Abstract
Background
Cancers of the ampulla of Vater, distal common bile duct, and pancreas are known to have dismal prognosis. It is often reported that ampullary cancers are less aggressive relative to the other periampullary carcinomas. We sought to evaluate predictors of survival for periampullary cancers following pancreaticoduodenectomy to identify biologic behavior.
Methods
We reviewed the records of all patients who underwent pancreaticoduodenectomy for periampullary carcinoma between 1992 and 2007 at the Ohio State University Medical Center. Demographics, treatment, and outcome/survival data were analyzed. Kaplan–Meier survival curves were created and compared by log-rank analysis. Multivariate analysis was undertaken using Cox proportional-hazards method.
Results
346 consecutive periampullary malignancies (249 pancreatic cancers, 79 ampullary carcinomas, 18 extrahepatic cholangiocarcinomas) treated by pancreaticoduodenectomy were identified. Pancreatic cancer histology correlated with the shortest median survival (17.1 months), followed by cholangiocarcinoma (17.9 months) and ampullary carcinoma (44.3 months) (P < 0.001). Potential predictors of decreased survival on univariate analysis included site of origin, preoperative jaundice, microscopic positive margin, nodal metastasis, lymphovascular invasion, neural invasion, and poor differentiation. Only nodal metastasis (median 16.2 versus 29.9 months, P < 0.001) and neural invasion (median 17.7 versus 47.9 months, P < 0.00001) significantly predicted outcome on multivariate analysis.
Conclusions
Although ampullary cancers have the best prognosis overall, when controlled for tumor stage, only presence of neural invasion and nodal metastasis predict poor survival following pancreaticoduodenectomy. Biological behavior remains the most important prognostic indicator in periampullary cancers amenable to resection, regardless of site of origin.
Periampullary cancers include adenocarcinomas arising from the pancreas, ampulla of Vater, and distal common bile duct. The exact site of origin of periampullary tumors is often difficult to ascertain preoperatively. Hence, surgeons treating patients with these tumors have favored an aggressive approach toward resection to benefit those patients harboring cancers with better prognosis. Although the perioperative outcomes for these tumors are similar, the long-term survival has traditionally varied.1–3 It is unknown why outcome varies for adenocarcinomas arising from anatomic sites in such close proximity. It is also uncertain whether this discrepancy in survival is due to different biologic behavior among tumor types or simply secondary to stage bias. Given that the majority of periampullary structures originate embryologically from the foregut, one would expect biologic behavior and pattern of cancer spread to be similar for pancreatic, ampullary, and distal bile duct cancers.4 However, anatomic or embryologic factors are likely to contribute little to differences in outcome.1,4
Regardless of the site of origin of these tumors, most patients undergo a similar operative procedure for extirpation of their disease: radical pancreaticoduodenectomy. In this study, we sought to evaluate our experience with pancreaticoduodenectomy for periampullary cancers to identify predictors of survival and to compare their biologic behavior.
METHODS
The study protocol was reviewed and approved by the Institutional Review Board at the Ohio State University. We retrospectively reviewed the records of all patients who underwent pancreaticoduodenectomy for periampullary carcinoma between 1992 and 2007. Patient cases were selected by reviewing the archives of the James Cancer Hospital tumor registry for patients diagnosed with periampullary tumor (i.e., pancreas, distal bile duct, ampulla) who subsequently underwent surgical exploration with curative intent. Demographics, treatment, recurrence, and outcome/survival data were collected. Data were pooled from three sources: the Ohio State University Medical Center information warehouse, the Ohio State University James Cancer Center Tumor Registry, and by review of patients’ electronic charts. Data obtained included patient demographics, clinical presentation, operative findings, tumor pathologic characteristics, perioperative outcome, and long-term survival. Overall survival was determined from date of operation until date of death from any cause, as determined by hospital records and/or the social security death index as of August 31, 2008 (http://ssdi.rootsweb.ancestry.com).
Data comparisons of the primary groups were made by analysis of variance (ANOVA) and contingency tables analysis (chi-square test or Fisher’s exact test) where appropriate. Statistical significance was accepted at P < 0.05. Overall survival curves were created using the Kaplan–Meier method and compared by log-rank analysis. Multivariate analysis was undertaken using variables from univariate analysis most likely to impact survival (i.e., P < 0.2) by Cox proportional-hazards method. For this multivariate analysis, perioperative deaths, defined as patients surviving less than 2 months after the operation, were excluded. The purpose of this exclusion was to allow the Cox regression model to accurately identify predictors of survival related to the malignancy itself rather than the operative risk. Statistical analyses were performed using STATA 10.1 for Macintosh (StataCorp LP, College Station, TX). Survival analysis was performed and related graphics created by using SPSS Statistics 17.0 for Macintosh (SPSS Inc., Chicago, IL).
RESULTS
Between 1992 and 2007, 398 pancreaticoduodenectomies were undertaken at our institution; 346 of these were for periampullary malignancies (249 pancreatic cancers, 79 ampullary carcinomas, 18 extrahepatic cholangiocarcinomas). No differences were seen in gender, age, comorbidities or proportion presenting with jaundice (Table 1). Patients with pancreatic cancer were more likely to present with abdominal pain, while pain was rarely described in patients with ampullary cancer. Biliary decompression was not routinely undertaken as only 25–41% had preoperative stents placed. Preoperative CA19-9 levels varied widely, with cholangiocarcinoma tending to have the lowest measured values, though not significantly different from pancreatic or ampullary cancers. The vast majority of patients with pancreatic cancer presented with abnormally elevated CA19-9 levels, whereas less than 25% with cholangiocarcinoma had elevated CA19-9.
TABLE 1.
Clinicopathologic characteristic of patients with periampullary cancers undergoing pancreaticoduodenectomy, by tumor type
| Cholangiocarcinoma | Ampullary cancer | Pancreatic cancer | P | |
|---|---|---|---|---|
| Number of patients | 18 | 79 | 249 | |
| Male | 13 (55%) | 47 (59%) | 151 (61%) | 0.893 |
| Mean age (SD), years | 66.1 (10.7) | 63.4 (11.2) | 64.4 (10.7) | 0.6 |
| Comorbidities | 9 (50%) | 44 (55%) | 163 (66%) | 0.1 |
| Presentation | ||||
| Jaundice | 15 (83%) | 53 (67%) | 182 (73%) | 0.283 |
| Abdominal pain | 5 (28%) | 15 (6%) | 90 (36%) | 0.014 |
| Preoperative stent | 4 (22%) | 30 (38%) | 103 (41%) | 0.6 |
| Mean (SD) preoperative CA19-9, U/mL | 134 (282) | 1,867 (7,158) | 1,083 (2,807) | 0.37 |
| Elevated CA19-9 (>35 U/mL) | 4 (23%) | 54 (68%) | 214 (86%) | <0.001 |
| Pylorus preservation | 5 (28%) | 32 (41%) | 63 (25%) | 0.029 |
| Size, mean (SD), cm | 2.2 (1.0) | 2.4 (1.6) | 3.4 (1.4) | <0.001 |
| Poor differentiation | 5 (28%) | 20 (25%) | 85 (34%) | 0.3 |
| Positive nodes | 6 (33%) | 35 (45%) | 161 (68%) | <0.001 |
| Neural invasion | 6 (33%) | 16 (20%) | 206 (74%) | <0.001 |
| Lymphovascular invasion | 2 (11%) | 24 (30%) | 117 (48%) | 0.01 |
| Negative margins | 15 (83%) | 76 (96%) | 205 (82%) | 0.007 |
| Complications | 3 (17%) | 40 (50%) | 102 (41%) | 0.027 |
| Perioperative mortality | 1 (5.5%) | 2 (2.5%) | 12 (4.8%) | 0.2 |
At resection, patients with ampullary carcinoma were more likely to undergo pylorus preservation compared with those with cholangiocarcinoma or pancreatic cancer (Table 1). Resected pancreatic cancers tended to be larger than cholangiocarcinomas or ampullary cancers and displayed more pathologic features of tumor aggression, i.e., poor differentiation, nodal metastasis, neural invasion, and lymphovascular invasion. As such, complete (i.e., R0) resection was less likely to be achieved for pancreatic cancer (Table 1), whereas only three patients with ampullary primary had a positive margin (R1 resection), one at the distal bile duct and two in the posterior surface of the pancreas.
In all, complications occurred in 145 (42%), resulting in perioperative mortality of 4.6%. The cholangiocarcinoma group had significantly fewer perioperative complications than either the pancreatic or ampullary cancer groups. However, perioperative mortality was similar for the three tumor types. The most common complication following pancreaticoduodenectomy was infection, occurring in 68 patients (19.7%). Pancreatic fistula, as defined in accordance with the International Study Group on Pancreatic Fistula, occurred in 26 patients (7.6%%) and was similar between tumor types (P = 0.52).5
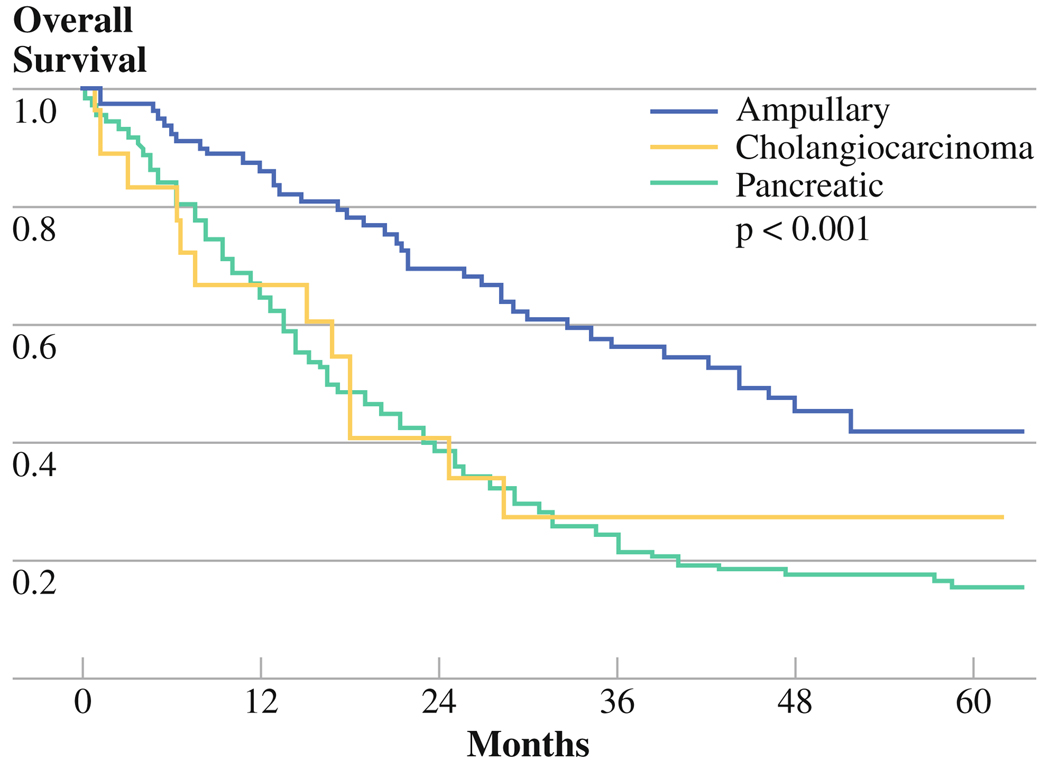
Overall, median survival for all periampullary cancers was 21.9 months, with ampullary cancers having the longest survival (Fig. 1, Table 2). Pancreatic cancer portended the worst prognosis, though not significantly different from that of cholangiocarcinoma. All demographic and clinicopathologic variables were considered to identify potential predictors of survival on univariate analysis, after exclusion of perioperative mortality. Site of origin, margin positive resection, nodal metastasis, lymphovascular invasion, and neural invasion were all significant predictors of poor survival on univariate analysis (Table 3). Those with the greatest potential to impact survival (i.e., P < 0.2 on univariate analysis) were entered into the multivariate Cox proportional-hazards model. On multivariate analysis, only neural invasion and nodal status significantly predicted survival. It is noteworthy that, when controlling for clinicopathologic variables, site of origin was not predictive of survival.
FIG. 1.
Overall survival by tumor site
TABLE 2.
Overall survival following pancreaticoduodenectomy for periampullary cancers, by tumor type (P < 0.001)
| Cholangiocarcinoma | Ampullary cancer |
Pancreatic cancer |
|
|---|---|---|---|
| Number of patients |
18 | 79 | 249 |
| Median | 17.9 months | 44.3 months | 17.1 months |
| Two-year | 34.1% | 68.2% | 38.2% |
| Five-year | 27.3% | 42.1% | 14.4% |
TABLE 3.
Cox proportional-hazards analysis of variables of 346 patients with periampullary cancer, undergoing pancreaticoduodenectomy
| Variable | Univariate analysis |
Multivariate analysis |
Hazard ratio | Confidence interval |
|---|---|---|---|---|
| Site of primary | <0.001 | 0.322 | ||
| Preoperative jaundice | 0.096 | 0.078 | ||
| Positive margins | 0.023 | 0.373 | ||
| Nodal metastasis | <0.001 | 0.014 | 1.53 | (1.09, 2.16) |
| Size | 0.068 | 0.717 | ||
| Lymphovascular invasion | <0.001 | 0.130 | ||
| Neural invasion | <0.001 | <0.001 | 2.49 | (1.56, 3.97) |
| Poor differentiation | 0.6 | 0.6 |
Data represent P values. Perioperative mortality is excluded
The results on the two far left columns represent p-values from a Cox proportional hazards regression model. The results on the two far right columns represent the hazard ratios of the significant by multivariate analysis variables and their respective confidence intervals
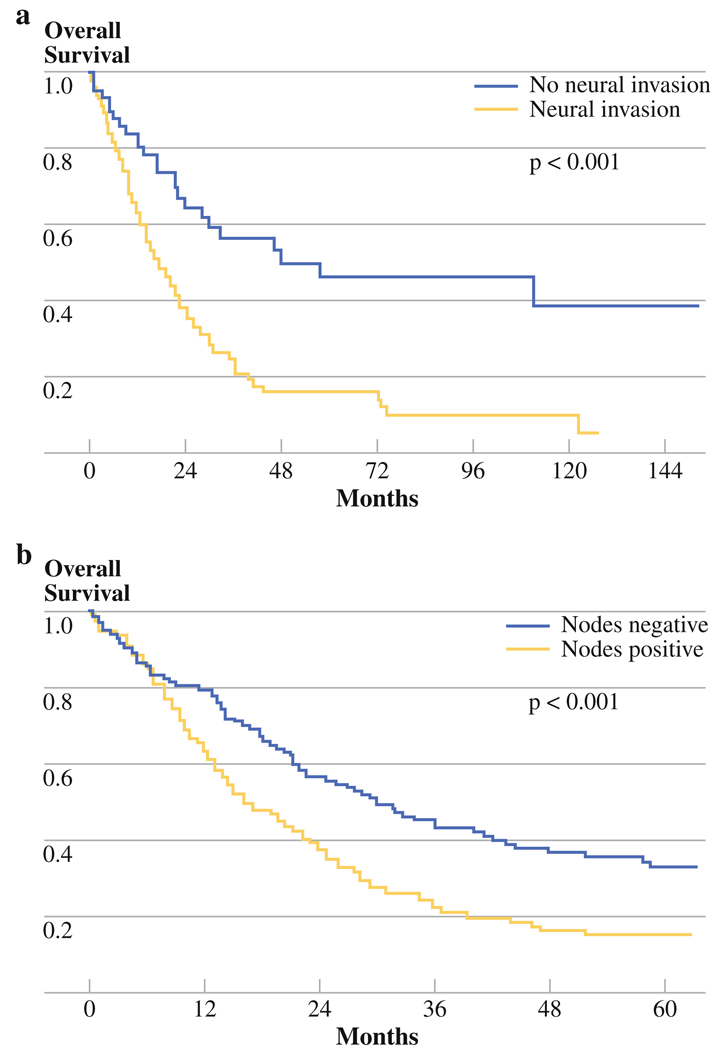
As a single variable, neural invasion had the most dramatic differential effect on survival, resulting in a nearly threefold reduction in median survival (Table 4, Fig. 2a). Nodal metastasis decreased survival by nearly one-half (Table 4, Fig. 2b). The presence of neural invasion was almost uniformly associated with nodal metastases, whereas nodal metastases were significantly less common in the absence of neural invasion (96% versus 58%, P < 0.001). Neural invasion was not associated with poor differentiation, nor was poor differentiation associated with nodal metastasis. Despite positive microscopic margins being associated with nodal metastasis (96% versus 81%, P = 0.007), margin-negative resection was not a predictor of outcome on multivariate analysis (Table 3). Nodal metastasis was also associated with presence of lymphovascular invasion (P < 0.001); however, lymphovascular invasion did not reach statistical significance in the multivariate model (Table 3).
TABLE 4.
Overall survival in patients with periampullary cancers undergoing pancreaticoduodenectomy
| Median | 2-Year | 5-Year | P | |
|---|---|---|---|---|
| Neural invasion | ||||
| Present | 17.7 | 37.8% | 16.0% | 0.02 |
| Absent | 47.9 | 64.1% | 46.1% | |
| Positive nodes | ||||
| Present | 16.2 | 38.2% | 15.5% | <0.001 |
| Absent | 29.9 | 56.5% | 33.2% |
FIG. 2.
Overall survival by: a neual invasion and b nodal metastasis
Next we carried out independent multivariate analysis for each tumor type. Similar to above, all measured variables were considered in univariate analyses for each tumor type and only those variables most likely to impact survival (i.e., P < 0.2 on univariate analysis) were entered into the multivariate model. For ampullary tumors, extent of resection and differentiation were predictive of survival. For pancreatic cancer, neural invasion and elevated CA19-9 (>35 U/ mL) were the only predictors of survival. For cholangiocarcinoma, univariate analysis showed a favorable trend for margin-negative resection (P = 0.25); however, when entered into the multivariate model, no variable reached statistical significance.
DISCUSSION
Though of similar embryologic origin and receiving similar surgical treatment, cancers of the ampulla of Vater are typically considered to have favorable outcomes relative to other periampullary malignancies. In this study we analyzed a large cohort of periampullary malignancies that underwent curative resection at our institution. As expected, the majority of our 346 patients who underwent pancreaticoduodenectomy had pancreatic cancer. We found that ampullary cancers, while presenting earlier than cholangiocarcinoma and pancreatic cancers, had similar biologic behavior when controlled for stage. Each tumor type carries its own set of prognostic indicators, calling for a tailored approach to management.
Our cohort of patients consisted predominantly of men in their seventh decade of life. Commensurate with their age, the majority had significant comorbidities. As expected, demographics were similar between the subtypes of periampullary cancers, although significantly fewer patients with ampullary cancers presented with abdominal pain. Other factors such as preoperative biliary decompression and elevated tumor markers were also similar between the groups. As one might expect, pancreatic cancers presented with larger tumors, more nodal metastases, and higher incidence of neural and lymphovascular invasion.
The most pronounced clinicopathologic difference was the proportion of margin-negative resections between the three subgroups, which was 86% overall. All but three operations for ampullary cancer resulted in R0 resection, which was significantly higher than for pancreatic or bile duct cancers. This difference was not predictive of outcome in the multivariate model for survival. In the independent multivariate analysis for the pancreatic primary, R0 resection was not a significant predictor of survival either. Size was also significantly different, with pancreatic tumors presenting with nearly 40% larger tumors. This is possibly a surrogate of late presentation of pancreatic cancer. Nevertheless, size was neither a significant predictor of survival on cumulative multivariate analysis nor on independent multivariate analysis for pancreatic cancer alone.
Biologic behavior in this cohort of periampullary tumors was the most predictive of survival. Nodal metastases were common in pancreatic cancer, with 68% of tumors harboring nodal disease on presentation versus 33% and 45%% for cholangiocarcinoma and ampullary cancer, respectively. Nodal metastasis was strongly associated with poor survival on multivariate analysis. In addition, the proportion of neural and lymphovascular invasion was significantly different in the three subgroups, with neural invasion showing strong association with poor survival. The presence of neural invasion was significantly associated with nodal metastasis (P < 0.001), another strong predictor of outcome on multivariate analysis. Given the relative subjectivity in the distinction between histologic grades, differentiation as a predictor of survival in these inherently aggressive cancers has not been universal; hence, poor differentiation was not independently linked to poor survival on multivariate analysis.
Tumors with neural invasion had significantly higher incidence of nodal metastasis, but neural invasion was not associated with poor differentiation, nor was poor differentiation associated with nodal metastasis. Positive margin was not a predictor of outcome on multivariate analysis (0.373), despite positive microscopic margins being associated with nodal metastasis (P = 0.007). Nodal metastasis was also associated with presence of lymphovascular invasion (P < 0.001); however, lymphovascular invasion was not a significant predictor of survival in the multivariate model.
Predicting survival and outcomes after pancreaticoduodenectomy is a common topic in hepatopancreatobiliary literature. Several authors have described their experience with the identification of possible predictors of outcome. Tumor biology appears to carry the strongest weight of evidence in the literature.2,3,5–13 This study correlates with the existing evidence in that tumor biology is the most predictive of outcome.
Some authors maintain that lymphovascular invasion is highly associated with, if not equivalent to, nodal metastasis. 1 In published series, nodal metastases are present at time of resection in 55–80% of pancreatic cancers, 30–50% of ampullary cancers, and 55–70% of distal cholangiocarcinomas. 1,10–12 In our study, lymphovascular invasion was highly associated with nodal metastasis, but was not a predictor of survival. At the same time, nodal metastasis was strongly associated with poor survival. Similarly, neural invasion has been described to be a predictor of survival for periampullary as well as other gastrointestinal cancers, including gastric, esophageal, and colorectal. 9,13–17 In a study of 60 periampullary tumors, Chan et al. identified absence of neural invasion as the sole biological marker of survival.18
The applicability of resection margins in predicting survival has long been debated.19 We found that microscopically positive margins did not significantly impact survival when controlling for tumor type. However, positive margin was a predictor of poor survival in ampullary cancers. This might be expected, since ampullary cancers tended to present at an earlier stage, thus emphasizing the potential benefit of complete resection. However, in our largest cohort of patients (i.e., those with pancreatic cancer), positive resection margin was not associated with poorer outcome. Whereas Kang et al. observed that the rate of complications was higher with attempts at obtaining negative margins, which diminished the slim oncologic benefit of R0 resection, others have shown margin-negative resection to be predictive of survival.7,19 The lack of impact of R1 resection on overall survival in pancreatic cancer is not a unique finding. Raut et al. analyzed 360 patients who underwent resection in the setting of pancreatic head cancer over 15 years at M.D. Anderson Cancer Center.20 R0 resection was achieved in 300 (83.3%), while 60 (16.7%) had R1 resection. Patients who underwent R1 resection had median overall survival of 21.5 months, compared with 27.8 months in patients who underwent R0 resection (P = 0.027). However, after controlling for other variables in multivariate analysis, resection status did not independently affect survival. Similarly, Butturini et al., in a meta-analysis of four randomized controlled trials evaluating resection margins [875 patients total, 278 (32%) with R1 and 591 (68%) with R0 resection], found that resection margin involvement was not a statistically significant prognostic factor [hazard ratio (HR): 1.10; 95% confidence interval (CI): 0.94–1.29], although there was a trend for R1 patients to do worse, with median survival of 14.1 versus 15.9 months for patients with R0 resections (P = 0.24).21 We have shown previously that intraoperative assessment of surgical neck margin at time of pancreaticoduodenectomy increases the likelihood of achieving R0 resection, yet margin status was not a predictor of survival for pancreatic cancer.22 Still, margin-negative resection should be sought whenever possible, although aggressive resection should not be abandoned in anticipation of a microscopically positive margin for pancreatic cancer or cholangiocarcinoma.23–25 On the other hand, our data show that R1 resection has a negative impact on survival, in the setting of ampullary primary, hence all efforts should be made to assure R0 resection in the face of ampullary malignancy. R2 resections were very rare and not included in our analyses, as these were clearly noncurative operations and thus represented a different patient population. Our high rate of R0 resection could reflect the impact of a substantial number or resections being done for nonpancreatic primaries or incomplete evaluation of the superior mesenteric artery (SMA)/uncinate margin by our pathologists. We currently ink and evaluate retroperitoneal and uncinate margins as described by modern reports and continue to see high rates of R0 resection. Positive margin as a sole variable does seem to influence survival but is more likely a marker of more advanced disease. As such, significance is lost on multivariate analysis when more influential variables such as nodal metastasis and neural invasion are included. Our data do not suggest that margin status is unimportant, just that it is not a single predictor of poor outcome and, thus, should not be a deciding factor when considering a patient for potentially curative resection.
This study has several potential weaknesses. It is a retrospective cohort review of patients undergoing radical resection in a single institution. As such, outcomes may be reflective of selection bias that cannot be measured. As well, as in the case of most studies evaluating these tumors, this study may also be underpowered to identify subtle differences between the three tumor sites evaluated herein. This was particularly evident when performing independent Cox proportional-hazards analysis on the extrahepatic cholangiocarcinoma group, where the regression model did not yield any significant variables. However, despite these potential weaknesses, the cumulative effects of neural invasion, nodal metastasis, and poor differentiation were pronounced. Also noteworthy is the affirmation that ampullary cancers, though presenting at earlier stages, are similar to pancreatic cancer and cholangiocarcinoma in lethality.
In conclusion, the limitations of this study notwithstanding, the data presented herein show that, overall, ampullary cancers present at an earlier stage and, thus, have better prognosis than pancreatic cancer and cholangiocarcinoma. However, when controlled for stage, tumor type is not predictive of overall survival. Based upon our results, radical resection for ampullary cancers mandates margin-negative resection, whereas anticipation of microscopically positive margins should not preclude resection in pancreatic and distal bile duct cancers.
Acknowledgments
DISCLOSURES Mark Bloomston, M.D. is supported as Paul Cabrezi scholar on NIH/NCI 1 K12 CA133250.
Footnotes
Presented at the Society of Surgical Oncology 2009 Annual Cancer Symposium, Phoenix, AZ, March 5–8, 2009.
REFERENCES
- 1.Sarmiento JM, Nagomey DM, Sarr MG, Farnell MB. Periampullary cancers: are there differences? Surg Clin North Am. 2001;81(3):543–555. doi: 10.1016/s0039-6109(05)70142-0. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JL, Crist DW, Sitzmann JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161(1):120–124. doi: 10.1016/0002-9610(91)90371-j. discussion 124–5. [DOI] [PubMed] [Google Scholar]
- 3.Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227(6):821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Androulakis J, Colborn GL, Skandalakis PN, et al. Embryologic and anatomic basis of duodenal surgery. Surg Clin North Am. 2000;80(1):171–199. doi: 10.1016/s0039-6109(05)70401-1. [DOI] [PubMed] [Google Scholar]
- 5.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140(5):764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Kang CM, Kim DH, Choi GH, et al. Detrimental effect of postoperative complications on oncologic efficacy of R0 pancreatectomy in ductal adenocarcinoma of the pancreas. J Gastrointest Surg. 2009;13:907–914. doi: 10.1007/s11605-009-0823-9. [DOI] [PubMed] [Google Scholar]
- 8.Kayahara M, Nagakawa T, Ueno K, et al. Lymphatic flow in carcinoma of the distal bile duct based on a clinicopathologic study. Cancer. 1993;72(7):2112–2117. doi: 10.1002/1097-0142(19931001)72:7<2112::aid-cncr2820720709>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1(3):469–476. [PubMed] [Google Scholar]
- 10.Nakao A, Harada A, Nonami T, et al. Lymph node metastases in carcinoma of the head of the pancreas region. Br J Surg. 1995;82(3):399–402. doi: 10.1002/bjs.1800820340. [DOI] [PubMed] [Google Scholar]
- 11.Monson JR, Donohue JH, McEntee GP, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991;126(3):353–357. doi: 10.1001/archsurg.1991.01410270099016. [DOI] [PubMed] [Google Scholar]
- 12.Kurosaki I, Tsukada K, Hatakeyama K, Muto T. The mode of lymphatic spread in carcinoma of the bile duct. Am J Surg. 1996;172(3):239–243. doi: 10.1016/S0002-9610(96)00156-0. [DOI] [PubMed] [Google Scholar]
- 13.Nakai T, Koh K, Kawabe T, et al. Importance of microperineural invasion as a prognostic factor in ampullary carcinoma. Br J Surg. 1997;84(10):1399–1401. [PubMed] [Google Scholar]
- 14.Tomazic A, Pegan V, Ferlan-Marolt K, et al. Cyclin D1 and bax influence the prognosis after pancreatoduodenectomy for periampullary adenocarcinoma. Hepatogastroenterology. 2004;51(60):1832–1837. [PubMed] [Google Scholar]
- 15.Mori M, Adachi Y, Kamakura T, et al. Neural invasion in gastric carcinoma. J Clin Pathol. 1995;48(2):137–142. doi: 10.1136/jcp.48.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15(11):3278–3288. doi: 10.1245/s10434-008-0065-1. [DOI] [PubMed] [Google Scholar]
- 17.Horn A, Dahl O, Morild I. The role of venous and neural invasion on survival in rectal adenocarcinoma. Dis Colon Rectum. 1990;33(7):598–601. doi: 10.1007/BF02052215. [DOI] [PubMed] [Google Scholar]
- 18.Chan C, Herrera MF, de la Garza L, et al. Clinical behavior and prognostic factors of periampullary adenocarcinoma. Ann Surg. 1995;222(5):632–637. doi: 10.1097/00000658-199511000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10(10):1338–1345. doi: 10.1016/j.gassur.2006.09.008. discussion 1345–6. [DOI] [PubMed] [Google Scholar]
- 20.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143(1):75–83. doi: 10.1001/archsurg.2007.17. [DOI] [PubMed] [Google Scholar]
- 22.Dillhoff M, Yates R, Wall K, et al. Intraoperative assessment of pancreatic neck margin at the time of pancreaticoduodenectomy increases likelihood of margin-negative resection in patients with pancreatic cancer. J Gastrointest Surg. 2009;13(5):825–830. doi: 10.1007/s11605-009-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verbeke CS, Menon KV. Redefining resectionmargin status in pancreatic cancer. HPB (Oxford) 2009;11(4):282–289. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15(6):1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 25.Howard TJ, Krug JE, Yu J, et al. A margin-ngative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10(10):1338–1345. doi: 10.1016/j.gassur.2006.09.008. [DOI] [PubMed] [Google Scholar]