Abstract
Premenopausal women have less cardiovascular disease and lower cardiovascular morbidity and mortality than men the same age. Our previous studies showed that female mice have lower mortality and better preserved cardiac function after myocardial infarction. However, the precise cellular and molecular mechanisms responsible for such a sex difference are not well established. Using cultured adult mouse cardiomyocytes (ACMs), we tested the hypothesis that the survival advantage of females stems from activated estrogen receptors (ER) and Akt survival signaling pathways. ACMs were isolated from male and female C57BL/6J mice and treated with hydrogen peroxide (H2O2, 100 μM) for 30 min. Cell survival was indicated by rod ratio (rod shaped cells/total cells) and cell death by lactate dehydrogenase (LDH) release and positive staining of Annexin-V (AV+, a marker for apoptosis) and propidium iodide (PI+, a marker for necrosis). In response to H2O2, female ACMs exhibited a higher rod ratio, lower LDH release and fewer AV+ and PI+ cells compared to males. Phospho-Akt was greater in females both at baseline and after H2O2 stimulation. The downstream molecule of Akt, phosphor-GSK-3β (inactivation), was also higher while caspase-3 activity was lower in females in response to H2O2. Bcl-2 did not differ between genders. ERα was the dominant isoform in females, whereas ERβ was low but similar in both genders. Our findings demonstrate that female ACMs have a greater survival advantage when challenged with oxidative stress-induced cell death. This may be attributable to activation of Akt and inhibition of GSK-3β and caspase-3 through an ERα-mediated mechanism.
Keywords: mice, estrogen, myocytes, oxidative stress, apoptosis, signal transduction
INTRODUCTION
Premenopausal women have less cardiovascular disease and lower cardiovascular morbidity and mortality than men the same age; however, these cardioprotective benefits disappear after menopause.1 Experimental and clinical evidence of sex differences in myocardial remodeling and heart failure favors women.2 We previously showed that female mice had lower mortality, better preserved cardiac function, less inflammation and augmented healing responses after myocardial infarction (MI),3 suggesting that females have an inherent cardioprotective advantage. However, the precise underlying mechanisms behind this advantage are not fully understood.
Compelling evidence indicates that cardiomyocyte death via apoptosis and necrosisplays a critical role in a wide range of cardiovascular diseases, including ischemic heart disease, myocarditis, dilated cardiomyopathy and atherosclerosis.4 Since adult cardiomyocytes (ACMs) possess minimal capacity to re-enter the cell cycle or divide and/or proliferate, control of myocyte loss through suppression of cell death pathways represents a logical strategy for cardioprotection. Estrogen has also been shown to inhibit apoptosis by blocking transcription factor NF-κB and caspase activation,5 which may conserve contractile myocytes and delay or prevent development of left ventricular dysfunction and heart failure.
Three estrogen receptor (ER) isoforms, ERα, ERβ, and GPR30, have been identified.6 ERα and ERβ are found in the heart and vasculature.7 The anti-oxidative and anti-apoptotic actions of estrogen have been shown to be mediated by activation of Akt either via ERα and ERβ or in some ER-independent fashion.8 Several downstream targets of Akt have been recognized as cell survival regulatory molecules, including glycogen synthase kinase-3β (GSK-3β), caspase-3 and Bcl-2. GSK-3β is a serine/threonine kinase and one of the few protein kinases known to be inactivated by phosphorylation. It has multiple functions in heart tissue 9 and has recently attracted attention because of its association with both cell apoptosis and survival.10 Caspases, which are cysteine-aspartic proteases, play essential roles in apoptosis, necrosis and inflammation.11 Eleven caspases have been found in humans.12 They are activated by removal of the pro-domain upon apoptotic stimuli and other caspases.12 Akt (acting via phosphorylation) has been shown to inhibit the initiator caspase-9, which in turn blocks the effector caspase-3, suppressing apoptosis. Bcl-2 is an anti-death gene that functions as an intracellular antioxidant.13 However, we still do not know whether these Akt-associated signaling molecules are involved in the sex difference favoring females in cell survival.
In the present study, we treated cultured mouse ACMs with hydrogen peroxide (H2O2) to test the hypothesis that improved cardiomyocyte survival in females is mediated by enhanced anti-apoptotic and anti-necrotic signaling pathways. This involves elevated expression of ERα, activation of AKT/GSK3β, AKT/Bcl-2 and inhibition of caspase-3.
MATERIALS AND METHODS
Animals
Male and female C57BL/6J mice 8 – 10 weeks old were obtained from Jackson Laboratories (Bar Harbor, ME). They were housed in anair-conditioned room with a 12/12hour dark-light cycle and given standard chow with free access to tap water. The study was approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System in accord with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Isolation of adult mouse cardiomyocytes (ACMs)
Male (n = 43) and female (n = 41) C57B16/J mice were anti-coagulated with 50 units heparin (100 IU/mL, 0.5 ml i.p.) and anesthetized with pentobarbital sodium (50 mg/kg i.p.). Hearts were rapidly excised and arrested in Ca2+-free perfusion buffer consisting of (in mmol/l) 120 NaCl, 5.4 KCl, 1.2 NaH2PO4, 1.2 MgSO4 · 7 H2O, 5.6 glucose, 20 NaHCO3, 10 2,3-butanedione monoxime (BDM; Sigma) and 5 taurine (Sigma), pH 7.2, gassed with 95% O2-5%CO2. The aorta was cannulated and the heart perfused retrograde withCa2+-free perfusion buffer at a constant flow of 1 ml/min at 37°C for 4 min14 to flush out blood from the vasculature and remove extracellular calcium to stop contraction. The perfusion buffer was then switched to myocyte digestion buffer, which consisted of perfusion buffer and0.5 mg/ml collagenase type 2 (Worthington) and 0.02 mg/ml proteinase type D, and maintained for 5 min. Calcium concentration was raised to 50 μM in the myocyte digestion buffer for 8–10 min. The heart appeared swollen, pale, and flaccid. The ventricles were removed and cut into several chunks in the same enzyme solution. Using a wide-open pipette, dispersed myocytes were filtered through a 100-μm mesh and centrifuged for 3 min at 500 rpm. The supernatant was removed and the cell pellet promptly resuspended in perfusion buffer containing 125 μM Ca++ and 5 mg/ml BSA. The cells were allowed to sediment by gravity in a cell culture incubator for 10 min, after which they were washed sequentially with perfusion buffer containing 250 and 500 μM Ca++. Half of the perfusion buffer from the last wash was replaced by phenol red-free MEM (washing medium) containing 2.5 % fetal bovine serum (FBS, Gibco) and 1250 μM Ca++. ACMs were incubated at 37°C for 10 min. Then half of the medium was replaced by washing medium to gradually increase Ca++ to 1 mM (final concentration). Finally the cells were counted and plated.
Culture of adult mouse cardiomyocytes
The entire culture process was performed under a biological safety hood. Culture plates were pre-coated with 8–10 μg/ml mouse laminin (Sigma) in phosphate-buffered saline (PBS; Hyclone) with 1% penicillin-streptomycin (PS; Gibco) and pre-incubated at 37°C for 1 hr. Freshly isolated ACMs were plated at 0.5 × 104 cells/cm2 in washing medium and incubated at 37°C with 5% CO2 and saturated water moisture for 1 hr. Then the medium was gently changed to culture medium, which was washing medium without FBS, and maintained for 1 hr.
Cell viability and H2O2-induced cell death
After 1 hr starvation, ACMs on 24-well plates in 500 μl medium per well were treated with either culture medium (CTL) or 100 μM H2O2 for 30 min to induce cell death.15,16 Cell viability was determined by the ratio of rod-shaped (typical viable adult cardiomyocytes with length: width > 1 – 1.5) to total number of cells. Apoptosis and necrosis were detected using an annexin-V–FITC/propidium iodide stain detection kit (BioVision). Briefly, ACMs were incubated with annexin-V–FITC (AV-FITC) and propidium iodide (PI-FITC) for 10 min at room temperature in the dark. Plates were photographed under both phase contrast and fluorescence microscopes and rod-shaped, sphere-shaped (length: width < 1 – 1.5) and total cells were counted. Apoptotic myocytes were defined as AV-FITC positive (AV+, green stained cells) and necrotic myocytes as PI positive (PI+, red stained cells) + AV+. Images were processed and surface area of freshly isolated ACMs measured with MicroSuite software (Biological Suite). For each parameter, 200–300 myocytes were counted in randomly selected fields by three independent researchers. Each experiment was conducted in triplicate and averaged as one experiment.
Caspase-3 activity assay
Caspase-3 activity was measured using an ApoTarget caspase-3 protease assay kit (BioSource International). Briefly, at the end of the experiment the medium was centrifuged and the supernatant removed to collect detached cells as a pellet. Attached cells were scraped and combined with the pellet in ice cold lysis buffer (from the kit). Samples were centrifuged and the supernatant collected. Protein concentration was determined with a BCA assay kit (Pierce). The cell lysate (30 μg) from each sample was combined with 200 μM DEVD-pNA substrate and diluted with 2 × reaction buffer containing 10 mM DTT. After incubation at 37°C in the dark for 1 hr, samples were read at 405 nm in a microplate reader. Measurements were repeated after 1 hr. Values were compared with known standards to determine enzymatic activity. Caspase-3 activity was expressed as p moles per microgram (protein) per hour.
Cytotoxicity
Cytotoxicity was measured by lactate dehydrogenase (LDH) release using an LDH assay kit (Bio Vision, CA). Briefly, ACMs were plated at 2 × 104 cells/well in 24-well plates. Cells were treated with control medium (CTL) or 100 μM H2O2 for 30, 60, and 90 min. At the end of each time point, the plates were gently shaken to make sure LDH was evenly distributed in the medium. Medium were centrifuged and the supernatant transferred to a 96-well plate (10 μl per well). 100 μl LDH WST substrate mixtures (4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) were added and incubated for 30 min at room temperature. Absorbance was measured with a plate reader at 450 nm. Cytotoxicity (expressed as a percentage) was calculated as an index of cell death using the formula:
Where test sample is experimental sample (cells) with or without H2O2 treatment; the low-control is sample taken from culture medium containing freshly isolated cells without any treatment; high control is sample taken from culture medium containing cells treated with lysis solution. Each experimental sample had its own low- and high- controls.
Western blot
Cells were harvested in lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF. After 20 min incubation at 4°C, the lysate was centrifuged at 13,500 rpm for 15 min and protein concentrations in the supernatant determined as described above (details please see Methods in Data Supplement, http://hyper.ahajournals.org)
Statistics
Data are expressed as mean ± SE. All parameters were analyzed using Student’s t-test, taking p < 0.05 as significant. The number of experiments (n) refers to independent cultures from different hearts.
RESULTS
Sex difference in cell survival in response to H2O2
Freshly isolated viable ACMs were rod-shaped with rectangular “stepped” ends and clear cross-striations. The surface area of female ACMs was smaller than in males; however, this difference disappears when it is corrected by body weight (Data Supplement, Figure S1. Please see http://hyper.ahajournals.org). In contrast to rod-shaped viable ACMs, non-viable cells were spherical Viability was indicated by the rod ratio (rod-shaped cells/total cells). Under control conditions (CTL), the rod ratio was similar between female and male ACMs. H2O2 decreased the rod ratio in both sex; however, the reduction was significantly greater in males (Fig. 1). We further confirmed the survival differences between male and female ACMs by measuring LDH released into the medium. As shown in Fig. 2, H2O2 increased LDH release in a time-dependent manner, and the increase was significantly greater in male ACMs at all time points.
Figure 1.
Sex difference in ACMs viability as indicated by the rod ratio in response to H2O2. A: Representative images showing viable ACMs (rod-shaped cells, indicated by arrows) and dead cells (spherical, indicated by sparkles) under control conditions (CTL) or treated with H2O2 (100 μM). B: Quantitative analysis of the rod ratio (rod-shaped/total cells, upper panel) and change from CTL in response to H2O2 (lower panel). n = 5.
Figure 2.
Sex difference in H2O2-induced cytotoxicity as indicated by LDH release. LDH was calculated using the formula: [(test sample − low control)/(high control − low control)] × 100 and expressed as % LDH release. n = 4
Sex difference in H2O2-induced apoptosis and necrosis
Apoptotic and necrotic cell death were shown respectively by annexin-V-FITC (A-V+, green-stained) and propidium iodide (PI+, red-stained) (Fig. 3A). Under control conditions, there were a few apoptotic and necrotic cells and no sex difference was found (Fig. 3B). H2O2 increased apoptosis and necrosis (as indicated by more numerous A-V+ and PI+ cells) in both male and female ACMs; however, the increase was significantly greater in males (Fig. 3B and C).
Figure 3.
Sex difference in H2O2-induced ACMs apoptosis and necrosis. A: Representative images showing typical apoptotic (Annexin-V positive, A-V+, green stained) and necrotic cells (Propidium Iodide positive, PI+, red stained). B: Representative images showing sex effect on apoptotic and necrotic cell death either under control conditions (CTL) or in response to H2O2. C: Quantitative analysis of A-V+ and PI+ cells expressed as a percentage of the total number of cells. n = 6–7.
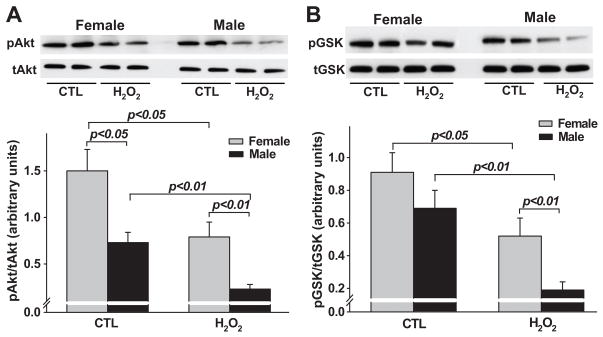
Sex difference in Akt and GSK-3β phosphorylation
Under basal conditions, activated Akt (phosphor-Akt) was significantly higher in female ACMs H2O2 reduced Akt phosphorylation in both males and females; however, the reduction was significantly greater in males (Fig. 4A). Basal phosphor-GSK-3β (inactivated form) tended to be higher in females, although the difference did not reach statistical significance. H2O2 reduced phosphorylation of GSK-3β in both male and female ACMs and this reduction was significantly greater in males (Fig. 4B).
Figure 4.
Sex difference in Akt and GSK-3β phosphorylation in response to H2O2. A: representative Western blots of phosphorylated Akt (pAkt) and total Akt (tAkt) protein (upper panel) and semi-quantitative analysis of pAkt corrected by tAkt; n = 8. B: representative Western blots of phosphorylated GSK3β) (pGSK3β) and total GSK3β (tGSK3β) protein (upper panel) and semi-quantitative analysis of pGSK3β corrected by tGSK3β); n = 6. CTL: control medium.
Sex difference in caspase-3 activity and Bcl2 expression
As shown in Fig. 5, basal caspase-3 levels were similar between male and female ACMs. H2O2 activated caspase-3 in males but not in females. Bcl2 protein expression was similar in both males and females. H2O2 reduced Bcl2 expression to a similar extent in both males and females (data not shown).
Figure 5.
Sex difference in caspase-3 activation in response to H2O2. CTL: control medium. n = 5–7.
Estrogen receptor (ER) α and β expression
We found that ERα and ERβ were expressed by both male and female ACMs. ERα was the dominant isoform in females, 4-fold higher than in males (Fig. 6A). The much higher level of ERα may be responsible for the improved survival of female ACMs challenged with H2O2 induced cell death. ERβ was low but similar in both males and females (Fig. 6B).
Figure 6.
Semi-quantitative analysis of estrogen receptor α (ERα) and β (ERβ) protein expression in male and female ACMs. ERα and ERβ proteins were corrected by actin. n = 8.
DISCUSSION
Using cultured adult mouse cardiomyocytes (ACMs), we demonstrated a significant sex difference in favor of females in H2O2-induced cell death. ACMs from female mice showed a clear survival advantage over males, as indicated by a greater rod ratio, lower LDH release, and fewer apoptotic and necrotic cells in response to H2O2. We also showed that female ACMs treated with H2O2 had less reduction of Akt and GSK-3β phosphorylation and less activation of caspase-3, whereas Bcl2 protein expression did not differ between genders. We further demonstrated that ERα was the dominant form in females, whereas ERβ was low and similar in both genders. Although our culture system is estrogen-free, the freshly isolated ACMs from mice have been exposed to the estrogen environment during their lifetime, and their estrogen receptors (ERs) may have already become activated. Our findings support the hypothesis that higher ERα and activated Akt survival signaling pathways play significant roles in protecting female ACMs against oxidative stress-induced cell death. They also support the cardioprotective advantage of females found in human and animal models.
Survival advantage and functional recovery that favor females in response to cardiac ischemic/hypoxic stimuli have been evidenced both in vivo and in vitro,1,3 however, the molecular mechanisms responsible for such protection are not entirely known. In addition, there are limitations to in vivo experiments with animal models and in vitro studies of cultured neonatal cardiomyocytes (NCM) conducted to uncover sex-dependent signaling pathways. For example, in the whole animal it is difficult to define whether cardiomyocytes are the essential cell types being protected. Whereas in NCM ERs are inactivated in situ since NCM has not been exposed to the endogenous estrogen environment, making NCM a less than ideal tool for studying sex-dependent mechanisms. The advantage of using ACMs is that they have been exposed to sexual hormones during adulthood and express mature ERs, which help overcome the above limitations. Indeed, we found that female ACMs exhibited higher levels of ERα associated with a better survival rate and activated Akt survival signaling pathways, consistent with published studies showing that female hearts are protected from trauma. 17
Three ERs, ERα, ERβ, and GPR30, have been identified.18 It is generally agreed that ERα is expressed in the heart, whereas the existence and functional role of ER-β in the heart remain controversial.19 In our study, ER-α expression was 4-fold higher in females than in males. Low levels of ER-β were detected in both female and male ACMs, but no sex difference was apparent. Thus dominant ERα expression may be responsible for the survival advantage of female ACMs in our study. Using an isolated heart preparation, Wang et al 20 found that female mouse hearts demonstrated better functional recovery from ischemic/reperfusion injury than males but this advantage was lost in ER-α knockout females. In our preliminary study using 17β-extradiol (E2), we found that E2 significantly improved myocyte survival in response to H2O2 in both sex (Data Supplementation, Figure S2. Please see http://hyper.ahajournals.org). We then used a specific ERα agonist propylpyrazole triol (PPT) and confirmed that activation of ERα increased myocyte survival and this effect was abolished by the ER antagonist fulvestrant, also known as ICI 182780, (Data Supplementation, Figure S3. Please see http://hyper.ahajournals.org). Taken together, these data suggest that ERα may be the major ER isoform that protects ACMs against cell death and is responsible for the cardioprotective effect of estrogen.
Estrogen and ER signaling pathways have not been fully elucidated. In the classical pathway, estrogen was thought to diffuse into the cell and bind to nuclear ERs, which are referred to as transcription factors, interacting with cis elements of the estrogen response gene to activate gene transcription.21 Accumulated data support a second estrogen signaling pathway in which estrogen binds to G-protein-coupled membrane-bound ERs and generates a rapid signaling response via a nongenomic mechanism;22 for example, estrogen causes isolated coronary artery relaxation within 10 minutes.23 In our study we used freshly isolated ACMs and experiments were completed within 2 hours. We observed a significant sex difference in Akt and GSK3β phosphorylation and caspase-3 activation in response to H2O2, supporting the possibility that these effects may be mediated through a rapid nongenomic mechanism.
Cell survival has been shown to be mediated in part by an Akt signaling pathway.24 Estrogen protects against oxidative stress-induced cell death via activation of Akt in cardiac H9c2 cells.25 It also reportedly reduces cardiomyocyte apoptosis, preserves cardiac function and enhances cardiac myocyte survival via activation of Akt in MI, myocardial ischemia-reperfusion injury and cultured NCM treated with daunorubicin,26,27 Akt enhanced cell survival by inhibition of apoptosis can be mediated by transcription-independent and -dependent mechanisms.24 In the transcription-independent mechanism, Akt phosphorylates Bad, which prevents Bad from interacting with Bcl2 to release cytochrome C, thereby preventing apoptosis. Akt also phosphorylates the initiator caspase-9 and in turn prevent activation of the effector caspase-3, blocking apoptosis.28 In the transcription-dependent mechanism, Akt phosphorylates Fork head box transcription factors of the class O subfamily (FOXOs), reducing apoptosis.29 Camper-Kirby reported that young women have higher levels of nuclear phosphor-Akt than age-matched men and postmenopausal women.17 Consistent with these findings, our study showed that female ACMs inherently had high levels of phosphor-Akt. When stimulated with H2O2, Akt phosphorylation was reduced much less in female ACMs than in males, associated with better survival and diminished apoptotic and necrotic cell death, supporting the role of Akt activation in the survival advantage observed in females.
GSK-3β has been reported to be the immediate downstream substrate of Akt 30 and is inactivated by phosphorylation. GSK-3β promotes apoptosis in neuroblastoma cells 31 and cardiomyocytes.10 Akt inactivates GSK-3β by phosphorylation at Ser9.9 Our data showed that phosphorylation-GSK-3β (the inactive form) tended to be higher in female ACMs under basal conditions. H2O2 reduced GSK-3β phosphorylation; however, this reduction was much less in females than in males. Thus inactivation of GSK-3β by Akt may contribute to improved survival of female ACMs.
In the present study, we showed that H2O2 increased caspase-3 activity in male ACMs but not in females. Caspases are known to play a critical role in apoptosis.32 Caspase-9, an upstream initiator of caspases, triggers a proteolytic cascade that activates other pro-apoptotic caspases including caspase-3.33 The caspase-3 then activates caspase-2 and -6, promoting apoptosis.32 Akt has been shown to inactivate caspase-9 by phosphorylation,34 which in turn inhibits downstream effector caspase-3. Our findings that Akt phosphorylation is enhanced and caspase-3 activity low in females may indicate that the Akt/caspase-3 signaling pathway plays an important role in protecting female ACMs against H2O2-induced cell death.
Bcl-2 is an anti-death gene and functions as an intracellular antioxidant.13 Akt has been shown to interact with Bcl2 and suppress apoptosis.35 In the present study, we found that basal Bcl2 protein expression did not differ between female and male ACMs. H2O2 reduced Bcl2 protein to a similar extent and no sex difference was found. Thus our data do not support a significant role for Bcl2 in this process.
Perspectives
We have demonstrate here that female cardiomyocytes are more resistant to oxidative stress-induced cell death. These data agree with clinical findings that women are protected against cardiovascular disease. Thus understanding the underlying mechanisms responsible for this gender difference in favor of females is of significant clinical relevance. Our data indicates that the ERα/Akt pro-survival signaling pathways play an important role in protecting female cardiomyocytes against cell death. Activation of Akt promotes cell survival pathways, including phosphorylation and thus inhibition of GSK-3β and decreases in caspase activity. These findings may help explain the cardioprotective advantage favoring females.
Supplementary Material
Acknowledgments
Sources of funding: 1) NIH Grant HL-078951 (XPY); 2) Henry Ford Health System institutional funds (XPY).
Footnotes
Disclosures: Fangfei Wang, NONE
Quan He, NONE
Ying Sun, NONE
Xiangguo Dai, NONE
Xiao-Ping Yang, NIH Grant HL-078951
REFRENCES
- 1.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 2.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Keimig T, He Q, Ding J, Zhang Z, Pourabdollah-Nejad S, Yang XP. Augmented healing process in female mice with acute myocardial infarction. Gend Med. 2007;4:230–247. doi: 10.1016/s1550-8579(07)80043-x. [DOI] [PubMed] [Google Scholar]
- 4.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- 5.Pelzer T, Schumann M, Neumann M, deJager T, Stimpel M, Serfling E, Neyses L. 17beta-estradiol prevents programmed cell death in cardiac myocytes. Biochem Biophys Res Commun. 2000;268:192–200. doi: 10.1006/bbrc.2000.2073. [DOI] [PubMed] [Google Scholar]
- 6.Peter I, Huggins GS, Shearman AM, Pollak A, Schmid CH, Cupples LA, Demissie S, Patten RD, Karas RH, Housman DE, Mendelsohn ME, Vasan RS, Benjamin EJ. Age-related changes in echocardiographic measurements: association with variation in the estrogen receptor-alpha gene. Hypertension. 2007;49:1000–1006. doi: 10.1161/HYPERTENSIONAHA.106.083790. [DOI] [PubMed] [Google Scholar]
- 7.Shearman AM, Cooper JA, Kotwinski PJ, Miller GJ, Humphries SE, Ardlie KG, Jordan B, Irenze K, Lunetta KL, Schuit SC, Uitterlinden AG, Pols HA, Demissie S, Cupples LA, Mendelsohn ME, Levy D, Housman DE. Estrogen receptor alpha gene variation is associated with risk of myocardial infarction in more than seven thousand men from five cohorts. Circ Res. 2006;98:590–592. doi: 10.1161/01.RES.0000210578.62102.a6. [DOI] [PubMed] [Google Scholar]
- 8.Sugden PH, Clerk A. Akt like a woman. Gender differences in susceptibility to cardiovascular disease [editorial] Circ Res. 2001;88:975–977. doi: 10.1161/hh1001.091864. [DOI] [PubMed] [Google Scholar]
- 9.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol. 2008;153 (Suppl 1):S137–S153. doi: 10.1038/sj.bjp.0707659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon B, Johnson JN, Ross RS, Singh M, Singh K. Glycogen synthase kinase-3beta plays a pro-apoptotic role in beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes: Role of beta1 integrins. J Mol Cell Cardiol. 2007;42:653–661. doi: 10.1016/j.yjmcc.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das DK, Maulik N. Conversion of death signal into survival signal by redox signaling. Biochemistry (Mosc ) 2004;69:10–17. doi: 10.1023/b:biry.0000016345.19027.54. [DOI] [PubMed] [Google Scholar]
- 14.Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 15.Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK. IL-10 attenuates TNF-alpha-induced NF kappaB pathway activation and cardiomyocyte apoptosis. Cardiovasc Res. 2009;82:59–66. doi: 10.1093/cvr/cvp040. [DOI] [PubMed] [Google Scholar]
- 16.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J Biol Chem. 2008;283:35622–35629. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 18.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol. 2006;290:H2204–H2209. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- 20.Brem AS, Cashore WJ, Pacholski M, Tetreault J, Lawler RG. Effects of bilirubin on transepithelial transport of sodium, water, and urea. Kidney Int. 1985;27:51–57. doi: 10.1038/ki.1985.9. [DOI] [PubMed] [Google Scholar]
- 21.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 22.Haynes MP, Li L, Russell KS, Bender JR. Rapid vascular cell responses to estrogen and membrane receptors. Vascul Pharmacol. 2002;38:99–108. doi: 10.1016/s0306-3623(02)00133-7. [DOI] [PubMed] [Google Scholar]
- 23.Mugge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- 24.Sugden PH. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circ Res. 2003;93:1179–1192. doi: 10.1161/01.RES.0000106132.04301.F5. [DOI] [PubMed] [Google Scholar]
- 25.Urata Y, Ihara Y, Murata H, Goto S, Koji T, Yodoi J, Inoue S, Kondo T. 17Beta-estradiol protects against oxidative stress-induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J Biol Chem. 2006;281:13092–13102. doi: 10.1074/jbc.M601984200. [DOI] [PubMed] [Google Scholar]
- 26.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 27.Matsui T, Tao J, del MF, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 28.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 29.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 30.Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 31.Bijur GN, De SP, Jope RS. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J Biol Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 32.Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. J Cell Mol Med. 2005;9:51–58. doi: 10.1111/j.1582-4934.2005.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duguay D, Deblois D. Differential regulation of Akt, caspases and MAP kinases underlies smooth muscle cell apoptosis during aortic remodelling in SHR treated with amlodipine. Br J Pharmacol. 2007;151:1315–1323. doi: 10.1038/sj.bjp.0707334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 35.Clerk A, Cole SM, Cullingford TE, Harrison JG, Jormakka M, Valks DM. Regulation of cardiac myocyte cell death. Pharmacol Ther. 2003;97:223–261. doi: 10.1016/s0163-7258(02)00339-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.