Abstract
Sjogren syndrome (SS) is one of the most common autoimmune diseases. Early clinical manifestations of SS are primarily decreased tear and saliva secretion, leading to dry eye and dry mouth syndromes, but in its later stages, it can become systemic, even resulting in B cell lymphomas. The use of new animal models, coupled with new technologies, is providing exciting insights into the pathogenesis, genetic predisposition, and, possibly, early diagnosis of SS. This article reviews newly described features of SS identified in experimental animal models and their relationship to human disease. New technologies, such as genomics and proteomics, may permit identification of potential candidate genes and biomarkers for disease diagnosis. Current studies using appropriate animal models in parallel with studies of human subjects is rapidly establishing a foundation for new intervention strategies that go beyond merely treating symptoms.
Keywords: animal models, autoimmune disease, genomics, keratoconjunctivitis sicca, proteomics, Sjogren syndrome, stomatitis sicca
I. INTRODUCTION
Sjogren syndrome (SS) is a systemic autoimmune disease that initially targets primarily the lacrimal and salivary glands, resulting in keratoconjunctivitis sicca (KCS [dry eye disease]) and/or stomatitis sicca (dry mouth disease).1 Despite expanding efforts to define the genetic, environmental, and immunological bases of SS, the underlying etiology of this disease remains poorly understood. SS is only one of the many dry eye/dry mouth diseases, but it is considered to be among the most severe. Although no prevalence studies have been conducted in the USA, the National Arthritis Data Workgroup, using the Olmsted County, Minnesota, and 2005 USA population prevalence estimates from the Census Bureau, has estimated that the prevalence of primary SS (pSS) in the USA approaches 1.3 million, with a range of 0.4–3.1 million; this potentially makes SS the most common rheumatic disease.2
Primary SS is characterized by a chronic autoimmune attack involving both lacrimal and salivary glands. Secondary SS is marked by an autoimmune attack against the lacrimal and/or salivary glands concomitantly with another autoimmune disease, most often a connective tissue disease like rheumatoid arthritis (RA), systemic lupus erythmatosus (SLE) or scleroderma.3,4 In addition to the apparent primary sites, other organs are also affected, including the entire gastrointestinal tract, skin, lungs, vasculature, kidneys, bladder, and vagina. Involvement of the musculature often leads to fibromyalgia-like symptoms and chronic fatigue. Between 4% and 10% of patients with SS will develop non-Hodgkin’s B cell lymphomas, some of which become high-grade malignancies.5–8 As with many autoimmune connective tissue diseases, a sexual dimorphism exists in SS, with women affected 10–20 times more frequently than men, indicating hormonal involvement.9–13
In this review, we compare SS disease with experimental SS-like disease present in murine models and explore possible etiologies of disease development. We discuss how new technologies are beginning to provide better insights into the SS pathogenic molecular events and uncover possible diagnostic biomarkers.
II. DEFINITION OF SJOGREN SYNDROME AS A DRY EYE/DRY MOUTH AUTOIMMUNE DISEASE
A. Classification Criteria for Sjogren Syndrome
The revised European-American Consensus Group criteria for diagnosis of SS is based on several assessments, including the patient’s subjective description of ocular and oral symptoms, objective signs of ocular and oral dryness, detection of infiltrating lymphocytes within minor salivary glands (quantified by histopathological evaluation), and the presence in serum of autoantibodies, specifically anti-Ro/SSA and anti-La/SSB.14 Recently, considerable attention has been focused on serological evaluations that show the presence of rheumatoid factor, elevated immunoglobulin levels (hypergammaglobulinemia) and the presence of antibodies to the muscarinic acetylcholine receptors, especially the type 3 receptor (M3R).15–23 In addition, new technologies, such as genomics and proteomics, are beginning to define markers that may prove useful for identifying not only patients with SS, but also individuals who are either genetically susceptible or in the early stages of disease.
B. Genetic Predisposition
1. Human Disease
It is widely accepted that two elements are necessary for development and onset of an autoimmune disease: a host genetic susceptibility and an environmental trigger for activating the immune system. Although environmental triggers responsible for initiating SS remain elusive, it is intuitively obvious that genes of the major histocompatibility complex (MHC) are involved, as MHC genes regulate development of the immune system and immune responses.
SS appears to be a multifactorial autoimmune disease encompassing many critical causal elements, but whether linked to MHC or non-MHC genes, these elements remain ill-defined.24 This is, in part, due to the relatively weak tendency toward familial aggregation resulting from the lack of large twin and/or large cohort studies. Nevertheless, early observations reported by Harley et al suggested that SS susceptibility was linked to HLA-DQ genes, specifically DQ1 and DQ2, when associated with the presence of anti-SSA/Ro and anti-SS-B/La autoantibodies.25
Similarly, Gottenberg et al have reported that the frequency of the HLA-DRβ1*15 allele in Caucasians with SS was 13% higher than in healthy, non-diseased individuals or patients with anti-SSA/Ro antibody positive sera.26 In addition, patients with both anti-SSA/Ro and anti-SSB/La serum antibodies displayed a 44% frequency of HLA-DRβ1*3, compared to 12% in patients negative for anti-La and anti-Ro, 19% in anti-Ro-positive patients, and 10% in healthy controls. Furthermore, a direct correlation between anti-La and HLA-A1, B8, DR3 has been reported in SS patients.27
As anticipated, different MHC genetic linkages have been identified in different ethnic groups. Northern and Western European Caucasians and North Americans showed higher frequency of B8, DRw52 and DR3 genes, while Scandinavians and Greeks manifested predominantly DR2 and DR5 genes, respectively.24
During the past few years, an increasing number of reports have identified non-MHC genes that appear to be linked to SS susceptibility and/or pathogenesis. These include the genes encoding carbonic anhydrases (Car1, Car2 & Car6), Mbl, IL-10 promoter, Fas antigen, tumor necrosis factor (TNF)-α, ApoE and GSTM1, the latter of which has been identified to contribute to production of anti-SSA.28 One interesting linkage involves the Tap1 and Tap2 genes critical for antigen processing and presentation. A large cohort study of 108 Japanese SS patients identified polymorphisms in both Tap1 and Tap2, but a unique base-pair substitution at codon 577 of Tap2 correlated with a higher prevalence in SS patients manifesting elevated anti-Ro autoantibody.29
2. Murine Disease
In recent years, increasing numbers of genetically diverse mouse strains have been advanced as models of SS. These models have provided surprisingly strong support for observations already reported for SS patients. Over the past 15 years, we have utilized the Non-Obese Diabetic (NOD) mouse as a model to investigate both the genetic and immunopathophysiological basis of SS. One advantage of this mouse model is that a large collection of congenic and gene knock-out (KO) mice are available from studies defining the insulin-dependent diabetes susceptibility (Idds) loci that genetically predispose these mice to type 1 diabetes (T1D).
Unlike the genetic predisposition for T1D in NOD mice, which is dependent on specific genes mapping to the MHC, the genetic predisposition for SS-like disease in these mice appears to be independent of—or, at best, only weakly dependent on—MHC-associated genes. This was first observed in studies of the congenic strain, NOD. B10-H2b, in which the MHC of NOD was replaced with the MHC of C57BL/10 mice.30 NOD.B10-H2b mice, while failing to exhibit insulitis and development of diabetes, continued to show a full SS-like syndrome, including lacrimal and salivary gland dysfunction subsequent to leukocytic infiltration of the glands.
Similarly, replacing a number of other diabetes susceptibility loci in the NOD mouse (eg, Idd10, Idd9, Idd13, etc.) proved to have little effect on development and onset of SS-like disease. However, when both Idd3 and Idd5 were replaced with the corresponding genetic intervals derived from C57BL/6 mice, the severity of the biological markers of epithelial cell pathology was reduced and the loss of secretory function reversed.31
In a reciprocal approach, the introduction of both the Idd3 and Idd5 genetic regions derived from NOD mice into SS nonsusceptible C57BL/6 mice resulted in the appearance of SS-like disease, confirming the contributions of these two genetic loci to development and onset of disease.32,33 This newly generated mouse strain is referred to as C57BL/6. NOD-Aec1Aec2, where Aec1 corresponds approximately to Idd3 on chromosome 3 and Aec2 to Idd5 on chromosome 1 of NOD mice.
Importantly, it should be pointed out that C57BL/6. NOD-Aec1Aec2 mice are very different from NOD mice and, thus, portray a very different overall mouse strain phenotype than NOD. C57BL/6.NOD-Aec1Aec2 mice are of a C57BL/6J genetic background carrying only two small genetic segments derived from NOD mice. The C57BL/6J genetic background alleviates virtually all the irrelevant problems noted for NOD mice (eg, diabetes, abnormal tissue development, lack of complement, problems in myeloid cell development, etc.). In addition, these mice do not exhibit abnormal development of lacrimal glands, and histological examinations indicate normal architecture prior to onset of SjS-like disease.
While the Aec1 and Aec2 genetic regions are relatively large in C57BL/6.NOD-Aec1Aec2 mice, we have now narrowed the genetic region of Aec1 from a 48.5 cM segment to a centromeric piece spanning 19.2 cM (Aec1.1) plus a second piece located between 33.0 and 45.8 cM (Aec1.2). Similarly, we have narrowed genetic region Aec2 from a 73.3 cM segment to a small telomeric piece spanning about 10 cM lying around 79.0 cM. Interestingly, the Aec1.1 region contains the IL2 and IL7 genes, as well as the carbonic anhydrase genes, Car1, Car2 and Car3, whereas the Aec1.2 region contains cathepsin-S, four genes associated with systemic lupus erythmatosus (Sles3 and Sle11), experimental allergic encephalomyelitis (Eae3), collagen-induced arthritis (Cia5) and T1D (Idd17), and two genes associated with cytokine activity (Il12a and Il6ra).
Car2 is of particular interest, as its encoded protein plays a role in fluid secretions of the salivary glands via conversion of CO2 to HCO3− and bicarbonate ions and has been shown to be associated with SS-susceptibility. This genetic region also encodes genes associated with regulation of lipids/fatty acids (Npy2r, Chldq2, and MgII-rs1) and neural functions (Glrb, Npy2r, Cnpl, Sfrp2, Iapls1-7, S100a10, Chrnb2, Nes, Hapln2 and Mtx1). Disease profiling now suggests that genes lying in Aec1.2 not only act as QTL genes involved in the disease severity, but also contribute to gender differences in autoimmune diseases (eg, RA and T1D),34,35 and SS in the C57BL/6.NOD-Aec1Aec2 mice.
In contrast to the Aec1 region, Aec2 primarily regulates the pathophysiological abnormalities that activate the autoimmune attack against lacrimal and salivary glands. Genes located within the shortened Aec2 region of chromosome 1 include a large number of lipid, lipoprotein, cholesterol, and fatty acid regulatory and processing elements, an area that has recently received attention in SS, because lipid depositions36 and changes in lipid rafts37 appear to influence the pathology in both lacrimal and salivary glands.
Genes of interest include Hdlq14, Hdlq5 or Apoa2, Gpa33, Cq1, Soat1, prdx6, and Tnfsf4. These genes control free fatty acid, lipid, and lipoprotein homeostasis, and dysfunction of this homeostasis can result in considerable pathology, including inflammation and apoptotic cell death. This region also contains genes specific to the ocular/lacrimal gland etiology, eg, Pdc (phosducin), which is a protein of the retinal photoreceptor cells,38 and Myoc (myocilin), whose product interacts with olfactemedin involved in glaucoma.39 Two genetic elements in this region that are hypothesized to be directly involved in the immunopathology of human SS are FasL40 and Cypr2.41 Thus, like SS in humans, SS-like disease in NOD, NOD-derived congenic, and C57BL/6. NOD-Aec1Aec2 mice shows a weak linkage to MHC genes, but a strong linkage to multiple non-MHC genes.
C. Pathological and Clinical Consequences
KCS and stomatitis sicca result, respectively, from basic changes in the flow rates of tear and saliva, their composition, and/or combinations thereof. Underlying causes of KCS include the natural aging process, physical injury, surgical procedures, meibomian gland dysfunction, use of medications, and/or autoimmune attack against one or more of the multiple secretory tissues/glands of the eye. Manifestations of KCS brought on by decreased tear fluid secretion in conjunction with an increase in tear fluid evaporation are progressively debilitating for the patient.
Complaints from patients with dry eyes include burning sensations, grittiness, itching, fatigue, blurred vision, and, surprisingly, watery eyes, which result from increased reflex tear secretion. Over time, there is eye surface deterioration and ulceration, leading to small, red-appearing eyes with crusts in the ciliae, debris in the tear film, meibomitis, mucus strands adhering to the corneal surfaces, reduced light reflectivity, and irregular blinking.1
Similarly, underlying causes of stomatitis sicca include the natural aging process, use of medications, asthma and mouth breathing, chemotherapy, radiation therapy, autoimmune attack against secretory tissues/glands of the mouth, thyroid dysfunction, kidney dialysis, and/or stroke. Considerable emphasis has been placed on manifestations of xerophthalmia, but manifestations of xerostomia can be just as debilitating. Saliva is a critical factor in oral health; thus, patients with dry mouth can have increased caries and tooth decay, increased oral microbial infections, halitosis, cracked lips and bleeding gums, taste disturbances, difficulty in eating and swallowing, and even difficulty in talking. In addition, these patients can suffer from esophageal dysphagia, epigastric pain, and dyspepsia, possibly due, in part, to decreased levels of digestive enzymes, anti-microbial substances, and factors such as epidermal growth factor (EGF) in saliva, as well as poor nutritional uptake.
Overall, manifestations of dry eyes and dry mouth, especially in SS patients, appear to correlate with an onset of exocrine cell senescence or refractivity, loss of neural regulation of ocular and salivary secretory function, and a loss of exocrine cell mass, first from antagonistic autoantibodies then from effector T lymphocytes.1 The consequence for the patient is a significant reduction of quality of life.
III. MOUSE MODELS OF SJOGREN SYNDROME
In an effort to identify the underlying pathogenesis of SS in humans, numerous mouse models in which various aspects of SS appear spontaneously or are experimentally induced are being intensively investigated.1 Typically, these mouse models show lymphocyte infiltration of the exocrine glands, increased expressions of proinflammatory cytokines, generation of unique autoantibodies (especially ANAs, anti-α-fodrin, and anti-M3R antibodies), and eventually impairment of secretory function. Strains that have been studied extensively include NZB/NZW F1-hybrids, MRL/lpr, NOD and NFS/sld. More recently, several new strains have been added, including the Id3 gene KO mouse, the aromatase gene KO mouse, the Baff gene knock-in (KI) mouse, as well as the IQI/Jic mouse and C57BL/6.NOD-Aec1Aec2 congenic line. A listing of these models and their general disease profiles are presented in Table 1. While each strain is reported to have features resembling those of SS in human patients, no one model represents completely the pathological characteristics of the human disease.
Table 1.
Examples of mouse strains used in the study of Sjogren syndrome
| Mouse strains | Characteristics | Disease Manifestations/Phenotypes |
|---|---|---|
| NZB/W [(NZB × NZW)F1 hybrid] | Naturally occurring mouse model by crossing NZB and NZW | Lacrimal gland involvement A greater percentage of B cells compared with MRL/lpr mouse |
| MRL/lpr | Mutation in lpr that encodes Fas protein | Diffuse lymphocytic infiltration Autoantibodies (against ssDNA, RNPs, IgG) |
| NOD | Spontaneous insulitis and diabetes | SS-like disease phenotype; Loss of secretion, anti-M3R Abs, focal lymphocytic infiltration |
| C57BL/6.NOD-Aec1.Aec2 | C57BL/6 carrying Aec1 (Idd3) genetic region on Chr 3 and Aec2 (Idd5) genetic region on Chr 1 | SS-like disease phenotype in a C57BL/6 genetic background |
| IQI/Jic | Inbred strain originating from ICR | Anti-kallikrein 1 and -13 Abs |
| NFS/sld | Autosomal recessive gene with sublingual gland differentiation arrest | Anti-α-fodrin Abs |
| Id3 gene KO | Id3; basic helix-loop-helix transcription factor, a dominant negative inhibitor of gene expression | Impaired TCR-mediated T cell selection Loss of secretion Anti-Ro and anti-La Abs |
| Aromatase gene KO | Estrogen deficiency due to absence of enzyme catalyzing the conversion of testosterone to estradiol | B cell hyperplasia in the BM and spleen Anti-α-fodrin Abs |
| BAFF transgenic | Transgenic for B-cell survival factor | Loss of secretion by 15–17 months of age Lymphocytic infiltration w/majority of B cells |
One extensively studied murine model is the MRL/lpr mouse. MRL/lpr mice carry a mutation in the lpr gene that causes a defect in the Fas protein involved in the Fas/FasL apoptotic pathway. This defect leads to impairment of normal lymphocyte apoptosis, resulting in an abnormal proliferation and survival of lymphocytes, especially B cells.42
Although the MRL/lpr mouse was developed for the study of SLE, it manifests an autoantibody pattern found, in part, in SS, including anti-dsDNA, anti-ssDNA, ANA, and rheumatoid factor.43 An early report suggested that MRL mice do not synthesize anti-SSA/Ro and anti-SSB/La autoantibodies44; however, more recent reports indicate that nearly 30% of mice develop anti-52 KDa SSA/Ro antibodies, 6% develop anti-60 KDa SSA/Ro antibodies, and 6% develop anti-SSB/La antibodies45 but not SSA/Ro.
MRL/lpr mice also develop dacryoadenitis of the lacrimal glands and sialadenitis of the submandibular, parotid, and lingual glands.46 The infiltrates are generally comprised of CD4+ T cells, with lesser numbers of CD8+ T cells and B cells. In addition, scattered patterns of macrophages and dendritic cells are present.47 However, the infiltrates appear diffuse, somewhat dissimilar from the compact, tightly packed foci found in NOD mice. Although lymphocyte infiltrations may cause destruction of exocrine gland tissues, perhaps by iNOS/nitric oxide (NO),48 TNF-α and/or various cytokines,49 it would be of interest to determine if these mice also produce antineural autoantibodies, especially those that are reactive with muscarinic acetylcholine receptors and/or β-adrenergic receptors.
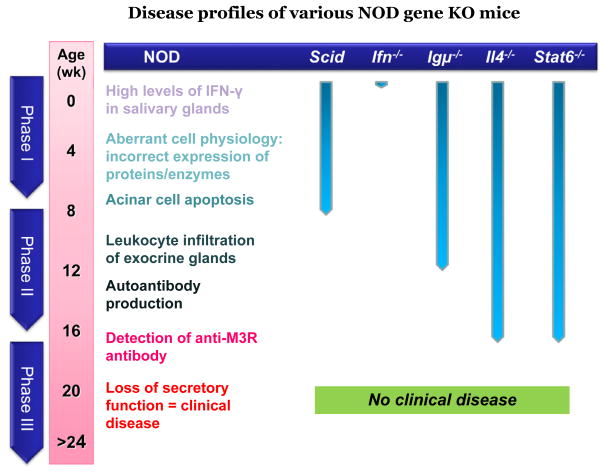
One of the more interesting and well-studied models of SS is the NOD mouse, which closely mimics the human disease. Even though the NOD mouse is a popular animal model to study spontaneous type-1 diabetes, its value in studying SS is sometimes questioned because it lacks anti-SSA/Ro or anti-SSB/La autoantibodies and because male mice exhibit a more severe lacrimal gland disease than females, neither of which is characteristic of human SS.50 A major strength of this model, however, is that it allows the study of a large number of congenic partner gene KO strains, eg, NOD-scid, NOD.Ifnγ−/−, NOD.IL2−/−, NOD.IL4−/−, NOD.IL10−/−, NOD.Igμ−/−, and NOD. Stat6−/−, permitting investigations into the role of individual genes in the development and onset of SS-like disease.50–53
Results of these studies suggest that the disease can be divided into three distinct, yet interactive, consecutive phases.53–56 In phase 1 (initiation of glandular pathology), a number of aberrant genetic, physiological, and biochemical activities associated with retarded salivary gland organogenesis and increased acinar cell apoptosis occur sequentially prior to and independent of detectable autoimmunity.56 In phase 2 (onset of autoimmunity believed to result from the acinar cell apoptosis), leukocytes expressing proinflammatory cytokines infiltrate the exocrine glands, establishing lymphocytic foci, first of T cell clusters followed by recruitment of B lymphocytes.57,58 In phase 3 (onset of clinical disease), loss of salivary and lacrimal gland secretory functions occur, most likely the result of antagonistic (auto)-antibodies reactive with the M3Rs.16,50,59,60 These three phases are shown in Figure 1.
Figure 1.
Events depicting the three phases and associated immunopathological manifestations that were determined by using various gene knockout models of NOD mice.
IV. COMPARISON OF MOUSE MODELS AND HUMAN DISEASE
No animal model represents perfectly its corresponding human autoimmune disease, and this is clearly true for SS. Nevertheless, the NOD mouse model, and especially the genetically derived C57BL/6. NOD-Aec1Aec2 mouse model, exhibit many features similar to those of human SS, as shown in Table 2. While both NOD and C57BL/6.NOD-Ae-c1Aec2 mice exhibit infiltration of the lacrimal and salivary glands with concomitant decreased tear and saliva flow rates, additional similarities exist, particularly with respect to production of autoantibodies. One important difference appears to be in the production of antibodies reactive with Ro/SSA and La/SSB; no convincing evidence demonstrates that NOD mice can produce such antibodies. Interestingly, however, recent data suggest that C57BL/6.NOD-Aec1Aec2 mice may produce anti-Ro/SSA and anti-La/SSB antibodies (unpublished data from our laboratory).
Table 2.
Comparison of general symptoms of Sjogren syndrome patients and mice
| Characteristic | Sjogren syndrome | NOD miceb | C57BL/6.NOD-Aec1Aec2 |
|---|---|---|---|
| Dacryoadentitis | (Yes)a | Yes | Yes |
| Sialadenitis | Yes | Yes | Yes |
| Decreased tear & saliva flow rates | Yes | Variablec | Yes |
| Altered proteins in tears & saliva | Yes | Yes | Yes |
| Pro-inflammatory cytokine production | Yes | Yes | Yes |
| Autoantibodies | |||
| Anti-Ro/SS-A, Anti-La/SS-B | Yes | Probably notd | UDe |
| Anti-DNA (ANAs) | Yes | Yes | Yes |
| Anti-α-fodrin | Yes | Yes | NDf |
| Anti-β-adrenergic receptor | Yes | Yes | ND |
| Anti-type-3 muscarinic ACh receptor | Yes | Yes | Yes |
| Keratoconjunctivitis sicca | Yes | (?) | Yes |
| Ocular epithelium dessication (Rose-Bengal Dye) | Yes | Yes | ND |
| Break-up time testing | Decreased | (?) | ND |
| Lysozyme & Lactoferrin activity | Decreased | Decreased | (?) |
| Stomatitis sicca | Yes | Yes | Yes |
| Buccal epithelium dessication | Yes | (?) | ND |
| Serine protease activity against PSP | (?) | Yes | Yes |
| Amylase & EGF activity | Decreased | Decreased | Decreased |
Biopsies of lacrimal glands not often performed.
Discussed in text.
Male and female mice differ, depending on severity of disease in glands (males have more severe lacrimal gland disease, females more severe salivary gland disease.
Possibly detected as part of ANAs, but very rare in NOD background mice.
Unpublished data.
Not determined.
A second distinctive feature of these two mouse models may be in the development of KCS. Although ocular dryness is often observed, it is uncertain whether this results from autoimmunity or from the cage environment; most modern cage systems have rapid airflow rates that could severely desiccate the animal ocular surface while blowing irritants into the eyes of the mice (personal communication and experience). When we reduced the airflow rates to the cages of our C57BL/6.NOD-Aec1Aec2 mice, the number of eyes actually lost decreased significantly. Under standard airflow rates implemented by Animal Care Services at University of Florida, our C57BL/6.NOD-Aec1Aec2 mice would begin losing their eyeballs at about 10 weeks of age. At first, the globes appeared to develop opaqueness, with subsequent shrinkage followed by full loss of the globe (phthisis bulbi). Eyeball loss could be unilateral or bilateral. However, following reduced airflow rates (from 60 cage changes per minute to 30 cage changes per minute), the mice exhibited a significant reduction in globe loss.
Based on these observations, we believe that the rapid airflow may cause irritation in the eyes, followed by inflammation of the ocular surface. Morphological diagnoses have not yet been performed, but they might provide information as to the underlying cause.
V. ETIOLOGY OF SJOGREN SYNDROME AND SJOGREN SYNDROME-LIKE DISEASE
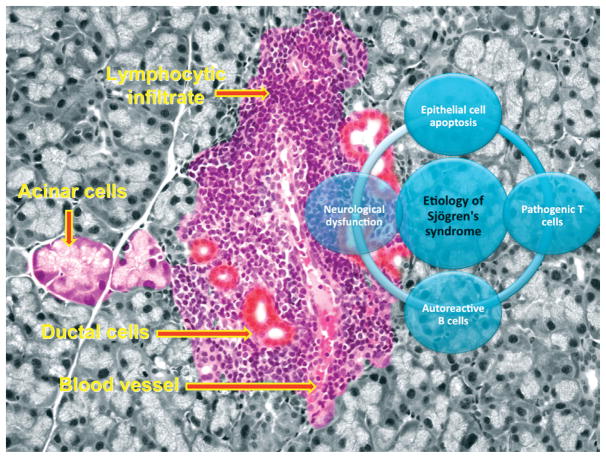
In 1933, Dr. Henrik Sjögren (1899–1986) presented his findings on KCS that formed the basis for SS. Since that time, extensive research has focused on identifying specific etiologies that may be directly involved in SS development. A number of hypotheses have been postulated to explain the complexity of SS-related dry eyes, including androgen deficiency,61–63 meibomian gland dysfunction,64,65 viral infection,66–68 and many more. However, in this review, we have focused on four mechanisms that we believe will provide better understanding of the etiologies for both ocular and salivary gland dysfunction observed in humans and animal models of SS. These include 1) the role of epithelial cell apoptosis, 2) the function of autoreactive T lymphocytes, 3) the role of autoreactive B cells, and 4) the clinical observation of neurological dysfunction. Each mechanism is summarized in Figure 2 and described below.
Figure 2.
Proposed etiologies of SS and SS-like disease manifested in the exocrine glands.
A. Role of Apoptosis
The role of apoptosis in SS remains controversial. Whether directly or indirectly involved in the development of secretory dysfunction in the lacrimal and salivary glands, acinar cell apoptosis is a histological feature of SS. Whether it results from glandular destruction by inflammation or by malfunction of acinar and ductal epithelial cells, apoptosis appears to affect acinar cell mass. Two factors associated with acinar tissue apoptosis are α-fodrin proteolysis and Fas/Fas-ligand interaction. Recent studies suggest that α-fodrin, a 240 kDa membrane-associated cytoskeleton protein, is cleaved into three fragments of 150, 120 and 23 kDa following the activation of caspase-3.69 The 120 kDa fragment, initially isolated from salivary glands of NFS/sld mice, behaves as an organ-specific autoantigen.70 Proteolysis of α-fodrin is pathogenic to cells, due to its direct involvement in membrane malfunction and cell contraction.69 Purified 120 kDa fragments are capable of stimulating T cell proliferation and secretion of IL-2 and IFN-γ in vitro.71 In addition, specific autoantibodies against α-fodrin have been detected in NOD mice, and these antibodies correlated with the levels of sialadenitis.1 Furthermore, adoptive transfer of CD4+ T cells reactive against synthetic α-fodrin could induce SS in normal syngeneic mice, whereas injection of anti-α-fodrin into neonate mice promoted tolerance by enhancing the numbers and function of regulatory T cells capable of suppressing the development of SS-like disease.72
Although the proteolysis of α-fodrin was initially identified in SS mouse models, accumulating evidence indicates a similar phenomenon in human SS. Studies have shown that IgA antibodies reactive with α-fodrin are detectable in 64% of patients with pSS, 47% of patients with secondary SS and SLE, and 86% of patients with secondary SS and RA, compared to only 1 in 160 sera obtained from normal controls, 1 in 50 sera from SLE patients, and 2 in 12 sera from RA patients without sicca syndrome. Similarly, the prevalence of IgG antibodies reactive with α-fodrin was significantly higher in sera obtained from patients with pSS and secondary SS compared to sera from normal controls. Interestingly, anti-α-fodrin antibody was detected before anti-Ro/SSA or anti-La/SSB autoantibodies and showed a positive correlation with the severity of dry eye disease.73 Therefore, it has been suggested that apoptosis initiated by activation of α-fodrin results in the exposure of cryptic epitopes against which the immune system can respond, leading to the subsequent production of antibodies against Ro/SSA and La/SSB.
The interaction of Fas (TNFRSF6) and FasL (TNFSF6) can facilitate a cascade of events that lead to the activation of caspases and proteinases that serve to fragment DNA. Various studies have indicated that Fas antigen is expressed in ductal epithelial cells of SS patients with severe sialadenitis, but not in patients with mild sialadenitis, suggesting that Fas-positive ductal cells provide a good target for effector T cells.74 In addition, autoreactive T cells tend to be more pathogenic if they are highly reactive against Fas antigen.75
A study involving 243 patients diagnosed with KCS with or without SS found a higher percentage of Fas-positive cells in peripheral blood and increased amounts of soluble FasL in sera from SS patients compared to non-SS cohorts.76 However, the presence of Fas/FasL does not necessarily imply their direct role in inducing apoptosis. Minor salivary gland biopsies from a subset of primary SS patients stained positive for Fas and Bcl2, but negative for TUNEL; this has been interpreted as a lack of apoptosis due to an over-expression of Bcl2 that continues to promote infiltrating lymphocyte proliferation.77
These findings were further supported by Ohlsson et al, who showed that Fas and FasL were expressed on SS infiltrating cells and epithelial cells of human salivary glands, but that, at the same time, a low level of TUNEL+ cells was detected.78
In the animal models of SS, Fas-mediated cell death is well documented. We have shown that Fas and FasL mRNAs, as well as their proteins, are expressed on salivary and lacrimal glands of NOD mice at 8 and 18 weeks of age, even in the absence of lymphocytic infiltrates.40 Second, Ishimaru et al have revealed that Fas antigen and TUNEL staining, but not FasL staining in CD4+ T cells in salivary glands, showed a positive correlation with age of NFS/sld mice.79 In addition, Fas apparently could mediate proteolysis of α-fodrin to its immunogenic 120 kDa fragment in NFS/sld mice, resulting in an increase in anti-α-fodrin autoantibodies. However, the underlying pathology is, no doubt, complex, considering the fact the expansion of autoreactive lymphocytes may be due to a defect in apoptosis of lymphocytes in secondary lymphatics, leading to expansion of the autoreactive T cells infiltrating the lacrimal glands.80
An intriguing question in autoimmune diseases like SS pertains to how intracellular self-proteins become autoantigens, presented as dominant neoantigens and recognized by immune cells. Rosen et al suggested that cellular apoptosis is one of the early events in SS development.81–83 They described the redistribuion of molecules within the subcelluar compartment in cells undergoing apoptosis. Small membrane blebs were shown to contain the Ro-52 kDa molecule, along with other molecules, such as calreticulin, normally present within the ER lumen. Nuclear antigens are also redistributed during apoptosis, exhibiting an increase in localization of Ro/SSA, La/SSB, the snRNPs, Ku and PARP as a rim around the condensing chromatin.83,84 Such clustering of potential autoantigens occurs during apoptosis, but not during necrosis.85,86
B. Role of Autoreactive T Lymphocytes
Antigen-presenting cells (APCs) function to present these antigens in the context of MHC to T cells and initiate specific immune responses. This immune response can be autoimmune when it initiates in the presence of autoantigens derived from self-cellular components, exposure of cryptic/immunogenic epitopes, or even viral mimicry. Studies in both humans and animal models have shown that infiltrates appear generally as periductal foci within the glandular architecture of the lacrimal and salivary glands consisting of CD4+ T cells, CD8+ T cells, B cells, and macrophages. The T cells exhibit a preferential antigen receptor repertoire, whereas the overall infiltrating cells express various cytokines (including IL-1β, IL-6, IL-10, TNF-α, and IFN-γ).87–90
The importance of T lymphocytes in the activation, proliferation, and differentiation of antigen-reactive B cells in autoimmune-prone mice is nicely replicated in the Id3-gene KO mouse.91 Id3-deficient mice carrying different genetic backgrounds develop a SS-like disease, including the synthesis of anti-Ro/SSA and anti-La/SSB antibodies, at approximately 1 year of age.92 In the absence of T cells, these Id3-deficient mice failed to exhibit development of a SS-like disease. One fascinating observation in this mouse model is the appearance of secretory dysfunction as early as 6 weeks of age, before other disease symptoms are apparent. This raises the possibility that organogenesis of the salivary glands may be impaired in this Id3-gene KO mouse, similar to NOD mice, resulting in aberrant antigen presentation and autoreactive T cell activation. Id3 is known to be a Smad4-dependent TGF-β responsive gene whose pathway is important for salivary gland development.93
Previous studies carried out in both NOD.IFNγ−/− and NOD.IL4−/− mice revealed that both IFN-γ and IL-4 are critical cytokines for development of SS-like disease in the NOD mouse model.94,95 IFN-γ appears to be essential at both an early stage (the early innate response period) and a late stage (development of the adaptive response), while IL-4 is essential during the early adaptive immune response. These observations suggest the need for both TH1 and TH2 cell-associated cytokines for onset of clinical disease. However, the recent identification of the CD4+ TH17 memory cell population96 challenges the long-standing TH1/TH2 cell paradigm, and our more recent data now indicate that the TH17 cell population is present within the lymphocytic infiltrations appearing in exocrine glands of both SS patients and C57BL/6.NOD-Aec1Aec2 mice.
The TH17 cell population is a subset of CD4+ memory effector T cells that appears to be distinct and unrelated to either the TH1 or TH2 cell lineage.97–101 Differentiation of TH17 cells is mediated by a balanced stimulation of TGF-β and IL-6.99 TH17 effector cells are characterized by their unique ability to secrete IL-17 in response to stimulation by TGF-β and IL-6, which induces expression of the master regulator, RORγt (T cell-specific orphan nuclear receptor-γ).102
Although the IL-17 family of cytokines consists of six members (Il-17A (IL-17), IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F), most of the attention has focused on IL-17A. These molecules are potent proinflammatory cytokines, which are actively involved in tissue inflammation via induction of proinflammatory cytokine and chemokine expression, such as IL-6, TNF and MIP-2.103 As part of the local inflammatory response, IL-17 is involved in the proliferation, maturation, and migration of neutrophils.96
Most importantly, the TH17 cell population per se is a tissue-seeking T cell population that is intimately involved in autoimmune diseases, eg, Crohn’s disease,104,105 experimental autoimmune encephalomyelitis,106 collagen-induced arthritis,107 and others. Tissue destruction appears to result, in part, from the upregulation of MMPs known to be expressed in inflamed lacrimal and salivary glands during development of SS and SS-like disease.
Despite the apparent involvement of TH17 in the development and/or onset of SS, one cannot forget the importance of both the TH1 and TH2 pathways. In our previous studies, elimination of INF-γ in SS-susceptible mice ameliorated all pathological and clinical signs of disease, while elimination of IL-4 prevented loss of secretory function.94,95 Furthermore, the TH1 proinflammatory cytokine, IL-18, has been shown to be present in CD68+ macrophages and in ductal and acinar cells of SS salivary glands108–110 and to be secreted at significantly higher levels in sera and saliva of SS patients and NOD mice.110,111 Therefore, one might postulate that IL-18 produced by activated macrophages and T cells can stimulate the production of other inflammatory cytokines, chemokines, and adhesion molecules to attract inflammatory cells to the site of injury; in SS, this phenomenon is probably associated with the activation of the inflammasome.
These observations indicate an essential role for both TH1 and TH2 cell-associated cytokines and raise several important questions concerning the interplay between TH1, TH2, and TH17 cells. When does each become involved in the autoimmune response? Do TH17 cells act directly through secretion of inflammatory IL-17 family cytokines or by activating autoimmune T and B cells? If TH17 cells act in SS, as is reported in EAE, then IL-17 may not be required for initiation of SS, but, rather, may represent a critical regulator of TH1 cells and their production of IFN-γ,112 which would be consistent with our data from the study of IFN-γ gene knockout mice.94
C. Role of Autoreactive B Cells
It was initially thought that the decline in tear and saliva flow rates in SS patients was a consequence of the progressive loss of exocrine tissue mass from acinar cell apoptosis induced by effector T lymphocytes; however, this concept is challenged by the lack of correlation between the degree of lymphocytic infiltration (focus score) and/or the amount of intact acinar tissues. A search for a more consistent explanation led to our observation in NOD mice that onset of clinical disease required B lymphocytes and autoantibodies, especially those reactive against the parasympathetic neurons.60 First, NOD.Igμnull mice lacking mature B lymphocytes failed to develop lacrimal and salivary gland secretory dysfunction. Second, sera IgG fractions from SS patients, when infused into NOD-scid or normal C57BL/6 mice, induced a transient stimulation or inhibition of saliva secretory rates. Therefore, attention is now directed toward understanding the role of the B lymphocyte in SS.
Accumulating evidence suggests that disturbances in B lymphocyte homeostasis, including their role in ectopic germinal center formation in lacrimal and salivary gland tissue, are major features of SS and SS-like disease. B cell hyper-reactivity is clearly documented in SS, leading to hypergammaglobulinemia, production of an expanding set of autoreactive antibodies, and at times the monoclonal expansion of B cells capable of transformation to B-cell lymphomas in a subset of patients6,7; however, only recently have some of the underlying molecular events been revealed.
Perhaps the most important observations have been those reporting the increased levels of the B cell activating factors, BAFF(Blys) and APRIL, in salivary and lacrimal glands, respectively.113–120 Overexpression of BAFF (and possibly APRIL) in both transgenic mouse models and SS patients appears to correlate with B cell hyperactivity leading to excessive immunoglobulin production and prolonged B cell expansion.
In recent years, numerous studies have reported an expanding list of autoantibodies in the sera of SS patients and mouse models. These include antibodies reactive with nuclear antigens, membrane proteins, cytoplasmic proteins, and secreted products. Many autoantigens are intracellular enzymes and regulatory factors required for cellular function, especially gene replication, transcription, RNA processing, and protein synthesis.121 Interestingly, these molecules have little in common in terms of structure, subcellular localization, or biological function.
More importantly, few data are available that indicate that antibodies directed against the vast majority of these molecules have any direct effect in the initial or overall pathogenesis, ie, loss of fluid secretion by exocrine glands. Nevertheless, antibodies targeting nuclear proteins (ANAs) in SS, eg, the ribonuclear proteins Ro/SSA and La/SSB,122 have long been used as diagnostic markers of SS in both humans and animal models. Furthermore, the various immunofluorescence patterns of ANAs have, over time, led to the identification of additional nuclear proteins targeted by autoantibodies, such as Sm, dsDNA, the nuclear mitotic apparatus (NuMA), proteasomes, mitotic chromosomal autoantigens (MCAs), and poly-(ADP-ribose) polymerase.123
Autoantibodies detected in SS patients and various animal models are not limited to targeting nuclear proteins, as illustrated above with the intracellular cytoskeletal protein, α-fodrin.124 As NFS/sld mice age, they produce autoantibodies against ssDNA, IgG1 and IgG2a subclasses of IgG, rheumatoid factor (RF), and type II collagen.125 Other examples of intracellular autoantigens are tissue kallikreins-1 (Kik-1) and -13 (Kik-13), as defined by detectable autoantibodies in sera of IQI/Jic mice after 12 weeks of age. However, only Kik-13 could induce a proliferative response by splenic T cells.126
The fact that Kik-13 is highly expressed in the salivary gland ductal cells may explain the target tissue specificity of SS, as it is consistent with periductal infiltration of immune cells. Furthermore, since only reduced forms of Kik-13 are recognized by the sera of IQI/Jic mice, one might conclude that cryptic epitopes are involved, not unlike the case proposed for the potential development of autoantibody responses to parotid secretory protein (PSP) in NOD mice.127 Lastly, both SS patients and NOD mice form antibodies reactive with islet cell autoantigen-69 (ICA-69) expressed in pancreatic islets, the brain, and both lacrimal and salivary glands.128 Disruption of the ICA69 locus in the NOD mouse prevents lacrimal gland disease and greatly reduces salivary gland disease, suggesting that immunoreactivity against ICA-69 might play a role in disease progression.128
The disease profile observed in NOD.IL4−/− or NOD. B10-H2b.IL4−/− mice raises a number of questions pertaining to the role of B lymphocytes in development and onset of SS-like disease.1 These IL4−/− mice exhibit all aspects of SS-like disease seen in parental NOD mice except decreased tear and saliva flow rates. This observation leads to the hypothesis that an autoimmune B cell population exists in NOD mice that is activated by IL-4 to produce a specific IgG1 against the muscarinic acetylcholine receptors (AchRs), specifically M3R, and that this antibody blocks the parasympathetic signal to the acinar cells. However, additional considerations, which require further examination, include 1) whether B cells act as APCs for initiation of SS, as has been proposed for initiation of T1D in NOD mice; 2) whether B cells in NOD mice respond abnormally to the presence of IL-4, resulting in circumvention of normal homeostatic mechanisms leading to hyperproliferation, survival, and escape from negative selective pressures; and/or 3) whether IL-4 interacts with BAFF/APRIL, leading to a synergistic response by B cells.
Unfortunately, it may be difficult to determine whether these B cell populations act as APCs, because SS is generally characterized as a hypersensitivity type 2 (or antibody-mediated) immunological response, yet at the same time, IL-4 appears to be important in IgG isotype switching to promote production of IgG1 anti-M3R autoantibody.52 Furthermore, B lymphocytes infiltrating the salivary glands appear to be marginal zone B cells, a population known to participate in innate immunity, the maintenance of germinal centers in the target tissue, and subsequent antibody production in SS.116
D. Sjogren Syndrome-Associated Neurological Dysfunction
Exocrine glands are highly innervated by neural tissue. Of special interest are the sympathetic and parasympathetic nerves that interact with the β-adrenergic and muscarinic AchRs. Although several reviews have focused on the neural dysregulation of the lacrimal secretion in dry eye diseases,129,130 limited attention has been devoted to understanding the neurological dysfunction associated with SS, even though sensory, peripheral, cranial, and myelopathic neuropathies131 develop in approximately 20% of SS patients.132 In addition, cognitive impairments, such as dementia, lack of concentration, memory loss, and various psychiatric disorders (ranging from depression to anxiety), are often noted in SS patients during clinic visits.133–135
A variety of possible mechanisms leading to secretory dysfunction in the lacrimal and salivary glands are under intensive investigation. Recent attention has focused on the role of anti-M3R autoantibodies acting as blocking or antagonistic antibodies that inhibit fluid secretion. Other possibilities include: 1) impairment or damage of the parasympathetic neurons; 2) release of cytokines by infiltrating lymphocytes that prevents release of neurotransmitters; 3) internalization of the muscarinic receptors following interaction with autoantibodies, resulting in decreased expression on acinar cells; and 4) constant stimulation of the muscarinic receptors by autoantibody binding, resulting in a state of desensitization and reduced capacity for secretion.1,129,136,137
Our studies suggest that anti-M3R antibodies probably act by blocking the neural signal to the acinar tissue, even though we cannot rule out a progressive desensitization.138 Of the many autoantibodies identified in SS patients to date, the association between anti-M3R autoantibodies and secretory dysfunction seems most likely, as the M3R is considered to be the major receptor mediating secretion in the lacrimal and salivary glands in response to parasympathetic stimuli. This is supported by the fact that purified IgG from SS patients downregulate carbachol-evoked bladder muscle contraction by 50%, while anti-idiotypic antibodies neutralize this inhibition of cholinergic neurotransmission.15,18,139 Furthermore, anti-idiotypic antibodies are capable of neutralizing autoantibodies that inhibit cholinergic neurotransmission.139
Similarly, studies using the human salivary gland ductal cells showed that pretreatment of the cells with IgG from SS patients reduced the magnitude of subsequent carbachol-induced intracellular calcium release.17 Lastly, M3R desensitization occurs in mice with anti-M3R autoantibodies, as Cha et al demonstrated in a comparison of carbachol-evoked responses in NOD mice >20 weeks of age versus either age-matched C57BL/6 or antibody-negative 8–10-week-old NOD mice.16
Taken together, the above observations are consistent with the hypothesis that chronic stimulation by anti-M3R antibody induces an inhibitory effect on M3Rs. This concept is supported by the fact that NOD mice with overt SS-like disease initially respond well to pilocarpine-stimulation for saliva secretion, but exhibit a downregulated response following repeated injections. Extrapolating these data to a clinical setting, in human patients positive for anti-M3R autoantibodies, chronic intake of pilocarpine might enhance salivary gland secretion initially, an effect that is then followed by rapid desensitization. The relevance of this scenario to the lacrimal gland should be investigated in detail, as there are possibly conflicting reports regarding the ability of pilocarpine and/or cevimeline to stimulate tear secretion in the lacrimal glands of SS patients.140–144
VI. NEW TECHNOLOGY TO CHARACTERIZE AND DIAGNOSE SJOGREN SYNDROME
The increasing database of information about the pathogenesis of SS will, no doubt, influence diagnosis and treatment of this disease. Many of these data are derived from studies using murine models and single-gene KO mice, eg, Il4−/−, Il10−/−, Ifnγ−/−, C3−/−, Igμ−/−, Id3−/− and Stat6−/− mice, which have revealed the importance of individual factors in SS-like disease.1 However, this approach is time-consuming and suboptimal for discovering new genes and/or molecular networks underlying the development and onset of SS-like disease. Furthermore, since silencing a specific factor for the life of the animal is known to result in global effects on the immune system and/or general physiology, identification of the precise mode of action on the autoimmune disease per se is greatly complicated. For this reason, new approaches have come into vogue, including the use of siRNA, inducible gene expressions, and inducible gene silencing. However, the application of these technologies depends on identification of genes and gene products important in the disease process. For this reason, the use of both genomic and proteomic analysis is rapidly increasing in an attempt to identify the genetic basis and susceptibility for autoimmunity, the molecular processes involved, and biomarkers specific for disease diagnosis. The utility of these technologies, which are still in their infancy, is discussed below.
A. Genomics Approach Using Microarray
To date, the use of microarray to identify differentially expressed genes during development and onset of SS has been very limited and applied mostly to the salivary gland. One notable exception is the study of Kawasaki et al,145 in which the iAFLP microarray system was used to identify differentially expressed genes in conjunctival epithelial cells isolated from 26 SS patients compared to 30 normal healthy controls. Due to limitations in iAFLP microarray technology, the authors were able to examine only 931 genes, yet 34 genes were found to be upregulated and 12 genes downregulated. The upregulated genes encoded such proteins as kallikrein-7, s-PRP, HLA-DR, IL-6, c-fos, defensin-β2, keratin-16, -6b and -6c. The downregulated genes encoded for such proteins as INFR-1, fatty acid binding protein 5, collagen type-Iα2, c-Myc and aquaporin. This gene profile for conjunctival epithelial cells in SS patients was consistent with cell cycling events, keratin-associated remodeling, and ocular inflammation, as suggested by the authors.
The use of this technology to study human samples is limited, in that, to date, it is not possible to identify individuals susceptible to developing SS or individuals with early-stage disease. Thus, a global genomic analysis of genes differentially expressed temporally during the development and onset of clinical disease in humans remains a goal for the future. In contrast, murine models whose disease profiles have been well defined provide excellent opportunities to identify the changing molecular events and biological processes based on gene expressions during disease progression.
In a recent study, we carried out a full-genomic analysis for the development of SS-like disease in the lacrimal glands of our C57BL/6.NOD-Aec1Aec2 mice at five time points (4, 8, 12, 16, and 20 weeks of age) encompassing the complete time frame for development of SS-like disease. Each hybridization was carried out using Affymetrix GeneChip Mouse Genome 430 2.0 Arrays containing 45,000 probe sets permitting analyses of 39,000 transcripts and variants from over 34,000 genes. Software analyses identified 552 genes whose expression profiles were statistically altered over the time frame for disease development. These differentially expressed genes could be assigned to a number of pathways, functions, or biological processes using Panther software,146 a few of which are presented in conjunction with salivary glands (Table 3). Of special note, these analyses of lacrimal glands identified genes associated with T and B cell activation, Fas signaling, and apoptosis, as well as IFN-γ and integrin signaling pathways. Each of these areas represents a potentially important process for exocrine gland dysfunction in this mouse model, and possibly for human SS.
Table 3.
Selected pathways, biological processes and molecular functions represented by differential-expressed genes of the lacrimal and salivary glands during development and onset of SjS-like disease in C57BL/6.NOD-Aec1Aec2 mice
| Lacrimal Glands | Salivary Glands |
|---|---|
| A. Enriched Pathways | |
| B cell activation | Muscarinic AchR signaling pathway |
| T cell activation | Cell cycle |
| Fas/apoptosis signaling pathway | Angiogenesis |
| Interferon-γ signaling pathway | FGF signaling pathway |
| Integrin signaling pathway | Integrin signaling pathway |
| B. Biological Processes | |
| Apoptosis | Apoptosis |
| Lipid, fatty acid and steroid metabolism | Homeostasis |
| Protein metabolism/targeting/localization | Protein/carbohydrate metabolism |
| Cell cycle | VEGF signaling pathway |
| C. Molecular Functions | |
| Oxidoreductase | Oxidoreductase |
| Membrane traffic protein | Extracellular matrix |
| Kinase | Kinase |
| Transferase | Transferase |
Although microarray data permits cataloging of a multitude of genes that are differentially expressed, perhaps one of the more interesting results highlighted by our microarray studies is the ability of the microarray analyses to demonstrate the rapidly changing gene expressions during the chronic progression of disease development and subsequent onset of clinical SS, or exocrine gland dysfunction. Somewhat surprisingly, most genes exhibiting a statistically significant differential expression were upregulated not only at a specific point during disease development but also for a very limited time frame. As a result, a quick look at the gene expression profiles revealed that at the time of clinical disease, few genes remained upregulated. Nevertheless, these gene expression patterns mimicked our previous studies delineating disease in our NOD and NOD-derived mouse models, where the SS-like disease could be divided into several phases of disease development based on predictable pathophysiological activities, initiation of glandular inflammation, and subsequent immune-mediated acinar cell dysfunction. Thus, a necessary task for the future will be to correlate temporal changes in gene expressions with specific temporal changes in the pathophysiological and immunological observations. Unfortunately, extrapolating these findings to SS patients, examination of gene expressions in patients who already have clinical disease would, predictably, provide a very limited picture.
While microarray technology permits the listing of differentially expressed genes and even the grouping of genes with respect to function or interactions, perhaps even more important is the potential to build hypothetical models based on gene expression data describing molecular pathogenic events that can subsequently be tested experimentally. One example of this, based on genomic microarray data obtained using submandibular gland tissue from C57BL/6. NOD-Aec1Aec2 mice at 8 and 12 weeks of age, has demonstrated the involvement of several pathways of innate and adaptive immunity.147
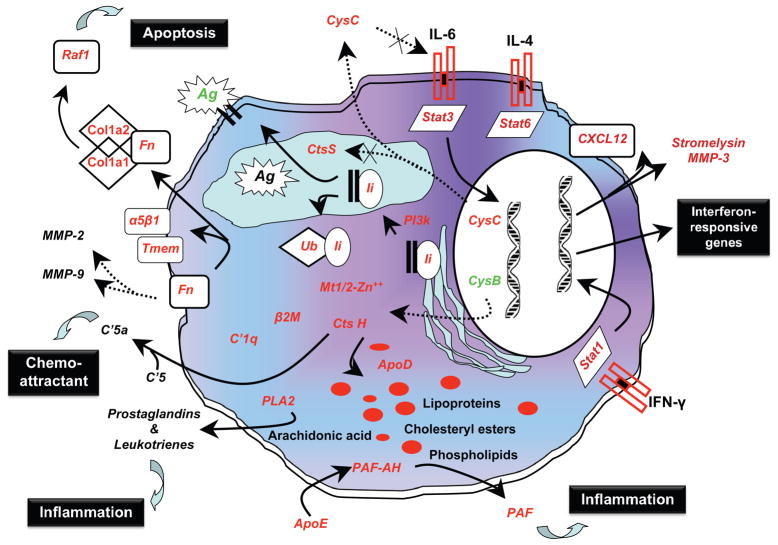
Similar pathways showing some important biological processes for the lacrimal gland can now be constructed. An example of this type of model building is presented in Figure 3, where the focus is on the possible interaction of a diverse set of genes. While acinar cell apoptosis has long been considered an important event in onset of SS, recent gene mapping data have indicated that the genetic region of chromosome 1 conferring SS-susceptibility on NOD and NOD-derived mice, like C57BL/6. NOD-Aec1Aec2, contains multiple genes regulating homeostasis of fatty acids, high-density lipids, and lipoproteins. Because these mice develop lipid deposits within the lacrimal glands, often correlating with onset of dacryoadentitis,148 we present a testable model for this aspect. Similar models can be built for any of the biological processes listed in Table 3.
Figure 3.
A hypothetical model linking multiple biological pathways in the development of SS-like disease based on differentially expressed genes identified in lacrimal glands by global microarray analyses. Starting with 27 genes identified as differentially expressed, a model for cellular dysfunction, initiation of inflammation, and altered antigen presentation has been designed that forms a basis for lacrimal gland pathology in SS. This model focuses considerable attention on reduced antigen-presentation by dendritic cells and macrophages in favor of B-cell antigen presentation considered more likely to result in autoimmune responses. Microarray data shows that transcripts for retinoic acid receptors RXR-α and RAR, as well as prostaglandin-α-activated PPAR-γ, are highly downregulated. These factors help regulate fatty acid, lipoprotein, and cholesterol homeostasis within cells and tissues, plus differentiation of DC; therefore, we hypothesize that an imbalance in the retinoic acid pathway leading to fatty acid, lipoprotein, and cholesterol dysregulation results in DC populations in NOD and C57BL/6.NOD-Aec1Aec2 mice that exhibit reduced responses to CSF, as previously reported.154
At the same time, there is apparently dysregulation in the interaction of the cathepsins and cystatins, especially the regulation of cathepsin-S by cystatin-C. Cystatin-C is a major inhibitor of the cathepsin-S in macrophages and DC. Gene expression of cathepsin-S indicates that it is highly upregulated during the early phases of disease development, but unlike the other cathepsins, eg, cathepsin-B, which is down-regulated, its expression remains elevated as the SS-like disease progresses. Since cathepsin-S (and IL-6) remains resistant to regulation by cystatin-C, we suggest that the levels of this protein become too high in the lysosomes, resulting in the breakdown of the MHC-class II binding with the invariant chain, CD74. This is known to prevent proper loading of the MHC-class II molecules with antigen for proper presentation by macrophages and DC, thereby permitting increased antigen presentation by B-cells, increasing the chance for autoimmunity. In contrast, cathepsin-B is strongly downregulated, and this results in the increased expression of multiple genes, eg, cathepsin-H, metallothionein, β-2-microglobulin, C1q, fibronectin and apolipoprotein-D.
While each of these factors can play an important role in the onset and propagation of inflammation,155,156 fibronectin is involved in multiple pathways. In addition to promoting association of integrins with transmembrane proteins (encoded by Tmem), thereby influencing cell migration, fibronectin can bind to collagen type-1α1 and α2 (precursors of the more prominent collagen of the eye) with subsequent activation of Raf1, a molecule that is highly pro-apoptotic.
B. Proteomics Approach Using 2D DIGE/MALDI-TOF/MS
Similar to genomics, proteomics to identify differentially expressed proteins during development and onset of SS is quite limited and has been applied mostly to the salivary glands. Collating proteomics with genomics is important, as protein profiles may or may not correlate with corresponding gene profiles. Nevertheless, during the development and onset of inflammation in SS, protein levels and protein profiles probably change dramatically, not only because of altered gene expressions, but also because of progressive loss of secretory function in salivary glands and the various tissues that drain into the tears (eg, meibomian gland, conjunctiva, and nasal-pharyngeal lacrimal system).
The use of routine techniques, such as ELISA, have, to date, measured a variety of proteins, eg, cytokines, chemokines, pro- and anti-inflammatory factors, enzymes, and antibodies that are present in tears, saliva, and sera of SS patients with KCS. For example, tear fluids from SS patients have shown reduced levels of lactoferrin, EGF,149 and the goblet cell-specific mucin MUC5AC,150 compared to tears of non-SS control patients. In addition, Ohashi et al reported that aquaporin-5 is elevated in tears of SS patients compared to tears from control or non-SS patients.149 Recently, Tsubota reported that clusterin levels are also upregulated in SS patients.151 Whether or not protein expression levels will become biomarkers of SS disease remains unknown.
Over the past ten years, technological advances in proteomic platforms have allowed us to examine full tear protein profiles, as well as to identify specific proteins present in these profiles, using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), a technique that measures mass-to-charge ratios of ionizable compounds.
In one of the earliest reports attempting to determine protein biomarkers in tear fluids from primary SS patients, Tomosugi et al,152 using surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS), identified 10 potential novel proteins that differed between SS patients and control subjects. Of these proteins, seven were downregulated and three correlated significantly with SS scores and epithelial damage of the ocular surface. Although these investigators have not yet identified the proteins, this study clearly demonstrates how such techniques can be applied to identify specific proteins involved in the pathophysiological processes associated with SS and how protein profiles might be developed to serve as a highly definitive diagnostic test.
To determine whether temporal changes in the protein composition of tears occur during the development and onset of SS, we have once again turned to the mouse model, which permits direct comparison of time points for SS-like disease. In our initial study, tear protein profiles were compared between tear fluids collected from NOD mice as the SS-like disease model and CD1 mice as the normal control at 9, 14 and 21 (± 2) weeks of age, using 2D DIGE separation and MALI-TOF/MS protein identification. Significant differences and temporal changes were demonstrated between NOD and CD1 mice in the number of protein spots with respect to both age and onset of SS-like disease in NOD mice.
Distinct differences in spot distribution at each time point examined during development of SS-like disease were unique, suggesting that the progression of the disease involves different physiological processes that translate to specific markers of disease development. For example, of 180 spots, 63 spots from the gel representing older mice were picked for positive identification by tandem mass spectrometry. Table 4 lists a few of these differentially expressed tear proteins. Several proteins are of particular interest, including the lipid-binding antibacterial proteins lipocalin-11, seminal vesicle autoantigen, and prolactin-inducible protein, because these proteins show direct correlation with results from microarrays. Other proteins of interest include prostatic steroid-binding protein, lactoperoxidase, the odorant-binding proteins, triacylglycerol lipase, cystatin Ap5 and DNase. Overall, these findings are in agreement with previously published data showing increases in lipocalin and lysozyme in dry eye patients.40
Table 4.
Selected major proteins identified by tandem MS/MS from the gel of tear proteins from older CD1 and NOD Lt/J mice
| Spot No. | Protein Descriptive Name | Accession No. |
|---|---|---|
| 2 | Lacrimal androgen-binding protein | IPI00466514 |
| 3 | Similar to prostatic steroid-binding protein C1 chain precursor | IPI00664264 |
| 4 | Cystatin Ap5 precursor | IPI00420893 |
| 18 | Lactoperoxidase | IPI00133770 |
| 19 | Keratin, type I cytoskeletal 15 | IPI00225378 |
| 21 | Similar to triacylglycerol lipase, gastric precursor | IPI00348851 |
| 25 | Odorant binding protein 1a | IPI00187557 |
| 26 | Seminal vesicle autoantigen | IPI00118165 |
| 32 | Lipocalin 11 | IPI00275210 |
| 35 | Major urinary protein 4 | IPI00115241 |
| 38 | Major urinary protein 4 precursor | IPI00115241 |
| 48 | Prolactin-inducible protein homolog precursor | IPI00113711 |
| 57 | Deoxyribonuclease 1 precursor | IPI00308684 |
Results of such proteomic studies, designed to identify changes in tear proteins, clearly indicate that detectable biochemical changes in protein compositions are strongly correlated with the progressive inflammation of lacrimal glands and the temporal change in tear flow rates observed in SS-like disease. Furthermore, observations on tear fluids using developing proteomic technologies and advancing analyses demonstrate temporal changes in tear protein compositions that most likely reflect a progressive lacrimal gland dysfunction. The data summarized here are also in agreement with recent studies of human SS patients by Hu et al153 and NOD mice by Delaleu et al,111 which showed protein changes in saliva. It is anticipated that with continuing advances in these technologies, identification of protein changes in tears and saliva will be facilitated, the bases for these changes will be understood, and their utility in defining SS will be realized.
VII. SUMMARY AND CONCLUSIONS
This review has discussed various aspects of SS found in both SS disease of humans and experimental SS-like disease in murine models. The feasibility and applicability of animal models have permitted us to explore potential pathogenic mechanisms in the development of SS. Advances in technological development have raised different perspectives and insights into the global, but pathway-specific, nature of the disease and, more importantly, have provided better tools to investigate potential biomarkers, which was not possible just a few years ago.
SS is a complex disease whose susceptibility appears to involve both MHC and non-MHC genetic components. How many of these components represent normal inflammatory responses to environmental challenges versus immune dysregulation remains to be discovered. The study of murine models of SS, both with spontaneous disease and induced disease, permit insightful genome-wide and temporal analyses of multiple factors covering genetics, epigentics, gender, and environment, which are difficult to mimic in the human situation. Clearly, the ability to link specific genes to different stages of SS disease or to identify candidate genes that control pathogenic events will provide new targets for potential intervention therapies. Thus, it is critical for human and rodent research of SS to continue together as a means to improve treatment of SS and eventually to be able to delay or prevent this disease.
Acknowledgments
We would like to express our deepest appreciation for Mrs. Janet G. Cornelius, who has been instrumental in many of the studies and findings presented in this review.
Supported in part by: PHS grant DE-014344 (to A.B.P.) from the National Institutes of Health and the University of Florida’s Center for Orphaned Autoimmune Disorders. C.Q.N. is supported by a post-doctoral fellowship from PHS grant T32 DE07200.
Footnotes
The authors have no commercial or proprietary interest in any concept or product discussed in this article.
Single-copy reprint requests to: Cuong Q. Nguyen, PhD (address below).
References
- 1.Nguyen CQ, Cha SR, Peck AB. Sjögren’s syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Front Biosci. 2007;12:1767–89. doi: 10.2741/2187. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Fox RI, Kang HI. Pathogenesis of Sjögren’s syndrome. Rheum Dis Clin North Am. 1992;18:517–38. [PubMed] [Google Scholar]
- 4.Fox RI, Michelson P. Approaches to the treatment of Sjögren’s syndrome. J Rheumatol Suppl. 2000;61:15–21. [PubMed] [Google Scholar]
- 5.Voulgarelis M, Moutsopoulos HM. Lymphoproliferation in autoimmunity and Sjögren’s syndrome. Curr Rheumatol Rep. 2003;5:317–23. doi: 10.1007/s11926-003-0011-y. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosetti A, Zanotti R, Pattaro C, et al. Most cases of primary salivary mucosa-associated lymphoid tissue lymphoma are associated either with Sjögren’s syndrome or hepatitis C virus infection. Br J Haematol. 2004;126:43–9. doi: 10.1111/j.1365-2141.2004.04993.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Casals M, Trejo O, Garcia-Carrasco M, et al. Triple association between hepatitis C virus infection, systemic autoimmune diseases, and B cell lymphoma. J Rheumatol. 2004;31:495–9. [PubMed] [Google Scholar]
- 8.De Vita S, Boiocchi M, Sorrentino D, et al. Characterization of prelymphomatous stages of B cell lymphoproliferation in Sjögren’s syndrome. Arthritis Rheum. 1997;40:318–31. doi: 10.1002/art.1780400217. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan DA. Sex hormones and Sjögren’s syndrome. J Rheumatol. 1997;24(Suppl 50):17–32. [PubMed] [Google Scholar]
- 10.Sullivan DA. Androgen deficiency and dry eye syndromes. Arch Soc Esp Oftalmol. 2004;79:49–50. [PubMed] [Google Scholar]
- 11.Toda I, Wickham LA, Sullivan DA. Gender and androgen treatment influence the expression of proto-oncogenes and apoptotic factors in lacrimal and salivary tissues of MRL/lpr mice. Clin Immunol Immunopathol. 1998;86:59–71. doi: 10.1006/clin.1997.4466. [DOI] [PubMed] [Google Scholar]
- 12.Warren DW, Azzarolo AM, Huang ZM, et al. Androgen support of lacrimal gland function in the female rabbit. Adv Exp Med Biol. 1998;438:89–93. doi: 10.1007/978-1-4615-5359-5_11. [DOI] [PubMed] [Google Scholar]
- 13.Wickham LA, Rocha EM, Gao J, et al. Identification and hormonal control of sex steroid receptors in the eye. Adv Exp Med Biol. 1998;438:95–100. doi: 10.1007/978-1-4615-5359-5_12. [DOI] [PubMed] [Google Scholar]
- 14.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavill D, Waterman SA, Gordon TP. Antibodies raised against the second extracellular loop of the human muscarinic M3 receptor mimic functional autoantibodies in Sjögren’s syndrome. Scand J Immunol. 2004;59:261–6. doi: 10.1111/j.0300-9475.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 16.Cha S, Singson E, Cornelius J, et al. Muscarinic acetylcholine type-3 receptor desensitization due to chronic exposure to Sjögren’s syndrome-associated autoantibodies. J Rheumatol. 2006;33:296–306. [PubMed] [Google Scholar]
- 17.Li J, Ha YM, Ku NY, et al. Inhibitory effects of autoantibodies on the muscarinic receptors in Sjögren’s syndrome. Lab Invest. 2004;84:1430–8. doi: 10.1038/labinvest.3700173. [DOI] [PubMed] [Google Scholar]
- 18.Waterman SA, Gordon TP, Rischmueller M. Inhibitory effects of muscarinic receptor autoantibodies on parasympathetic neurotransmission in Sjögren’s syndrome. Arthritis Rheum. 2000;43:1647–54. doi: 10.1002/1529-0131(200007)43:7<1647::AID-ANR31>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Dawson LJ, Allison HE, Stanbury J, et al. Putative anti-muscarinic antibodies cannot be detected in patients with primary Sjögren’s syndrome using conventional immunological approaches. Rheumatology (Oxford) 2004;43:1488–95. doi: 10.1093/rheumatology/keh389. [DOI] [PubMed] [Google Scholar]
- 20.Dawson LJ, Field EA, Harmer AR, Smith PM. Acetylcholine-evoked calcium mobilization and ion channel activation in human labial gland acinar cells from patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2001;124:480–5. doi: 10.1046/j.1365-2249.2001.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith AJ, Jackson MW, Wang F, et al. Neutralization of muscarinic receptor autoantibodies by intravenous immunoglobulin in Sjogren syndrome. Hum Immunol. 2005;66:411–6. doi: 10.1016/j.humimm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Jackson MW, Maughan V, et al. Passive transfer of Sjögren’s syndrome IgG produces the pathophysiology of overactive bladder. Arthritis Rheum. 2004;50:3637–45. doi: 10.1002/art.20625. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Cha S, Jonsson R, et al. Detection of anti-type 3 muscarinic acetylcholine receptor autoantibodies in the sera of Sjögren’s syndrome patients by use of a transfected cell line assay. Arthritis Rheum. 2004;50:2615–21. doi: 10.1002/art.20371. [DOI] [PubMed] [Google Scholar]
- 24.Bolstad AI, Jonsson R. Genetic aspects of Sjögren’s syndrome. Arthritis Res. 2002;4:353–9. doi: 10.1186/ar599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harley JB, Reichlin M, Arnett FC, et al. Gene interaction at HLA-DQ enhances autoantibody production in primary Sjögren’s syndrome. Science. 1986;232:1145–7. doi: 10.1126/science.3458307. [DOI] [PubMed] [Google Scholar]
- 26.Gottenberg JE, Busson M, Loiseau P, et al. In primary Sjögren’s syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum. 2003;48:2240–5. doi: 10.1002/art.11103. [DOI] [PubMed] [Google Scholar]
- 27.Ricchiuti V, Isenberg D, Muller S. HLA association of anti-Ro60 and anti-Ro52 antibodies in Sjögren’s syndrome. J Autoimmun. 1994;7:611–21. doi: 10.1006/jaut.1994.1045. [DOI] [PubMed] [Google Scholar]
- 28.Morinobu A, Kanagawa S, Koshiba M, et al. Association of the glutathione S-transferase M1 homozygous null genotype with susceptibility to Sjögren’s syndrome in Japanese individuals. Arthritis Rheum. 1999;42:2612–5. doi: 10.1002/1529-0131(199912)42:12<2612::AID-ANR15>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai S, Kanagawa S, Morinobu A, et al. Association of a new allele of the TAP2 gene, TAP2*Bky2 (Val577), with susceptibility to Sjögren’s syndrome. Arthritis Rheum. 1997;40:1685–92. doi: 10.1002/art.1780400919. [DOI] [PubMed] [Google Scholar]
- 30.Robinson CP, Yamachika S, Bounous DI, et al. A novel NOD-derived murine model of primary Sjögren’s syndrome. Arthritis Rheum. 1998;41:150–6. doi: 10.1002/1529-0131(199801)41:1<150::AID-ART18>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Cha S, Nagashima H, Brown VB, et al. Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjögren’s syndrome) on a healthy murine background. Arthritis Rheum. 2002;46:1390–8. doi: 10.1002/art.10258. [DOI] [PubMed] [Google Scholar]
- 32.Brayer J, Lowry J, Cha S, et al. Alleles from chromosomes 1 and 3 of NOD mice combine to influence Sjögren’s syndrome-like autoimmune exocrinopathy. J Rheumatol. 2000;27:1896–904. [PubMed] [Google Scholar]
- 33.Cha S, Nagashima H, Brown VB, et al. Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjögren’s syndrome) on a healthy murine background. Arthritis Rheum. 2002;46:1390–8. doi: 10.1002/art.10258. [DOI] [PubMed] [Google Scholar]
- 34.Podolin PL, Denny P, Lord CJ, et al. Congenic mapping of the insulin-dependent diabetes (Idd) gene, Idd10, localizes two genes mediating the Idd10 effect and eliminates the candidate Fcgr1. J Immunol. 1997;159:1835–43. [PubMed] [Google Scholar]
- 35.Griffiths MM, Wang J, Joe B, et al. Identification of four new quantitative trait loci regulating arthritis severity and one new quantitative trait locus regulating autoantibody production in rats with collagen-induced arthritis. Arthritis Rheum. 2000;43:1278–89. doi: 10.1002/1529-0131(200006)43:6<1278::AID-ANR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Lodde BM, Sankar V, Kok MR, et al. Serum lipid levels in Sjögren’s syndrome. Rheumatology (Oxford) 2006;45:481–4. doi: 10.1093/rheumatology/kei190. [DOI] [PubMed] [Google Scholar]
- 37.Dykstra M, Cherukuri A, Sohn HW, et al. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–81. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 38.Schulz R. The pharmacology of phosducin. Pharmacol Res. 2001;43:1–10. doi: 10.1006/phrs.2000.0757. [DOI] [PubMed] [Google Scholar]
- 39.Gobeil S, Letartre L, Raymond V. Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res. 2006;82:1017–29. doi: 10.1016/j.exer.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Kong L, Robinson CP, Peck AB, et al. Inappropriate apoptosis of salivary and lacrimal gland epithelium of immunodeficient NOD-scid mice. Clin Exp Rheumatol. 1998;16:675–81. [PubMed] [Google Scholar]
- 41.Llorente L, Richaud-Patin Y, Fior R, et al. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjögren’s syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–55. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 42.Skarstein K, Nerland AH, Eidsheim M, et al. Lymphoid cell accumulation in salivary glands of autoimmune MRL mice can be due to impaired apoptosis. Scand J Immunol. 1997;46:373–8. doi: 10.1046/j.1365-3083.1997.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 43.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman RW, Alspaugh MA, Waggie KS, et al. Sjögren’s syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984;27:157–65. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- 45.Wahren M, Skarstein K, Blange I, et al. MRL/lpr mice produce anti-Ro 52,000 MW antibodies: detection, analysis of specificity and site of production. Immunology. 1994;83:9–15. [PMC free article] [PubMed] [Google Scholar]
- 46.Jonsson R, Tarkowski A, Backman K, et al. Sialadenitis in the MRL-l mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987;60:611–6. [PMC free article] [PubMed] [Google Scholar]
- 47.van Blokland SC, van Helden-Meeuwsen CG, Wierenga-Wolf AF, et al. Two different types of sialoadenitis in the NOD- and MRL/lpr mouse models for Sjögren’s syndrome: a differential role for dendritic cells in the initiation of sialoadenitis? Lab Invest. 2000;80:575–85. doi: 10.1038/labinvest.3780062. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg JB. Nitric oxide as an inflammatory mediator in autoimmune MRL-lpr/lpr mice. Environ Health Perspect. 1998;106 (Suppl 5):1131–7. doi: 10.1289/ehp.98106s51131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi Y, Haneji N, Hamano H. Cytokine gene expression and autoantibody production in Sjögren’s syndrome of MRL/lpr mice. Autoimmunity. 1996;23:269–77. doi: 10.3109/08916939608995349. [DOI] [PubMed] [Google Scholar]
- 50.Brayer JB, Cha S, Nagashima H, et al. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD. IL-4-gene knockout mouse model of Sjögren’s syndrome. Scand J Immunol. 2001;54:133–40. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 51.Cha S, Brayer J, Gao J, et al. A dual role for IFN-gamma in the pathogenesis of Sjögren’s syndrome-like autoimmune exocrinopathy in the NOD mouse. Scand J Immunol. 2004;60:552–65. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 52.Gao J, Killedar S, Cornelius JG, et al. Sjögren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. 2006;26:90–10. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Robinson CP, Yamamoto H, Peck AB, Humphreys-Beher MG. Genetically programmed development of salivary gland abnormalities in the NOD (nonobese diabetic)-scid mouse in the absence of detectable lymphocytic infiltration: a potential trigger for sialoadenitis of NOD mice. Clin Immunol Immunopathol. 1996;79:50–9. doi: 10.1006/clin.1996.0050. [DOI] [PubMed] [Google Scholar]
- 54.Brayer JB, Humphreys-Beher MG, Peck AB. Sjögren’s syndrome: immunological response underlying the disease. Arch Immunol Ther Exp. 2001;49:353–60. [PubMed] [Google Scholar]
- 55.Cha S, Peck AB, Humphreys-Beher MG. Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: an update. Crit Rev Oral Biol Med. 2002;13:5–16. doi: 10.1177/154411130201300103. [DOI] [PubMed] [Google Scholar]
- 56.Cha S, van Blockland SC, Versnel MA, et al. Abnormal organogenesis in salivary gland development may initiate adult onset of autoimmune exocrinopathy. Exp Clin Immunogenet. 2001;18:143–60. doi: 10.1159/000049194. [DOI] [PubMed] [Google Scholar]
- 57.Robinson CP, Cornelius J, Bounous DE, et al. Characterization of the changing lymphocyte populations and cytokine expression in the exocrine tissues of autoimmune NOD mice. Autoimmunity. 1998;27:29–44. doi: 10.3109/08916939809008035. [DOI] [PubMed] [Google Scholar]
- 58.Robinson CP, Cornelius J, Bounous DI, et al. Infiltrating lymphocyte populations and cytokine production in the salivary and lacrimal glands of autoimmune NOD mice. Adv Exp Med Biol. 1998;438:493–7. doi: 10.1007/978-1-4615-5359-5_68. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen KH, Brayer J, Cha S, et al. Evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in nod mice. Arthritis Rheum. 2000;43:2297–306. doi: 10.1002/1529-0131(200010)43:10<2297::AID-ANR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 60.Robinson CP, Brayer J, Yamachika S, et al. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren’s syndrome. Proc Natl Acad Sci U S A. 1998;95:7538–43. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan DA. Tearful relationships? Sex, hormones, the lacrimal gland and aqueous-deficient dry eye. Ocul Surf. 2004;2:92–123. doi: 10.1016/s1542-0124(12)70147-7. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan DA, Krenzer KL, Sullivan BD, et al. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci. 1999;40:1261–5. [PubMed] [Google Scholar]
- 63.Sullivan DA, Wickham LA, Rocha EM, et al. Androgens and dry eye in Sjögren’s syndrome. Ann N Y Acad Sci. 1999;876:312–24. doi: 10.1111/j.1749-6632.1999.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 64.Shimazaki J, Goto E, Ono M, et al. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology. 1998;105:1485–8. doi: 10.1016/S0161-6420(98)98033-2. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan DA, Sullivan BD, Evans JE, et al. Androgen deficiency, meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002;966:211–22. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 66.Pflugfelder SC, Tseng SC, Pepose JS, et al. Epstein-Barr virus infection and immunologic dysfunction in patients with aqueous tear deficiency. Ophthalmology. 1990;97:313–23. doi: 10.1016/s0161-6420(90)32595-2. [DOI] [PubMed] [Google Scholar]
- 67.Crouse CA, Pflugfelder SC, Cleary T, et al. Detection of Epstein-Barr virus genomes in normal human lacrimal glands. J Clin Microbiol. 1990;28:1026–32. doi: 10.1128/jcm.28.5.1026-1032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox RI, Luppi M, Pisa P, Kang HI. Potential role of Epstein-Barr virus in Sjögren’s syndrome and rheumatoid arthritis. J Rheumatol Suppl. 1992;32:18–24. [PubMed] [Google Scholar]
- 69.Ulbricht KU, Schmidt RE, Witte T. Antibodies against alpha-fodrin in Sjögren’s syndrome. Autoimmun Rev. 2003;2:109–13. doi: 10.1016/s1568-9972(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 70.Haneji N, Nakamura T, Takio K, et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjögren’s syndrome. Science. 1997;276:604–7. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 71.Yanagi K, Ishimaru N, Haneji N, et al. Anti-120-kDa alpha-fodrin immune response with Th1-cytokine profile in the NOD mouse model of Sjögren’s syndrome. Eur J Immunol. 1998;28:3336–45. doi: 10.1002/(SICI)1521-4141(199810)28:10<3336::AID-IMMU3336>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 72.Saegusa K, Ishimaru N, Haneji N, et al. Mechanisms of neonatal tolerance induced in an animal model for primary Sjögren’s syndrome by intravenous administration of autoantigen. Scand J Immunol. 2000;52:264–70. doi: 10.1046/j.1365-3083.2000.00777.x. [DOI] [PubMed] [Google Scholar]
- 73.Witte T, Matthias T, Arnett FC, et al. IgA and IgG autoantibodies against alpha-fodrin as markers for Sjögren’s syndrome. Systemic lupus erythematosus. J Rheumatol. 2000;27:2617–20. [PubMed] [Google Scholar]
- 74.Matsumura R, Kagami M, Tomioka H, et al. Expression of ductal Fas antigen in sialoadenitis of Sjögren’s syndrome. Clin Exp Rheumatol. 1996;14:309–11. [PubMed] [Google Scholar]
- 75.Sumida T, Matsumoto I, Murata H, et al. TCR in Fas-sensitive T cells from labial salivary glands of patients with Sjögren’s syndrome. J Immunol. 1997;158:1020–5. [PubMed] [Google Scholar]
- 76.Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41:1356–63. [PubMed] [Google Scholar]
- 77.Kong L, Ogawa N, Nakabayashi T, et al. Fas and Fas ligand expression in the salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum. 1997;40:87–97. doi: 10.1002/art.1780400113. [DOI] [PubMed] [Google Scholar]
- 78.Ohlsson M, Skarstein K, Bolstad AI, et al. Fas-induced apoptosis is a rare event in Sjögren’s syndrome. Lab Invest. 2001;81:95–105. doi: 10.1038/labinvest.3780215. [DOI] [PubMed] [Google Scholar]
- 79.Ishimaru N, Yoneda T, Saegusa K, et al. Severe destructive autoimmune lesions with aging in murine Sjögren’s syndrome through Fas-mediated apoptosis. Am J Pathol. 2000;156:1557–64. doi: 10.1016/S0002-9440(10)65027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jabs DA, Lee B, Whittum-Hudson J, Prendergast RA. The role of Fas-Fas ligand-mediated apoptosis in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest Ophthalmol Vis Sci. 2001;42:399–401. [PubMed] [Google Scholar]
- 81.Rosen A, Casciola-Rosen L. Altered autoantigen structure in Sjögren’s syndrome: implications for the pathogenesis of autoimmune tissue damage. Crit Rev Oral Biol Med. 2004;15:156–64. doi: 10.1177/154411130401500304. [DOI] [PubMed] [Google Scholar]
- 82.Hall JC, Casciola-Rosen L, Rosen A. Altered structure of autoantigens during apoptosis. Rheum Dis Clin North Am. 2004;30:455–71. doi: 10.1016/j.rdc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995;182:1625–34. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casciola-Rosen L, Andrade F, Ulanet D, et al. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohlsson M, Szodoray P, Loro LL, et al. CD40, CD154, Bax and Bcl-2 expression in Sjögren’s syndrome salivary glands: a putative anti-apoptotic role during its effector phases. Scand J Immunol. 2002;56:561–71. doi: 10.1046/j.1365-3083.2002.01168.x. [DOI] [PubMed] [Google Scholar]
- 87.Boumba D, Skopouli FN, Moutsopoulos HM. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren’s syndrome. Br J Rheumatol. 1995;34:326–33. doi: 10.1093/rheumatology/34.4.326. [DOI] [PubMed] [Google Scholar]
- 88.Fox RI, Kang HI, Ando D, et al. Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]
- 89.Ohyama Y, Nakamura S, Matsuzaki G, et al. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 1996;39:1376–84. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- 90.Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999;92:265–75. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- 91.Sugai M, Gonda H, Nambu Y, et al. Role of Id proteins in B lymphocyte activation: new insights from knockout mouse studies. J Mol Med. 2004;82:592–9. doi: 10.1007/s00109-004-0562-z. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjögren’s syndrome. Immunity. 2004;21:551–60. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Kowanetz M, Valcourt U, Bergstrom R, et al. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol. 2004;24:4241–54. doi: 10.1128/MCB.24.10.4241-4254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cha S, Brayer J, Gao J, et al. A dual role for interferon-gamma in the pathogenesis of Sjögren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60:552–65. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 95.Brayer JB, Cha S, Nagashima H, et al. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD. IL-4-gene knockout mouse model of Sjögren’s syndrome. Scand J Immunol. 2001;54:133–40. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 96.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 97.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 98.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 101.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 102.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 103.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 104.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hue S, Ahern P, Buonocore S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 107.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakai A, Sugawara Y, Kuroishi T, et al. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181:2898–906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 109.Manoussakis MN, Boiu S, Korkolopoulou P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren’s syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–88. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- 110.Bombardieri M, Barone F, Pittoni V, et al. Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjögren’s syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res Ther. 2004;6:R447–56. doi: 10.1186/ar1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delaleu N, Immervoll H, Cornelius J, Jonsson R. Biomarker profiles in serum and saliva of experimental Sjögren’s syndrome: associations with specific autoimmune manifestations. Arthritis Res Ther. 2008;10:R22. doi: 10.1186/ar2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 113.Hansen A, Gosemann M, Pruss A, et al. Abnormalities in peripheral B cell memory of patients with primary Sjögren’s syndrome. Arthritis Rheum. 2004;50:1897–908. doi: 10.1002/art.20276. [DOI] [PubMed] [Google Scholar]
- 114.Hansen A, Lipsky PE, Dorner T. New concepts in the pathogenesis of Sjogren syndrome: many questions, fewer answers. Curr Opin Rheumatol. 2003;15:563–70. doi: 10.1097/00002281-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 115.Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 2003;48:3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 116.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavie F, Miceli-Richard C, Quillard J, et al. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren’s syndrome. J Pathol. 2004;202:496–502. doi: 10.1002/path.1533. [DOI] [PubMed] [Google Scholar]
- 118.Mariette X, Roux S, Zhang J, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren’s syndrome. Ann Rheum Dis. 2003;62:168–71. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szodoray P, Jellestad S, Teague MO, Jonsson R. Attenuated apoptosis of B cell activating factor-expressing cells in primary Sjögren’s syndrome. Lab Invest. 2003;83:357–65. [PubMed] [Google Scholar]
- 120.Szodoray P, Jellestad S, Alex P, et al. Programmed cell death of peripheral blood B cells determined by laser scanning cytometry in Sjögren’s syndrome with a special emphasis on BAFF. J Clin Immunol. 2004;24:600–11. doi: 10.1007/s10875-004-6240-7. [DOI] [PubMed] [Google Scholar]
- 121.Mimori T. Autoantibodies in connective tissue diseases: clinical significance and analysis of target autoantigens. Intern Med. 1999;38:523–32. doi: 10.2169/internalmedicine.38.523. [DOI] [PubMed] [Google Scholar]
- 122.Harley JB, Alexander EL, Bias WB, et al. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren’s syndrome. Arthritis Rheum. 1986;29:196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- 123.Muller S, Briand JP, Barakat S, et al. Autoantibodies reacting with poly(ADP-ribose) and with a zinc-finger functional domain of poly(ADP-ribose) polymerase involved in the recognition of damaged DNA. Clin Immunol Immunopathol. 1994;73:187–96. doi: 10.1006/clin.1994.1187. [DOI] [PubMed] [Google Scholar]
- 124.Hayashi Y, Arakaki R, Ishimaru N. Apoptosis and estrogen deficiency in primary Sjogren syndrome. Curr Opin Rheumatol. 2004;16:522–6. doi: 10.1097/01.bor.0000135450.78047.78. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi M, Yasui N, Ishimaru N, et al. Development of autoimmune arthritis with aging via bystander T cell activation in the mouse model of Sjögren’s syndrome. Arthritis Rheum. 2004;50:3974–84. doi: 10.1002/art.20679. [DOI] [PubMed] [Google Scholar]
- 126.Takada K, Takiguchi M, Konno A, Inaba M. Autoimmunity against a tissue kallikrein in IQI/Jic Mice: a model for Sjögren’s syndrome. J Biol Chem. 2005;280:3982–8. doi: 10.1074/jbc.M410157200. [DOI] [PubMed] [Google Scholar]
- 127.Robinson CP, Bounous DI, Alford CE, et al. Aberrant expression and potential function for parotid secretory protein (PSP) in the NOD (non-obese diabetic) mouse. Adv Exp Med Biol. 1998;438:925–30. doi: 10.1007/978-1-4615-5359-5_131. [DOI] [PubMed] [Google Scholar]
- 128.Winer S, Astsaturov I, Cheung R, et al. Primary Sjögren’s syndrome and deficiency of ICA69. Lancet. 2002;360:1063–9. doi: 10.1016/S0140-6736(02)11144-5. [DOI] [PubMed] [Google Scholar]
- 129.Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul Surf. 2004;2:76–91. doi: 10.1016/s1542-0124(12)70146-5. [DOI] [PubMed] [Google Scholar]
- 130.Dartt DA, Hodges RR, Zoukhri D. Signal transduction pathways activated by cholinergic and alpha 1-adrenergic agonists in the lacrimal gland. Adv Exp Med Biol. 1998;438:113–21. doi: 10.1007/978-1-4615-5359-5_15. [DOI] [PubMed] [Google Scholar]
- 131.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 132.Delalande S, de Seze J, Fauchais AL, et al. Neurologic manifestations in primary Sjogren syndrome: a study of 82 patients. Medicine (Baltimore) 2004;83:280–91. doi: 10.1097/01.md.0000141099.53742.16. [DOI] [PubMed] [Google Scholar]
- 133.Malinow KL, Molina R, Gordon B, et al. Neuropsychiatric dysfunction in primary Sjögren’s syndrome. Ann Intern Med. 1985;103:344–50. doi: 10.7326/0003-4819-103-3-344. [DOI] [PubMed] [Google Scholar]
- 134.Belin C, Moroni C, Caillat-Vigneron N, et al. Central nervous system involvement in Sjögren’s syndrome: evidence from neuropsychological testing and HMPAO-SPECT. Ann Med Interne (Paris) 1999;150:598–604. [PubMed] [Google Scholar]
- 135.Valtysdottir ST, Gudbjornsson B, Lindqvist U, et al. Anxiety and depression in patients with primary Sjögren’s syndrome. J Rheumatol. 2000;27:165–9. [PubMed] [Google Scholar]
- 136.Dawson LJ, Fox PC, Smith PM. Sjogrens syndrome--the non-apoptotic model of glandular hypofunction. Rheumatology (Oxford) 2006;45:792–8. doi: 10.1093/rheumatology/kel067. [DOI] [PubMed] [Google Scholar]
- 137.Sullivan DA. Possible mechanisms involved in the reduced tear secretion in Sjögren’s Syndrome: state of the art. Amsterdam: Kugler Press; 1994. pp. 13–9. [Google Scholar]
- 138.Cha S, Singson E, Cornelius J, et al. Muscarinic acetylcholine type-3 receptor desensitization due to chronic exposure to Sjögren’s syndrome-associated autoantibodies. J Rheumatol. 2006;33:296–306. [PubMed] [Google Scholar]
- 139.Cavill D, Waterman SA, Gordon TP. Antiidiotypic antibodies neutralize autoantibodies that inhibit cholinergic neurotransmission. Arthritis Rheum. 2003;48:3597–602. doi: 10.1002/art.11343. [DOI] [PubMed] [Google Scholar]
- 140.Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169–77. doi: 10.1097/01.rhu.0000135553.08057.21. [DOI] [PubMed] [Google Scholar]
- 141.Petrone D, Condemi JJ, Fife R, et al. A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 2002;46:748–54. doi: 10.1002/art.510. [DOI] [PubMed] [Google Scholar]
- 142.Tsifetaki N, Kitsos G, Paschides CA, et al. Oral pilocarpine for the treatment of ocular symptoms in patients with Sjögren’s syndrome: a randomised 12 week controlled study. Ann Rheum Dis. 2003;62:1204–7. doi: 10.1136/ard.2002.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ono M, Takamura E, Shinozaki K, et al. Therapeutic effect of cevimeline on dry eye in patients with Sjögren’s syndrome: a randomized, double-blind clinical study. Am J Ophthalmol. 2004;138:6–17. doi: 10.1016/j.ajo.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 144.Aragona P, Di Pietro R, Spinella R, Mobrici M. Conjunctival epithelium improvement after systemic pilocarpine in patients with Sjögren’s syndrome. Br J Ophthalmol. 2006;90:166–70. doi: 10.1136/bjo.2005.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawasaki S, Kawamoto S, Yokoi N, et al. Up-regulated gene expression in the conjunctival epithelium of patients with Sjögren’s syndrome. Exp Eye Res. 2003;77:17–26. doi: 10.1016/s0014-4835(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 146.Thomas PD, Campbell MJ, Kejariwal A, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–41. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Killedar SY, Eckenrode SE, McIndoe RA, et al. Early pathogenic events associated with Sjögren’s syndrome (SjS)-like disease of the nod mouse using microarray analysis. Lab Invest. 2006;86:1243–60. doi: 10.1038/labinvest.3700487. [DOI] [PubMed] [Google Scholar]
- 148.Nguyen C, Singson E, Kim JY, et al. Sjögren’s syndrome-like disease of C57BL/6. NOD-Aec1 Aec2 mice: gender differences in keratoconjunctivitis sicca defined by a cross-over in the chromosome 3 Aec1 locus. Scand J Immunol. 2006;64:295–307. doi: 10.1111/j.1365-3083.2006.01828.x. [DOI] [PubMed] [Google Scholar]
- 149.Ohashi Y, Ishida R, Kojima T, et al. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003;136:291–9. doi: 10.1016/s0002-9394(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 150.Argueso P, Balaram M, Spurr-Michaud S, et al. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–11. [PubMed] [Google Scholar]
- 151.Tsubota K, Mishima K, Obara K, et al. Reactive oxygen species can be controlled by the secretory glycoprotein, clusterin, from side population cells in the lacrimal gland: a new intervention for age-related dry eye disorders. Presentation: 5th International Conference on the Tear Film and Ocular Surface: Basic Science and Clinical Relevance; Taormina, Sicily, Italy. Sept 5–8, 2007. [Google Scholar]
- 152.Tomosugi N, Kitagawa K, Takahashi N, et al. Diagnostic potential of tear proteomic patterns in Sjögren’s syndrome. J Proteome Res. 2005;4:820–5. doi: 10.1021/pr0497576. [DOI] [PubMed] [Google Scholar]
- 153.Hu S, Wang J, Meijer J, et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007;56:3588–600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Serreze DV, Gaskins HR, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993;150:2534–43. [PubMed] [Google Scholar]
- 155.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–51. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- 156.Szatmari I, Torocsik D, Agostini M, et al. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood. 2007;110:3271–80. doi: 10.1182/blood-2007-06-096222. [DOI] [PubMed] [Google Scholar]