Abstract
To assess the effect of rapid individual growth on trace element concentrations in fish, we measured concentrations of seven trace elements (As, Cd, Cs, Hg, Pb, Se, Zn) in stream-dwelling Atlantic salmon (Salmo salar) from 15 sites encompassing a 10-fold range in salmon growth. All salmon were hatched under uniform conditions, released into streams, and sampled ~120 days later for trace element analysis. For most elements, element concentrations in salmon tracked those in their prey. Fast-growing salmon had lower concentrations of all elements than slow-growers, after accounting for prey concentrations. This pattern held for essential and non-essential elements, as well as elements that accumulate from food and those that can accumulate from water. At the sites with the fastest salmon growth, trace element concentrations in salmon were 37% (Cs) to 86% (Pb) lower than at sites where growth was suppressed. Given that concentrations were generally below levels harmful to salmon and that the pattern was consistent across all elements, we suggest that dilution of elements in larger biomass led to lower concentrations in fast-growing fish. Streams that foster rapid, efficient fish growth may produce fish with lower concentrations of elements potentially toxic for human and wildlife consumers.
Keywords: bioaccumulation, growth dilution, mercury, stream food web, trace metal, trophic transfer
Introduction
Accumulation of potentially toxic trace elements in fish is a serious ongoing concern for human and wildlife health. In addition to direct toxic effects of elevated trace element concentrations on fish in heavily contaminated areas (1), fish from thousands of inland streams, rivers, and lakes have been declared as unsafe for unrestricted human consumption because of concerns about exposure to toxic trace elements and other contaminants (2). Wildlife species that consume contaminated fish are also at risk. Studies have linked elevated concentrations of Hg and other toxic trace elements in blood or tissue of fish, birds and mammals with negative effects such as reduced reproductive success, hormonal changes, and motor skill impairment (3,4).
Elevated trace elements concentrations in fish are often associated with industrial pollution, residual contamination from past mining activity (1), or other human land use (5,6). However, even where inputs of trace elements are similar, concentrations in fish and other aquatic organisms vary tremendously due to variation in biological and environmental factors that drive trace element uptake and accumulation (7,8). The overall goals of our work are to identify the most important ecological factors responsible for increasing trace element accumulation in fish under natural conditions in the field. This will improve our capacity to model and predict trace element concentrations in situ, and to identify sites and environmental conditions which are most likely to produce fish with elevated concentrations of elements of concern for human and wildlife health.
Variation in individual growth rate of fish should, in principle, dramatically affect trace element concentrations. All else equal, increased growth will reduce whole-body trace element concentrations in fast-growing fish relative to slow growers by somatic growth dilution (SGD). SGD occurs when fast growers assimilate more biomass relative to the specific trace element than slow growers; hence, the element becomes diluted by the extra body mass (9,10). However, translating this principle to the field is complicated and at present there is little consensus on the overall influence of rapid growth on trace element concentration. For example, for trace elements accumulated from food, increased growth due solely to increased intake of prey and associated trace elements will not lead to SGD (11). Despite clear experimental evidence for strong SGD of trace elements in some systems (10,12), empirical studies disagree on the magnitude (13) and even direction (14–16) of the relationship between fish growth rate and trace element concentration. Increasing our understanding of the effects of variation in growth rate on element accumulation is critical, because individual growth is one of the most variable biological parameters across fish populations, and growth is highly sensitive to fisheries management activities and anthropogenic impacts (17).
We used a unique field assay to measure concentrations of essential and non-essential potentially toxic trace elements (As, Cd, Cs, Hg, Pb, Se, and Zn) in free-living juvenile Atlantic salmon (Salmo salar) across a gradient in individual growth. We stocked newly-hatched Atlantic salmon from controlled initial conditions across multiple streams and collected samples after one growing season to measure trace element concentrations in salmon and their prey. Because we used stocked fish, we could ensure that exposure time, initial mean size, and initial mean element concentration were uniform across all sites. Therefore, our samples provide a standardized measure of trace element accumulation and growth across the study sites. We selected relatively pristine study sites that were not impacted by point-source contamination and that produced a wide range in salmon growth rate, associated with variation in the amount of prey available (17). We took this approach in order to isolate the effects of growth rate without confounding from high variation in trace element inputs or direct toxic effects of elevated trace element concentrations on fish physiology.
Methods
We sampled 15 study sites located on 5 small (< 7 m average summer width) tributary streams of the Connecticut River in New Hampshire and Massachusetts (3 sites per stream; site descriptions in (17,18). All of the streams were in predominantly forested watersheds, with no known mining activity or point sources of trace element contamination. We used juvenile Atlantic salmon, stocked as part of an ongoing population restoration program in the study basin (19), as a field assay of trace element accumulation. The salmon were produced at the White River National Fish Hatchery in Bethel, VT and stocked on 13–16 May 2005. All stocked salmon were unfed fry that were still utilizing yolk resources. We released 200, 600, or 1800 fry at each site for companion studies we conducted on density-dependent survival and growth of salmon (17,18). There is no natural Atlantic salmon reproduction in the study streams and the sites were separated sufficiently to minimize salmon moving among sites during the growing season (18). Therefore, we could ensure that the underyearling salmon we sampled at each site were from our controlled stocking events. We collected four underyearling salmon from each site for trace element analysis from 7–9 September 2005 (116–117 days after stocking). We collected fish with a backpack electrofisher, conducting a single pass through a 50–150 m reach immediately downstream of the release site. Salmon were immediately euthanized and transported on ice to a freezer for storage until processing.
We removed stomach contents of all fish prior to processing for trace elements. Undigested stomach contents from the foregut of all fish within a site were composited and processed separately from fish to estimate trace element concentration in salmon prey. Previous studies show that undigested stomach contents can provide a useful index of trace element concentrations in fish prey (20,21). While stomach samples from a single time point could potentially miss important seasonal variation, diets of underyearling salmon in our study area are overwhelmingly dominated by a few taxa of aquatic invertebrates (predominantly Ephemeroptera, Baetidae and Diptera, Chironomidae) throughout the summer growing season (21–23). These taxa accounted for >60% of the volume of salmon stomach contents at all of our sites. In subsequent years we have found nearly identical relationships between fish and prey Hg concentrations when prey were collected separately from fish throughout the growing season (D.M. Ward unpublished data). Thus, we treat the composited gut contents as a reliable index of site-specific trace element concentrations in prey.
Fish and prey samples were freeze dried and the whole sample (for samples < 1 g dry weight) or a homogenized subsample (for samples > 1 g dry weight) were digested in Optima grade nitric acid in sealed Teflon vessels in a microwave reaction accelerator (Mars5, CEM USA, Matthews, NC). Trace element concentrations in the digested solution were measured by inductively coupled plasma mass spectrometry (Agilent 7500cx, Agilent, Santa Clara, CA). Quality control was ensured by analysis of certified reference materials, duplicate samples, and digestion blanks with every processing batch of 20 samples.
We measured several additional biotic and abiotic characteristics of all study sites. At each site we measured mean stream water pH through the growing season (Oakton pH Testr 2, Oakton Instruments, Vernon Hills, IL; biweekly measurements in the field), base flow alkalinity (inflection point titration with sulfuric acid in 2007), mean water temperature (Onset Optic StowAway, Onset Computer Corporation, Pocasset, MA; hourly measurements by loggers anchored to the stream bed), the biomass of benthic invertebrate prey (6 Surber samples, 500 µm mesh, combined biomass of aquatic Ephemeroptera and Diptera), overhead canopy cover (tubular densitometer, mean of 6 measures per site), and the percent of wetland and forested area in the catchment (24).
Data analysis
The response variables for our primary analyses were trace element concentrations in individual salmon. We had two primary predictors of interest, individual growth (measured as final dry mass) and the concentration of the specific element in prey (measured from composited stomach contents at the site level). Some of the trace elements we measured (e.g. As, Cd, Pb, Zn) can accumulate in fish from water as well as from food. We did not measure aqueous metal concentrations. However, controlled laboratory exposures of juvenile salmonids to known food and water concentrations show that uptake from prey accounts for much of the variation in trace element concentrations of juvenile salmonids even across a wide range of water concentrations (1,25–27). Therefore, using prey concentrations to partially account for variation in trace element exposure across sites is a valid approach in this analysis.
To estimate the effect of suppressed growth on trace element concentrations in fish across individuals and sites while accounting for variation in prey concentrations, we used multi-level linear models with an additive random classification term indicating each site, mean salmon mass and prey concentration as covariates at the site level, and individual deviations from site-mean mass as a covariate at the individual level. To account for differences in stream water chemistry, we also tested whether adding stream-water pH or alkalinity as predictors at the site level improved model fit. For all covariates at the site level, we used the number of sites as the denominator degrees of freedom. In all analyses, trace element concentrations and fish mass were log10-transformed to yield approximately normal residual variance and linear relationships. In supplementary material (Table S1), we also present pair-wise correlations among all trace elements and correlations of element concentrations with measured site characteristics to aid in identification of habitat factors associated with increased trace element accumulation in stream food webs. Data analysis was conducted using JMP 5.0 and the R program for statistical computing (28,29).
Results
The study sites encompassed a wide range in salmon growth and trace element concentration. Mean individual growth (as final dry mass) varied more than 10-fold across sites (one-way analysis of variance (ANOVA): F14,45=37.0, P<0.0001, r2 =0.92), with low growth rates associated with low prey biomass (17). All element concentrations varied significantly across sites (one-way ANOVAs; all F14,45>4.5, P<0.0001, r2>0.58). Mean concentrations of Cd, Cs, Hg, and Pb all ranged more than 10-fold across sites (maximum/minimum site mean Cd: 14.9; Cs: 13.6; Hg: 10.3; Pb: 59.0), while mean concentrations of As and the essential elements Se and Zn varied less across sites (maximum/minimum site mean As: 2.8; Se: 6.6; Zn: 3.7). All element concentrations in prey and fish were well below levels associated with toxic effects in fish, except for the highest Se concentrations (ref. 30; Table S2). Se is an essential element with a very narrow range between deficient and toxic concentrations (31), so even though concentrations were not particularly high compared to Se contaminated areas, two of our sites and eight individual fish had Se concentrations associated with reduced fish health in sensitive species (2 ppm in prey, 4 ppm in fish; ref. 30). However, excluding the sites and individuals with elevated Se did not affect results. The element concentrations we observed in salmon exceeded no effects hazard concentrations for piscivorous wildlife (4) for Hg (10 sites), Se (2 sites), and Zn (1 site; Table S3).
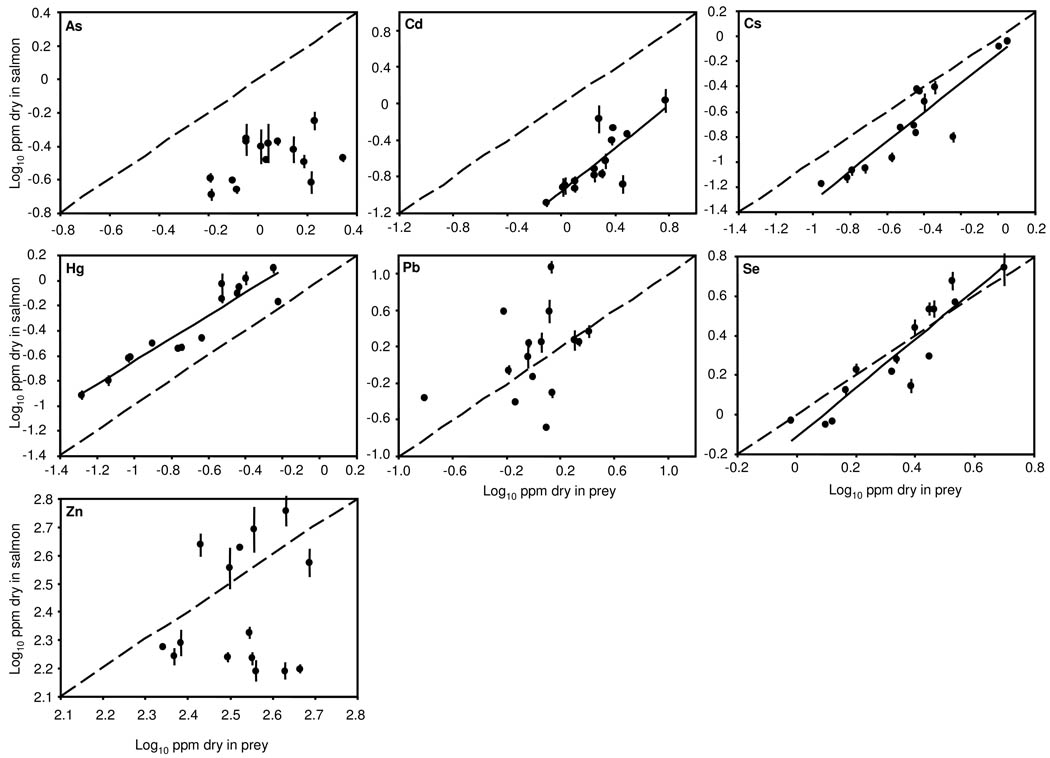
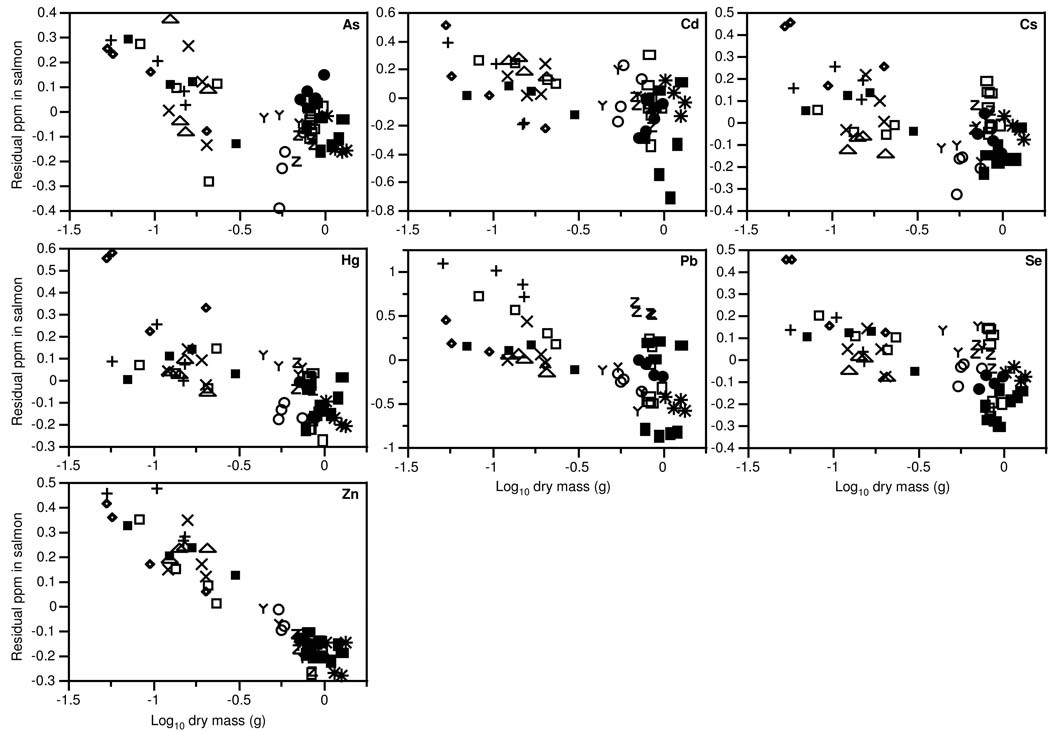
Mean Cd, Cs, Hg, and Se concentrations in salmon tracked those in their prey across sites, while As concentrations in salmon were marginally related to As in prey and Pb and Zn concentrations were unrelated in salmon and prey (Figure 1, Table 1). Only Hg biomagnified, resulting in element concentrations in salmon t hat were consistently higher than those in their prey; As, Cd, and Cs consistently biodiminished, resulting in element concentrations in salmon that were consistently lower than those in their prey (Figure 1). Fast-growing salmon had lower trace element concentrations for a given concentration in prey (Figure 2). In the regression model including prey concentrations, all trace element concentrations in salmon were significantly lower at sites with high mean individual salmon mass (Table 1). Across individuals, all element concentrations were lower in relatively large individuals within sites, although this relationship within sites was only marginally significant for Hg and Cs (Table 1). Adding pH or alkalinity to the model did not significantly increase the variance explained for any trace element (all P>0.21).
Figure 1.
Scatterplots of trace element concentrations in salmon and their prey across sites. Each point is one site; error bars are ± 1 SE for salmon concentrations. The solid lines are linear fits of salmon concentration to prey concentration and are only shown where this relationship is statistically significant (P<0.05). The dashed line is the 1:1 line, so points above this line indicate element concentration in fish exceed those in prey (biomagnifying), while points below indicate element concentration in fish are less than those in prey (biodimishing).
Table 1.
Regression results for all element concentrations in fish s amples. The “log10 prey ppm” predictor is the element concentration in prey for the same element as the response in each model. The “Mean log10 dry mass” predictor is the mean dry mass (g) of fish at the site, while “Individual log10 dry mass” is the deviation of each individual fish from the site mean dry mass. Slopes are the coefficient estimates for each predictor from the hierarchical linear model, SE is the standard error of the slope, P is the P-value.
| log10 prey ppm | Mean log10 dry mass | Individual log10 dry mass | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Response | Slope | SE | P | Slope | SE | P | Slope | SE | P |
| log10 As ppm | 0.33 | 0.13 | 0.02 | −0.21 | 0.05 | <0.01 | −0.50 | 0.11 | <0.01 |
| log10 Cd ppm | 0.71 | 0.17 | <0.01 | −0.44 | 0.09 | <0.01 | −0.53 | 0.15 | <0.01 |
| log10 Cs ppm | 1.23 | 0.12 | <0.01 | −0.20 | 0.08 | 0.03 | −0.13 | 0.08 | 0.10 |
| log10 Hg ppm | 0.59 | 0.07 | <0.01 | −0.40 | 0.05 | <0.01 | −0.16 | 0.08 | 0.05 |
| log10 Pb ppm | −0.68 | 0.63 | 0.30 | −0.86 | 0.26 | 0.01 | −0.72 | 0.13 | <0.01 |
| log10 Se ppm | 0.63 | 0.16 | <0.01 | −0.42 | 0.06 | <0.01 | −0.31 | 0.07 | <0.01 |
| log10 Zn ppm | 0.03 | 0.08 | 0.67 | −0.49 | 0.02 | <0.01 | −0.45 | 0.06 | <0.01 |
Figure 2.
Scatterplots of residual trace element concentrations, after accounting for variation in concentrations in prey, and salmon dry mass. Dry mass is an index of growth rate as fish started at uniform initial mean size across sites and all fish were similar age at sampling. Each point is one individual salmon, different symbols indicate the different sites.
Discussion
Fast growth was associated with lower trace element concentrations in fish for a given concentration in prey, consistent with SGD. This pattern held for essential (Se, Zn), non-essential (As, Cd, Cs, Hg, Pb), and potentially toxic elements (both essential and non-essential at elevated concentrations), as well as elements that accumulate largely from food (Cs, Hg, Se) and those that can accumulate from water (As, Cd, Pb, Zn). Based on the regression slopes, rapid growth potentially reduced mean element concentrations between 1.6 (Cs) and 7 (Pb) fold across the 10-fold range in growth at our study sites. This result is critically important for management and prediction of levels of trace elements and persistent contaminants in fish, as a common starting point for assessment of fish contamination risk is to assume constant accumulation from water or prey to fish regardless of fish growth rate (32). Further, our results support the general hypothesis that high biological productivity and rapid fish growth can reduce concentrations of toxic trace elements in aquatic food webs (11,33–35).
We suggest that fast growth reduced whole-body concentrations of trace elements in salmon regardless of whether they accumulate from food or water. For elements that accumulate from food, this means that fast growth is likely associated with high growth efficiency – a larger gain in biomass for each gram of food, and food-born trace elements, consumed (9,10). An increasing number of studies indicate that variation in growth efficiency, mediated by differences in prey quality or variation i n energetic costs, is a key driver of variation in growth rate for fish and other aquatic organisms (10,36,37). If variation in growth efficiency frequently drives variation in fish growth rate, SGD should be a common mechanism reducing element concentrations in fish, consistent with our observations.
For elements that accumulate from water, increased biomass should reduce element concentrations in fast growers via SGD as long as rapid growth is not associated elevated exposure. At an individual level, increased respiration rate can increase exposure to aqueous metals via the gills (38), but rapid respiration is not consistently associated with rapid growth of fish (37). Across our study sites, salmon growth was potentially spatially confounded with aqueous exposure because sites with low prey biomass and low fish growth also had relatively low pH and alkalinity (Table S1) – water quality factors associated with increased aqueous metal bioavailability and accumulation (39). However, adding pH or alkalinity as predictors did not explain significant additional variation concentration of any of the trace elements in our analysis. Further, the comparison across individual salmon within sites was not affected by this potential spatial confounding yet relatively fast-growing individuals also had lower trace element concentrations than slow growers within sites. Thus, while the analysis for water-born trace elements is not conclusive without aqueous concentration data, we suggest that SGD drove the similar relationships for elements that accumulate from either food or water.
We assessed the potential for SGD for whole-body trace element concentrations in salmon, but trace element concentrations vary across tissues within individual fish. For example, elements accumulated from water are often elevated in the gills, while those accumulated from food are elevated in the intestine (25). Whole-body SGD may not be associated with reduced trace element concentrations within individual organs, yet the toxic effects of trace elements on fish often depend on organ-specific concentrations (1,25–27). Therefore, it is unlikely that SGD will reduce toxic effects of trace elements on individual fish. Nonetheless, whole-body SGD can dramatically reduce potential trophic transfer of toxic trace elements to piscivorous fish and wildlife that consume whole fish (11).
Rapid growth was associated with lower element concentrations for all elements we analyzed, yet there were different patterns of accumulation in fish associated with the physiological role of specific elements. In particular, variation across sites in the concentrations of the essential elements Se and Zn was much lower than for all other elements except As. Arsenic is not generally recognized as essential, yet feeding trials indicate that As may have a beneficial or essential function at ultra trace concentrations in wide variety of taxa (31). Thus, lower variation in the concentration of As, Se, and Zn relative to Cd, Cs, Hg, and Pb is potentially due to tighter physiological regulation of essential or potentially beneficial trace elements (40). However, concentrations of all elements, including essential elements, varied substantially across sites, indicating that even the essential trace elements are not under strict homeostatic control.
The correlation between element concentration and body size is one of the most frequently reported metrics for trace elements in fish (41), yet the drivers of this scaling relationship are not known for most elements. Unlike our controlled assay, fish age and size are confounded in most field studies, so these relationships can not be used to assess SGD. Yet, controlled laboratory exposures have shown that allometric scaling relationships can generate concentration-body size relationships for trace elements in fish independent of age or SGD. For example, metal-accumulating tissues in the intestine, kidney, or gills may be a smaller proportion of total body mass in larger fish, yielding negative relationships between whole-body concentrations and body size. This is not the case for intenstine (42), kidney (43), and gills (44) in growing juvenile Atlantic salmon, suggesting that this was not mechanism driving the whole-body SGD that we observed. For many elements, assimilation efficiency from food increases with body size, while elimination rate and specific growth rate decline with body size – scaling relationships that should drive positive relationships between element concentrations and body size (45–47). These allometric scaling relationships may partly account for the elevated trace element concentrations in larger, older fish typical for some elements (9), but can not explain the SGD pattern we observed. In contrast, negative allometric scaling of mass-specific consumption rate and mass-specific uptake rate of water-born trace elements could drive negative concentration-body size relationships consistent with those we observed (45,47). Thus, these mechanisms may have contributed to the SGD pattern of reduced trace element concentrations in large, fast-growing fish that we observed.
Rapid growth can reduce concentrations of trace elements and other contaminants in fish, relative to prey or water exposure sources (11,48,49). Yet, the resultant concentrations in the fish clearly depend critically on the concentrations in the source of exposure. Our results confirm earlier studies showing that, comparing across sites, many trace elements in fish track the concentrations in their prey (50,51). Given the key role of prey concentrations, managing for increased individual growth rate and SGD alone can not mitigate trace element contamination in fisheries (16). However, whereas contaminant inputs and concentrations in prey may not be amenable to short-term, local292 scale control, individual growth is one of the most variable characteristics of fish populations (52) and fish growth is sensitive to anthropogenic manipulations (16,17,49). In fact, increasing individual growth of fish is a frequent target for fisheries management, yet the potential for this widespread fisheries management goal to reduce trace element concentrations in fish remains largely unknown.
Supplementary Material
Acknowledgement
We thank Brian Jackson, Vivien Taylor, and Arthur Baker at the Dartmouth Trace Element Analysis Core for sample analysis and Roxanne Karimi for discussions of growth dilution. This project was funded by NIEHS-SBRP grant ES07373 and the USFS Northern Research Station.
Footnotes
Supporting Information Available
This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Farag AM, Woodward DE, Brumbaugh W, Goldstein JN, MacConnell E, Hogstrand C, Barrows FT. Dietary effects of metals-contaminated invertebrates from the Coeur D'Alene River, Idaho, on cutthroat trout. Trans. Am. Fish. Soc. 1999;128:578–592. [Google Scholar]
- 2.US EPA. 2005/2006 national listing of fish advisories. Washington, DC: U.S. EPA; Technical report EPA 823-F-07-003. 2007
- 3.Scheulhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio. 2007;36:12–18. doi: 10.1579/0044-7447(2007)36[12:eoemot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Hinck JE, Schmitt CJ, Chojnacki KA, Tillitt DE. Environmental contaminants in freshwater fish and their risk to piscivorous wildlife based on a national monitoring program. Environ. Monit. Assess. 2009;152:469–494. doi: 10.1007/s10661-008-0331-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen CY, Folt CL. Bioaccumulation and diminution of arsenic and lead in a freshwater food web. Environ. Sci. Technol. 2000;34:3878–3884. [Google Scholar]
- 6.Renshaw CE, Bostick BC, Feng XH, Wong CK, Winston ES, Karimi R, Folt CL, Chen CY. Impact of land disturbance on the fate of arsenical pesticides. J. Environ. Qual. 2006;35:61–67. doi: 10.2134/jeq2005.0096. [DOI] [PubMed] [Google Scholar]
- 7.Ward DM, Nislow KH, Folt CL. Bioaccumulation syndrome: identifying factors that make some stream food webs prone to elevated mercury bioaccumulation. Ann. N. Y. Acad. Sci. 2010 doi: 10.1111/j.1749-6632.2010.05456.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CY, Dionne M, Mayes BM, Ward DM, Sturup S, Jackson BP. Mercury bioavailability and bioaccumulation in estuarine food webs in the Gulf of Maine. Environ. Sci. Technol. 2009;43:1804–1810. doi: 10.1021/es8017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trudel M, Rasmussen JB. Bioenergetics and mercury dynamics in fish: a modelling perspective. Can. J. Fish. Aquat. Sci. 2006;63:1890–1902. [Google Scholar]
- 10.Karimi R, Chen CY, Pickhardt PC, Fisher NS, Folt CL. Stoichiometric controls of mercury dilution by growth. Proc. Natl. Acad. Sci. USA. 2007;104:7477–7482. doi: 10.1073/pnas.0611261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward DM, Nislow KH, Chen CY, Folt CL. Rapid, efficient growth reduces mercury concentrations in stream-dwelling Atlantic salmon. Trans. Am. Fish. Soc. 2010;139:1–10. doi: 10.1577/T09-032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surette C, Lucotte M, Doire J, Tremblay A. Mercury bioaccumulation in fish: effects of intensive fishing in three natural lakes of northern Quebec, Canada. J. Phys. IV. 2003;107:1443. [Google Scholar]
- 13.Stafford CP, Haines TA. Mercury contamination and growth rate in two piscivore populations. Environ. Toxicol. Chem. 2001;20:2099–2101. [PubMed] [Google Scholar]
- 14.Rodgers DW, Beamish FWH. Dynamics of dietary methylmercury in rainbow trout, Salmo gairdneri. Aquat. Toxicol. 1982;2:271–290. [Google Scholar]
- 15.Dutton MD. Methyl mercury bioaccumulation: a study of factors influencing uptake and elimination in fish. Ph.D. Thesis. Waterloo, Ontario: University of Waterloo; 1997. [Google Scholar]
- 16.Lepak JM, Robinson JM, Kraft CE, Josephson DC. Changes in mercury bioaccumulation in an apex predator in response to removal of an introduced competitor. Ecotoxicology. 2009;18:488–498. doi: 10.1007/s10646-009-0306-5. [DOI] [PubMed] [Google Scholar]
- 17.Ward DM, Nislow KH, Folt CL. Increased population density and suppressed prey biomass: relative impacts on juvenile Atlantic salmon growth. Trans. Am. Fish. Soc. 2009;138:135–143. [Google Scholar]
- 18.Ward DM, Nislow KH, Folt CL. Predators reverse the direction of density dependence for juvenile salmon mortality. Oecologia. 2008;156:515–522. doi: 10.1007/s00442-008-1011-4. [DOI] [PubMed] [Google Scholar]
- 19.Folt CL, Nislow KH, Power ME. Implications of temporal and spatial scale for Atlantic salmon (Salmo salar) research. Can. J. Fish. Aquat. Sci. 1998;55:9–21. [Google Scholar]
- 20.Tucker S, Rasmussen JB. Using cs-137 to measure and compare bioenergetic budgets of juvenile Atlantic salmon (Salmo salar) and brook trout (Salvelinus fontinalis) in the field. Can. J. Fish. Aquat. Sci. 1999;56:875–887. [Google Scholar]
- 21.Kennedy BP, Klaue B, Blum JD, Folt C. Integrative measures of consumption rates in salmon: expansion and application of a trace element approach. J. Appl. Ecol. 2004;41:1009–1020. [Google Scholar]
- 22.Kennedy BP, Nislow KH, Folt CL. Habitat-mediated foraging limitations drive survival bottlenecks for juvenile salmon. Ecology. 2008;89:2529–2541. doi: 10.1890/06-1353.1. [DOI] [PubMed] [Google Scholar]
- 23.Grader M, Letcher BH. Diel and seasonal variation in food habits of Atlantic salmon parr in a small stream. J. Freshwat. Ecol. 2006;21:503–517. [Google Scholar]
- 24.Moore RB, Johnston CM, Robinson KW, Deacon JR. Scientific Investigations Report 2004–5012. Washington, DC: USGS; 2004. Estimation of total nitrogen and phosphorus in New England streams using spatially referenced regression models. [Google Scholar]
- 25.Farag AM, Boese CJ, Woodward DF, Bergman HL. Physiological changes and tissue metal accumulation in rainbow trout exposed to foodborne and waterborne metals. Environ. Toxicol. Chem. 1994;13:2021–2029. [Google Scholar]
- 26.Woodward DF, Farag AM, Bergman HL, Delonay AJ, Little EE, Smith CE, Barrows FT. Metals-contaminated benthic invertebrates in the Clark Fork River, Montana: effects on age-0 brown trout and rainbow trout. Can. J. Fish. Aquat. Sci. 1995;52:1994–2004. [Google Scholar]
- 27.Woodward DF, Brumbaugh WG, Delonay AJ, Little EE, Smith CE. Effects on rainbow trout fry of a metals-contaminated diet of benthic invertebrates from the Clark Fork River, Montana. Trans. Am. Fish. Soc. 1994;123:51–62. [Google Scholar]
- 28.JMP user's guide, version 5. Cary, NC: SAS Institute Inc.; 2002. SAS Institute Inc. [Google Scholar]
- 29.R Development Core Team. R 2.10.2. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 30.Hamilton SJ. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004;326:1–31. doi: 10.1016/j.scitotenv.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 31.National Research Council. Mineral tolerance of animals. Washington, DC: National Acadamies Press; 2005. [Google Scholar]
- 32.Kleinow KM, Nichols JW, Hayton WL, McKim JM, Barron MG. Toxicokinetics in fishes. In: Schlenk D, Giulio RTD, Hinton DE, Giulio RTD, editors. The toxicology of fishes. Boca Raton, FL: CRC Press; 2008. pp. 55–152. [Google Scholar]
- 33.Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK. Mercury contamination in forest and freshwater ecosystems in the northeastern united states. Bioscience. 2007;57:17–28. [Google Scholar]
- 34.Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc. Natl. Acad. Sci. USA. 2002;99:4419–4423. doi: 10.1073/pnas.072531099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill WR, Larsen IL. Growth dilution of metals in microalgal biofilms. Environ. Sci. Technol. 2005;39:1513–1518. doi: 10.1021/es049587y. [DOI] [PubMed] [Google Scholar]
- 36.Trudel M, Tremblay A, Schetagne R, Rasmussen JB. Why are dwarf fish so small? An energetic analysis of polymorphism in lake whitefish (Coregonus clupeaformis) Can. J. Fish. Aquat. Sci. 2001;58:394–405. [Google Scholar]
- 37.Rennie MD, Collins NC, Shuter BJ, Rajotte JW, Couture PA. comparison of methods for estimating activity costs of wild fish populations: more active fish observed to grow slower. Can. J. Fish. Aquat. Sci. 2005;62:767–780. [Google Scholar]
- 38.Veltman K, Huijbregts MAJ, Van Kolck M, Wang WX, Hendriks AJ. Metal bioaccumulation in aquatic species: quantification of uptake and elimination rate constants using physicochemical properties of metals and physiological characteristics of species. Environ. Sci. Technol. 2008;42:852–858. doi: 10.1021/es071331f. [DOI] [PubMed] [Google Scholar]
- 39.Alsop DH, McGeer JC, McDonald DG, Wood CM. Costs of chronic waterborne zinc exposure and the consequences of zinc acclimation on the gill/zinc interactions of rainbow trout in hard and soft water. Environ. Toxicol. Chem. 1999;18:1014–1025. [Google Scholar]
- 40.Karimi R, Folt CL. Beyond macronutrients: element variability and multielement stoichiometry in freshwater invertebrates. Ecol. Lett. 2006;9:1273–1283. doi: 10.1111/j.1461-0248.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 41.Canli M, Atli G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003;121:129–136. doi: 10.1016/s0269-7491(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 42.Sanden M, Berntssen MHG, Krogdahl A, Hemre GI, Bakke-McKellep AM. An examination of the intestinal tract of Atlantic salmon, Salmo salar L., parr fed different varieties of soy and maize. J. Fish Dis. 2005;28:317–330. doi: 10.1111/j.1365-2761.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 43.Sissener NH, Sanden M, Bakke AM, Krogdahl A, Hemre GI. A long term trial with Atlantic salmon (Salmo salar L.) fed genetically modified soy; focusing general health and performance before, during and after the parr-smolt transformation. Aquaculture. 2009;294:108–117. [Google Scholar]
- 44.Wells PR, Pinder AW. The respiratory development of Atlantic salmon 1: morphometry of gills, yolk sac and body surface. J. Exp. Biol. 1996;199:2725–2736. doi: 10.1242/jeb.199.12.2725. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Wang WX. Size-dependence of the potential for metal biomagnification in early life stages of marine fish. Environ. Toxicol. Chem. 2007;26:787–794. doi: 10.1897/06-348r.1. [DOI] [PubMed] [Google Scholar]
- 46.Trudel M, Rasmussen JB. Modeling the elimination of mercury by fish. Environ. Sci. Technol. 1997;31:1716–1722. [Google Scholar]
- 47.Mathews T, Fisher NS, Jeffree RA, Teyssie JL. Assimilation and retention of metals in teleost and elasmobranch fishes following dietary exposure. Marine Ecology-Progress Series. 2008;360:1–12. [Google Scholar]
- 48.Simoneau M, Lucotte M, Garceau S, Laliberte D. Fish growth rates modulate mercury concentrations in walleye (Sander vitreus) from eastern Canadian lakes. Environ. Res. 2005;98:73–82. doi: 10.1016/j.envres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Stow CA, Carpenter SR, Madenjian CP, Eby LA, Jackson LJ. Fisheries management to reduce contaminant consumption. Bioscience. 1995;45:752–758. [Google Scholar]
- 50.Chen CY, Stemberger RS, Klaue B, Blum JD, Pickhardt PC, Folt CL. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol. Oceanogr. 2000;45:1525–1536. [Google Scholar]
- 51.Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the northeast us. Ecotoxicology. 2005;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- 52.Nislow KH. International symposium on the implications of salmonid growth variation. Rev. Fish Biol. Fish. 2001;10:521–527. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.