Abstract
Background
Previous studies indicated that chronic alcohol drinking increased the sensitivity of the posterior ventral tegmental area (p-VTA) to the reinforcing effects of ethanol. The current study tested the hypothesis that local exposure of the p-VTA to ethanol would increase the sensitivity of dopamine (DA) neurons to the stimulating effects of ethanol.
Methods
Experiment 1 examined the stimulating effects of ethanol in the p-VTA after a 7-day ethanol pretreatment in the p-VTA. Adult female Wistar rats were pretreated with microinjections of 200 mg% ethanol or artificial cerebrospinal fluid (aCSF) into the p-VTA once a day for 7 days. On the eighth day, rats received a challenge injection of ethanol (100, 200, or 300 mg%) or aCSF into the p-VTA, and extracellular DA levels were measured in the nucleus accumbens (NAc) shell with microdialysis. Experiment 2 examined the stimulating effects of ethanol (200 mg%) after a 3- or 5-day ethanol (200 mg%) pretreatment in the p-VTA. Experiment 3 examined the stimulating effects of ethanol (200 mg%) 7 days after the last of the 7-day ethanol (200 mg%) pretreatments in the p-VTA.
Results
Experiment 1: in both aCSF- and ethanol-pretreated rats, the challenge microinjection of ethanol dose-dependently increased DA release in the NAc shell, with significantly greater increases in ethanol-pretreated groups. Experiment 2: the 5-day, but not 3-day, ethanol pretreatment protocol increased the response of p-VTA dopamine neurons to the ethanol challenge. Experiment 3: the increased stimulating effects of ethanol were still evident after 7 days.
Conclusions
The results indicate that repeated local ethanol exposure of the p-VTA produced neuroadaptations in DA neurons projecting to the NAc shell, resulting in a persistent increase in the sensitivity of these neurons to the stimulating effects of ethanol.
Keywords: Dopamine, Ethanol, Microdialysis, Nucleus Accumbens, Sensitization, Ventral Tegmental Area
The Mesolimbic Dopamine (DA) system projects from DA neurons in the midbrain ventral tegmental area (VTA) to several limbic forebrain regions, mainly the nucleus accumbens (NAc) (Oades and Halliday, 1987). A number of studies have associated this pathway with mediating the reinforcing and rewarding effects of drugs of abuse, including ethanol (Gonzales et al., 2004; Koob, 1992; Koob et al., 1998; McBride et al., 1999; Wise, 1996).
Electrophysiological and neurochemical studies indicate that ethanol activates the mesolimbic DA system. Ethanol increased the firing rates of VTA DA neurons both in vivo (Foddai et al., 2004; Gessa et al., 1985) and in vitro (Brodie et al., 1990, 1999). Studies using the in vivo microdialysis technique demonstrated that experimenter-administered ethanol increased extracellular DA levels in the VTA (Campbell et al., 1996; Kohl et al., 1998) and NAc (Campbell and McBride, 1995; Imperato and Di Chiara, 1986; Yoshimoto et al., 1991). Operant self-administration of ethanol was also shown to enhance NAc DA levels (Gonzales and Weiss, 1998; Melendez et al., 2002; Weiss et al., 1993, 1996), although recent findings (Doyon et al., 2004, 2005) suggested that the increase of NAc DA levels after self-administration might not be due solely to the local pharmacological effects of ethanol. A number of studies testing the effects of dopaminergic agents on alcohol drinking in animals also suggest an association between the mesolimbic DA system and alcohol drinking. Nonspecific blockade of DA receptors, either globally or locally within the NAc, decreased oral ethanol intake and operant responding for ethanol (Rassnick et al., 1992; Samson et al., 1993). Specific activation of VTA D2 autoreceptors also decreased voluntary oral ethanol intake and operant responding (Hodge et al., 1993; Nowak et al., 2000). Furthermore, physiologically relevant concentrations of ethanol were self-infused directly into the posterior VTA (p-VTA) (Gatto et al., 1994; Rodd-Henricks et al., 2000), suggesting this region is a neuroanatomical substrate for the reinforcing effects of ethanol. Subsequent studies indicated that activation of p-VTA DA neurons is involved in the local reinforcing effects of ethanol (Rodd et al., 2004, 2005c).
Systemic ethanol pre-exposure in rodents produces neuroadaptations within the mesolimbic system. Repeated intermittent exposure to ethanol increased the responsiveness of VTA DA neurons to the excitation of a subsequent ethanol challenge in vitro (Brodie, 2002), elevated basal extracellular DA concentrations in the NAc (Smith and Weiss, 1999), decreased DA D2 autoreceptor function in the NAc (Engleman et al., 2003), and increased ethanol intake (Camarini and Hodge, 2004). Chronic ethanol exposure, via liquid diet, vapor chamber or continuous access, increased operant responding for ethanol (Schulteis et al., 1996) and subsequent voluntary oral ethanol intake (Lopez and Becker, 2005; Melis et al., 2002). Furthermore, chronic alcohol drinking and exposure to repeated deprivations enhanced the reinforcing effects of ethanol in the p-VTA (Rodd et al., 2005a,2005b). However, it remains unknown, given the global effects with systemic ethanol exposure, whether ethanol-induced neuroadaptations were initiated within the VTA, NAc, or even other brain regions modulating the activity of the mesolimbic system.
The electrolytic microinfusion transducer (EMIT) system (Bozarth and Wise, 1980; Goeders and Smith, 1987) has been used to test the local reinforcing effects of ethanol within the VTA (Gatto et al., 1994; Rodd et al., 2004, 2005c; Rodd-Henricks et al., 2000), and in a preliminary study to test the local stimulating effects of ethanol in the VTA on DA release in the NAc. In the later study, pulse injections of ethanol into the p-VTA increased DA release in the NAc shell. The use of the EMIT system provided a feasible approach to investigate the effects of direct ethanol exposure of the p-VTA on subsequent ethanol effects on VTA DA neurons. The hypothesis to be tested in the current study was that direct repeated exposure of the p-VTA to ethanol would produce neuroadaptations that would increase the sensitivity of VTA DA neurons to the stimulating effects of ethanol. Rats were given microinjections of ethanol into the p-VTA over multiple days, then microdialysis was conducted, during which rats received challenge injections of ethanol into the p-VTA. The change in extracellular DA levels in the NAc shell was used as an index of the local stimulating effects of ethanol within the p-VTA.
MATERIALS AND METHODS
Animals
Adult female Wistar rats (weight 270 to 320 g, Harlan, Indianapolis, IN) were doubly housed in temperature- and humidity-controlled rooms for at least 2 weeks prior to the experiment. Rats were maintained in a regular 12-hour light–dark cycle room (light on 7:00 AM) and food and water were available ad libitum. Female rats were used because these rats appeared to maintain their head size better than male rats for more accurate stereotaxic placement (Rodd-Henricks et al., 2000). The estrous cycle was not monitored in the present study. However, counterbalanced experiments were conducted on different days so that any effect of a given phase of the estrous cycle was distributed across experimental conditions. All experimental procedures were conducted during the light phase. Protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were conducted in accordance with principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Stereotaxic Surgery Procedures
Rats were stereotaxically implanted with 2 guide cannulae (Plastics One, Inc., Roanoke, VA) under 2% isoflurane anesthesia. One 22-gauge cannula was aimed above the p-VTA for microinjections, and the other 18-gauge cannula above the ipsilateral NAc shell for microdialysis. The coordinates for the target areas were: the NAc shell: AP +1.7 mm, ML +2.3 mm, DV −8.2 mm; the anterior VTA (a-VTA): AP −4.8 mm, ML +2.1 mm, DV −9.0 mm; the p-VTA: AP )5.6 mm, ML +2.1 mm, DV −9.0 mm (Paxinos and Watson, 1998). The incisor bar was set at −3.3 mm. Both cannulae were implanted at a 10° angle to the vertical. Stylets were inserted into cannulae when no experiments were being conducted. Rats were singly housed following surgery, and were allowed to recover from surgery for at least 5 days, during which they were habituated in the microdialysis chambers and handled everyday. Loop-style dialysis probes (active length 1.5 mm, Spectra/Por RC, inner diameter 200 µm, molecular weight cut-off: 13,000, Spectrum Laboratories, Inc, Rancho Dominguez, CA) were constructed (Benveniste and Huttemeier, 1990; Engleman et al., 2003) and inserted into the NAc shell, as previously described (Kohl et al., 1998).
EMIT System
The EMIT system has been utilized in intracranial self-administration experiments to identity the neuroanatomical substrates supporting the reinforcement of drugs of abuse (Goeders and Smith, 1987; McBride et al., 1999; Wise and Hoffman, 1992). It consists of a current generator (MNC Shreveport, LA), a lead attached with 2 electrodes, and a drug reservoir (28 mm in length × 6 mm in diameter) attached with an injector cannula (28-gauge) inserted into the brain site (Bozarth and Wise, 1980; Goeders and Smith, 1987). The EMIT system produces an electric current between the 2 electrodes that are immersed in the drug reservoir. The current produces hydrogen gas, which in turn forces a controlled amount of solution out of the reservoir into the targeted brain region (Bozarth and Wise, 1980; Goeders and Smith, 1987). The volume of injected solution depends on the current between the 2 electrodes. In the current study, the EMIT system was calibrated to produce a reliable and reproducible injection of 100 nl solution over a 5-second period, as described before (Bozarth and Wise, 1981; Gatto et al., 1994; Rodd-Henricks et al., 2000). A 10-µA quiescent current was used between injections to maintain the electrodes in an active state without ejecting solution. A cable and swivel (Model 205, Mercotac, Inc., Carlsbad, CA) connecting the current generator and lead allowed free movement of animals during experiments.
Pretreatment Procedure
The desired concentrations of ethanol were made by mixing 95% ethanol with artificial cerebrospinal fluid (aCSF: 120 mM NaCl, 4.75 mM KCl, 1.2 mM KH2PO4, 1.2mM MgSO4, 25mM NaHCO3, 2.5 mM CaCl, 10 mM D-glucose, pH 7.2 to 7.4). For 3, 5, or 7 consecutive days, depending on the experiment, aCSF or 200 mg% ethanol was injected into the VTA once a day. Each day, 30 pulse injections were conducted during a 10-minute period. Each pulse injection infused 100-nl solution over 5 seconds into the VTA, which was followed by a 15-second timeout period with no infusion. A total of 3 µl of solution was injected each day. After injection, the injector was left in the brain for 30 seconds before being removed.
General Microdialysis Procedure
Microdialysis probes were inserted into the NAc shell 16 to 18 hours before microdialysis. General dialysis procedures were described previously (Engleman et al., 2003; Kohl et al., 1998). Briefly, rats were placed into Plexiglas chambers (40 cm × 28 cm × 40 cm) and connected to a Harvard pump with PE20 tubing (inner diameter 0.38 mm; Becton Dickinson & Co., MD). Microdialysis aCSF (140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2H-PO4·7H2O, 1.0 mM MgCl2, and 0.2 mM ascorbate, pH 7.2 to 7.4) was perfused through probes at a rate of 1.0 µl/min. After a 90-minute washout period, four to five 20-minute baseline samples were collected. Then, rats received challenge microinjections of either ethanol or aCSF for 10 minutes following the same injection procedure as applied during pretreatments. Six 20-minute samples were collected thereafter. The “dead time” of the microdialysis probe outlet was approximately 20 minutes and has been taken into account in all of the analyses. All samples were collected into vials containing 5 µl of perchloric acid (0.1 N), frozen immediately on dry ice, and stored at −70°C. A previous study indicated no degradation of DA up to 1 month using this procedure (Campbell and McBride, 1995).
Experiment 1: The Effect of Challenge Microinjections of Ethanol in the p-VTA on Extracellular DA Levels in the NAc Shell After a 7-Day Pretreatment With Ethanol or aCSF
Four different sub-experiments were conducted at distinct time periods, and were therefore analyzed separately. Experiment 1a included 4 groups of rats (n = 5to 7/group) with 2 groups pretreated with aCSF and the other 2 pretreated with 200 mg% ethanol from day 1 (D1) to D7. Microdialysis was conducted on D8, during which the aCSF- and ethanol-pretreated rats received challenge microinjections of aCSF or 200 mg% ethanol, and extracellular DA levels were measured in the NAc shell. Experiment 1b was conducted at a later time after the results of experiment 1a had been analyzed. For experiment 1b, there were 2 groups (n =5 to 6/group). One group was pretreated with aCSF and the other was pretreated with 200 mg% ethanol for 7 days. During microdialysis on D8, both groups were challenged with 100 mg% ethanol injections. Experiment 1c was conducted at a later time after the results of experiment 1b had been analyzed. For experiment 1c, there were 2 groups (n = 4 or 7/group). One group was pretreated with aCSF and the other was pretreated with 200 mg% ethanol for 7 days. During microdialysis on D8, both groups were challenged with 300 mg% ethanol injections. Experiment 1d included 2 control groups (n = 4/group) receiving microinjections of aCSF or 200 mg% ethanol into the a-VTA following the same microinjection procedure used for the p-VTA; extracellular DA levels were determined in the NAc shell.
These concentrations of ethanol are physiologically relevant and can be obtained in blood by rats under different drinking conditions (Murphy et al., 1986; Waller et al., 1984). Wistar rats selfadministered ethanol into the p-VTA at these concentrations (Rodd-Henricks et al., 2000). These concentrations (in mM: 200 mg% = 44 mM) are within the range of concentrations that were used in in vitro slice experiments (up to 100 to 200 mM; Brodie et al., 1990; Lovinger et al., 1989). The 7-day pretreatment regimen was based upon the protocols used for intracranial self-administration of ethanol, in which 7 self-infusion sessions were typically performed (Rodd et al., 2004; Rodd-Henricks et al., 2000).
Experiment 2: The Effects of the 3- or 5-Day Ethanol Pretreatment on the Effects of Ethanol Challenge on Extracellular DA Levels in the NAc Shell
There were 3 groups in this experiment (n = 5 to 6/group). One group received aCSF pretreatment from D1 to D5; a second group received aCSF pretreatment from D1 to D2 and 200 mg% ethanol pretreatment from D3 to D5; and the third group received 200 mg% ethanol pretreatment from D1 to D5. Microdialysis was conducted on D6, during which all rats received challenge microinjections of 200 mg% ethanol and extracellular DA levels were measured in the NAc shell.
Experiment 3: The Persisting Effects of 7-Day Ethanol Pretreatment
Two groups of rats (n = 5/group) received either aCSF or 200 mg% ethanol pretreatment from D1 to D7. All rats were maintained in the home cage from D8 to D14 without ethanol exposure. Microdialysis was conducted on D15, during which all rats received challenge microinjections of 200 mg% ethanol, and extracellular DA levels were measured in the NAc shell.
Sample Analysis
DA in samples was analyzed with a reversed-phase high performance liquid chromatography system with electrochemical detection, as described previously (Engleman et al., 2006). Briefly, samples were loaded into a 10-µl loop and injected onto an analytical column (BDS Hypersil C18, 3 µm, 150 mm × 2 mm, Keystone Scientific, Bellefonte, PA). The mobile phase (sodium phosphate 9.0 g/l, EDTA 190 mg/l, sodium octyl-sulfate 350 mg/l, and 10% acetonitrile at pH 3.0) was delivered by an ESA 582 solvent delivery system (Chelmsford, MA). Two 3-mm dual glassy carbon electrodes were connected in series (oxidizing potentials set at + 720 and +100 mV, respectively) and coupled to an amperometric detector (EG&G Princeton Applied Research, Princeton, NJ). DA was detected at the second electrode with sensitivity set at 0.5 nA/V. The output from the detector was sent to a ChromPerfect (Ver 4.4.0, Justice Innovations, Inc., Palo Alto, CA) chromatography data analysis program. The lower limit for DA detection was approximately 0.1 nM.
Histology
At the end of each experiment, rats were killed with an overdose of CO2 inhalation; 1% bromophenol blue was then perfused through probes in the NAc shell and also injected into the p-VTA. Brains were removed quickly and frozen immediately on dry ice and stored at −20°C. Sections (40 µm) were sliced on a cryostat microtome and stained with cresyl violet for verification of injection sites and placements of probes with reference to the rat brain atlas of Paxinos and Watson (1998).
Statistical Analysis
The last 3 baseline samples prior to challenge microinjections were averaged and used to normalize data. Effects on extracellular DA levels are normalized as percent of baseline. ANOVAs with repeated measures on time were employed on normalized data. ANOVAs were also conducted on raw time-course data and yielded similar significant effects as observed with the normalized data. If significant differences were detected with ANOVAs (p <0.05), post-hoc LSD or Student’s t-tests were performed to determine the individual differences. In experiment 1, the values of area under the curve for each group were calculated and analyzed with two-way ANOVA to determine the dose-response effect.
RESULTS
Histology
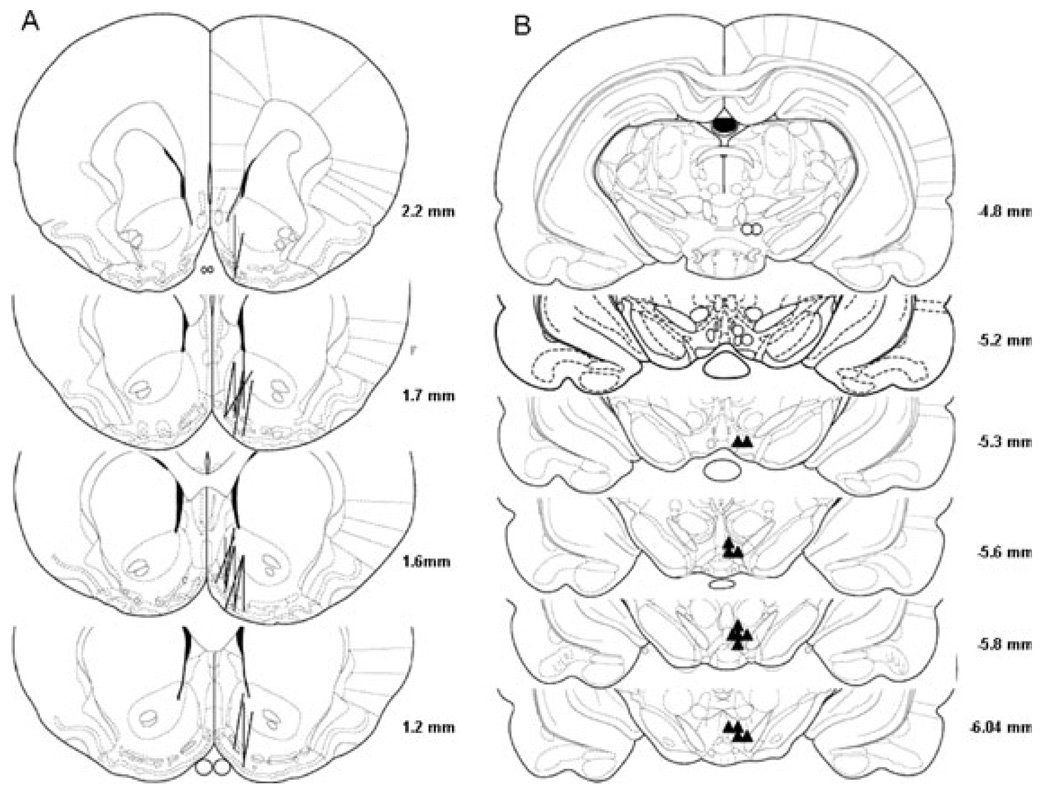
Figure 1 shows the representative placements of probes and injection sites. Overlapping probes and injection sites are not shown for clarity purposes. The correct placement for probes should have at least 75% of the active membrane within the NAc shell. Some probes also covered a portion of the olfactory tubercle. The p-VTA is at the level of the interpeduncular nucleus from 5.3 mm to 6.0 mm posterior to bregma, and the a-VTA is at the level of the mammillary body from 4.8 mm to 5.2 mm posterior to bregma (Rodd-Henricks et al., 2000). Only rats with correct placements in both the NAc shell and VTA were included for analysis, which accounted for about 80% of rats that underwent surgery. The injection sites outside of the VTA were mainly in the red nucleus and substantia nigra and injection of ethanol into these areas did not alter extracellular DA levels in the NAc shell (data not shown).
Fig. 1.
Representative placements of microdialysis probes in the NAc shell and microinjection sites in the VTA. (A) The lines represent the 1.5-mm length of microdialysis probes in the NAc shell. (B) The open circles and filled triangles represent microinjection sites within the a-VTA and p-VTA, respectively.
Experiment 1: Effect of Challenge Microinjections of Ethanol in the p-VTA on Extracellular DA Levels in the NAc Shell After Pretreated for 7 Days With 200 mg% Ethanol or aCSF
The basal extracellular DA levels in aCSF-pretreated rats (0.9 ± 0.2 nM) were not different than levels for the 200 mg% ethanol-pretreated rats (0.9 ± 0.1 nM). The levels in the current study are within the range of basal extracellular DA levels reported for the NAc (Engleman et al., 2000; Melendez et al., 2002).
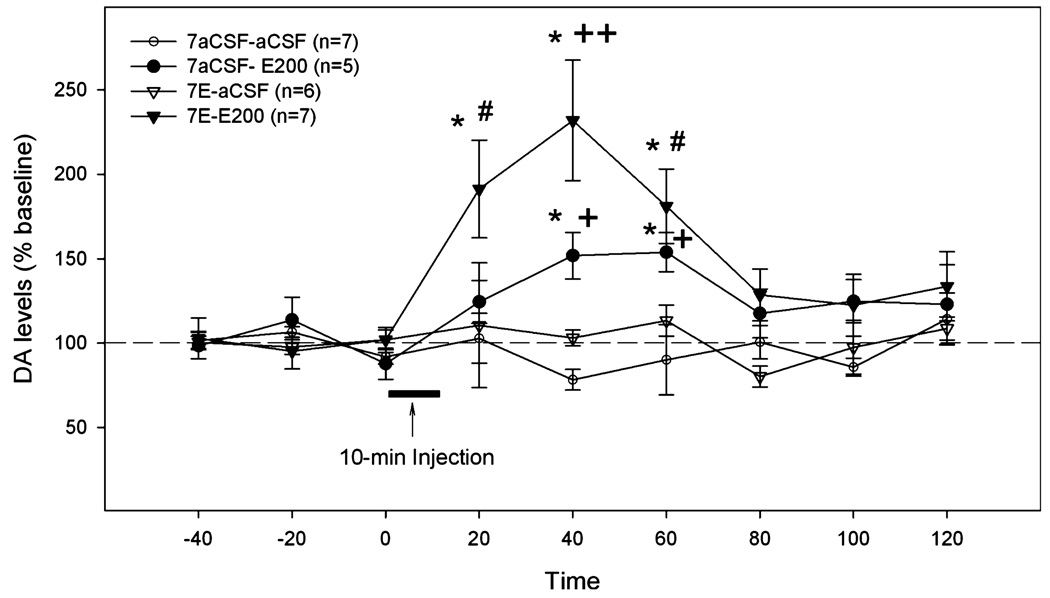
Figure 2 represents results from experiment 1a showing the effects of the challenge injection of aCSF or 200 mg% ethanol on extracellular DA levels in the NAc shell of rats pretreated with either aCSF or 200 mg% ethanol for 7 days. An ANOVA with repeated measures on time revealed significant effects of time, group, and time × group interaction (all F-values >1.93, all p-values <0.05). The challenge microinjection of 200 mg% ethanol into both the aCSF- and 200 mg% ethanol-pretreated rats increased DA levels in the NAc shell, with a significantly greater increase in the ethanol-pretreated rats. In the aCSF-pretreated rats, extracellular DA levels started to increase within 20 minutes after injection (124 ± 12% of baseline), reached a peak level 20 minutes later (151 ± 11% of baseline, p <0.05), and remained elevated for another 20 minutes before returning to baseline levels. In ethanol pretreated rats, the extracellular DA levels increased sharply to 191 ± 28% of baseline (p < 0.05) within 20 minutes after the challenge injection, reached a peak level of 232 ± 35% of baseline (p < 0.05) at 40 minutes, then gradually returned to baseline level at 80 minutes after the challenge microinjection. On the other hand, the challenge microinjection of aCSF in both aCSF- and ethanol-pretreated rats did not alter extracellular DA levels in the NAc shell. Because of this, the aCSF-challenged group was not included in the following experiments.
Fig. 2.
The effects of the challenge microinjection of aCSF or 200 mg% ethanol into the p-VTA on extracellular DA levels in the NAc shell of rats pretreated for 7 days with aCSF or 200 mg% ethanol. “7aCSF-aCSF” = pretreated for 7 days with aCSF and challenged with aCSF; “7aCSF-E200” = -pretreated for 7 days with aCSF and challenged with 200 mg% ethanol; “7E-aCSF” = pretreated for 7 days with 200 mg% ethanol and challenged with aCSF; “7E-E200” = pretreated for 7 days with 200 mg% ethanol and challenged with 200 mg% ethanol. *p < 0.05, different from baseline levels; #p < 0.05, different from “7aCSF-aCSF” and “7E-aCSF” groups; +p < 0.05, different from “7aCSF-aCSF” group; ++p < 0.05, different from all other groups.
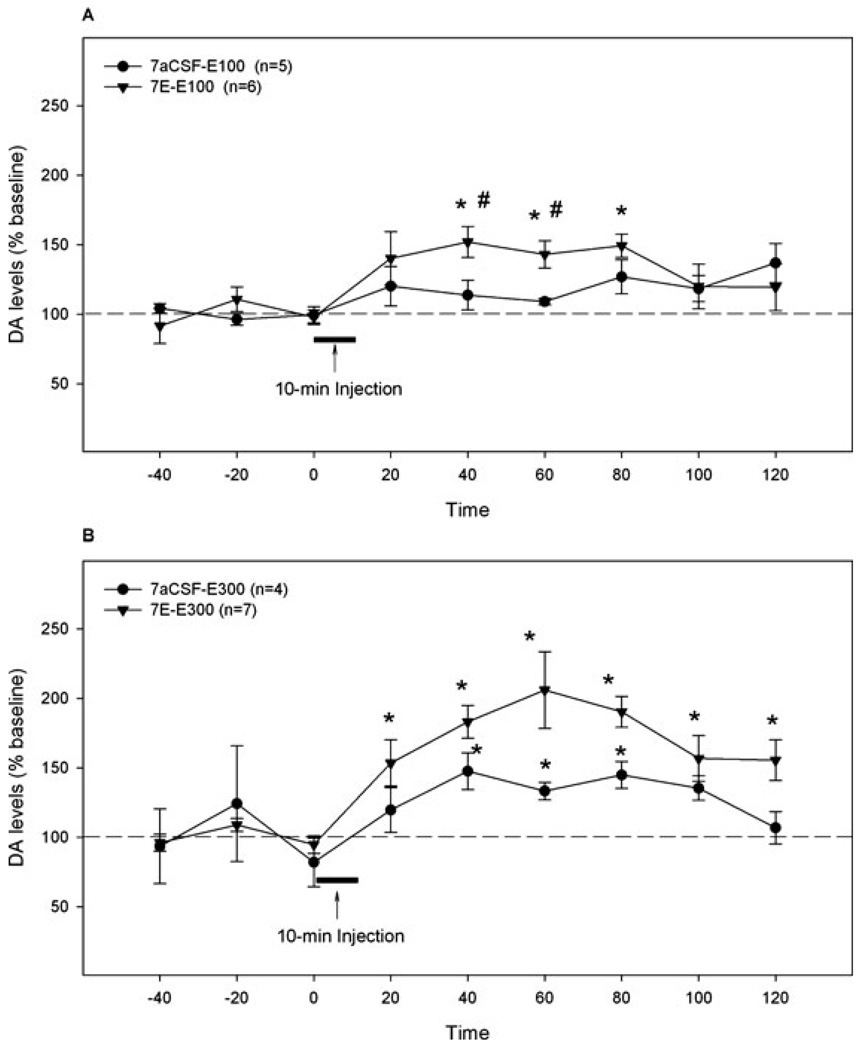
Figure 3A represents results from experiment 1b showing the effects of a challenge microinjection of 100 mg% ethanol on extracellular DA levels in the NAc shell after 7 days of aCSF or 200 mg% ethanol pretreatment. Repeated measures ANOVA revealed significant effects of time and interaction (all F-values >9.48, all p-values < 0.05), but no significant effect of group (F1,9 = 2.93, p = 0.12). The 100 mg% ethanol challenge microinjection in the aCSF-pretreated rats produced a small (but not statistically significant) increase of DA levels in the NAc shell (120 ± 14% of baseline), whereas the same challenge treatment in the 200 mg% ethanol-pretreated rats significantly increased DA levels in the NAc shell. Extracellular DA levels in ethanol-pretreated rats increased significantly within 40 minutes after the challenge injection (152 ± 11% of baseline, p < 0.05), remained at this level for another 40 minutes before returning to baseline. The extracellular DA levels in ethanol-pretreated rats were significantly higher at 40 and 60 minutes after the challenge ethanol injection than those in aCSF-pretreated rats (p < 0.05).
Fig. 3.
The effects of the challenge microinjection of 100 mg% (A) or 300 mg% (B) ethanol into the p-VTA on extracellular DA levels in the NAc shell of rats pretreated for 7 days with aCSF or 200 mg% ethanol. “7aCSF-E100” = pretreated for 7 days with aCSF and challenged with 100 mg% ethanol; “7E-E100” = pretreated for 7 days with 200 mg% ethanol and challenged with 100 mg% ethanol; “7aCSF-E300” = pretreated for 7 days with aCSF and challenged with 300 mg% ethanol; “7E-E300” = pretreated for 7 days with 200 mg% ethanol and challenged with 300 mg% ethanol. *p <0.05, different from baseline levels; #p < 0.05, different from the “7aCSF-E100” group.
Figure 3B shows results from experiment 1c illustrating the effects of a challenge microinjection of 300 mg% ethanol on extracellular DA levels in rats pretreated with aCSF or 200 mg% ethanol for 7 days. Repeated measures ANOVA revealed significant effects of time and group (all F-values >6.60, all p-values <0.05), but no significant interaction term (F8,72 = 1.2, p = 0.288). The challenge microinjection of 300 mg% ethanol increased DA levels significantly in the NAc shell of both aCSF- and 200 mg% ethanol-pretreated rats. In aCSF-pretreated rats, extracellular DA levels in the NAc shell increased significantly at 40 minutes after the challenge microinjection (147 ± 13% of baseline p < 0.05), remained elevated for the next 60 minutes before returning to baseline. In the 200 mg% ethanol-pretreated rats, extracellular DA levels increased significantly within 20 minutes after the challenge microinjection (154 ± 16% of baseline, p < 0.05), gradually reached a peak level (206 ± 27% of baseline, p < 0.05) at 60 minutes after the challenge microinjection, then slowly returned toward but stayed above the baseline throughout the collection. The average extracellular DA level during 40 to 80 minutes after the challenge microinjection in ethanol-pretreated rats was significantly higher than that in aCSF-pretreated rats (193 ± 15 vs. 142 ± 8%, p < 0.05).
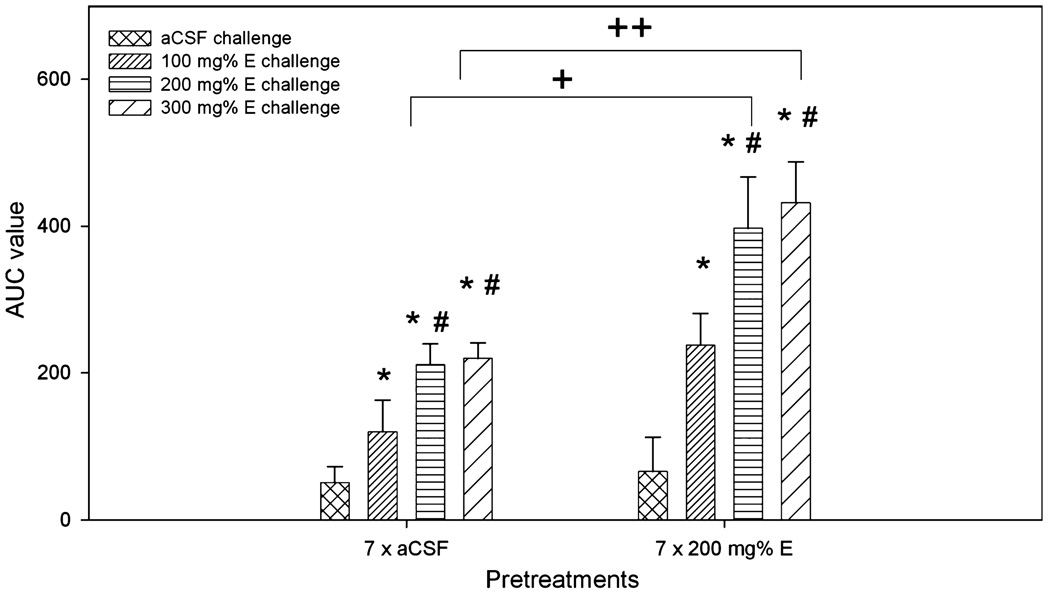
Figure 4 shows the values for the area under the curve (AUC) for each group to demonstrate the dose-response effects of different challenge doses of ethanol in aCSF- and ethanol-pretreated rats. Two-way ANOVA analysis revealed significant effects of pretreatment (F1,39 = 14.5, p < 0.001) and challenge treatment (F3,39 = 13.3, p < 0.001), but no effect of interaction (F3,39 = 1.7, p = 0.188). In both aCSF-and 200 mg% ethanol-pretreated rats, 3 concentrations of challenge ethanol produced a dose-dependent increase in the AUC values. The challenge doses of 200 and 300 mg% ethanol produced significantly greater effects after being injected into ethanol-pretreated rats compared to aCSF-pretreated rats.
Fig. 4.
Area under the curve (AUC) analysis for the dose–response effect of different challenge doses of ethanol after 7 days of pretreatment with aCSF or 200 mg% ethanol. *p < 0.05, different from aCSF-challenged groups; #p < 0.05, different from both aCSF- and 100 mg% ethanol-challenged groups; +,++p < 0.05, difference between the aCSF- and ethanol-pretreated groups.
Figure 5 represents results from experiment 1d showing the effects of microinjections of aCSF and 200 mg% ethanol into the a-VTA on extracellular DA levels in the NAc shell (n = 4/group). Repeated measures ANOVA indicated that there was no significant effect of time, group or interaction (all F-values <0.3, all p-values >0.9). Ethanol injection into the a-VTA did not alter extracellular DA levels in the NAc shell.
Fig. 5.
The effects of microinjections of aCSF or 200 mg% ethanol (n = 4 / group) in the a-VTA on extracellular DA levels in the NAc shell of rats. Ethanol did not alter extracellular DA levels in the NAc shell.
Experiment 2: Effects of the 3- or 5-Day Pretreatment With 200 mg% Ethanol on the Effects of the 200 mg%-Ethanol Challenge on Extracellular DA Levels in the NAc Shell
The basal extracellular DA levels were 0.8 ± 0.2 nM for the aCSF-pretreated group (n = 5), 0.9 ± 0.2 nM for the 3-day ethanol-pretreated group (n = 6), and 1.1 ± 0.3 nM for the 5-day ethanol-pretreated group (n = 5). The basal levels were not different among the 3 groups (one-way ANOVA, F2,15 = 0.47, p = 0.64).
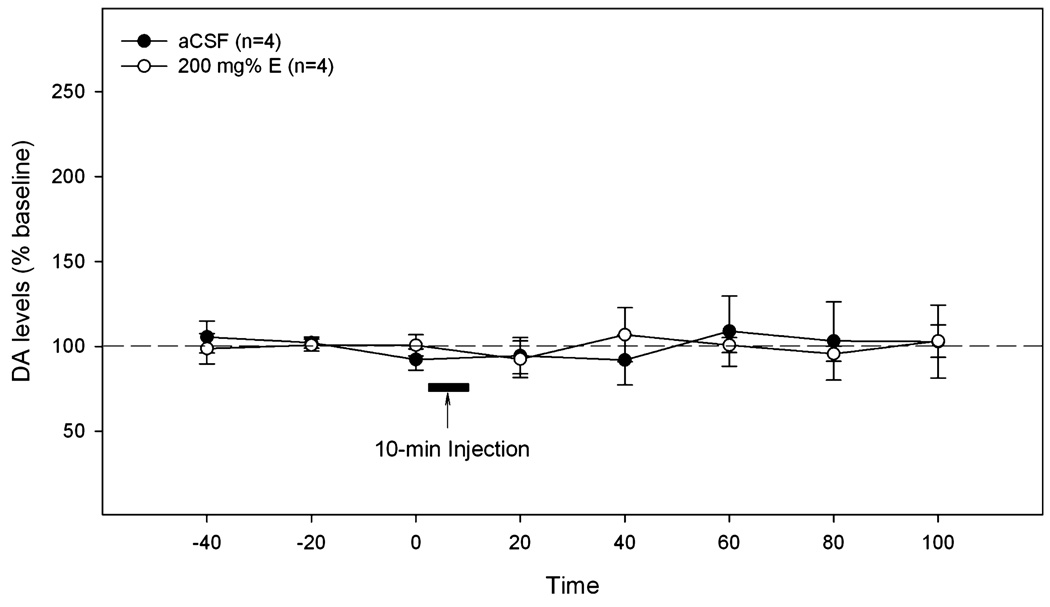
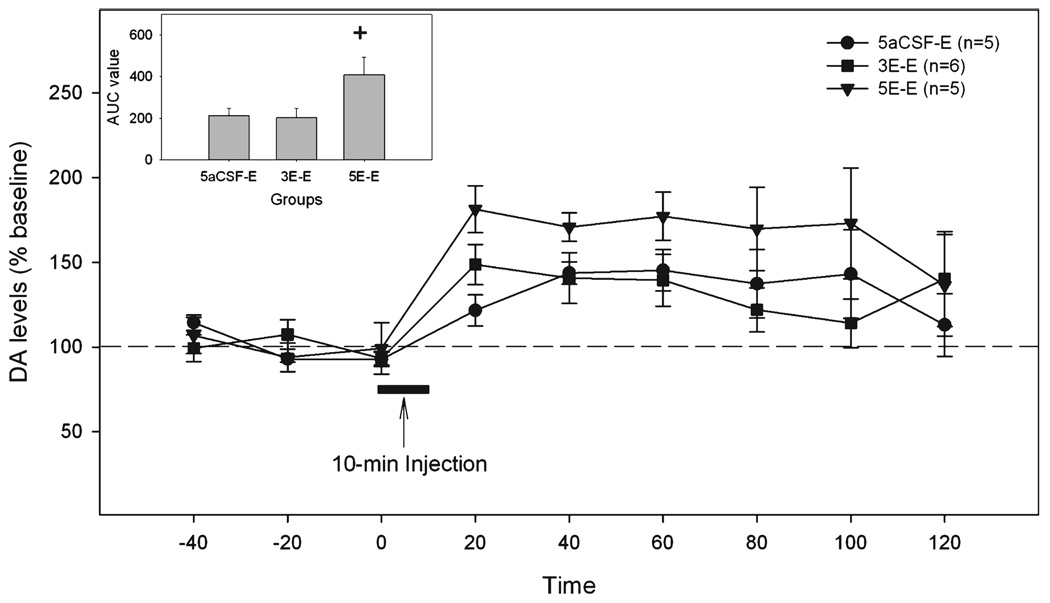
The time-course of extracellular DA levels in the NAc shell is shown in Fig. 6. Repeated measures ANOVA revealed a significant effect of time (F8,104 = 7.7, p < 0.001), but no effect of group or interaction (all F-values <2.7, all p-values >0.1). The significant time effect indicated that the 200 mg% ethanol challenge injection increased DA release in the NAc shell. The AUC analysis (Fig. 6 insert) indicated that the challenge microinjection of 200 mg% ethanol produced a significantly greater effect in rats pretreated for 5 days with 200 mg% ethanol than in rats either pretreated for 3 days with 200 mg% ethanol or pretreated for 5 days with aCSF (one-way ANOVA, F2,13 = 3.97, p < 0.05).
Fig. 6.
The effects of the challenge microinjection of 200 mg% ethanol in the p-VTA on extracellular DA levels in the NAc shell of rats pretreated for 3 or 5 days with 200 mg% ethanol. “5aCSF-E” = pretreated for 5 days with aCSF and challenged with 200 mg% ethanol; “3E-E” = pretreated for 3 days with 200 mg% ethanol and challenged with 200 mg% ethanol; “5E-E” = pretreated for 5 days with 200 mg% ethanol and challenged with 200 mg% ethanol. The insert is the area under the curve (AUC) analysis for 3 groups. +p < 0.05, difference between the “5E-E” group and the other 2 groups.
Experiment 3: The Persisting Effects of the 7-Day 200 mg% Ethanol Pretreatment
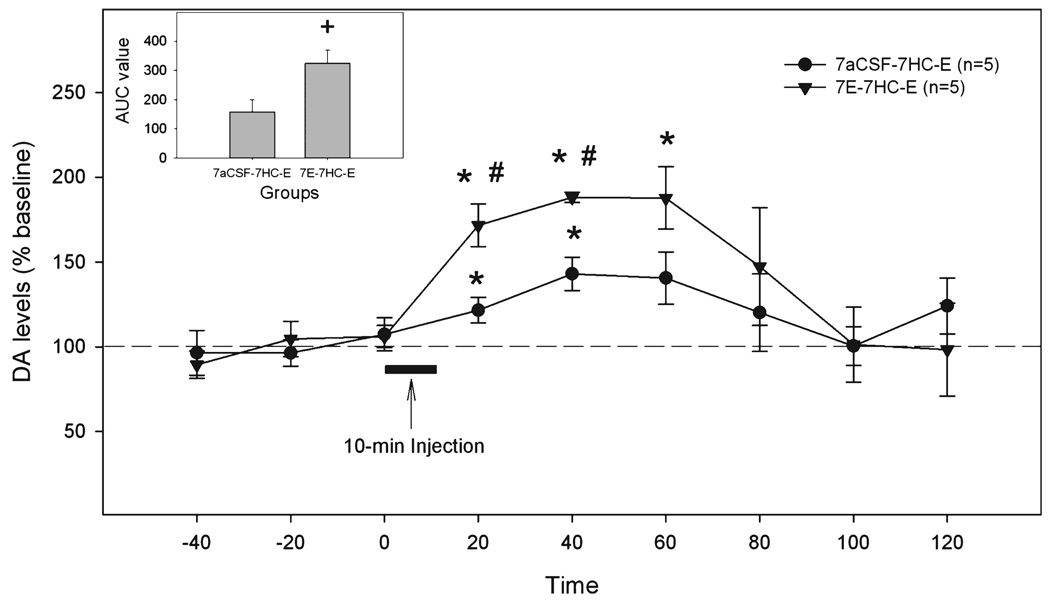
There was no difference in the basal extracellular DA levels in the NAc shell between aCSF-pretreated and ethanol-pretreated rats 7 days after the last pretreatment (1.1 ± 0.3 nM and 1.0 ± 0.3 nM, respectively). The time course of extracellular DA levels in the NAc shell is shown in Fig. 7. The overall analysis revealed significant effects of time, group and interaction (all F-values >6.0, all p-values < 0.05). Seven days after the last pretreatment, the 200 mg% ethanol challenge injection into aCSF-pretreated rats (n = 5) increased extracellular DA levels in the NAc shell within 20 minutes (122 ± 8% of baseline, p < 0.05), which peaked at 40 minutes (143 ± 9% of baseline, p < 0.05), and then returned to baseline. In the rats (n = 5) pretreated with 200 mg% ethanol for 7 days, and then untreated for 7 days, the extracellular DA levels after the 200 mg% ethanol challenge increased sharply within 20 minutes (172 ± 12% of baseline, p < 0.05), remained elevated for another 40 minutes after the challenge injection, and then returned to baseline. The extracellular DA levels were significantly higher at 20 and 40 minutes in ethanol-pretreated rats compared to aCSF-pretreated rats (p < 0.05). The AUC analysis also demonstrated a significant difference between 2 groups (Fig. 7 insert, p < 0.05).
Fig. 7.
The effects of the challenge injection of 200 mg% ethanol in the p-VTA on extracellular DA levels in the NAc shell of rats pretreated for 7 days with aCSF or 200 mg% ethanol then maintained in the home cage for 7 days. “7aCSF-7HC-E” = pretreated for 7 days with aCSF, maintained in home cage for 7 days, and challenged with 200 mg% ethanol; “7E-7HC-E” = pretreated for 7 days with 200 mg% ethanol, maintained in home cage for 7 days, and challenged with 200 mg% ethanol. *p < 0.05, different from baseline levels; #p < 0.05, different from the other group. The insert is area under the curve (AUC) analysis for 2 groups. + indicates difference between two groups, p < 0.05.
DISCUSSION
The major findings of the current study are administration of ethanol within the p-VTA increased the extracellular levels of DA in the NAc shell (Fig. 2 and Fig. 3); repeated locally administered ethanol pretreatments within the p-VTA, compared to vehicle pretreatments, produced greater DA release in the NAc shell to a challenge dose of ethanol (Fig. 2 and Fig. 3); and this greater DA release with repeated ethanol microinjections was still evident 7 days after the last pretreatment (Fig. 7). These results suggest that ethanol can have a local activating effect on DA neurons within the p-VTA and that repeated local ethanol pretreatments induced neuroadaptations in the p-VTA, resulting in a persistent increase in the sensitivity of local DA neurons to the stimulating actions of ethanol.
On the other hand, microinjections of ethanol into the a-VTA did not alter DA release in the NAc shell (Fig. 5), suggesting that the effects observed with microinjections of ethanol into the p-VTA were not a result of diffusion of ethanol into the a-VTA. The present results are consistent with previous findings that ethanol was self-infused in the p-VTA but not the a-VTA (Rodd et al., 2004, 2005c; Rodd-Henricks et al., 2000). In addition, although repeated injections into the p-VTA will likely produce some tissue damage, the finding that the p-VTA still responded to ethanol after both vehicle and ethanol pretreatments, suggests that the site is still viable. Moreover, studies with repeated intracranial self-administration indicated that ethanol still produced reinforcing effects after 7 sessions (Rodd et al., 2004, 2005c; Rodd-Henricks et al., 2000), supporting the contention that the p-VTA is still viable.
The stimulating effects of ethanol on VTA DA neurons observed in the current study are in agreement with in vitro electrophysiological studies suggesting a direct activation of ethanol on VTA DA neurons (Brodie et al., 1990, 1999). One mechanism of direct effects may involve several excitatory receptors in DA neurons, such as serotonin-3 (5-HT3) and/or nicotinic receptors. Studies indicate that ethanol enhances the excitatory effects of 5-HT at the 5-HT3 receptor (Lovinger and White, 1991), and can increase certain nic-otinic receptor-mediated functions (Cardoso et al., 1999; Narahashi et al., 1999). This idea is supported by findings that antagonists of 5-HT3 (Campbell et al., 1996) and nicotinic receptors (Blomqvist et al., 1997) attenuated ethanol-stimulated DA release. Another possible mechanism may involve several voltage-dependent ion channels in DA neurons. Recent studies suggest that ethanol enhancement of hyperpolarization-activated cation channels (Ih channels) (Brodie and Appel, 1998; Okamoto et al., 2006) and inhibition of M-channels (IM) (Koyama et al., 2007) may contribute to the excitation of ethanol on DA neurons. In addition to these direct effects, ethanol could indirectly stimulate DA neurons via suppression of GABA interneuron (Gallegos et al., 1999; Stobbs et al., 2004). Ethanol inhibition of GABA interneurons could occur through either increasing neurotransmission of opioid receptors (Gysling and Wang, 1983; Johnson and North, 1992) and/or decreasing NMDA receptor functions (Dildy and Leslie, 1989; Lovinger et al., 1989; Steffensen et al., 1998; Stobbs et al., 2004). Furthermore, the GABAA receptor is one major target of ethanol (Allan et al., 1987; Ticku et al., 1986). However, the involvement of VTA GABAA receptors in the stimulating effects of ethanol is likely to be complex, because some studies suggested inhibition of DA neurons by GABAA receptors (Johnson and North, 1992; Westerink et al., 1996; Yim and Mogenson, 1980), whereas other studies suggested stimulation of DA neurons by GABAA receptors (Beart and McDonald, 1980; Kalivas et al., 1990; Klitenick et al., 1992; Waszczak and Walters, 1980). This discrepancy may reflect the possibility that different neural circuitries and functions of GABAA receptors may be found within different VTA subregions. This idea is supported by behavioral studies suggesting GABAA receptors mediate inhibitory effects in the a-VTA but excitatory effects in the p-VTA (Arnt and Scheel-Kruger, 1979; Ikemoto et al., 1997, 1998). It is possible that, in the p-VTA, activation of GABAA receptors stimulates DA neurons by inhibiting GABA interneurons, and ethanol further enhances the stimulation, resulting in increased DA neuronal activity reported in the aforementioned and current studies. Because the ethanol concentrations (100 to 300 mg% or about 20 to 70 mM) used in the current study are within the reported optimal concentrations for acting at 5-HT3 (50 to 100 mM, Lovinger and White, 1991), NMDA (50 to 100 mM, Dildy and Leslie, 1989; Lovinger et al., 1989), and GABAA receptors (15 to 50 mM, Allan et al., 1987; Ticku et al., 1986), it is likely that a combination of multiple receptor mechanisms is involved in the observed ethanol effects (Fig. 2 and Fig. 3).
In the current study, ethanol was delivered over a 10-minute period, whereas the enhanced DA levels in the NAc shell remained elevated for 40 to 100 minutes (Fig. 2, Fig. 3, Fig. 6, and Fig. 7). The time-courses of DA increase in the current study are similar to those induced by systemic ethanol injection (Imperato and Di Chiara, 1986; Yim et al., 2000), which produces a prolonged increase of ethanol in the brain (Yim et al., 2000). In the current study, the effective ethanol concentrations in the p-VTA would decrease rapidly after injection due to diffusion and clearance (Gonzales et al., 1998). Therefore, the prolonged increase of extracellular levels of DA in the NAc shell may not be a direct effect of ethanol on DA neurons and could be due to certain neuronal changes induced by ethanol, e.g., ethanol-induced alteration of the phosphorylation states of certain receptor subtypes and/or membrane proteins.
Results from the current study indicate that repeated ethanol exposure of the p-VTA produced a persistent increase in the sensitivity of VTA DA neurons to the stimulating effects of ethanol (Fig. 2, Fig. 3, and Fig. 7), and that at least 5 ethanol pre-treatments are required to produce the increased sensitivity (Fig. 6). Mechanisms of these ethanol-induced neuroadaptations remain unknown, but could be due to up-regulated excitatory effects and/or down-regulated inhibitory effects on DA neurons after repeated ethanol pretreatments. One possible mechanism may involve ethanol-induced neuroadaptations in VTA glutamate transmission. Ethanol exposure was observed to enhance excitatory synaptic transmission on VTA DA neurons by increasing the ratio of AMPA receptor-mediated synaptic currents to NMDA receptor-mediated synaptic currents (Saal et al., 2003; Stuber et al., 2008), which might result from enhanced function and/or number of AMPA receptors (Ortiz et al., 1995; Stuber et al., 2008). Moreover, ethanol exposure can decrease glutamate uptake (Melendez et al., 2005). These adaptations in glutamate transmission may lead to further enhanced excitability of DA neurons. Another mechanism may involve neuroadaptations in VTA GABA neurons. Ethanol exposure has been shown to enhance subsequent ethanol inhibition of GABA interneurons, leading to potentially greater disinhibition of DA neurons (Gallegos et al., 1999; but see Melis et al., 2002).
Increased sensitivity of the mesolimbic DA system to the effects of ethanol has been reported in several studies with different ethanol administration paradigms. Repeated i.p. injections of ethanol in mice increased the stimulating effects of ethanol on VTA DA neurons in midbrain tissue slices (Brodie, 2002), and enhanced basal extracellular DA concentrations in the NAc (Smith and Weiss, 1999). Chronic continuous alcohol drinking produced a persistent increase in the sensitivity of the p-VTA to the reinforcing effects of ethanol measured with intracranial self-administration technique (Rodd et al., 2005a,b). Furthermore, limited scheduled daily access of alcohol drinking reduced D2 autoreceptor function in the NAc (Engleman et al., 2003). Reduced D2 autoreceptor function would increase basal DA neuronal activity and could contribute to the increased sensitivity of the mesolimbic DA system to the reinforcing and stimulating effects of ethanol.
The greater enhanced release of DA in the NAc shell after repeated ethanol administration may be a critical factor contributing to the development of alcohol abuse and addiction. In the “incentive-sensitization” theory of addiction (Robinson and Berridge, 1993), DA release in the NAc is proposed to mediate the assignment of “incentive salience” to rewards and reward-related cues. In the present study, repeated ethanol microinjections produced sensitization of DA release to ethanol challenge, which may increase the “incentive salience” of ethanol and promote high alcohol drinking behavior.
In summary, the present study demonstrated that the local microinjection of ethanol into the p-VTA stimulates local DA neuronal activity, as indicated by increased DA release in the NAc shell. Moreover, repeated local exposure of the p-VTA to ethanol produced alterations that increased the sensitivity of DA neurons to the stimulating effects of ethanol.
ACKNOWLEDGMENTS
This study was supported in part by research grants AA 10717, AA 10721, and AA 12262. We thank Michelle R. Davis and Cynthia M. Ingraham for their technical assistance.
REFERENCES
- Allan AM, Huidobro-Toro JP, Bleck V, Harris RA. Alcohol and the GABA receptor-chloride channel complex of brain. Alcohol Alcohol Suppl. 1987;1:643–646. [PubMed] [Google Scholar]
- Arnt J, Scheel-Kruger J. GABA in the ventral tegmental area: differential regional effects on locomotion, aggression and food intake after micro-injection of GABA agonists and antagonists. Life Sci. 1979;25:1351–1360. doi: 10.1016/0024-3205(79)90402-8. [DOI] [PubMed] [Google Scholar]
- Beart PM, McDonald D. Neurochemical studies of the mesolimbic dopaminergic pathway: somatodendritc mechanisms and GABAergic neurones in the rat ventral tegmentum. J Neurochem. 1980;34:1622–1629. doi: 10.1111/j.1471-4159.1980.tb11253.x. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Huttemeier PC. Microdialysis-theory and application. Prog Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Engel JA, Soderpalm B. Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Electrolytic microinfusion transducer system: an alternative method of intracranial drug application. J Neurosci Methods. 1980;2:273–275. doi: 10.1016/0165-0270(80)90016-3. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–244. [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Camarini R, Hodge CW. Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav. 2004;79:623–632. doi: 10.1016/j.pbb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav. 1995;51:835–842. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noreiga LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Dildy JE, Leslie SW. Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res. 1989;499:383–387. doi: 10.1016/0006-8993(89)90789-0. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra V, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentration in the nucleus accumbens. J Neurochem. 2005;93:1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Ramachandra V, Samson HH, Czachowski CL, Gonzales RA. Accumbal dopamine concentration during operant self-administration of a sucrose or a novel sucrose with ethanol solution. Alcohol. 2004;34:261–271. doi: 10.1016/j.alcohol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Li T-K, Lumeng L, Murphy JM. Ethanol drinking experience attenuates (-)sulpiride-induced increase in extracellular dopamine levels in the nucleus accumbens of alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2003;27:424–431. doi: 10.1097/01.ALC.0000056618.57931.A5. [DOI] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Wilber AA, Shaikh SR, Eha RD, Lumeng L, Li T-K, Murphy JM. Reverse microdialysis of a dopamine uptake inhibitor in the nucleus accumbens of alcohol-preferring rats: effects on dialysate dopamine levels and ethanol intake. Alcohol Clin Exp Res. 2000;24:795–801. [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC. Adaptive responses of γ-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 1999;291:1045–1053. [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gessa G, Muntoni AL, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Intracranial self-administration methodologies. Neurosci Biobehav Rev. 1987;11:319–329. doi: 10.1016/s0149-7634(87)80017-9. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, McNabb J, Yim HJ, Ripley T, Bungay PM. Quantitative microdialysis of ethanol in rat striatum. Alcohol Clin Exp Res. 1998;22:858–867. [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10672. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–127. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, Samson HH. Vental tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993;17:370–375. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusions of GABAA antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-administrations. Pharmacol Biochem Behav. 1998;61:87–92. doi: 10.1016/s0091-3057(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–239. [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther. 1990;253:858–866. [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioid and GABA: an in vivo microdialysis study. J Neurosci. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology. 1998;139:79–85. doi: 10.1007/s002130050692. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol. 2007;97:1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effects of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li T-K, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li T-K. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int. 1999;35:131–141. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li T-K, Murphy JM. Involvement of dopamine D2 autoreceptors in the ventral tegmental area on alcohol and saccharin intake of the alcohol-preferring P rat. Alcohol Clin Exp Res. 2000;24:476–483. [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res Rev. 1987;12:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. New York: Academic Press; 1998. [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li T-K, Lumeng L, McBride WJ. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005a;29:358–366. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li T-K, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005b;315:648–657. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li T-K, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005c;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull. 1993;30:133–141. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytia P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by wistar rats. Alcohol Clin Exp Res. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticku MK, Lowrimore P, Lehoullier P. Ethanol enhances GABA-induced 36Cl-influx in primary spinal cord cultured neurons. Brain Res Bull. 1986;17:123–126. doi: 10.1016/0361-9230(86)90168-1. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Gatto GJ, Lumeng L, Li T-K. Intragastric self-infusion of ethanol by ethanol-preferring and -nonpreferring lines of rats. Science. 1984;225:78–80. doi: 10.1126/science.6539502. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Walters JR. Intravenous GABA agonist administration stimulates firing of A10 dopaminergic neurons. Eur J Pharmacol. 1980;66:141–144. doi: 10.1016/0014-2999(80)90308-8. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accum-bens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–267. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, De Vries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Electrophysiological studies of neurons in the ventral tegmental area of Tsa1. Brain Res. 1980;181:301–313. doi: 10.1016/0006-8993(80)90614-9. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, Gonzales RA. Dissociation between the time course of ethanol and extracellular dopamine concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol Clin Exp Res. 2000;24:781–788. [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li T-K. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1991;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]