Abstract
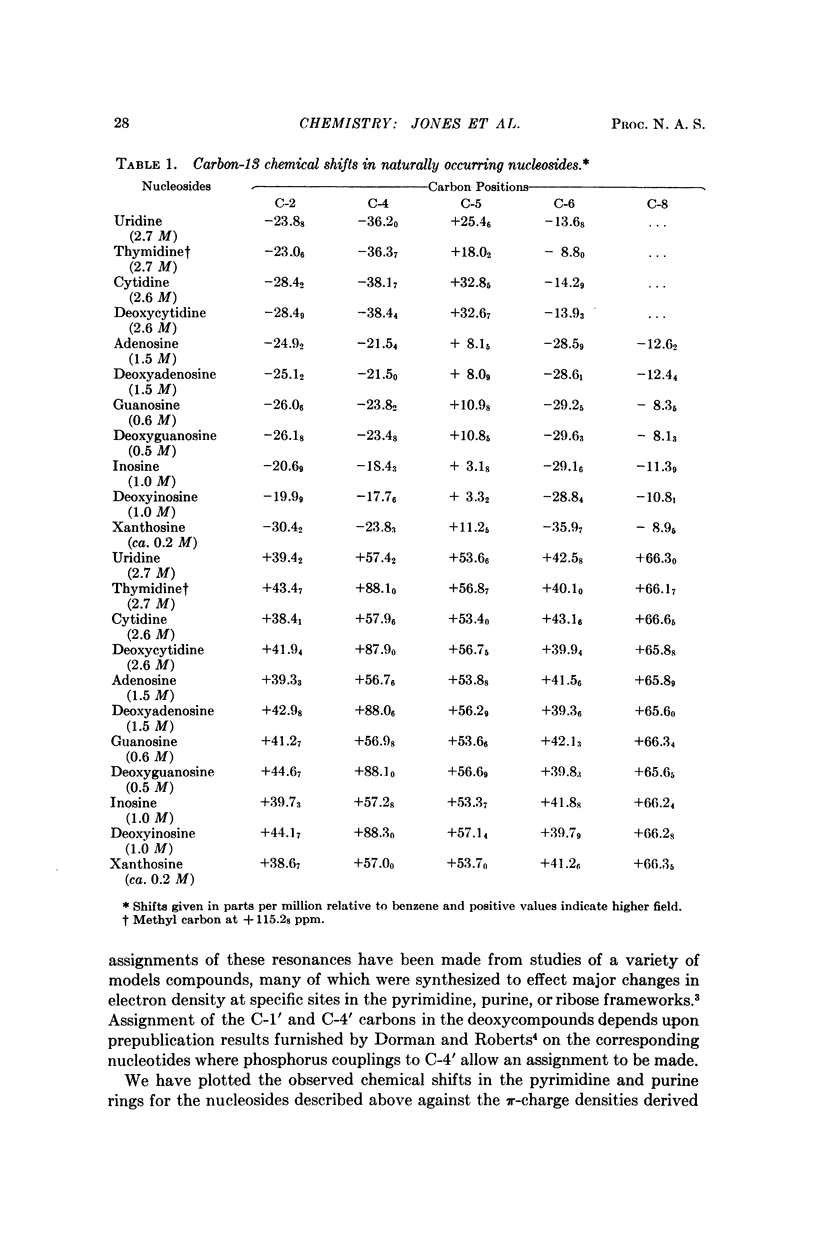
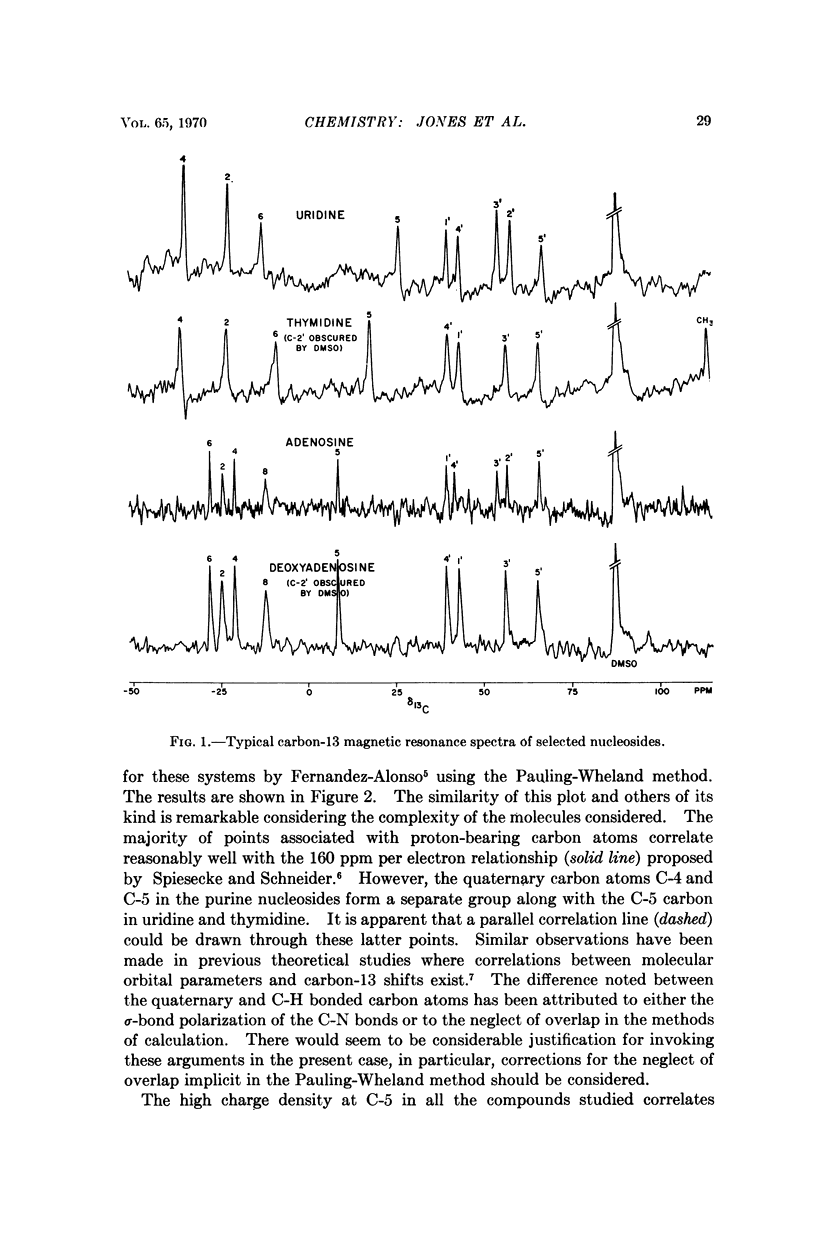
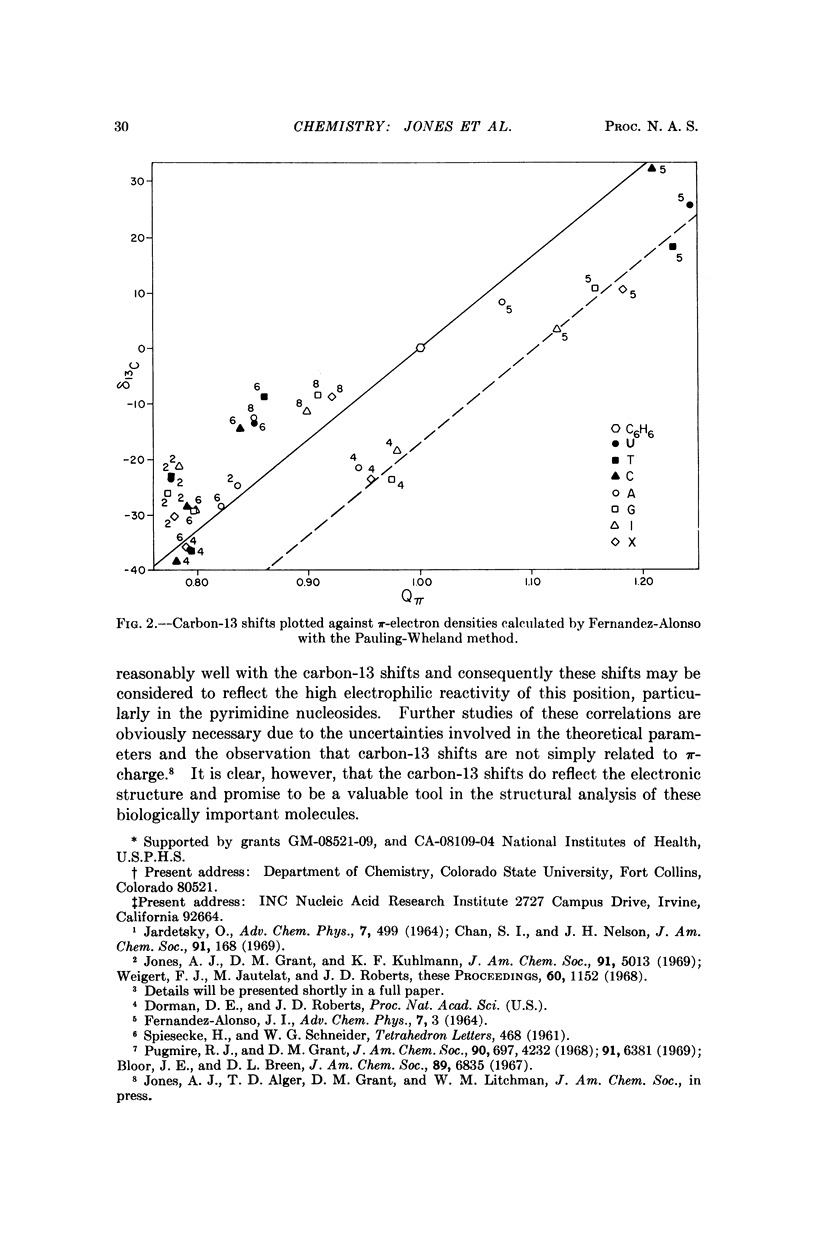
The natural-abundance carbon-13 nuclear magnetic resonance spectra of the naturally occuring pyrimidine and purine nucleosides have been determined and completely analyzed. The carbon-13 chemical shifts obtained provide information on the electronic structure and qualitatively at least correlate well with the known chemical reactivity of these molecules. The carbon-13 shifts are in general far more informative than proton shifts for structural analysis of the nucleosides.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Weigert F. J., Jautelat M., Roberts J. D. Natural-abundance C nuclear magnetic resonance spectra of medium-molecular-weight organic compounds. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1152–1155. doi: 10.1073/pnas.60.4.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]


