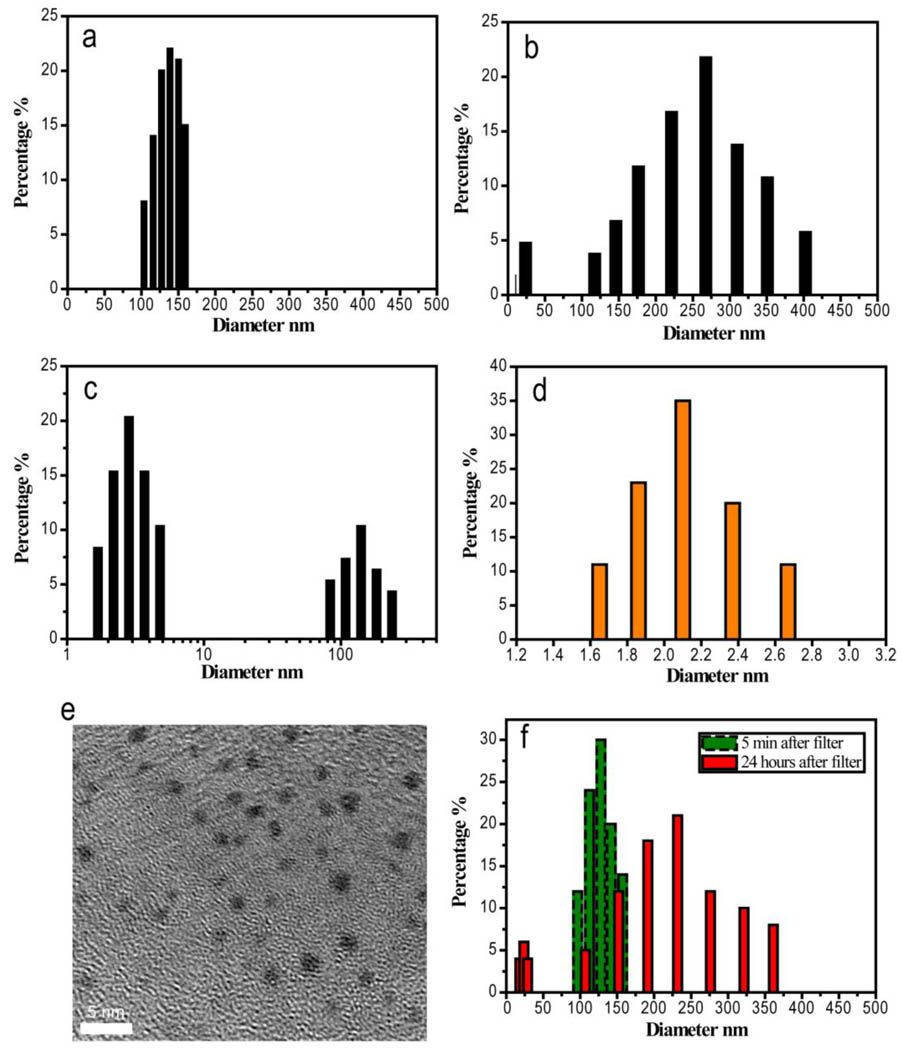
Consistent with previous observations,1–10 polymeric GS-Au(I) NPs were immediately formed after mixing HAuCl4 with GSH at 1:1 ratio, but the sizes of the NPs changed with time. Figure 1 shows the size distributions of the polymeric NPs in aqueous solution measured at the different time points. Right after mixing GS and gold ions, we found that only one polymeric GS-Au(I) component with a mean hydrodynamic diameter (HD) of 120±20 nm was observed (Fig. 1a). After 48 hours, not only a larger polymer GS-Au(I) component with a mean HD of 270±60 nm but also a small polymer component with a mean HD around 20 nm were detected (Fig. 1b). After two weeks, very large polymers were eventually precipitated out of the solution, and the dominating components left in the solution were~ 2 nm particles and ~50–100 nm polymeric NPs (Fig. 1c). After removing 50–100 nm polymeric NPs using centrifugation, we added a small amount of ethanol in the solution and obtained yellowish precipitates. The precipitates were then re-suspended in aqueous solution and were further purified using a size exclusive column to ensure that free gold ions or GS were completely removed. DLS studies indicated the final yellow product created at 1:1 ratio of Au and GS was extremely small NPs with a HD of 2.1±0.4 nm (Fig. 1d). The mean diameter of these NPs was measured to be 1.7±0.3 nm using TEM (Fig. 1e). To further confirm that formation of the small polymer component was due to the dissociation of the large GS-Au(I) polymer, we used a 0.1 µm size cutoff filter to remove small polymers, free GS and gold ions, and only retained a large component with sizes above ~100 nm in the solution (Fig. 1f). After 24 hours, the small GS-Au(I) polymer with size of ~20 nm emerged again, confirming that small nanoparticles were indeed formed due to the dissociation of the large polymeric NPs (Fig. 1f). Element analysis of carbon and nitrogen in a dried GS-AuNP sample was used to determine average ratio between gold and GSH ligand. The result showed that GS-AuNPs were composed by 16.52 % carbon and 5.73 % nitrogen. With assumption that only Au and GSH are existed in GS-AuNPs, the ratio of Au and GSH turned out to be 22:10, which is larger than those of Au25(SR)18,12,21–24 Au38(SR)24 25–28 but comparable to Au-thiol ratios ofAu144(SR)60. 29–30
Figure 1.
DLS and TEM studies on the formation of GS-Au(I) polymeric NPs and orange-emitting GS-AuNPs (OGS-AuNPs) in aqueous solution. The mean hydrodynamic diameter (HD) of GS-Au(I) polymeric NPs right after mixing HAuCl4 and GS in aqueous solution was around 120 nm (a); Two components with HDs around 20 nm and 270 nm respectively were observed in the solution after 48 hours (b). After two weeks, two components with HDs around 2 nm and 120 nm were observed in the solution (c). After purification, the HD of OGS-AuNPs in aqueous solution is around 2.1±0.4 nm (d). A typical TEM image of orange emitting GS-AuNPs shows that the mean size of the NPs is around 1.7±0.3 nm (e). To remove the possibility of 2nm component directly grown from free gold ions and glutathione in the solution, we used a size cutoff filter to remove gold ions and glutathione molecules in the solution but retained ~120 nm GS-Au(I) polymeric NPs in the solution. After 24 hours, two polymeric NPs with mean HDs of 20 nm and 220 nm were observed from the solution (f).
Photophysical properties of orange-emitting GS-AuNPs
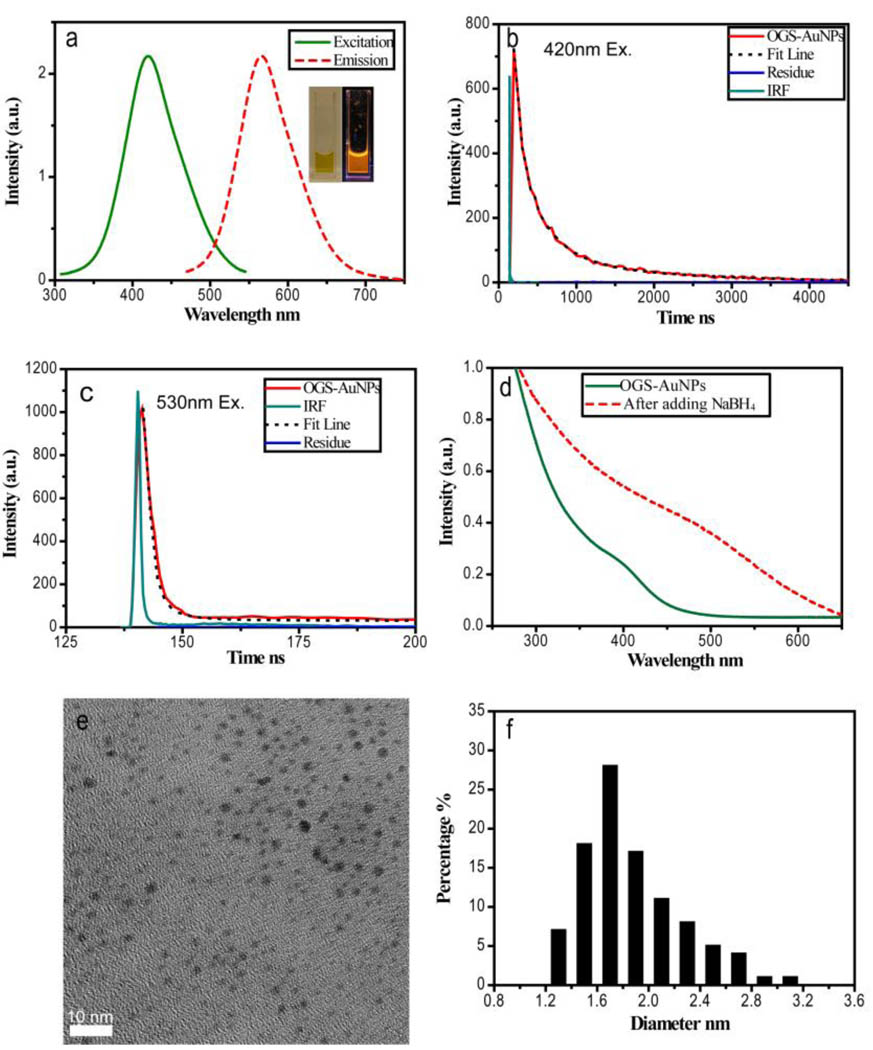
Orange-colored emission with a maximum at 565 nm was observed from these 1.7 nm GS-AuNPs created at a 1:1 ratio of Au to GSH (Fig. 2a). Quantum efficiency of these orange-emitting GS-AuNPs was measured to be 4.0±0.4%. The maximum excitation is localized at 420 nm and a Stokes shift of 145 nm is much larger than those of fluorescent gold nanoclusters (~50 nm).13,19,31–32 Similar large Stokes shifts were observed from luminescent gold (I) complexes/clusters such as gold(I) sulfido complexes where ligand-metal charge transfer (S→Au) involves luminescence transitions.33–40 Similar to those luminescent gold(I) complexes, orange-emitting GS-AuNPs at 420 nm excitation also exhibited microsecond luminescence lifetime (1.7µs(79%)/0.35µs(21%)), suggesting that 565 nm emission obtained at 420 nm excitation is derived from triplet excited states (Fig. 2b). However, different from luminescent gold(I) complexes,33–40 the lifetimes of 565 nm emission of orange-emitting NPs significantly decreased to 2.8ns(81%)/33ns(19%) when the excitation wavelength was shifted to 530 nm, which is on the same order of fluorescent lifetime of fluorescent gold(0) nanoclusters (Fig. 2c), where nanosecond emission originates from singlet excited states.13,19,31–32,41–42 Observation of dramatic decrease in luminescence lifetimes at the different excitation wavelengths implies that triplet and singlet excited states in the luminescent gold nanoparticles are degenerate in energy. This observation is consistent with the previously theoretically reported degeneracies between singlet and triplet states in Au56 clusters.43
Figure 2.
Photophysical properties of orange emitting GS-AuNPs (OGS-AuNPs). (a) The excitation and emission spectra of OGS-AuNPs in aqueous solution. Inset: pictures of OGS-AuNPs taken without and with excitation of a handheld long-wave UV lamp (365 nm). (b) The lifetime of OGS-AuNPs excited at 420 nm showing 1.7µs(79%)/0.35µs (21%). (c) The lifetime of OGS-AuNPs excited at 530 nm showing 2.8ns(81%)/33ns(19%). (d) UV-Vis absorption spectra of OGS-AuNPs before and after adding reducing agent NaBH4 into the solution. Reduction leads to a weak and broad plasmon absorption at 520 nm. (e) EM image of reduced OGS-AuNPs. (f) Size distribution of reduced OGS-AuNPs showing that the mean size of the reduced nanoparticles is around 1.7nm.
No surface plasmon absorption was observed from these orange-emitting gold nanoparticles (Fig. 2d), which is different from the same sized but NaBH4 reduced gold nanoparticles. Since surface plasmon arises from coherent oscillations of free electrons,11 the lack of surface plasmon in orange emitting GS-AuNPs suggests that some gold atoms are in high oxidation states and fail to provide free electrons. After further reducing GS-AuNPs with NaBH4, luminescence of the NPs vanished and a very weak surface plasmon absorption of gold NPs started emerging even though very little change in particle size before and after adding NaBH4 was observed (Fig. 2d~f).
XPS Studies of orange-emitting GS-AuNPs
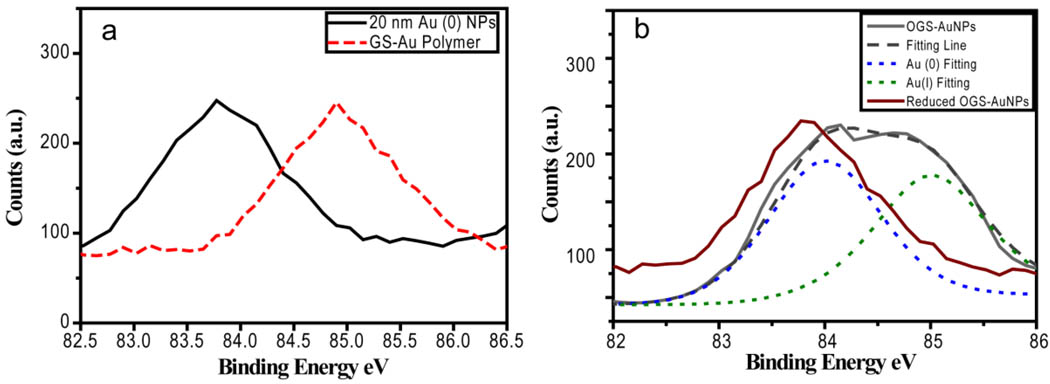
To further confirm the existence of Au(I) in the GS-AuNPs, we used XPS to investigate the valence states of gold atoms in the NPs. As shown in Figure 3, Au 4f7/2 binding energy (BE) of the GS-AuNPs is 84.4 eV, which falls in the middle between Au (0) BE (83.8 eV) of reduced ~20 nm Au (0) NPs and Au (I) BE (85.0 eV) of polymeric GS-Au(I) NPs, suggesting coexistence of Au (I) and Au (0) in the GS-AuNPs. Once the GS-AuNPs were further reduced using NaBH4, the Au 4f7/2 BE was shifted to 83.9 eV, indicating that a large amount of Au(I) ions are indeed in the NPs and significantly influence BE of Au atoms. After deconvoluting the XPS peak of GS-AuNPs, we obtained two peaks at 84.0 and 85.0 eV, which were assigned to Au(0) and Au(I) respectively. Since BE of metal NPs is size-dependent and BE shifts are inversely proportional to the particle size,42,44–45 Au 4f7/2 BE of Au(0) of luminescent GS-AuNPs is 0.1eV blue shifted compared to that of reduced ones, suggesting that Au(0) core of luminescent GS-AuNPs is smaller than that of reduced ones. Because intensities of Au 4f2/7 BE are independent on the valence states, we found that nearly 49% of Au atoms in the orange-emitting GS-AuNPs are in Au (I) oxidation state. Nearly 1:1 ratio between Au(I) and Au(0) atoms in luminescent GS-AuNPs NPs implies that the NPs might be composed of a small Au(0) nano-core coated by GS-Au(I) oligomers.24 However, further studies are needed to confirm this hypothesized structural model.
Figure 3.
(a)XPS spectra of 20 nm colloidal Au (0) NPs standard (black) and polymeric GS-Au(I) thiolates showing that Au 4f7/2 binding energies (BE) are 83.8 eV and 85.0 eV respectively. (b) Au 4f7/2 BE of orange emitting GS-AuNPs(OGS-AuNPs) and reduced OGS-AuNPs were measured to be 84.4 eV and 83.9 eV respectively. After deconvolution of BE peak of OGS-AuNPs, two peaks at 84.0 and 85.0 eV were found, which were assigned to Au(0) and Au(I) respectively.
Synthesis and characterizations of yellow-emitting GS-AuNPs (YGS-AuNPs)
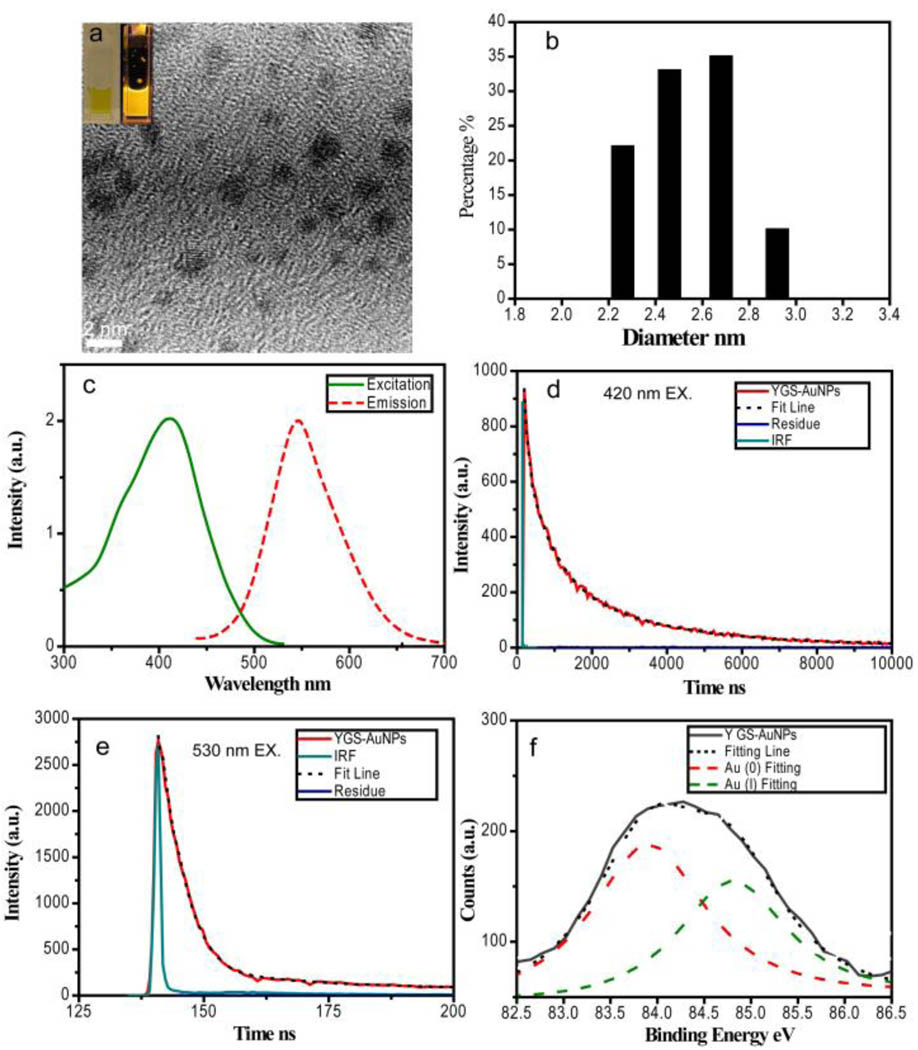
Emission of GS-AuNPs can be partially tuned by changing the ratio between Au3+ ions and GS. By adjusting the mixture ratio of GS:Au3+ to 2:1., we were able to create YGS-AuNPs which exhibited an emission maximum at 545 nm and an excitation maximum around 415 nm(Fig. 4a). The quantum efficiency of these NPs is about 4.3±0.3%. The mean particle size and HD of the YGS-AuNPs are 2.1±0.4 nm and 2.6±0.3 nm respectively, a little bit larger than orange emitting ones (Fig. 4b–c). We found that emission lifetimes of YGS-AuNPs were also dependent on the excitation wavelengths: the emission lifetimes were 2.77(78%)/0.70(22%) µs at 420 nm excitation and 4.4(72%)/57.7(28%) ns at 530 nm excitation respectively (Fig. 4e–f), suggesting that both triplet and singlet excited states can be involved in the emission and they are degenerate in energy levels. XPS studies showed the Au 4f7/2 BE of this yellow emitting NPs is 84.3 eV, indicating coexistence of gold(I) and gold(0) in the NPs (Fig. 4d). After deconvolution of the BE peak of YGS-AuNPs, two peaks at 83.9 and 84.8eV were found, which were assigned to Au(0) and Au(I) respectively. Based on the area ratio between these two peaks, nearly 40% gold atoms in YGS-AuNPs were in the gold(I) state, further indicating that valence states of Au atoms indeed influence the emission of luminescent gold NPs.
Figure 4.
Characterizations on yellow emitting GS-AuNPs (YGS-AuNPs). (a) TEM image of YGS-AuNPs showing the particle size is around 2.1±0.4 nm. Inset: pictures of YGS-AuNPs aqueous solution taken without and with excitation of a handheld long-wave UV lamp (365 nm). (b) Size distribution of YGS-AuNPs measured using DLS showing that the hydrodynamic diameter (HD) of the NPs in the solution is around 2.6 ± 0.3 nm. (c) Excitation and emission maxima of YGS-AuNPs located at 415 and 545nm respectively. (d) The luminescence lifetimes on YGS-AuNPs at 420 nm excitation are 2.77(78%)/0.70(22%)µs. (e) The luminescence lifetimes on YGS-AuNPs at 530 nm excitation are 4.4(72%)/57.7(28%)ns. (f) Au 4f7/2 BE of YGS-AuNPs and reduced YGS-AuNPs were measured to be 84.3 eV. After deconvolution of the BE peak of YGS-AuNPs, two peaks at 83.9 and 84.8eV were found, which were assigned to Au(0) and Au(I) respectively.
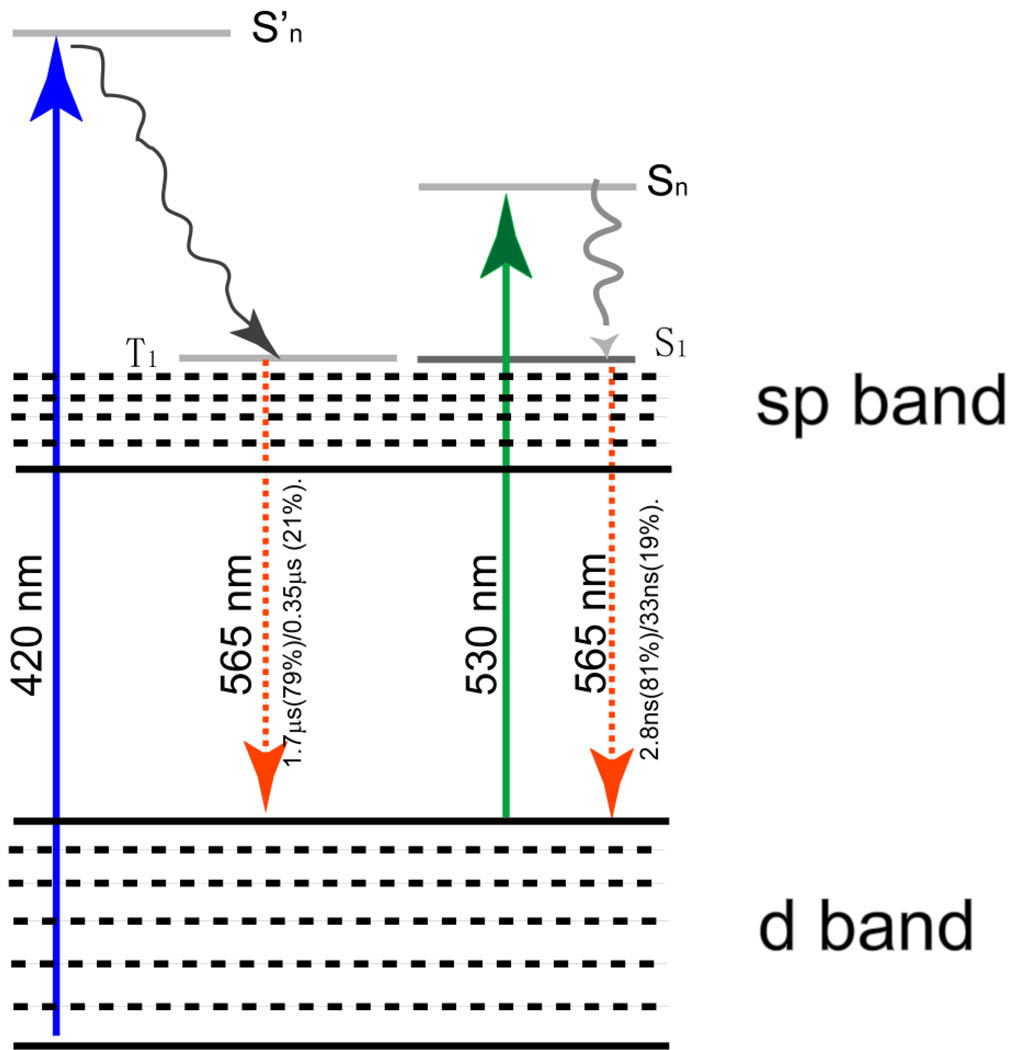
A hypothesized optical scheme for luminescent gold nanoparticles
Based on spectroscopic, XPS and EM studies, we hypothesized a possible optical transition scheme for luminescent GS-AuNPs (Fig. 4–5). Similar to previous reported gold nanoclusters,33–38 luminescent GS-AuNPs are expected to exhibit d and sp bands. Since the sizes of both orange-emitting and yellow-emitting GS-AuNPs are around 2 nm, the energy level spacing within sp band are too small to give visible emission.11 As a result, the emission unlikely originates from transitions within sp band but more likely results from transitions between LUMO levels in the sp band and HOMO levels in the d band. Since RS group is a π-acceptor ligand and its p orbitals are higher in energy than the d orbitals of gold(I),46 the overlapping of these orbitals lead to the formation of ligand-charge transfer excited states.47–50 Therefore, the dominant microsecond emission obtained from luminescent GS-AuNPs at 420 nm excitation likely originates from the triplet excited states in the sp band which is mixed with p orbitals of sulfur. However, when the excitation wavelength was shifted from 420 nm to 530 nm, the dominant lifetimes of emission with the same energy decreased to 2.4 and 4.4 ns for orange- and yellow-emitting gold NPs respectively, suggesting the emission results from the recombination of singlet excited states and ground states. Dependence of lifetimes on excitation-wavelength suggest that singlet and triplet excited states are degenerate. Differences in emission energies and valence states between orange and yellow emitting gold NPs suggest that the ground and excited states involving in the luminescence are dependent on valence states of gold atoms in the NPs.
Figure 5.
A possible optical transition scheme for orange-emitting GS-AuNPs where the luminescence originates from transitions between d and sp bands. When the NPs are excited at 420 nm, the electrons will be relaxed from triplet states in sp bands to some ground states in d bands, leading to microsecond emission. Once the excitation wavelength is shifted to 530 nm, the electrons will be decayed from singlet excited states in the sp band to singlet states in ground states and give out nanosecond emission. The triplet and singlet excited states in the luminescent gold nanoparticles are degenerate in energy.
While we observed significant influence of gold valence states on optical properties of NPs, quantitative understanding of relationships between valence states and luminescence properties such as emission energy, lifetime in the NPs still needs more investigation. One strategy is to create luminescent gold nanoparticles coated with different ligands. So far, we have tried two ligands: mercaptosuccinic acid and 2-aminoethanethiol. However, we found that neither of them can be used to synthesize luminescent gold NPs with the same quality as glutathione did. A possible reason is that glutathione is a better ligand than those two ligands in partially reducing gold atoms and stabilizing luminescent gold NPs in aqueous solution. We are currently exploring an alternative strategy for the control of surface chemistry of luminescent gold NPs, which will be reported in the future.
Triplet and singlet excited states have been observed from luminescent gold(I) complexes47–50 and fluorescent gold(0) clusters respectively;13,19,31–32,41–42 therefore, it is reasonable to observe both triplet and singlet excited states from these luminescent gold NPs with mixed valence states. However, origin of degeneracy of triplet and singlet states in such luminescent gold NPs is still not clear, which might be addressed using ultrafast transient absorption spectroscopy. Since triplet and singlet excited states play important roles in catalysis and bioimaging applications of gold nanoparticles,51–52 these luminescent gold NPs with mixed valence states are expected to serve as a new platform for probing these different spin states in a single nanosystem.
Conclusion
Polymeric GS-AuNPs as intermediate states have been widely observed during the synthesis of GS coated gold NPs. We found that these polymer NPs were not stable and dissociated in aqueous solution. The dissociation process resulted in the formation of luminescent gold NPs, which are composed of a large percentage of gold(I) atoms. Dependent on the excitation, both triplet and singlet transitions can involve in the emission processes and they are degenerate in energy levels. These luminescent NPs with mixed valence states can be considered an intermediate state between luminescent gold(I) complexes/clusters and nonluminescent gold NPs, and serve as a new platform for probing optical properties of noble metals on the nano scale.
Acknowledgement
J.Z would like to thank Dr. Robert M. Dickson at Georgia Institute of Technology for his support on the preliminary studies. Authors would like to thank Dr. Mohammad Omary at the University of North Texas for using his time-resolved spectroscopic system. This work was supported in part by NIH (R21EB009853 to J.Z.) and the start-up fund from the University of Texas at Dallas (J.Z.)
References
- 1.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Journal of the Chemical Society-Chemical Communications. 1994:801. [Google Scholar]
- 2.Schaaff TG, Whetten RL. Journal of Physical Chemistry B. 2000;104:2630. [Google Scholar]
- 3.Templeton AC, Wuelfing MP, Murray RW. Accounts of Chemical Research. 2000;33:27. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 4.Huang T, Murray RW. Journal of Physical Chemistry B. 2001;105:12498. [Google Scholar]
- 5.Schaaff TG, Shafigullin MN, Khoury JT, Vezmar I, Whetten RL. Journal of Physical Chemistry B. 2001;105:8785. [Google Scholar]
- 6.Song Y, Harper AS, Murray RW. Langmuir. 2005;21:5492. doi: 10.1021/la0503606. [DOI] [PubMed] [Google Scholar]
- 7.Brinas RP, Hu MH, Qian LP, Lymar ES, Hainfeld JF. Journal of the American Chemical Society. 2008;130:975. doi: 10.1021/ja076333e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whetten RL, Price RC. Science. 2007;318:407. doi: 10.1126/science.1150176. [DOI] [PubMed] [Google Scholar]
- 9.Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Science. 2007;318:430. doi: 10.1126/science.1148624. [DOI] [PubMed] [Google Scholar]
- 10.Walter M, Akola J, Lopez-Acevedo O, Jadzinsky PD, Calero G, Ackerson CJ, Whetten RL, Gronbeck H, Hakkinen H. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9157. doi: 10.1073/pnas.0801001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Vol. 25. Springer; 1995. [Google Scholar]
- 12.Schaaff TG, Knight G, Shafigullin MN, Borkman RF, Whetten RL. Journal of Physical Chemistry B. 1998;102:10643. [Google Scholar]
- 13.Link S, Beeby A, FitzGerald S, El-Sayed MA, Schaaff TG, Whetten RL. Journal of Physical Chemistry B. 2002;106:3410. [Google Scholar]
- 14.Negishi Y, Takasugi Y, Sato S, Yao H, Kimura K, Tsukuda T. Journal of the American Chemical Society. 2004;126:6518. doi: 10.1021/ja0483589. [DOI] [PubMed] [Google Scholar]
- 15.Shibu ES, Radha B, Verma PK, Bhyrappa P, Kulkarni GU, Pal SK, Pradeep T. Acs Applied Materials & Interfaces. 2009;1:2199. doi: 10.1021/am900350r. [DOI] [PubMed] [Google Scholar]
- 16.Wu ZW, Gayathri C, Gil RR, Jin RC. Journal of the American Chemical Society. 2009;131:6535. doi: 10.1021/ja900386s. [DOI] [PubMed] [Google Scholar]
- 17.Zhu MZ, Qian HF, Jin RC. Journal of the American Chemical Society. 2009;131:7220. doi: 10.1021/ja902208h. [DOI] [PubMed] [Google Scholar]
- 18.Muhammed MAH, Verma PK, Pal SK, Kumar RCA, Paul S, Omkumar RV, Pradeep T. Chemistry-a European Journal. 2009;15:10110. doi: 10.1002/chem.200901425. [DOI] [PubMed] [Google Scholar]
- 19.Lin CAJ, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI, Parak WJ, Chang WH. Acs Nano. 2009;3:395. doi: 10.1021/nn800632j. [DOI] [PubMed] [Google Scholar]
- 20.Schaaff TG, Whetten RL. Journal of Physical Chemistry B. 1999;103:9394. [Google Scholar]
- 21.Dass A, Dubay GR, Fields-Zinna CA, Murray RW. Analytical Chemistry. 2008;80:6845. doi: 10.1021/ac801259j. [DOI] [PubMed] [Google Scholar]
- 22.Akola J, Walter M, Whetten RL, Hakkinen H, Gronbeck H. Journal of the American Chemical Society. 2008;130:3756. doi: 10.1021/ja800594p. [DOI] [PubMed] [Google Scholar]
- 23.Zhu M, Aikens CM, Hollander FJ, Schatz GC, Jin R. Journal of the American Chemical Society. 2008;130:5883. doi: 10.1021/ja801173r. [DOI] [PubMed] [Google Scholar]
- 24.Jiang DE, Dai S. Inorganic Chemistry. 2009;48:2720. doi: 10.1021/ic8024588. [DOI] [PubMed] [Google Scholar]
- 25.Schaaff TG, Shafigullin MN, Khoury JT, Vezmar I, Whetten RL, Cullen WG, First PN, GutierrezWing C, Ascensio J, JoseYacaman MJ. Journal of Physical Chemistry B. 1997;101:7885. [Google Scholar]
- 26.Pei Y, Gao Y, Zeng XC. Journal of the American Chemical Society. 2008;130:7830. doi: 10.1021/ja802975b. [DOI] [PubMed] [Google Scholar]
- 27.Chaki NK, Negishi Y, Tsunoyama H, Shichibu Y, Tsukuda T. Journal of the American Chemical Society. 2008;130:8608. doi: 10.1021/ja8005379. [DOI] [PubMed] [Google Scholar]
- 28.Antonello S, Holm AH, Instuli E, Maran F. Journal of the American Chemical Society. 2007;129:9836. doi: 10.1021/ja071191+. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Acevedo O, Akola J, Whetten RL, Gronbeck H, Hakkinen H. Journal of Physical Chemistry C. 2009;113:5035. [Google Scholar]
- 30.Qian HF, Jin RC. Nano Letters. 2009;9:4083. doi: 10.1021/nl902300y. [DOI] [PubMed] [Google Scholar]
- 31.Zhou RJ, Shi MM, Chen XQ, Wang M, Chen HZ. Chemistry-a European Journal. 2009;15:4944. doi: 10.1002/chem.200802743. [DOI] [PubMed] [Google Scholar]
- 32.Zheng J, Zhang C, Dickson RM. Physical Review Letters. 2004;93:077402. doi: 10.1103/PhysRevLett.93.077402. [DOI] [PubMed] [Google Scholar]
- 33.Yam VWW, Cheng ECC, Cheung KK. Angewandte Chemie-International Edition. 1999;38:197. [Google Scholar]
- 34.Xiao H, Weng YX, Wong WT, Mak TCW, Che CM. Journal of the Chemical Society-Dalton Transactions. 1997:221. [Google Scholar]
- 35.White-Morris RL, Olmstead MM, Jiang FL, Tinti DS, Balch AL. Journal of the American Chemical Society. 2002;124:2327. doi: 10.1021/ja012397s. [DOI] [PubMed] [Google Scholar]
- 36.Yam VWW, Cheng ECC, Zhou ZY. Angewandte Chemie-International Edition. 2000;39:1683. doi: 10.1002/(sici)1521-3773(20000502)39:9<1683::aid-anie1683>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Assefa Z, McBurnett BG, Staples RJ, Fackler JP, Assmann B, Angermaier K, Schmidbaur H. Inorganic Chemistry. 1995;34:75. [Google Scholar]
- 38.Burini A, Bravi R, Fackler JP, Galassi R, Grant TA, Omary MA, Pietroni BR, Staples RJ. Inorganic Chemistry. 2000;39:3158. [PubMed] [Google Scholar]
- 39.Tzeng BC, Schier A, Schmidbaur H. Inorganic Chemistry. 1999;38:3978. [Google Scholar]
- 40.Lee YA, Eisenberg R. Journal of the American Chemical Society. 2003;125:7778. doi: 10.1021/ja034560k. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J, Petty J, Dickson RM. Journal of the American Chemical Society. 2003;125:7780. doi: 10.1021/ja035473v. [DOI] [PubMed] [Google Scholar]
- 42.Zheng J, Nicovich PR, Dickson RM. Annual Review of Physical Chemistry. 2007;58:409. doi: 10.1146/annurev.physchem.58.032806.104546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magyar RJ, Mujica V, Marquez M, Gonzalez C. Physical Review B. 2007;75 [Google Scholar]
- 44.Zheng J, Capadona LA, Petty JT, Zhang CW, Tzeng YL, Dickson RM. Abstracts of Papers of the American Chemical Society. 2004;228:U211. [Google Scholar]
- 45.Luo K, St Clair TP, Lai X, Goodman DW. Journal of Physical Chemistry B. 2000;104:3050. [Google Scholar]
- 46.Yam VWW, Cheng ECC. Angewandte Chemie-International Edition. 2000;39:4240. [Google Scholar]
- 47.Yam VWW, Chan CL, Li CK, Wong KMC. Coordination Chemistry Reviews. 2001;216:173. [Google Scholar]
- 48.Jiang DE, Tiago ML, Luo WD, Dai S. Journal of the American Chemical Society. 2008;130:2777. doi: 10.1021/ja710991n. [DOI] [PubMed] [Google Scholar]
- 49.Jiang DE, Luo W, Tiago ML, Dai S. Journal of Physical Chemistry C. 2008;112:13905. [Google Scholar]
- 50.Pei Y, Gao Y, Shao N, Zeng XC. Journal of the American Chemical Society. 2009;131:13619. doi: 10.1021/ja905359b. [DOI] [PubMed] [Google Scholar]
- 51.Coquet R, Howard KL, Willock DJ. Chemical Society Reviews. 2008;37:2046. doi: 10.1039/b707385m. [DOI] [PubMed] [Google Scholar]
- 52.Daniel MC, Astruc D. Chemical Reviews. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]