Abstract
Objective:
To compare the levels of plasma homocysteine (Hcy), vitamin B6 (vit-B6), serum vitamin B12 (vit-B12), and folate in healthy individuals and in patients with normal tension glaucoma (NTG), pseudoexfoliative glaucoma (PXG), or primary open-angle glaucoma (POAG).
Study design:
A prospective controlled trial.
Participants and methods:
Forty healthy subjects, 48 patients with NTG, 38 patients with PXG, and 34 patients with POAG were included in the study. Those who used vitamin supplements or medications affecting Hcy and vitamin levels were excluded from the study. The levels of Hcy and vit-B6 were measured by High Performance Liquid Chromatography (HPLC). The levels of serum vit-B12 and folic acid were measured by competitive chemiluminescent enzyme immunoassay (CEI). One-way analysis if variance (ANOVA), analysis of covariance (ANCOVA), and the Tukey honestly significant difference test were used for statistical analysis.
Results:
The mean Hcy level of the PXG group was 15.46 ± 9.27 μmol/L which was significantly higher (P = 0.03) than that of the control group. There were no statistical differences in serum vit-B12 and folate levels among control subjects and NTG, PXG and POAG groups (P > 0.05). It was found that the mean plasma vit-B6 level was significantly higher in subjects with NTG (P = 0.03) and POAG (P = 0.025) versus controls. Mean vit-B6 levels in NTG and POAG were 30.50 ± 11.29 μg/L and 30 ± 12.15 μg/L, respectively.
Conclusions:
The plasma level of Hcy was found to be increased only in PXG patients and the plasma levels of vit-B6 were found to increase in the NTG and POAG sample groups. Using homocysteine and vit-B6 levels as the determinants of hyperhomocysteinemia still needs further research.
Keywords: normal tension glaucoma, pseudoexfoliative glaucoma, primary open-angle glaucoma, homocysteine, vitamin B6
Introduction
Glaucoma, which is characterized by retinal ganglion cell (RGC) apoptosis, is the prominent cause of blindness. RGC apoptosis may be the result of either impaired optic nerve head blood supply or direct toxic action due to various cytotoxic agents.1,2
Homocysteine (Hcy), an amino acid, is of interest as it has cytotoxic and vasculopathic actions such as apoptosis of RGC, extracellular matrix alterations, oxidative stress, and ischemic vascular dysregulation.3–6 Recent focus on Hcy and glaucoma has yielded mixed results with some studies demonstrating an association with normal tension glaucoma (NTG), pseudoexfoliative glaucoma (PXG), and primary open-angle glaucoma (POAG), whilst others have not.7–15 Hcy metabolism is complex with both genetic and environmental factors. Differences in these factors could, in part, account for the disparity in reported results. Vitamin B12, vitamin B6, and folate act as cofactors in Hcy metabolism. These vitamins are the major environmental determinants of Hcy level. Low folate, vitamin-B12 status, or renal impairment account for the majority of cases where increased Hcy is observed.16–19
As vitamin-B6, vitamin-B12, and folate are among the most common environmental factors to influence Hcy, we aimed to determine whether they exert this influence on homocysteine levels in open-angle glaucoma patients.
Participants and methods
Forty-eight patients with NTG, 38 patients with PXG, 34 patients with POAG, and 40 healthy volunteers as controls were enrolled in this prospective controlled study. The study was designed according to the Helsinki Declaration and approved by the institutional ethic committee. Informed consents were obtained from the patients and the volunteers.
All patients and the control subjects underwent a complete ophthalmologic examination including best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement using Goldmann applanation tonometry, gonioscopy, dilated fundoscopy using a 90-diopter lens, and visual field examination with full-threshold strategy program G-1, by an Octopus perimeter. The systemic examination was performed and a detailed medical story was obtained to identify the patients with risk factors for vascular disease such as hypertension, diabetes mellitus, hyperlipidemia, cardiovascular, and cerebrovascular diseases. Blood samples were collected at 0800 hours after overnight fasting in all subjects.
Inclusion criteria
Group 1
Control subjects had no history of ocular disease (except refractive error) and had a normal eye examination including normal IOP (<22 mmHg), an open-angle, normal appearance of the optic disks and retinal nerve fiber layer, and normal visual fields.
Group 2
NTG patients were defined by the presence of glaucomatous cupping and visual field defect in at least one eye in two consecutive visits and an IOP lower than 22 mmHg.
Group 3
PXG was defined by the presence of typical pseudoexfoliative material on the anterior lens capsule, an open-angle, IOP higher than 22 mmHg, typical glaucomatous cupping, and visual field defects in at least one eye in two consecutive visits.
Group 4
POAG was defined by the presence of an open-angle, IOP higher than 22 mmHg, typical glaucomatous cupping, and visual field defects in at least one eye in two consecutive visits.
Patients with cardiomyopathy or prior myocardial infarction, diabetes mellitus, systemic hypertension, peripheral or coronary artery disease, cerebrovascular disease, ocular inflammation, retinal occlusive disease, vasculitis, renal or hepatic dysfunction, gastrointestinal malabsorption, pregnancy, psychiatric illness, and/or chronic alcohol abuse were excluded from the study. Likewise, patients who used anticonvulsants and immunosuppressives, hormone substitutes, cholesterol-lowering agents, antidepressants, recent antimicrobial therapy and vitamin supplements, plantary products, fish oil, or medications known to be associated with increased levels of Hcy, vitamins B6, B12, and folate, or patients with recent exposure (in the last three months) to anesthesia of nitrous oxide were also excluded.
Homocysteine, vitamin B6, vitamin B12, and folic acid assessment
Blood samples for vitamin-B12, vitamin-B6, folate, and Hcy were collected into a heparinized tube and transferred in ice blocks. The samples were then centrifuged at 4000 rpm for five minutes and stored at −20°C until the biochemical assay. Total Hcy (Recipe Chemicals, Munich, Germany) and vitamin-B6 levels were measured by HPLC (Shimadzu Corporation, Kyoto, Japan). The normal range for plasma Hcy and vitamin-B6 were 5–15 mol/L and 8.6–27.2 μg/L, respectively, in fasting subjects according to the manufacturers’ instruction.
The levels of serum vitamin-B12 and folate were measured by competitive CEI (Immulite 2000;-BIODPC, Los Angeles, CA. The normal serum values of vit-B12 and folate were 174–878 pg/mL and 3.1–17.5 ng/mL, respectively, according to manufacturer’s instruction.
Statistical analysis
Analysis of variance (one-way ANOVA) was performed to evaluate the differences in continuous variables (Hcy, vitamin-B6, vitamin-B12, and folate) among the groups. The Tukey honestly significant difference (HSD) test was also performed to evaluate the differences among the groups. Analysis of covariance (ANCOVA) was also used to adjust variables for vitamin-B12, vitamin-B6, and folate. A P value less than 0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS software (version 10.0; SPSS Inc, Chicago, IL).
Results
The parametric one-way ANOVA test showed that the mean Hcy (0.006) and vitamin B6 (0.004) levels were significantly different among the groups.
The levels of Hcy and vitamins in the study groups are given in Table 1. The study groups were matched in terms of age and gender and there was no significant difference between the groups (P > 0.05) (Table 2).
Table 1.
Homocysteine, vitamin B6, vitamin B12, and folate levels in the study groups
| Group | Number of subjects | Hcy (μmol/L) | Vitamin-B12 (pg/mL) | Vitamin-B6 (μg/L) | Folate (ng/mL) |
|---|---|---|---|---|---|
| Control | 40 | 8.68 ± 2.59 | 345.55 ± 201.75 | 20.09 ± 5.54 | 9.00 ± 6.39 |
| NTG | 48 | 11.27 ± 4.91 | 344.46 ± 247.84 | 30.50 ± 11.29a | 9.71 ± 3.86 |
| PXG | 38 | 15.46 ± 9.27a | 277.16 ± 139.08 | 22.81 ± 11.71 | 10.82 ± 4.92 |
| POAG | 34 | 11.28 ± 4.80 | 368.24 ± 262.65 | 30.22 ± 12.15a | 11.59 ± 9.40 |
Note: aIndicates statistically significant (P < 0.05).
Abbreviations: NTG, normal tension glaucoma; PXG, pseudoexfoliative glaucoma; POAG, primary open-angle glaucoma; Hcy, homocysteine.
Table 2.
Gender and age data for study groups
| Group | Mean age and range | Gender |
|---|---|---|
| Control | 62 ± 8.1 years (range: 50–71) | 18 males (45%) and 22 females (55%) |
| NTG | 56 ± 6.8 years (range: 45–69) | 24 males (50%) and 24 females (50%) |
| PXG | 63 ± 6.3 years (range: 55–76) | 20 males (52.63%) and 18 females (47.37%) |
| POAG | 58 ± 7.5 years (range: 45–75) | 14 males (41.18%) and 20 females (58.82%) |
Abbreviations: NTG, normal tension glaucoma; PXG, pseudoexfoliative glaucoma; POAG, primary open-angle glaucoma.
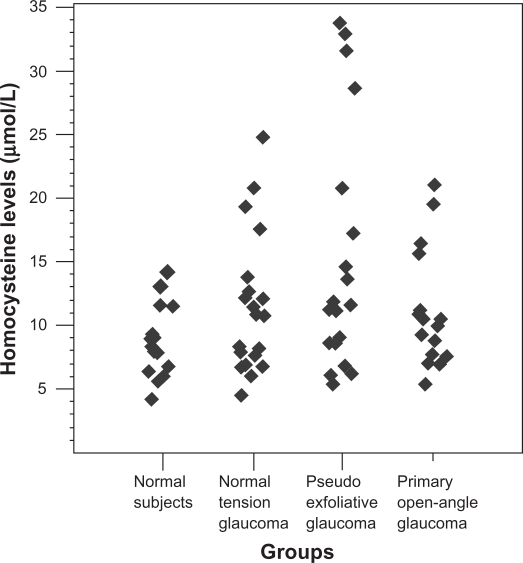
The mean Hcy level in the PXG group was 15.46 ± 9.27 μmol/L and was significantly higher than that of the control (P = 0.003). The mean Hcy level in the control, NTG, and POAG groups were 8.68 ± 2.59, 11.27 ± 4.91, 11.28 ± 4.80 μmol/L, respectively, and the mean Hcy level of the control group was not significantly different from those of the NTG and POAG groups (P > 0.05) (Table 1, Figure 1).
Figure 1.
Scatterplot demonstrating distribution of plasma homocysteine levels in the study groups.
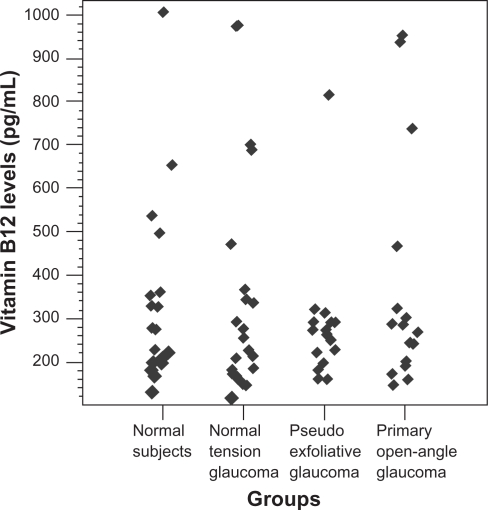
The data pertaining to the vit-B12 levels in the groups are presented in Table 1 and Figure 2. The mean serum levels of vitamin-B12 were not different among the groups (P > 0.05). Although it was not statistically significant, the PXG group had the lowest mean vitamin-B12 level among the study groups.
Figure 2.
Scatterplot demonstrating distribution of serum vitamin B12 levels in the study groups.
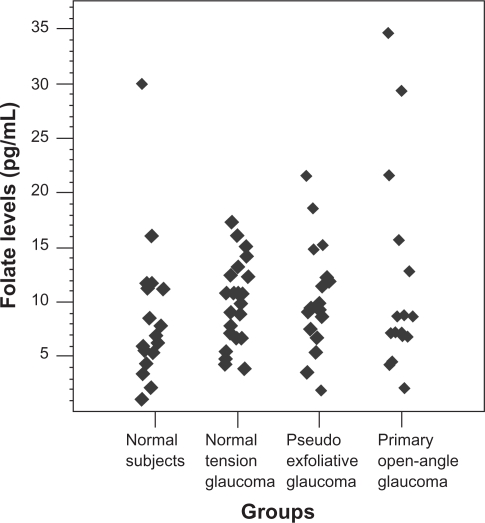
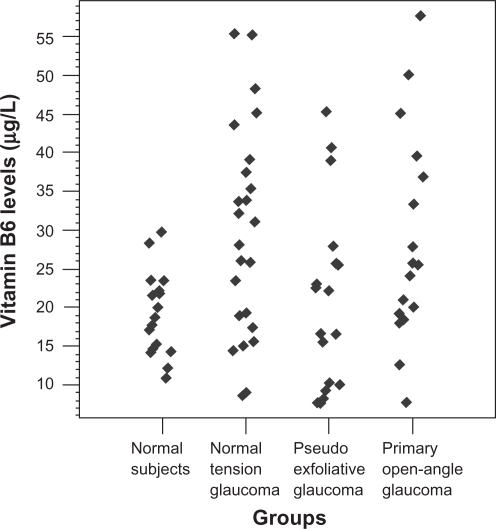
The data about folate in the groups are presented in Table 1 and Figure 3. There was no statistically significant difference among the groups (P > 0.05). Similarly, the data about vitamin B6 in the groups are presented in Table 1 and Figure 4 and it was found that the mean vitamin B6 levels of the NTG (P = 0.01) and POAG (P = 0.025) groups were significantly higher versus the control one.
Figure 3.
Scatterplot demonstrating distribution of serum folate levels in the study groups.
Figure 4.
Scatterplot demonstrating distribution of plasma vitamin B6 levels in the study groups.
Additionally, in our study, analysis of covariance (ANCOVA) was also used to adjust variables for vitamin B12, vitamin B6, and folate because the levels of vitamin B12 and vitamin B6 were shown to be relatively different in group 3 (PXG sample) compared with the other glaucoma groups. The levels of Hcy were adjusted for these vitamins. Differences were not detected after adjusting for vitamin B12 and vitamin B6 using ANCOVA.
Discussion
Elevated Hcy level is defined as an independent risk factor for atherosclerotic vascular disease and its plasma level is influenced by environmental and genetic factors. A high level of plasma Hcy is observed in disorders of Hcy metabolism, vitamin deficiencies, systemic arterial hypertension, diabetes mellitus, chronic renal insufficiency, and/or malign neoplasms. Additionally, habitual smoking and coffee intake, some medications, alcohol consumption, and physical activity may also affect Hcy levels.19,20
A number of studies have investigated the association of Hcy with different types of glaucoma including NTG, PXG, and POAG. However, the results of these studies are still controversial. In the present study, Hcy levels were found to be significantly higher only in the PXG group and similar results have been reported in previous studies.10,11,21–27
Previous data suggest that an elevation in one’s Hcy level may cause changes in the optic nerve head microvasculature and impair optic nerve blood flow via a vasoconstrictive effect, endothelial injury, smooth muscle proliferation, platelet activation, thrombogenesis, and apoptotic cell death in retinal ganglion cells.28–33 This data might explain the relationship between the elevated levels of Hcy and neuronal cell death.
Although it is not significant, vitamin B12 levels of the PXG sample were lowest among the groups in this study. Cumurcu et al and Puustjarvi et al have reported that the levels of serum vitamin B12 do not statistically differ between PXG and the other types of open-angle glaucoma.22,34 Conversely, Roedl et al have reported that the levels of serum vitamin B12 were significantly lower in the PXG group.35
In the case of Hcy metabolism, depression of vit-B12 is expected in the PXG group. According to previous data, it is well known that vitamin B12 deficiency may be one of the causes of hyperhomocysteinemia. This might be related to the fact that excess Hcy is converted to methionin by vitamin B12 in the organism and when vitamin B12 is deficient this above-mentioned conversion does not take place, resulting higher levels of Hcy. Similarly vitamin B12 levels of the POAG and the NTG groups are in the normal range. This might be related to the normal Hcy levels of these groups via the same metabolic process.35,36
The folate levels are in the normal range among all the groups in the present study. This is in accordance with the fact that Hcy metabolism is reversible via the pathway using folate21,35 and, therefore, the level of folate might not interfere with the level of Hcy.
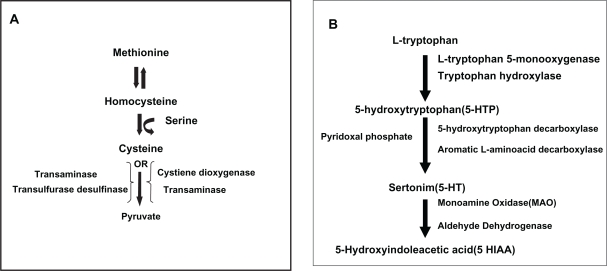
In terms of Hcy metabolism, depression but not elevation of vitamin B6 is expected in the various glaucoma types. Whilst Puustjarvi et al reported that the level of serum vitamin B6 does not differ statistically between the PXG and control groups,34 Roedl et al found that levels of vitamin B6 were significantly lower in their PXG patients.35 Vitamin B6, which is a cofactor in the catabolism of Hcy, increased in the POAG and NTG groups, whilst it is in the normal range in the PXG group. It might be speculated that the rate of the catabolism of vitamin B6 is probably high in the PXG patients resulting in a low catabolism rate of Hcy, causing higher Hcy levels. However, the molecular data about the catabolic utilization of vitamin B6 is lacking in this study. It is probable that actual vitamin B6 levels in PXG patients would be higher if vitamin B6 had not been used in Hcy catabolism.37 (Figure 5A).
Figure 5.
A) Homocysteine catabolism to pyruvate and vitamin B6 (pyridoxine)-transaminase. B) Pathway for the synthesis of serotonin from tryptophan and pyridoxine as a coenzyme.
High levels of plasma vitamin B6 in patients with POAG and NTG might be related, in part, with the deficiency or absence of enzymes such as 5-hydroxytryptophan decarboxylase and/or aromatic L-amino acid decarboxylase whose cofactor is vitamin B6, in the serotonin pathway37 (Figure 5B). Meanwhile, a possible negative feed-back mechanism related to high serotonin levels might play a partial role in the decrease of the consumption of vitamin B6. Serotonin is a possible mediator of vasospasm and it influences blood viscosity, platelet aggregation, and vaso-constriction. These actions are similar to those of Hcy. In addition to the above-mentioned actions, serotonin might lead to transient vasospasm of optic nerve head vessels and it may play a role in glaucomatous optic neuropathy in some eyes, particularly in NTG patients.38,39 However, serotonin, cysteine 5-hydroxytryptophan decarboxylase, and/or aromatic l-amino acid decarboxylase have not been measured in our study.
In conclusion, hyperhomocysteinemia was associated with PXG, while the vitamin B6 levels were increased in the NTG and POAG sample groups. We suggest that the levels of Hcy and B vitamins should be measured routinely in patients with glaucoma. This would aid to screen patients for life-threatening disorders related with hyperhomocysteinemia. We grant, the high levels of Hcy observed in the PXG group and the high levels of vitamin B6 detected in the NTG and POAG groups might be related with some compensatory mechanisms or biochemical events that we could not understand in either Hcy metabolism or genetic factors.
Acknowledgments
Involved in conduct of study were BT and MK Collection of data, typing and editing, preparation, review, and/or approval of the manuscript included BT, TD, SA, MK, MG, and MKK All authors read and approved the final manuscript.
Footnotes
Disclosures
The authors indicate no financial support or financial conflict of proprietary interest with this work.
References
- 1.Feldman F, Sweeney V, Drance S. Cerebro-vascular studies in chronic simple glaucoma. Can J Ophthalmol. 1969;4:358–364. [PubMed] [Google Scholar]
- 2.Broadway D, Drance S. Glaucoma and vasospasm. Br J Ophthalmol. 1998;82:862–870. doi: 10.1136/bjo.82.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritch R. Exfoliation syndrome: beyond glaucoma. Arch Ophthalmol. 2008;126:859–861. doi: 10.1001/archopht.126.6.859. [DOI] [PubMed] [Google Scholar]
- 4.Carroll JF, Tyagi SC. Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbit. Am J Hypertens. 2005;18:692–698. doi: 10.1016/j.amjhyper.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 5.Mujumdar VS, Aru GM, Tyagi SC. Induction of oxidative stress by homocysteine impairs endothelial function. J Cell Biochem. 2001;82:491–500. doi: 10.1002/jcb.1175. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan S, Sulochana KN, Lakshmi S, Selvi R, Angayarkanni N. Biochemistry of homocysteine in health and diseases. Indian J Biochem Biophys. 2006;43:275–283. [PubMed] [Google Scholar]
- 7.Vine AK, Stader J, Branham K, Musch DC, Swaroop A. Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology. 2005;112:2076–2080. doi: 10.1016/j.ophtha.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Pianka P, Almog Y, Man O, Goldstein M, Sela BA, Lowenstein A. Hyperhomocysteinemia in patients with nonarteritic anterior iscemic optic neuropathy, central retinal artery occlusion, and central retinal vein occlusion. Ophthalmology. 2000;107:1588–1592. doi: 10.1016/s0161-6420(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 9.Roedl JB, Bleich S, Schlötzer-Schrehardt U, von Ahsen N, Kornhuber J, Naumann GOH, et al. Increased homocysteine levels in tear fluid of patients with primary open-angle glaucoma. Ophthalmic Res. 2008;40:249–256. doi: 10.1159/000127832. [DOI] [PubMed] [Google Scholar]
- 10.Bleich S, Jünemann A, Von Ahsen N, et al. Homocysteine and risk of open angle glaucoma. J Neural Transm. 2002;109:1499–1504. doi: 10.1007/s007020200097. [DOI] [PubMed] [Google Scholar]
- 11.Vessani RM, Ritch R, Liebmann JM, Jofe M. Plasma homocysteine is elevated in patients with exfoliative syndrome. Am J Ophthalmol. 2003;136:41–46. doi: 10.1016/s0002-9394(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 12.Bousse V, Newman NJ, Sternberg P. Retinal vein occlusion and transient monocular visual loss associated with hyperhomocysteinemia. Am J Ophthalmol. 1997;124:257–260. doi: 10.1016/s0002-9394(14)70800-1. [DOI] [PubMed] [Google Scholar]
- 13.Cahill M, Karabatzaki M, Meleady R, et al. Raised plasma homocysteine as a risk factor for retinal vascular occlusive disease. Br J Ophthalmol. 2000;84:154–157. doi: 10.1136/bjo.84.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowenstein A, Goldstein M, Winder A, Lazar M, Eldor A. Retinal vein occlusion associated with methylenetetrahydrofolate reductase mutation. Ophthalmology. 1999;106:1817–1820. doi: 10.1016/S0161-6420(99)90357-3. [DOI] [PubMed] [Google Scholar]
- 15.Brown BA, Marx JL, Ward TP, et al. Homocysteine: a risk factor for retinal venous occlusive disease. Ophthalmology. 2002;109:287–290. doi: 10.1016/s0161-6420(01)00923-x. [DOI] [PubMed] [Google Scholar]
- 16.Welch GN, Lascalzos J. Homocysteine and atherothrombosis. N Eng J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 17.Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Medeiros FA, Barshop BA, Weinreb RN. Total plasma homocysteine and primary open-angle glaucoma. Am J Ophthalmol. 2004;137:401–406. doi: 10.1016/j.ajo.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Clarke R, Stansbie D. Assessment of homocysteine as a cardiovascular risk factor in clinical practice. Ann Clin Biochem. 2001;38:624–632. doi: 10.1258/0004563011901046. [DOI] [PubMed] [Google Scholar]
- 20.Junemann AG, von Ahsen N, Reulbach U, et al. C677T variant in the methylentetrahydrofolate reductase gene is a genetic risk factor for primary open-angle glaucoma. Am J Ophthalmol. 2005;139:721–723. doi: 10.1016/j.ajo.2004.09.081. [DOI] [PubMed] [Google Scholar]
- 21.Clement CI, Goldberg I, Healey PR, Graham SL. Plasma homocysteine, MTHFR gene mutation, and open-angle glaucoma. J Glaucoma. 2009;18:73–78. doi: 10.1097/IJG.0b013e31816f7631. [DOI] [PubMed] [Google Scholar]
- 22.Cumurcu T, Sahin S, Aydin E. Serum homocysteine, vitamin B12 and folic acid levels in different types of glaucoma. BMC Ophthalmol. 2006;6:6. doi: 10.1186/1471-2415-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leibovitch I, Kurtz S, Shemes G, Goldstein M, Sela BA, Lazar M, et al. Hyperhomocysteinemia in pseudoexfoliative glaucoma. J Glaucoma. 2003;12:36–39. doi: 10.1097/00061198-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Altintas O, Maral H, Yuksel N, Karabas VL, Dillioglugil MO, Caglar Y. Homocysteine and nitric oxide levels in plasma of patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2005;243:677–683. doi: 10.1007/s00417-004-1097-2. [DOI] [PubMed] [Google Scholar]
- 25.Fan BJ, Chen T, Grosskreutz C, et al. Lack of association of polymorphisms in homocysteine metabolism genes with pseudoexfoliation syndrome and glaucoma. Mol Vis. 2008;14:2484–2491. [PMC free article] [PubMed] [Google Scholar]
- 26.Fingert JH, Kwon YH, Moore PA, et al. The C677T variant in the methylenetetrahydrofolate reductase gene is not associated with disease in cohorts of pseudoexfoliation glaucoma and primary open-angle glaucoma patients from Iowa. Ophthalmic Genet. 2006;27:39–41. doi: 10.1080/13816810600677883. [DOI] [PubMed] [Google Scholar]
- 27.Turaçli ME, Tekeli O, Ozdemir F, Akar N. Methylenetetrahydrofolate reductase 677 C-T and homocysteine levels in Turkish patients with pseudoexfoliation. Clin Exper Ophthalmol. 2005;33:505–508. doi: 10.1111/j.1442-9071.2005.01070.x. [DOI] [PubMed] [Google Scholar]
- 28.Stamler JS, Osborne JA, Jaraki O, et al. Adverse vascular effects of homocysteine are modulated by endotheliumderived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91:308–318. doi: 10.1172/JCI116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai JC, Perrella MA, Yoshizumi M, et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci U S A. 1994;91:6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang L, Mamotte CD, Van Bockxmeer FM, Taylor RR. The effect of homocysteine on DNA synthesis in cultured human vascular smooth muscle. Atherosclerosis. 1998;136:169–173. doi: 10.1016/s0021-9150(97)00208-6. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers GM, Conn MT. Homocysteine, an atherogenic stimulus, reduces protein C activation by arterial and venous endothelial cells. Blood. 1990;75:895–901. [PubMed] [Google Scholar]
- 32.Rodgers GM, Kane WH. Activation of endogenous factor V by a homocysteine-induced vascular endothelial cell activator. J Clin Invest. 1986;77:1909–1916. doi: 10.1172/JCI112519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore P, El-sherbeny A, Roon P, Schoenlein PV, Ganapathy V, Smith SB. Apoptotic cell death in the mouse retinal ganglion cell layer is induced in vivo by the excitatory amino acid homocysteine. Exp Eye Res. 2001;73:45–57. doi: 10.1006/exer.2001.1009. [DOI] [PubMed] [Google Scholar]
- 34.Puustjarvi T, Blomster H, Kontkanen M, Punnonen K, Terasvirta M. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol. 2004;242:749–754. doi: 10.1007/s00417-004-0918-7. [DOI] [PubMed] [Google Scholar]
- 35.Roedl JB, Bleich S, Reulbach U, et al. Vitamin deficiency and hyperhomocysteinemia in pseudoexfoliation glaucoma. J Neural Transm. 2007;114:571–575. doi: 10.1007/s00702-006-0598-z. [DOI] [PubMed] [Google Scholar]
- 36.Dudman NP, Guo XW, Gordon RB, Dawson PA, Wilcken DE. Human homocysteine catabolism: three major pathways and their relevance to development of arterial occlusive disease. J Nutr. 1996;126(4 Suppl):1295S–1300S. doi: 10.1093/jn/126.suppl_4.1295S. [DOI] [PubMed] [Google Scholar]
- 37.Tower DB. Neurochemical aspects of pyridoxine metabolism and function. Am J Clin Nutr. 1956;4:329–345. doi: 10.1093/ajcn/4.4.329. [DOI] [PubMed] [Google Scholar]
- 38.Hayreh SS. Retinal and optic nerve head ischemic disorders and atherosclerosis: role of serotonin. Prog Retin Eye Res. 1999;18:191–221. doi: 10.1016/s1350-9462(98)00016-0. [DOI] [PubMed] [Google Scholar]
- 39.Lance JW. 5-Hydroxytryptamine and its role in migraine. Eur Neurol. 1991;31:279–281. doi: 10.1159/000116754. [DOI] [PubMed] [Google Scholar]