Abstract
This study examines the responses of heat shock protein transcripts, Hsp70 and Hsp90, to dehydration stress in three mosquito species, Aedes aegypti, Anopheles gambiae and Culex pipiens. We first defined the water balance attributes of adult females of each species, monitored expression of the hsp transcripts in response to dehydration, and then knocked down expression of the transcripts using RNA interference (RNAi) to evaluate potential functions of the Hsps in maintenance of water balance. Fully hydrated females of all three species contained nearly the same amount of water (66–68%), but water loss rates differed among the species, with A. aegypti having the lowest water loss rate (2.6%/h), followed by C.pipiens (3.3%/h), and A. gambiae (5.1%/h). In all three species water could be replaced only by drinking water (or blood). Both A. aegypti and C. pipiens tolerated a loss of 36% of their body water, but A. gambiae was more vulnerable to water loss, tolerating a loss of only 29% of its body water. Dehydration elicited expression of hsp70 in all three species, but only C. pipiens continued to express this transcript during rehydration. Hsp90 was constitutively expressed and expression levels remained fairly constant during dehydration and rehydration, except expression was not noted during rehydration of C. pipiens. Injection of dsRNA to knock down expression of hsp70 (83% reduction) and hsp90 (46% reduction) in A. aegypti did not alter water content or water loss rates, but the dehydration tolerance was lower. Instead of surviving a 36% water loss, females were able to survive only a 28% water loss in response to RNAi directed against hsp70 and a 26% water loss when RNAi was directed against hsp90. These results indicate a critical function for these Hsps in mosquito dehydration tolerance
Keywords: mosquito, water balance, heat shock proteins, RNAi
1. Introduction
Insect responses to dehydration are defined by two factors: maintaining the water balance necessary for activity and reducing stress from fluctuations of the internal water pool (Hadley, 1994). To maintain water balance, insects must both replenish water and prevent excessive water loss through retention. Water uptake is accomplished by ingesting water, absorbing water vapor, or the production of metabolic water (Hadley, 1994). Water loss is retarded by improving the water-proofing capacity of the cuticle, increasing water re-absorption in the alimentary canal, reducing respiratory water loss, and/or altering behavior (Gibbs, 1998; Gibbs et al., 2002; Chown and Nicolson, 2004). Stress reduction is accomplished by increasing protective osmolytes, such as trehalose, glycerol and proline, and the up regulation of stress proteins, particularly heat shock proteins (Benoit, 2009). Several studies have focused on the dehydration-resistance of adult mosquitoes (Gray and Bradley, 2005; Benoit and Denlinger, 2007), but little is known about the relationship between heat shock proteins and water stress.
Heat shock proteins (Hsps) respond to most types of environmental stress (Parsell & Lindquist, 1993; Feder & Hofmann, 1999; Rinehart et al., 2007). These proteins function as general molecular chaperones that maintain or return proteins to their functional state, minimize aggregation of proteins in their non-native conformation, and target unfolded or aggregated proteins for degradation or removal (Feder & Hofmann, 1999). Few studies have analyzed the expression of these proteins during insect dehydration (Hayward et al., 2004; Rinehart et al., 2006; Sinclair et al., 2007; Benoit et al., 2009; Lopez-Martinez et al., 2009), but based on these studies Hsp70 and Hsp90 are involved in dehydration for some, but not all, insects.
In this study, we examined the water balance characteristics of three mosquito (Culicidae) vectors of disease: Aedes aegypti L. (dengue, Chikungunya, and yellow fever), Anopheles gambiae Giles (malaria), and Culex pipiens L. (West Nile virus). How these mosquitoes tolerate dehydration is important to public health throughout the world since this physiological characteristic is linked to habitat preferences and activity (Hadley, 1994; Benoit, 2009). To examine water balance requirements we determined water content, water loss rates, dehydration, and we also monitored expression levels of transcripts encoding Heat shock protein 70 (Hsp70) and Hsp90 during dehydration. These two hsps are upregulated in response to a wide range of environmental stresses (Parsell and Lindquist, 1993; Feder and Hofmann, 1999; Rinehart et al., 2007), including dehydration (Tammariello et al., 1999; Hayward et al., 2004). Finally, we knocked down expression of hsp70 and hsp90 in A. aegypti to evaluate the functional role for these genes in the maintenance of water balance.
2. Materials and Methods
2.1. Mosquitoes
A. gambiae (Suakoko strain), A. aegypti (Rockefeller Strain), and C. pipiens (Buckeye strain) were reared according to protocol previously described (Gary and Foster, 2004; Mostowy and Foster, 2004; Robich and Denlinger, 2005). Briefly, all three species were held at 27°C under long daylength (15:9 L:D) to prevent diapause and to standardize rearing conditions (Robich and Denlinger, 2005). Larvae were fed a diet of ground fish food (Tetramin). Adults were provided access to 10% sucrose and water ad libitum. Adult females were used 8–10d post-ecdysis unless otherwise noted. Adults utilized for RNAi were injected 5–7d post-ecdysis and water balance experiments were initiated 8–10d post-ecdysis, i.e. 3 days post-injection.
2.2. Water balance characteristics
Mass changes in the mosquitoes were monitored gravimetrically using an electrobalance (CAHN 35, Ventron Co., Cerritos, CA). Each mosquito was weighed singly without enclosure after a brief CO2 knockdown according to Benoit and Denlinger (2007). Mosquitoes were transferred to weighing pans and returned to their experimental conditions within 1 min. Relative humidities were generated using saturated salt solutions (Winston and Bates, 1960), 0% RH was established with calcium sulfate, and double-distilled water was used to create 100% RH. All test relative humidities were validated with a hygrometer (Taylor Scientific, St. Louis, MO). All observations were conducted at 22–24°C in an environmental room to allow comparison to previous water balance literature (Hadley, 1994; Benoit and Denlinger, 2007).
The amount of water available for exchange (m, water mass) was determined according to standard methods (Wharton, 1985; Hadley, 1994; Benoit and Denlinger, 2007). Mosqui toes were placed at 0%, 22–24°C until a loss of 4–6% of their mass to ensure that mass changes reflected a shift in the water pool (Wharton, 1985). Subsequently, consecutive mass determinations (0% RH, 22–24°C) were made at hourly intervals for a total of six mass readings, and then individuals were transferred to 80° 0% RH to determined dry mass (d, denoted by five consecutive daily mass measurements with no changes). Water mass was determined by subtracting dry mass from the initial mass. Initial, immediate and final water mass values were analyzed according by Wharton (1985) for determining water loss rates:
where mt is the water mass at any time t, m0 is the initial water mass, and k is the rate water loss expressed as %/h. The dehydration level at which the mosquitoes can no longer right themselves and then subsequently fly when prodded was defined as the critical activity point. This denotes the dehydration tolerance limit, an irreversible lethal amount of water loss.
Drinking of liquid water was tested by allowing mosquitoes access to Evans blue (0.1%) dyed water for 24h. The mosquitoes were dissected in 10% NaCl using a light microscope and examined for the presence of blue coloration (Benoit and Denlinger, 2007). Water vapor was tested as an additional resource by measuring mass changes of mosquitoes held at multiple relative humidities (33, 75, 85, 93, 98 and 100% RH) every 12h until death. Maintenance of a stable water pool at any relative humidity, particularly below saturation, is evidence water loss is countered by water gain, and the lowest relative humidity where a stable water pool occurs is denoted as the critical equilibrium humidity (CEH). A lack of water uptake at relative humidities below saturation indicates the CEH is above the water activity within the insect (0.99aw), thus the CEH = 100% RH (Wharton, 1985).
2.3. Cloning and northern blots
hsp70 was cloned from all three mosquitoes using the TOPO TA cloning kit (Invitrogen, Carsbad, CA). Species-specific primers were designed from sequences found in online databases (GenBank, Vector Base). hsp90 was also cloned from all three species in the same manner. The control gene, 28S, was cloned using homology primers designed for dipterans (forward primer 5'-CTGTGGATGAACCAAACGTG-3'; reverse primer 5'-TGTACGCCAGCGGTAATGTA-3'). All three genes for the three species were matched (100%) to original sequences in the online databases.
Total RNA was extracted from groups of twenty adults using Trizol reagent (Invitrogen, Carlsbad, California). RNA for northern blots was extracted from all three mosquito species after a loss of 0 (control), 5, 15, and 25% of their water content and following rehydration (100% RH and access to free water for 24h). RNA (eight micrograms per treatment) samples were run on a 1.4% agarose, 0.41 M formaldehyde gel. The RNA was then transferred to a nylon membrane (Hybond-N+, Amersham Biosciences, Piscataway, NJ) with the Schleicher & Schuell’s Turboblotter transfer system. Labeled DNA clones for hybridization were generated overnight at 38°C with a Roche Diagnostic’s DIG-High Prime labeling kit. Northern blots were prepared according to the DNA Labeling and Detection Starter Kit II (Roche) and exposed to Blue Lite Film (ISC BioExpress, Kaysville, Utah) for 30 min. Membranes were stripped and re-hybridized with 28S (control); all northern blots were performed in technical triplicates.
2.4. dsRNA preparation and injection into mosquitoes
Hsp70 and Hsp90 dsRNA was prepared using a MEGAscript T7 transcription kit (Ambion, Austin, TX) according to Sim and Denlinger (2008) for A. aegypti. PCR-derived fragments were generated from primers (5’-TAATACGACTCACTATAGGGAGGCCGATATCAAACACTGG-3’ and 3’-TAATACGACTCACTATAGGGCCAGCTGTGGCTCTTACCTC-5’ for Hsp70 and 5’-TAATACGACTCACTATAGGGAAGCTCCTCGTTGAAAAGG-3’ and 3’-TAATACGACTCACTATAGGGTTCAGGTAGTCGGGGATCAG-5’ for Hsp90). β-galactosidase (β-gal) served as the control (Generated with 5’-TAATACGACTCACTATAGGGGCAATCAGCGTAGACCTGCT-3’ and 3’-TAATACGACTCACTATAGGGTAGTCAGCTGGCTGGGATCT). Sequences obtained were blasted to those from A. aegypti for validation of Hsp70, Hsp90, and β-gal. For knockdown experiments approximately 0.8–1 µl dsRNA Hsp70 (1.1µg/µl) or Hsp90 (1.2µg/µl) was injected into the thorax of cold-anesthetized female mosquitoes using a microinjector. Controls were injected with 0.9–1µl dsRNA β-gal (1.1µg/µl), and results from sham injections are also provided. Thus, for all treatments mosquitoes were injected with approximately 1µg dsRNA. Based on previous studies, our dsRNA targeted the entire expressed Hsp70 family (Gross et al., 2009; Zhou et al., 2009). The selected concentrations and injection volumes were based on previous studies using mosquitoes of similar size (Sim and Denlinger, 2008) and optimization experiments. Three days after injection, expression levels of hsp70 and hsp90 were evaluated using northern blots after exposing injected and control mosquitoes to dehydration at 0% RH for 5h. Females that lost 25% of their water content were tested for expression, and this was replicated three times. ImageJ (http://rsb.info.nih.gov/ij) was used for determining pixel intensity of the northern blots to assess percent reduction of expression. After verification of hsp70 and hsp90 knockdown, water content, survival during exposure to 0% RH, water loss rates, and dehydration tolerance were measured for RNAi-injected females.
2.5. Statistics
Each water balance experiment was replicated three times with 20 mosquitoes per replicate. Calculated means ± S. E. were compared using one- and two-away analysis of variance (ANOVA) followed by the Bonferroni-Dunn test. Percentages were arcsin transformed before analysis. Data from regression lines were assessed by testing for equality of slopes (Sokal and Rohlf, 1995).
3. Results
3.1. Water content
Water balance characteristics for adult females of A. aegypti, A. gambiae, and C. pipiens are presented in Table 1. Water mass correlated with dry mass in all cases ( r > 0.91; P < 0.05), indicating water flux is standardized according to size (Wharton, 1985). In relation to initial mass, C. pipiens was the largest of the three mosquitoes, followed by A. aegypti, with A. gambiae being the smallest (Table 1; ANOVA, P < 0.05). As with size, C. pipiens contained more water than A. aegypti or A. gambiae. Percent water content for the three mosquitoes was not significantly different; water content was 66–68% for each species (ANOVA; P > 0.05). Though the mosquitoes are of different sizes, all three species have a similar proportion of body water.
Table 1.
Water balance characteristics of thr ee female mosquitoes (Aedes aegypti,Anopheles gambiae, and Culex pipiens).
| A. aegypti | A. gambiae1 | C. pipiens2 | |
|---|---|---|---|
| Water content | |||
| Initial mass (mg) | 2.48 ± 0.11 | 1.82 ± 0.14 | 3.61 ± 0.19 |
| Dry mass (mg) | 0.83 ± 0.09 | 0.60 ± 0.12 | 1.21 ± 0.13 |
| Water mass (mg) | 1.65 ± 0.08 | 1.22 ± 0.11 | 2.40 ± 0.16 |
| Percent water (%) | 66.5 ± 3.2 | 67.0 ± 2.5 | 66.5 ± 2.6 |
| Water loss | |||
| Rate (%/h) | 2.56 ± 0.19 | 5.12 ± 0.34 | 3.26 ± 0.25 |
| Loss tolerance (%) | 36.4 ± 2.2 | 29.0 ± 2.1 | 34.4 ± 1.8 |
| Survival at 0% RH (h)* | 15.3 ± 0.8 | 5.4 ± 1.1 | 10.2 ± 0.8 |
| Water gain | |||
| Free water uptake | + | + | + |
| CEA (av) | 1.00 | 1.00 | 1.00 |
CEA, critical equilibrium activity; +, positive occurrence.
Superscripts indicate results are similar to those reported by (1) Gray and Bradley (2005) and (2) Benoit and Denlinger (2007). All values are expressed as the mean ± SE of 45 individuals.
3.2. Water loss
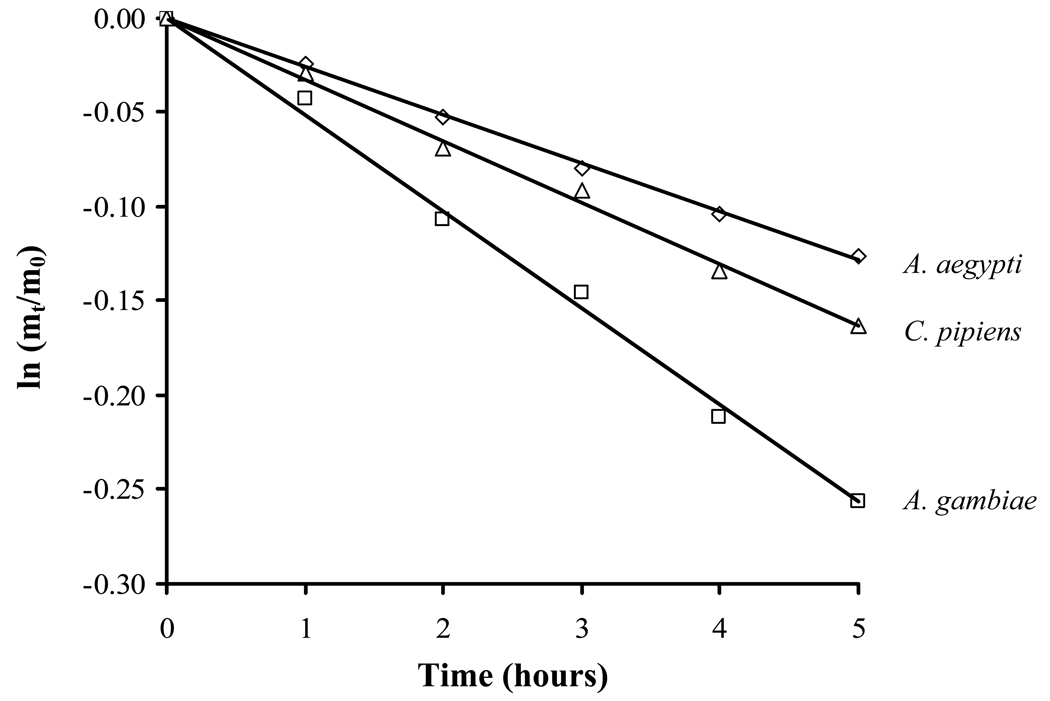
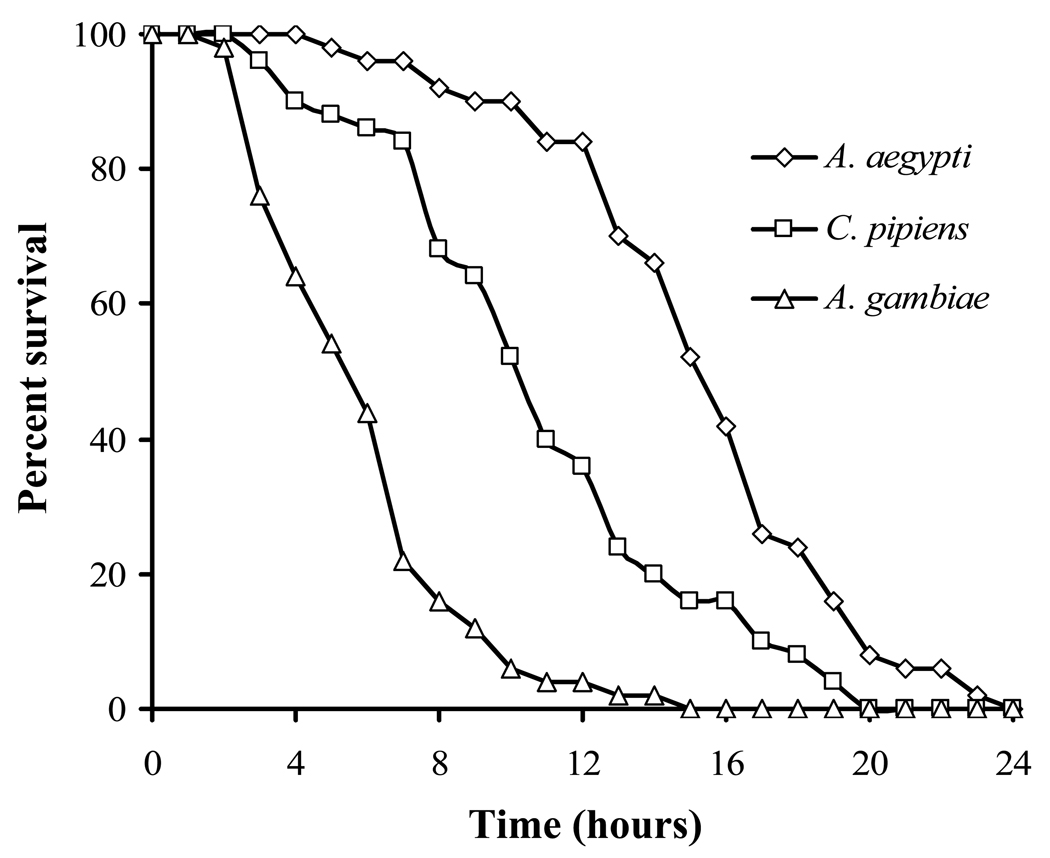
Significant differences in water loss rates were noted in the three species (Fig. 1; Table 1). Anopheles gambiae was the most porous, with a water loss rate of 5.1% loss of initial body water)/h, followed by C. pipiens (3.3%/h) and A. aegypti (2.6%/h). Rates were significantly different for each species (ANOVA; P < 0.05). A. aegypti and C. pipiens could tolerate a 34–36% loss of body water (ANOVA, P > 0.05, Table 1), but tolerance of A. gambiae was significantly lower, 29% (ANOVA; P < 0.05). Based on water loss rates and dehydration tolerances, A. aegypti is the most resistance to water loss, and this is evident by its higher survival at 0% RH (Fig. 2).
Fig. 1.
Net water loss rates for females of Aedes aegypti, Anopheles gambiae, and Culex pipiens. The slope represents the water loss rate in % h−1 . mt represents the water mass at anytime t and m0 is the initial water mass. Values represent the mean ± SE of 45 mosquitoes.
Fig. 2.
Percent survival of adult females of Aedes aegypti, Anopheles gambiae, and Culex pipiens when held at 0% RH. Each point represents the mean ± SE of 6 groups of 10 mosquitoes.
3.3. Water gain
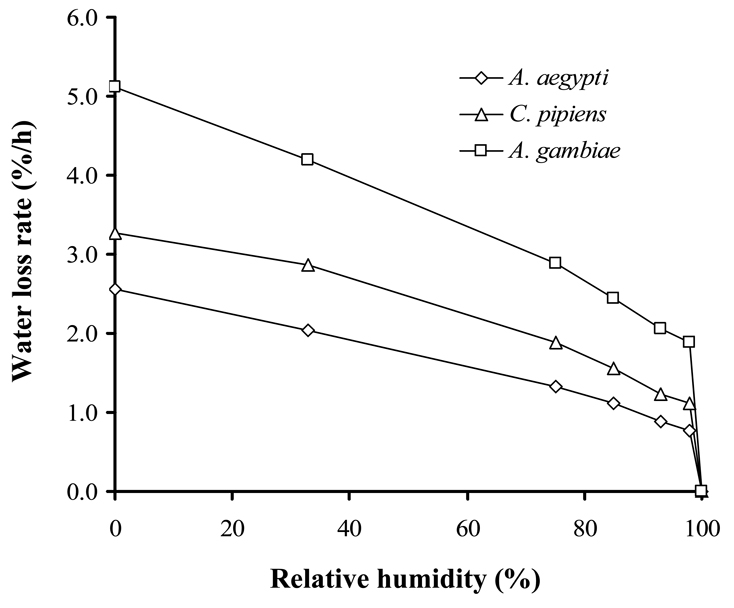
Water content declined at all relative humidities below saturation for all three species (Fig. 3), indicating that the CEH is at water vapor saturation (100% RH) for these mosquitoes. Water loss declined with an increase in RH, indicating an increase in passive gains, but these gains could not counter water loss at any RH but saturation. As seen previously (Benoit and Denlinger, 2007), water loss increased linearly below 98% RH, but a rapid increase was noted between 100% and 98% RH (Fig. 3). To determine if dehydration could prompt water vapor uptake in A. aegypti and A. gambiae (failure to do so was noted in C. pipiens previously; Benoit and Denlinger, 2007), mosquitoes were held at 75% RH until a loss of 10–15% of their water mass was noted; mosquitoes were then transferred to 98% RH. Water masses continued to decline for both mosquito species under these conditions, indicating that dehydration does not trigger an uptake mechanism. Thus, water vapor is not the primary source for replenishing moisture levels. When offered water dyed with Evans blue, dehydrated mosquitoes (10–15% water loss) moved to the periphery of water droplets and inserted their proboscis. After a 24h exposure to water droplets, mosquitoes were removed, killed by exposure to −20°C, and examined for the presence of blue coloration in their crop and midgut (N=30). Blue coloration was noted in all cases, indicating that liquid ingestion serves as the primary source used by these mosquitoes to increase their water pool.
Fig. 3.
Water loss rates at relative humidities ranging from complete dryness (0% RH) to water saturation (100% RH) for adult females of Aedes aegypti, Anopheles gambiae, and Culex pipiens. Rates were determined in the same manner as Fig. 1.
3.4. Heat shock proteins
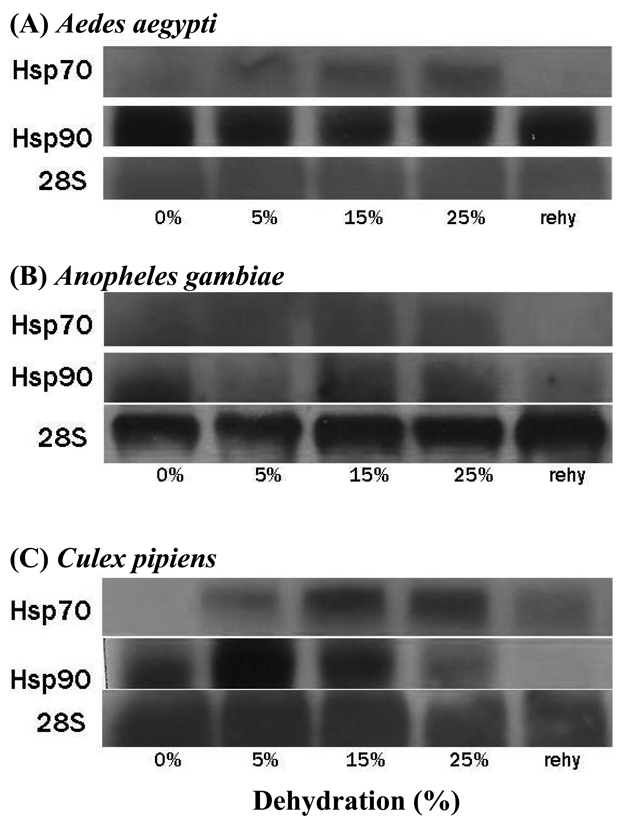
Expression patterns for hsp70 and hsp90 are presented in Fig 4. Hsp 70 was expressed during dehydration in A. aegypti but not during rehydration. Hsp90 expression in A. aegypti was high in the control, indicating constitutive expression, and remained high in dehydrated and rehydrated mosquitoes (Fig. 4a). Expression of hsp70 in A. gambiae was similar to that of A. aegypti (Fig. 4b). The pattern was a bit different for C. pipiens: both hsp70 and hsp90 were expressed during dehydration, but only hsp70 was expressed during rehydration (Fig. 4c).
Fig. 4.
Northern blot hybridizations displaying mRNA expression of hsp70 and hsp90 during dehydration in three mosquitoes (Aedes aegypti, Anopheles gambiae, and Culex pipiens). 28s was used as a control gene. Control, fully-hydrated mosquitoes; 5, 15, and 25% represent percent reduction in water content; rehy, mosquitoes that lost 15% of their water content and were then rehydration at 100% RH in the presence of liquid water. Each blot was replicated three times.
3.5. Hsp70 and Hsp 90 RNAi in A. aegypti
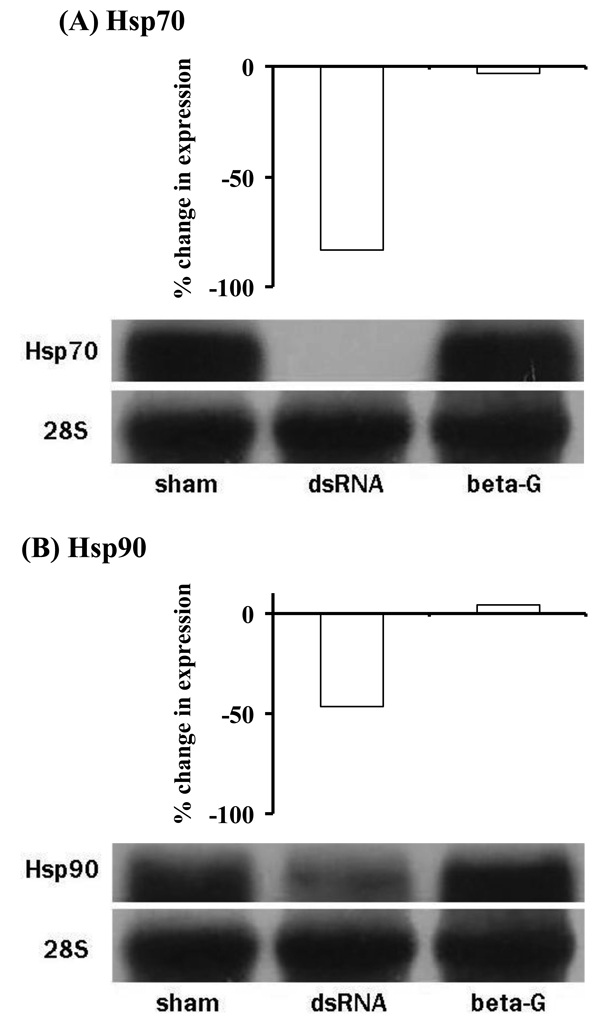
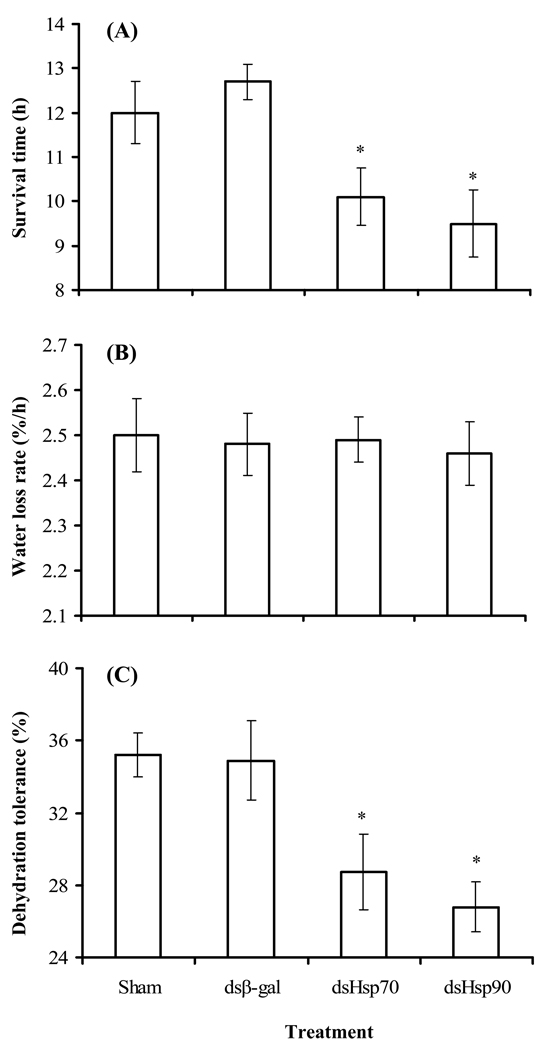
Injection of dsHsp70 and dsHsp90 into A. aegypti reduced the levels of hsp70 and hsp90 expression during dehydration by 85 and 46%, respectively (Fig. 5). Sham and dsβ-gal injections served as controls and did not alter the dehydration-induced expression of hsp70 or hsp90. Thus, expression of hsp70 and hsp90 was substantially reduced in A. aegypti by RNAi. Survival following dehydration at 0% RH was reduced by 2–3 days after injection of dsHsp70 and dsHsp90 compared to those injected with dsβ-gal (Fig. 6a, ANOVA, P < 0.05). dsHsp70, dsHsp90, dsβ-gal and sham injection had no effect on water loss rates of adult females (Fig 6b, ANOVA, P > 0.05). But, dehydration tolerance following injection of dshsp70 and dshsp90 was reduced by 6–9% (ANOVA; P < 0.05; Fig. 6c). Thus, hsp70 and hsp90 both appear to be essential for A. aegypti to maintain its maximum dehydration tolerance.
Fig. 5.
Northern blot hybridizations displaying knockdown of hsp70 and hsp90 transcripts after injection of dsHsp70, dsHsp90, and dsβ-galactosidase. A sham injection was conducted as an additional control. Percent expression changes are in comparison to the sham control. Each knockdown experiment was conducted three times.
Fig. 6.
Physiological changes in water balance features in adult females of Aedes aegypti following injection of dsHsp70, dsHsp90, and dsβ-galactosidase. A sham injection was also performed to control for possible cuticle damage that may have altered water vapor exchange. (A) Time to 50% mortality for mosquitoes held at 0% RH (B) water loss rates (% h−1) determined according to Fig. 1, and (C) dehydration tolerance displayed as percentage of water loss tolerated (mean of three groups of 20 mosquitoes each ). *, denotes significance difference from sham control.
4. Discussion
Basic water balance characteristics of these three mosquito species are similar to those found in most insect species (Hadley, 1994). The percent water content of the mosquitoes examined in our experiment was 66–68%, a value similar to that of most insects (Hadley, 1994) and similar to values obtained previously in A. gambiae (66%; Gray and Bradley, 2005) and C. pipiens (66.7%; Benoit and Denlinger, 2007). Dehydration tolerance of these three mosquito species also fell within the range typical of most insects, 25–40% (Hadley, 1994). However, the significantly smaller size of A. gambiae, compared to A. aegypti and C. pipiens, likely makes this mosquito more prone to dehydration as a result of its higher surface area to volume ratio.
Water loss rates of the mosquitoes were relatively high, indicating that these species are preferentially adapted for survival in moist environments (Hadley, 1994; Benoit and Denlinger, 2007). This study, along with the previous study by Gray and Bradley (2005) on A. gambiae and A. arabiensis and our study on C. pipiens (Benoit and Denlinger, 2007), suggests that most mosquitoes will likely be classified as hydrophilic or water sorption dependent in relation to their water loss rates (Hadley, 1994), but more species will need to be examined to establish a solid relationship between mosquito habitat preferences and water loss rates. Survival times of mosquitoes held at 0% RH correlated with water loss rates, and these were similar to those reported by Gray and Bradley (2005) for A. gambiae and Benoit and Denlinger (2007) for C. pipiens. Thus, based on water loss rates, A. aegypti is the most resistant to dehydration, followed by C. pipiens, then A. gambiae. To counter their high moisture requirements, these mosquito species likely prefer moist, protective microhabitats and host search only when atmospheric water levels are high or at night when temperatures are lower, features that reduce water loss, a prediction that is consistent with the recorded activity patterns for these species (Kessler and Guerin, 2008). Similar behavior has been demonstrated in other hematophagus arthropods including ticks (Crooks and Randolph, 2006) and Rhodnius prolixus (Heger et al., 2006), both of which vigorously search for hosts when their saturation deficits are low.
None of the mosquitoes examined were able to counter water loss at relative humidities below saturation, indicating that they are unable to uptake water vapor from the air below saturation (this study, Benoit and Denlinger, 2007). Additionally, it is important to note that mosquitoes dehydrated at lower relative humidities (i.e. 75% RH) and subsequently transferred to higher relative humidities (98% RH) below saturation failed to take up water vapor, thus we have no evidence to suggest that a water vapor uptake mechanism operates in these mosquitoes. As reported by Benoit and Denlinger (2007), water loss only occurred linearly from 0–100% RH, suggesting that mosquito activity may be higher at relative humidities approaching saturation, resulting in increased respiratory water loss at high RHs. To replenish their water stores, all three mosquito species rely on ingested fluids (free water or blood), indicating that most mosquitoes will need to replenish water orally as adults. Thus, adult mosquito survival is dependent on access to liquid water, either in a bloodmeal or as free water.
Regulation of Hsps during dehydration was reported in one previous mosquito study that investigated expression of hsp70 in C. pipiens (Rinehart et al., 2006). In our present study, we show that the responses of hsp70 and hsp90 can differ, a difference also noted in the Antarctic midge, Belgica antarctica (Lopez-Martinez et al., 2009). Increased expression of hsp70 in response to dehydration was reported in the flesh fly, S. crassipalpis (Tammariello et al., 1999; Hayward et al., 2004), and more recently in the bed bug, Cimex lectularius (Benoit et al., 2009), but expression was not influenced by dehydration in D. melanogaster (Sinclair et al., 2007). hsp90 is constitutively expressed at low levels throughout development in most insects and is elevated in some species by dehydration (Benoit et al., 2009; Lopez-Martinez et al., 2009), but not in others (Hayward et al., 2004). Few studies have been conducted on rehydration in insects, but from the expression patterns that have been observed, species variation is evident. In some cases the same expression pattern observed during dehydration persists during rehydration (Lopez-Martinez et al., 2008; Benoit et al., 2009), but in others different hsps respond to rehydration (Hayward et al., 2004). In this study, we show that hsp70 and hsp90 are both responsive to the hydration state, albeit with slight differences to dehydration and rehydration in the three mosquitoes we examined.
When RNAi was used to knockdown hsp70 and hsp90 expression in A. aegypti, no differences were noted in the basic water balance characteristics of dry mass, water mass, percent water content and water loss rate. Reduced survival time was noted after injection of dshsp70 and dshsp90, and this was most likely a direct result of a reduction in dehydration tolerance. While controls could tolerate a loss of 36% of their body water, the dsHs70 and dsHsp90 mosquitoes were less tolerant and survived a loss of only 26–28% of their body water. The cellular stress of dehydration intensifies as the amount of water lost approaches the dehydration tolerance limit of 36%. While in this low hydration state, increased protein aggregation and denaturation are likely to occur (Pestrelaski et al., 1993; Goyal et al., 2005), reactive oxygen species may be generated (França et al., 2007; Lopez-Martinez et al., 2009), and phase transitions in membranes may occur, influencing cellular ion homeostasis and altering transmembrane proteins (Crowe et al., 1992; Hayward et al., 2004). We hypothesize that Hsp70 and Hsp90 help ameliorate the stress incurred by cellular desiccation that occurs near the limit of dehydration tolerance, thus resulting in the decreased dehydration tolerance we noted in A. aegypti when the Hsps were knocked down. RNAi directed against Hsps in S. crassipalpis (Rinehart et al., 2007) and the linden bug, Pyrrhocoris apterus (Koštál and Tollarová-Borovanská, 2009) resulted in reduced heat and cold tolerance, but this is the first report demonstrating a functional role for Hsps during dehydration
Acknowledgements
Research was funded by a grant from the National Institutes of Health (R01 AI058279), and support for JBB was provided by a Mary S. Muelhaput Endowed Presidential Fellowship from the Ohio State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benoit JB. Water management by dormant insects: comparisons between dehydration resistance during estivation and winter diapause. In: Navas C, Eduardo J, editors. Progress in Molecular and Subcellular Biology. Vol. Estivation. Berlin: Springer; 2009. In press. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Denlinger DL. Suppression of water loss during adult diapause in the northern house mosquito, Culex pipiens. Journal of Experimental Biology. 2007;210:217–226. doi: 10.1242/jeb.02630. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Lopez-Martinez G, Teets NM, Phillips SA, Denlinger DL. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening, and expression of transcript encoding heat shock proteins. Medical and Veterinary Entomology. 2009 doi: 10.1111/j.1365-2915.2009.00832.x. (submitted) [DOI] [PubMed] [Google Scholar]
- Chown SL, Nicolson SW. Insect Physiological Ecology. Oxford: Oxford University Press; 2004. pp. 87–114. [Google Scholar]
- Crooks E, Randolph SE. Walking by Ixodes ricinus ticks: intrinsic and extrinsic factors determine attraction of moisture and host odour. Journal of Experimental Biology. 2006;209:2138–2142. doi: 10.1242/jeb.02238. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- França MB, Panek AD, Eleutherio ECA. Oxidative stress and its effect during dehydration. Comparative Biochemistry and Physiology A. 2007;146:621–631. doi: 10.1016/j.cbpa.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Gary RE, Jr, Foster WA. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Medical and Veterinary Entomology. 2004;18:102–107. doi: 10.1111/j.0269-283X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- Gibbs AG. Water-proofing properties of cuticular lipids. American Zoologist. 1998;38:471–482. [Google Scholar]
- Gibbs AG. Water balance in desert Drosophila: lessons from non-charismatic microfauna. Comparative Biochemistry and Physiology Part A. 2002;133:781–787. doi: 10.1016/s1095-6433(02)00208-8. [DOI] [PubMed] [Google Scholar]
- Goyal K, Walton LJ, Browne JA, Burnell AM, Tunnacliffe A. Molecular anhydrobiology: identifying molecules implicated in invertebrates anhydrobiosis. Integrative and Comparative Biology. 2005;45:702–709. doi: 10.1093/icb/45.5.702. [DOI] [PubMed] [Google Scholar]
- Gray EM, Bradley TJ. Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. American Journal of Tropical Medicine and Hygiene. 2005;73:553–559. [PubMed] [Google Scholar]
- Gross TL, Myles KM, Adelman ZN. Identification and characterization of heat shock 70 genes in Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2009;46:496–504. doi: 10.1603/033.046.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley NF. Water relations of terrestrial arthropods. New York: Academic Press; 1994. [Google Scholar]
- Hayward SAL, Rinehart JP, Denlinger DL. Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. Journal of Experimental Biology. 2004;207:963–971. doi: 10.1242/jeb.00842. [DOI] [PubMed] [Google Scholar]
- Heger T, Guerin PM, Eugster W. Microclimatic factors influencing refugium suitability for Rhodnius prolixus. Physiological Entomology. 2006;31:1–9. [Google Scholar]
- Kessler S, Guerin PM. Reponses of Anopheles gambiae, Anopheles stephani, Aedes aegypti, and Culex pipiens mosquitoes (Diptera: Culicidae) to cool and humid refugium conditions. Journal of Vector Ecology. 2008;33:145–149. doi: 10.3376/1081-1710(2008)33[145:roagas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Koštá V, Tollarová-Borovanská M. The 70kDa Heat shock protein assists during repair of chilling injury in the insect, Pyrrhocoris apterus. PLOS One. 2009:1–8. doi: 10.1371/journal.pone.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martinez G, Benoit JB, Rinehart JP, Elnitsky MA, Lee RE, Jr, Denlinger DL. Dehydration, rehydration and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. Journal of Comparative Physiology B. 2009;179:481–491. doi: 10.1007/s00360-008-0334-0. [DOI] [PubMed] [Google Scholar]
- Mostowy WM, Foster WA. Antagonistic effects of energy status on meal size and egg-batch size of Aedes aegypti. Journal of Vector Ecology. 2004;29:84–93. [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annual Review of Genetics. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pestrelski SJ, Tedeshi N, Arakawa T, Carpenter JF. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophysics Journal. 1993;65:661–671. doi: 10.1016/S0006-3495(93)81120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart JP, Robich RM, Denlinger DL. Enhanced cold and desiccation tolerance in diapausing adults of Culex pipiens, and a role for Hsp70 in response to cold shock but not as a component of the diapause program. Journal of Medical Entomology. 2006;43:713–722. doi: 10.1603/0022-2585(2006)43[713:ECADTI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SAL, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proceeding of the National Academy of Sciences USA. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proceeding of the National Academy of Sciences USA. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proceedings of the National Academy of Sciences USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Molecular Biology. 2007;16:435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: W. H. Freeman; 1995. [Google Scholar]
- Tammariello SP, Rinehart JP, Denlinger DL. Desiccation elicits heat shock protein transcription in the flesh fly, Sarcophaga crassipalpis, but does not enhance tolerance to high or low temperature. Journal of Insect Physiology. 1999;45:933–938. doi: 10.1016/s0022-1910(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Wharton GW. Water balance of insect. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 4. Oxford: Pergamon Press; 1985. pp. 565–603. [Google Scholar]
- Winston PW, Bates DS. Saturated solutions for the control of humidity in biological research. Ecology. 1960;41:232–237. [Google Scholar]
- Zhao L, Pridgeon JW, Becnel JJ, Clark GG, Linthicum KJ. Identification of genes differentially expressed during heat shock treatment in Aedes aegypti. Journal of Medical Entomology. 2009;46:490–495. doi: 10.1603/033.046.0312. [DOI] [PubMed] [Google Scholar]