Abstract
The purpose of this translational study was twofold: (1) to contrast behavioral and brain activity between pathological and nonpathological gamblers, and (2) to examine differences as a function of the outcome of the spin of a slot machine, focusing predominately on the “Near-Miss”—when two reels stop on the same symbol, and that symbol is just above or below the payoff line on the third reel. Twenty-two participants (11 nonpathological; 11 pathological) completed the study by rating the closeness of various outcomes of slot machine displays (wins, losses, and near-misses) to a win. No behavioral differences were observed between groups of participants, however, differences in brain activity were found in the left midbrain, near the substantia nigra and ventral tegmental area (SN / VTA). Near-miss outcomes uniquely activated brain regions associated with wins for the pathological gamblers and regions associated with losses for the nonpathological gamblers. Thus, near-miss outcomes on slot machines may contain both functional and neurological properties of wins for pathological gamblers. Such a translational approach to the study of gambling behavior may be considered an example that gives life to B. F. Skinner's conceptualization of the physiologist of the future.
Keywords: pathological gambling, fMRI, near-miss, slot machine, addiction
B. F. Skinner described gambling as perhaps one of the most naturalistic examples of human behavior under a given schedule of reinforcement (Skinner, 1974). He stated: “all gambling systems are based on variable-ratio schedules of reinforcement, although their effects are usually attributed to feelings” (p. 60). With regard to the slot machine, the apparatus resembles a simple operant chamber, as it consists of a single lever (the slot machine arm), a reinforcer hopper (the coin tray), and a series of visual stimuli (the slot reels and displays) that accompany the delivery of reinforcement. This latter component, the slot reel display, is often misconstrued by the gambler, however, as a discriminative stimulus that provides information regarding the delivery of upcoming reinforcement. Skinner noted this misconception on the part of the gambler by stating that when a losing display looks similar to a winning display a reinforcement effect may occur, while costing the casino nothing for its delivery (Skinner, 1953).
An increasing number of conceptual and experimental investigations have been conducted involving slot machine gambling from a behavioral perspective in the years that followed Skinner's initial comments. Weatherly and Dixon (2007) introduced a comprehensive conceptualization of excessive gambling that included additional variables beyond the programmed reinforcement of the gaming device. These authors noted that perhaps pathological gambling was a dynamic interaction between programmed contingencies, verbal behavior, and various contextual stimuli (i.e., financial status; race; comorbid psychological disorders). Although purely conceptual, this model has been noted by others as having great utility in understanding the complexity of pathological gambling (Catania, 2008; Fantino & Stolarz-Fantino, 2008). Fantino and Stolarz-Fantino have also developed a conceptual model of pathological gambling that stems from the discounting of delayed consequences, which has been supported by a number of researchers as a potential framework to guide empirical investigations (DeLeon, 2008; Madden, 2008). In summary, it appears that a contemporary behavior analytic account of gambling suggests that programmed contingencies alone within the gambling device are not sufficient to sustain the occasionally witnessed pathological behavior.
Empirical data supporting this assertion continue to emerge. When exposed to concurrent slot machines or computerized simulations of those devices, participants often do not allocate their responses to the relative rates of reinforcement (Weatherly, in press) and instead often alter preference based on various instructions (Dixon, 2000), or as the result of changes in stimulus functions that occur via conditional discrimination training and testing procedures (Hoon, Dymond, Hackson, & Dixon, 2008; Zlomke & Dixon, 2006). As a result, it appears that as additional data are generated which show changes in participants' behavior irrespective of programmed contingencies of the slot machine, Skinner's (1974) contingency analysis provides only a partial answer to why people gamble.
Perhaps the most provocative aspect of Skinner's (1953; 1974) description of slot machine play was the reference to almost winning. The almost-win, often termed a “near-miss” has been the focus of a wide range of investigations by gambling researchers over the past 20 years. This losing outcome occurs when two reels of a slot machine display the same symbol and the third wheel displays that symbol immediately above or below the payoff line. In games of skill, near-misses provide useful information for players to gauge their performance. In games of chance, however, such as slot machines, near-misses do not provide any useful information to the player, and in some instances can prove to be misleading such as when a gambler interprets the near-miss as a positive sign of their strategy or when it promotes the view that a win is “just around the corner” (Parke & Griffiths, 2004). Behaviorally speaking, the near-miss may serve a discriminative function that a reinforcer will be available in the near future. Superstitious reinforcement of such a behavior (i.e., the belief that a win is due) only strengthens the assumed discriminative control.
Previous research on the near-miss has shown that slot machine players will tend to play for longer periods of time if those machines contain occurrences of specific near-miss frequencies (Kassinove & Schare, 2001; MacLin, Dixon, Daugherty, & Small, 2007; Strickland & Grote, 1967). Too high a near-miss density (over 40% of all losses) may weaken the effects, and too low a density (less than 20%) may not produce the effect (MacLin et al.). Near-misses have been argued to have the same kind of conditioning effects on behavior as actual wins (Parke & Griffiths, 2004). Additionally, Dixon and Schreiber (2004) have shown that slot machine players will rate near-misses as closer to wins than traditional losses, and Clark et al. (2009) have shown that players rated near-misses as more aversive than traditional losses but gave higher ratings of wanting to continue to play after a near-miss than a traditional loss. These studies indicate that near-misses are not simply another form of loss and that gamblers' behavior can be altered and reinforced by near-misses in the same way that it can by wins.
While the majority of our understanding of gambling pathology and the near-miss effect has come from behavioral studies, behaviorists, cognitive psychologists, and cognitive neuroscientists have increasingly recognized that in order to develop a comprehensive understanding of pathological gambling and effective treatment options, it is necessary to understand how the brain responds to various types of gambling cues such as near-misses as well as how the brains of pathological gamblers differ from the brains of nonpathological gamblers while both are engaged in gambling. To this end, researchers have begun to utilize modern brain imaging tools such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) to study pathological gambling. In an early study, Potenza et al. (2003) compared brain activity between nonpathological and pathological gamblers. Their findings revealed that during the initial presentation of gambling cues, pathological gamblers demonstrated relative decreases in activity within cortical, striatal, and thalamic areas when compared to nonpathological gamblers. Reuter et al. (2005) observed a similar effect in the ventral striatum. Additionally, they noted that activity in this region was negatively correlated with severity of gambling pathology (i.e., as pathology increased, activity decreased). More recently, Clark et al. (2009) examined the neural correlates of the near-miss directly in a group of nonpathological gamblers. They found that relative to all forms of losses (near-misses and full losses), winning outcomes recruited the ventral striatum bilaterally, the anterior insula bilaterally, the rostral anterior cingulate, the thalamus, and a midbrain cluster near to the substantia nigra / ventral tegmental area. Within the set of regions that was activated after winning outcomes, Clark et al. (2009) observed greater activity for near-misses than losses in the ventral striatum bilaterally as well as in the right anterior insula. Together, these studies indicate that brain activity as a function of different gambling outcomes differs between pathological and nonpathological gamblers.
The main purpose of this study was to examine overt behavioral responding as well as brain activity when pathological and nonpathological gamblers experienced winning, near-miss, and losing spins on a computerized slot machine task. To date, no published study has been conducted using gambling stimuli that closely resemble an actual slot machine (i.e., three spinning reels, with symbols displayed above and below the payoff line). Furthermore no study to date has compared the near-miss effect on brain activation in both pathological and nonpathological gamblers. To the extent that pathological gamblers may experience near-misses as more win-like and nonpathological gamblers experience them as more loss-like, we hypothesized that brain activity to near-misses will be more similar to losses in nonpathological gamblers but more similar to wins in pathological gamblers. Through the combining of traditional behavioral procedures with the supplemental utilization of fMRI technology, we attempted to gain a greater comprehensive analysis of the behavior of the human organism when exposed to an actual slot machine task.
METHOD
Participants, Setting, and Apparatus
Potential pathological gambling was assessed by the South Oaks Gambling Screen (SOGS). Eleven healthy right-handed non-treatment seeking pathological gamblers (Male = 10; Age = 19–26; SOGS > 2) and 11 healthy right-handed nonpathological gamblers (Male = 4; Age = 19–27; SOGS < = 2) each received a $30 gift card for participation in the study. After complete description of the study to the subjects, written informed consent was obtained. The study was approved by the Human Subjects Committee of Southern Illinois University Carbondale.
The experiment took place in the Imaging Center of a comprehensive care hospital, Memorial Hospital of Carbondale. Participants were placed in a scanning room containing the fMRI scanner as well as various other medical equipment, including the equipment necessary for stimulus presentation and the recording of subject responses (MRI-compatible LCD screen, pneumatic headphones, and response buttons). The experimenter, technician, and graduate assistants were in the adjoining control room.
FMRI scans were acquired on a Philips Intera 1.5 T magnet with the following parameters: T2* single-shot EPI, TR = 2.5 s, TE = 50 ms, flip angle = 90°, FOV = 220 × 220 mm2, 64 × 64 matrix, 3.44 × 3.44×5.5 mm voxels, 26 × 5.5 mm axial slices, 0 mm gap, first eight images were discarded. Conventional high-resolution T1 weighted 3-D structural images were acquired at the end of the functional imaging stage. Data were analyzed with SPM 2 implemented in Matlab 6.51 (Mathworks). Images were (1) slice time corrected for acquisition order, (2) realigned and motion corrected to the first image of the session, (3) normalized to a common template (MNI EPI template), (4) resliced to 2 × 2 × 2 mm voxels, and (5) spatially smoothed with a 10 mm Gaussian filter. A 128-s high pass filter was applied to each time series in order to eliminate low frequency noise. Single-subject statistical contrasts were created using the general linear model (GLM). Conditions of interest (wins, near-misses, losses) for both nonpathological and pathological gamblers were modeled using a canonical hemodynamic response function. Group comparisons were created using a random effects model. Contrasts were thresholded at p < 0.001 uncorrected for multiple comparisons. Coordinates are presented in the Talairach and Tournoux (1988) coordinate system.
Prescanning Procedures
Prior to scanning all participants completed a series of informed consents, and demographic questionnaires that assessed overall health, medical, psychological, and neurological history, as well as recent substance use, dominant handedness, and the presence of any MRI contraindications. All participants were then asked to remove any metal objects (jewelry, etc.) from their bodies, and directed into a 9-m by 7.5-m room containing the fMRI scanner. Next participants were instructed to lie down on a 2.5-m table, and inserted into the scanner by the presiding technician. Participants viewed stimuli on an 18-cm (diagonal) MRI-compatible LCD screen through a mirror attached to the inside of the head coil at a distance of approximately 15 cm. The right hand of each participant was affixed to an MRI-compatible response pad consisting of five keys which were to be pressed by corresponding fingers at various points during the scanning activity. Participants read the following directions prior to the start of each scan: “Please rate how close to a win you feel the current slot machine display is on a scale from 1 (not at all) to 5 (a win) with your thumb being a 1 and your pinky a 5.”
Scanning Procedures
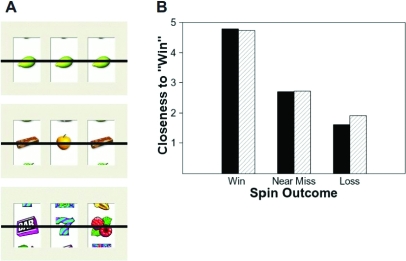
Pathological and nonpathological gamblers were scanned while viewing the wheels of a computerized slot machine. The wheels of the slot machine spun for 1.5 s, stopping (for 2.5 s) on one of three equally likely outcomes: (1) win (three identical symbols on the pay-off line), (2) near-miss (two identical symbols on the payoff line with the third matching symbol above or below the payoff line), and (3) loss (three different symbols on the payoff line; Figure 1a). The computerized slot machine task was programmed in E-Prime 1.0 software (Psychology Software Tools, Pittsburgh, PA). Each spin consisted of a sequence of static images presented in rapid succession in order to give the illusion of motion. The first seven images were shown for 30 ms, the next two for 45 ms, the next four for 50 ms, the next four for 100 ms, and the last three for 200 ms. This presentation rate gave the illusion of spinning slot machine wheels, gradually slowing down, and eventually stopping on an outcome. This image then remained on the screen for 2.5 s and participants, at this point, were required to indicate how “close” to a win they felt the outcome was using a five-point scale.
Fig 1.
(a) Sample of stimuli presented to subjects during each run. The top stimulus depicts a winning outcome; the middle stimulus depicts a near-miss outcome; the bottom stimulus depicts a losing outcome. (b) Mean closeness to a “win” response for each stimulus category for normal (N = 11; black bars) and pathological (N = 11; cross-hatched bars) gamblers.
A total of five functional runs were acquired. Each run lasted 5 min and 20 s, with the first 20 s necessary for stabilization of the magnetic field. The images from this portion were discarded. During each run, participants viewed 20 winning outcomes, 20 near-miss outcomes, and 20 losing outcomes, presented in a random order.
RESULTS
Behavioral Effects
On the behavioral task, subjects were required to indicate, on a 1-to-5 scale, how “close” to a win each type of spin outcome was. Both pathological and nonpathological gamblers rated near-miss outcomes as significantly “closer” (i.e. more win-like) to wins than the loss outcomes (F(2, 32) = 191.6, p <0.001; Figure 1b). No other behavioral effects reached significance. Thus, both groups demonstrated equally what has been reported previously in the literature as a “near-miss” effect.
Differences in Brain Activity Between Pathological and Nonpathological Gamblers
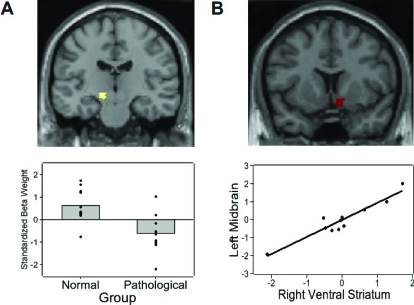
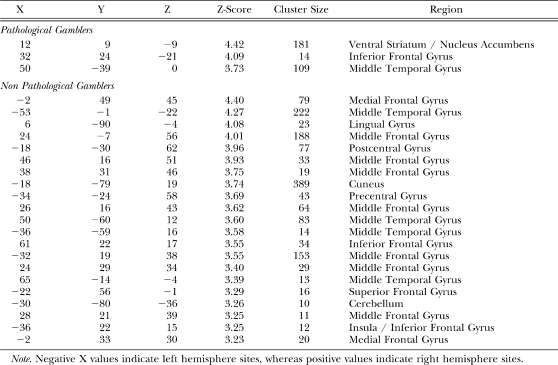
We first examined differences in brain activity between pathological and nonpathological gamblers irrespective of slot machine outcome. To achieve this, we contrasted BOLD (Blood Oxygenation Level Dependent) activity between pathological and nonpathological gamblers averaged across all three slot machine outcomes. This contrast revealed greater activity in the left midbrain region (xyz = −12 −20 −6; Z = 3.23; k = 6) for nonpathological compared to pathological gamblers (Figure 2a). This activity was in the vicinity of the substantia nigra and the ventral tegmental area. Because neurons from the substantia nigra and ventral tegmental area primarily project to the nucleus accumbens in the ventral striatum (Robbins & Everitt, 1999) we next examined whether activity in this left midbrain site correlated with activity in the ventral striatum. Using activity in the left midbrain as a covariate, we performed a whole-brain regression analysis that revealed that activity in the right ventral striatum correlated positively (r = .95) with activity in the left midbrain in pathological but not nonpathological gamblers (Figure 2b). Additional regions that correlated with the left midbrain site in pathological gamblers included the right inferior frontal gyrus and the right middle temporal gyrus. While no region in the ventral striatum correlated with activity in the left midbrain in nonpathological gamblers, numerous other sites did. These included the medial frontal gyrus, bilateral middle temporal gyrus, lingual gyrus, bilateral middle frontal gyrus, left superior frontal gyrus, and the left insula (for full list of coordinates, see Table 1).
Fig 2.
(a) Activity in the left midbrain, depicted on a coronal MRI slice, is greater for normal than pathological gamblers. Plot shows mean and individual subject standardized regression beta weights for normal (N = 11) and pathological (N = 11) gamblers in this region. (b) Activity in the right ventral striatum correlates with activity in left midbrain in pathological gamblers (N = 11). Axes of scatter plot represent standardized regression beta weights in these regions.
Table 1.
Coordinates of significant positive correlation with activity in left midbrain in pathological and nonpathological gamblers.
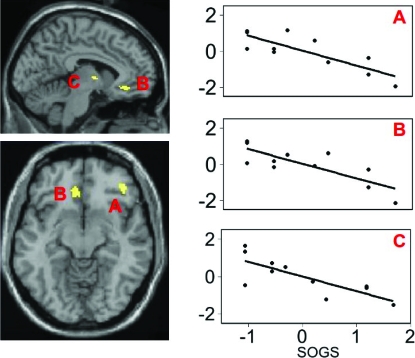
We also examined whether brain activity in pathological gamblers was related to severity of pathological gambling as determined by the SOGS. Using SOGS as a covariate, across all slot machine outcomes, we observed negative correlations with activity in the right middle frontal gyrus (xyz = 44 36 −14; Z = 3.13; k = 45; r = −.82), ventral medial frontal gyrus (xyz = −6 29 −10; Z = 2.85; k = 43; r = −.78), and the thalamus (xyz = −2 −2 2; Z = 2.99; k = 31; r = −.80; Figure 3). These correlations indicate that in pathological gamblers, as gambling severity increased, activity in these regions declined.
Fig 3.
Activity in right middle frontal gyrus (a), ventral medial frontal gyrus (b), and thalamus (c) correlates with scores on the South Oaks Gambling Survey (SOGS) in pathological gamblers. Ordinate on scatter plots represents standardized regression beta weights in corresponding brain regions.
Overall Effects of Winning, Near-Miss, and Losing Spins
We adopted a conservative approach to identifying group-independent activation related to win, near-miss, and loss spin outcomes. Rather than computing the main effect of wins (wins–losses), near-misses (near-misses–losses), and losses (losses–wins) across both groups, an analysis which may reveal activations largely driven by one group or the other, we adopted a conjunction analysis approach (Nichols et al., 2005) to identifying common win, near-miss, and loss networks across both groups. Conjunction analysis is more conservative than examining the spin-outcome main effects because an activation needs to surpass a statistical threshold in both groups before it is revealed in the conjunction contrast. Using this approach, we performed conjunction analyses to examine win (wins–losses), near-miss (near-misses–losses), and loss (losses–wins) networks that were common in both pathological and nonpathological gamblers.
The conjunction analysis on win outcomes revealed no significantly active voxels, indicating that the network of regions active for winning spins in nonpathological gamblers was entirely nonoverlapping with the network active in pathological gamblers. The conjunction analysis on near-miss outcomes revealed nearly the same finding. The only exceptions (i.e., regions common to both pathological and nonpathological gamblers) were observed in bilateral activations in the inferior occipital gyrus (left: xyz = −24 −99 −2; Z = 3.45; k = 21; right: xyz = 24 −99 −2; Z = 3.64; k = 41). The conjunction analysis of loss outcomes revealed greater common activation between pathological and nonpathological gamblers. The common loss network consisted of overlapping activations in bilateral precuneus (left: xyz = −12 −59 56; Z = 4.13; k = 125; right: xyz = 18 −63 60; Z = 5.63; k = 406), bilateral middle/superior occipital gyri (left: xyz = −26 −85 19; Z = 3.84; k = 262; right: xyz = 36 −80 30; Z = 4.07; k = 57), and bilateral superior frontal gyri (left: xyz = −26 6 49; Z = 3.11; k = 54; right: xyz = 30 8 56; Z = 3.67; k = 102).
Unique Effects of Winning, Near-Miss, and Losing Spins in Pathological and Nonpathological Gamblers
Having identified common (or lack thereof) win, near-miss, and loss activations in pathological and nonpathological gamblers, we turned next to examining unique win, near-miss, and loss activity in each group. In order to identify unique activity and exclude activity that was common to both groups, we excluded the regions active in one group when analyzing the same contrast in the other group. For example, to identify activity associated with winning spins (wins–losses) unique to pathological gamblers, we analyzed the wins–losses contrast in the nonpathological gamblers and then excluded the active regions from this contrast when examining the wins–losses in the pathological gamblers. That way, any activity in the wins–losses contrast in the pathological group would be unique to only that group. This procedure, referred to as exclusive masking, was carried out for all outcome-specific analyses in order to identify activity that was unique to each group. The contrast used for the exclusive mask was thresholded at p < 0.05 uncorrected for multiple comparisons. Because the mask contrast is used to identify regions to exclude from an analysis, this threshold serves to liberally exclude regions that may be active in each group, thus ensuring that the regions that are identified by the contrast are unique to each group.
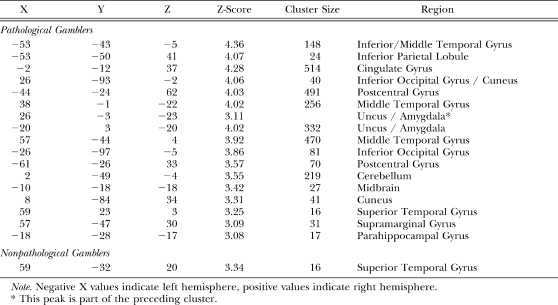
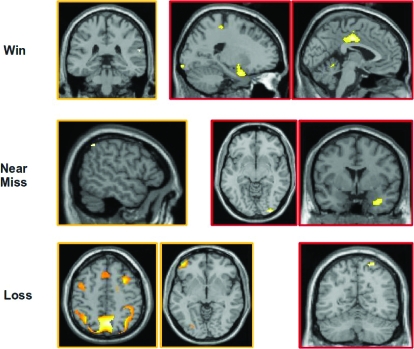
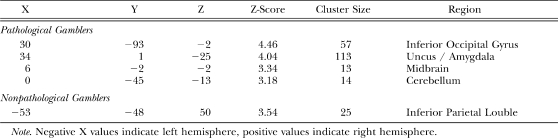
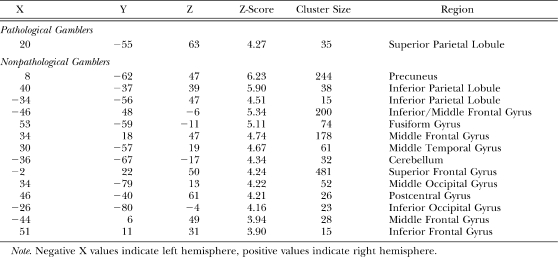
For wins (wins–losses), nonpathological gamblers uniquely activated the right superior temporal gyrus whereas pathological gamblers uniquely activated an extended network of regions including bilateral middle temporal gyrus, left inferior parietal lobule, the cingulate gyrus, bilateral cuneus, left postcentral gyrus, the uncus extending into the amygdala bilaterally, bilateral cerebellum, left brainstem, and right inferior frontal gyrus (see Table 2; Figure 4 top row). For near-misses (near-misses–losses), nonpathological gamblers uniquely activated the inferior parietal lobule, whereas pathological gamblers uniquely activated the right inferior occipital gyrus, the right uncus extending into the amygdala, the midbrain, and the cerebellum (see Table 3; Figure 4 middle row). For losses (losses–wins), nonpathological gamblers uniquely activated an extensive network of brain regions that included the precuneus in the medial parietal cortex, bilateral inferior parietal lobule, left inferior/middle frontal gyrus, bilateral middle frontal gyrus, as well as posterior visual areas including the right fusiform gyrus, right middle occipital gyrus, and left inferior occipital gyrus. Pathological gamblers only uniquely activated the superior parietal lobule (see Table 4; Figure 4 bottom row).
Table 2.
Coordinates of unique win-specific (wins–losses) activations in pathological and nonpathological gamblers.
Fig 4.
Unique activity for Wins–Losses (top row), Near Misses–Losses (middle row), and Losses–Wins (bottom row) in nonpathological (indicated by orange borders) and pathological gamblers (indicated by red borders). Top row: Activity in superior temporal gyrus is greater for Wins than Losses in nonpathological but not pathological gamblers, whereas activity in the anterior medial temporal lobe and cingulate gyrus is greater for Wins than Losses in pathological but not nonpathological gamblers (see also Table 2). Middle row: Activity in left inferior parietal lobule is greater for Near Misses than Losses in nonpathological gamblers but not pathological gamblers, whereas activity in the uncus and right inferior occipital gyrus is greater for Near Misses than Losses in pathological but not nonpathological gamblers (see also Table 3). Bottom row: Activity in medial parietal cortex, bilateral inferior parietal lobule, superior frontal gyrus, bilateral middle frontal gyrus, and left middle/inferior frontal gyrus is greater for Losses than Wins in nonpathological but not pathological gamblers, whereas activity in the superior parietal lobule is greater for Losses than Wins in pathological but not nonpathological gamblers (see also Table 4).
Table 3.
Coordinates of unique near miss-specific (near-misses–losses) activations in pathological and nonpathological gamblers.
Table 4.
Coordinates of unique loss-specific (losses–wins) activations in pathological and nonpathological gamblers.
Overlap between Near-Misses and Wins and Losses in Pathological and Nonpathological Gamblers
At the outset, we predicted that near-misses would show greater overlap with losses in nonpathological gamblers but they would have greater overlap with wins in the pathological group. This prediction implies that near-misses have both win-like and loss-like qualities. To identify the win-like qualities of near-misses, we contrasted near-misses with losses (near-misses–losses). Under the assumption of additivity, this contrast should reveal win-like near-miss activity by subtracting out the loss-like components of near-misses. Conversely, to identify the loss-like qualities of near-misses, we contrasted near-misses with wins (near-misses–wins). In this contrast, the win-like properties of near-misses should be subtracted out, revealing loss-like near-miss activity. Following Clark et al.'s (2009) approach, each of these contrasts was masked with their respective win (win–loss) or loss (loss–win) network in order to examine the overlap with that network.
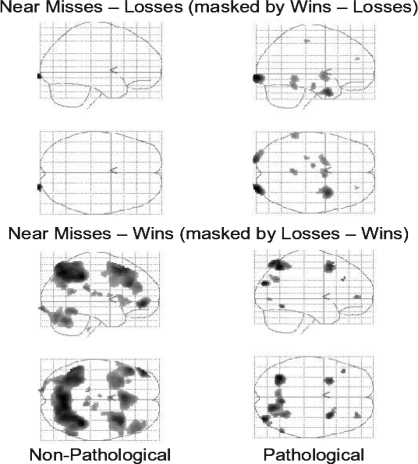
With regard to the win-like qualities of near-misses, to the extent that our hypothesis is correct, we should observe greater overlap between near-misses and wins in the pathological group than in the nonpathological group. Indeed, this is what we observed. In the pathological group, greater activity for near-misses than losses (masked by the wins–losses contrast) was observed in bilateral inferior occipital gyrus (right: xyz = 28 −97 −2; Z = 4.77; k = 171; left: xyz = −20 −99 −5; Z = 4.07; k = 126), right uncus (34 1 −25; Z = 4.04; k = 267), bilateral dorsal striatum (right: xyz = 6 −2 −2; Z = 3.34; k = 57; left: xyz = −22 −2 −3; Z = 3.17; k = 93), cerebellum (xyz = 0 −45 −13; Z = 3.18; k = 60), left middle temporal gyrus (xyz = −60 −43 −6; Z = 3.13; k = 75), and the left midbrain near the substantia nigra (xyz = −10 −18 −16; Z = 3.04; k = 27). This same contrast carried out in the nonpathological gamblers revealed only one significant peak located in the right occipital lobe (xyz = 24 −100 −2; Z = 3.64; k = 45; Figure 5 top row).
Fig 5.
Overlap between Near Miss activity and Win (top row) and Loss (bottom row) activity in pathological and nonpathological gamblers. Top Row: Pathological gamblers show greater overlap between Near Miss and Win activity than nonpathological gamblers. Bottom Row: Nonpathological gamblers show greater overlap between Near Miss activity and Loss activity than pathological gamblers.
We next examined the loss-like qualities of near-misses in each group. For these analyses, we had predicted that the overlap between near-misses and losses would be greater in the nonpathological than the pathological group. Again, the results confirmed our prediction. In the pathological group, greater activity for near-misses than wins (masked by the losses–wins contrast) was observed in the superior parietal lobule bilaterally (left: xyz = −32 −60 51; Z = 3.49; k = 181; right: xyz = 18 −67 59; Z = 3.30; k = 88), the superior middle frontal gyrus bilaterally (right: xyz = 30 12 51; Z = 3.25; k = 31; left: xyz = −28 12 45; Z = 3.17; k = 49), the right precuneus (xyz = 8 −57 −54; Z = 3.17; k = 27) extending into the superior parietal lobule (xyz = 30 −54 56; Z = 3.18; k = 12), and the right superior occipital gyrus (xyz = 38 −80 28; Z = 3.37; k = 38). In contrast, this same comparison carried out in the nonpathological group activated an extensive network that included bilateral inferior parietal lobule (right: xyz = 40 −40 40; Z = 5.42; k = 180; left: xyz = −28 −47 44; Z = 4.81; k = 166), the medial parietal/precuneus (xyz = −5 −68 49; Z = 5.42; k = 293), left inferior (xyz = −48 46 −6; Z = 4.81; k = 141), bilateral middle (right: xyz = 34 18 47; Z = 4.73; k = 569; xyz = 44 38 20; Z = 3.66; k = 217; left: xyz = −32 16 54; Z = 3.92; k = 301; xyz = −48 30 26; Z = 4.54; k = 345), and medial superior (xyz = −4 22 49; Z = 4.63; k = 605) frontal gyri, bilateral cerebellum (right: xyz = 30 −63 −24; Z = 4.10; k = 202; xyz = 4 −77 −16; Z = 3.75; k = 136; left: xyz = −38 −71 −15; Z = 3.25; k = 11), left inferior occipital gyrus (xyz = −18 −96 −7; Z = 3.87; k = 17), right inferior temporal gyrus (xyz = 59 −53 −12; Z = 3.91; k = 86), and the posterior cingulate (xyz = 6 −32 20; Z = 3.52; k = 12; Figure 5 bottom row).
DISCUSSION
The purpose of this study was twofold: (1) to contrast behavioral and brain activity between pathological and nonpathological gamblers, and (2) to examine differences as a function of the outcome of the spin of a slot machine, focusing specifically on the near-miss—when two reels stop on the same symbol, and that symbol is just above or below the payoff line on the third reel. Previous studies have examined differences in neural activity between pathological and nonpathological gamblers and between near-misses and wins and losses (Potenza et al., 2003; Reuter et al., 2005; Clark et al., 2009), however, no study that we are aware of has combined both aspects into a single study. Based on the conception of the near-miss as having topographical and/or functional properties of both wins and losses (see Dixon, Nastally, Jackson, & Habib, in press), we hypothesized that pathological gamblers would likely incline towards the win-like properties of the near-miss while nonpathological gamblers would more easily see the near-miss for what it truly is—a losing outcome. Although the behavioral data did not support this finding, that is, pathological and nonpathological gamblers rated near-misses closer to wins equally, the fMRI results provided additional insight as to the unique interaction of behavior and neurophysiology. The imaging data showed greater overlap between the win-like aspects of the near-miss (near-miss–losses) and the win network (wins–losses) in pathological than nonpathological gamblers. Conversely, the loss-like aspects of the near-miss (near-miss–wins) and the loss network (losses–wins) showed greater overlap in the nonpathological than pathological gamblers.
With respect to the specific win, near-miss, and loss networks that were active, our goal was to both identify regions that were common to both groups and regions that were unique to each group. For wins (wins–losses), the conjunction analysis carried out to identify common regions between the two groups failed to reveal any significant activation suggesting that the network underlying wins was completely separate for pathological and nonpathological gamblers. With regards to unique activations, we identified a region in the right superior temporal gyrus that was unique in nonpathological gamblers. In pathological gamblers, the win network consisted of unique activations in the uncus and the posterior cingulate gyrus, both regions within the extended medial temporal lobe system. For losses (losses–wins), common activations for pathological and nonpathological gamblers were noted in bilateral medial parietal region (precuneus), bilateral middle/superior occipital gyrus, and bilateral superior frontal gyri. Unique activations in nonpathological gamblers were noted in an extensive network that included the medial and bilateral lateral parietal cortices and the medial, bilateral middle frontal, and left inferior frontal gyri, amongst a broader network. This network was greatly reduced in pathological gamblers with the only region showing significant activation occurring in the right lateral parietal cortex. For near-misses (near-misses–losses), there was only minimal common activation. Activations in nonpathological gamblers occurred in a region in the left inferior parietal lobule near to a similar region activated when contrasting losses with wins. That is, in nonpathological gamblers, a similar region was activated when these individuals viewed losses and near-misses. Conversely, activations in pathological gamblers occurred in the uncus in the right anterior medial temporal lobe as well as the right inferior occipital gyrus. In contrast to the nonpathological gamblers, the near-miss activation in the pathological group overlapped more with activations seen in the wins–losses contrast. Together, these sets of analyses support our hypothesis that nonpathological gamblers are more likely to view near-misses for what they truly are—losing outcomes, whereas brain activity in pathological gamblers indicates that near-misses appear to activate some of the same brain regions that are activated in this group when they experience winning spins.
Two observations regarding the win network are noteworthy. First, this network was more extensive in pathological than nonpathological gamblers. Second, whereas the right superior temporal gyrus was activated in nonpathological gamblers, the network in pathological gamblers included regions of the medial temporal lobe including the uncus extending into the amygdala bilaterally and the cingulate gyrus, as well as the midbrain. These activations are especially interesting given that all subjects received the same monetary compensation for participating in the experiment and winning spins were not associated with any additional payout. Nevertheless, pathological but not nonpathological gamblers activated emotional regions of the brain as well as portions of the midbrain that are part of the brain's reward system (Robbins & Everitt, 1999). One potential interpretation may be that pathological gamblers found the winning spins more pleasant, positive, or rewarding, even though no additional payout was provided. Another possibility is that pathological gamblers have gambled considerably more during their lives than nonpathological gamblers, so that the function of the near-miss is comparatively well-learned (as reflected in the differing patterns of brain activation). A related thought is that gambling may enter into a much wider array of environment–behavioral relations in the pathological gambler (e.g., enabling relations, such as hiding gambling debts and lying about gambling activities), resulting in more extensive networks of brain activation under experimental conditions such as gambling, including those that alter the significance of the near-miss. These speculations, which require a substantial amount of research to even begin to address, highlight the likely bidirectional nature of brain–behavior interactions.
Indeed, the finding of greater activity during winning and near-miss spins in the anterior medial temporal region in pathological but not nonpathological gamblers is consistent with a role for structures in this region in the aberrant learning that is hypothesized to underlie various forms of addiction (Robbins & Everitt, 1999). Past studies have shown that the amygdala and the hippocampus receive dopaminergic projections from the mesolimbic reward pathway (Adinoff, 2004; Robbins & Everitt, 1999; Volkow, Fowler, Wang, & Goldstein, 2002) and send projections to the nucleus accumbens (Robbins & Everitt, 1999). Thus, the amygdala and hippocampus play an integral role in the dopaminergic mesolimbic reward system, the neural system that underlies experiences of pleasure and reward as well as addiction. Additionally, the amygdala has been implicated in the learning of associations between specific cues and drug-induced states (Robbins & Everitt, 1999; Kalivas & Volkow, 2005), as well as stress-induced drug seeking behavior (Kalivas & Volkow). Together, these findings suggest that activity in the anterior medial temporal region in the pathological gamblers may be associated with aberrant emotional highs to the winning slot machine outcomes, and in a casino environment, this type of brain response may increase the likelihood of pathological gambling, especially since a main motivator for gambling is as a means to deal with day-to-day stress (Petry, 2005).
Turning to the losses, two observations are also noteworthy about this set of results. First, the network of activated regions was more extensive in nonpathological than pathological gamblers, and secondly, the network in nonpathological gamblers involved medial and lateral parietal cortices, as well as bilateral frontal cortices. In pathological gamblers the only region uniquely active was the superior parietal cortex. The more extensive nature of the network may imply that nonpathological gamblers are more responsive to losses than pathological gamblers. The regions involved in the loss network are intriguing because similar regions have been associated with the less impulsive choice in the delayed discounting procedure. For example, McClure, Laibson, Loewenstein, and Cohen (2004) observed greater activity within dorsolateral prefrontal and posterior parietal cortices when subjects preferred trials with a larger delayed reward over a smaller immediate reward. Interestingly, when subjects indicated that they preferred the smaller immediate reward over the larger delayed reward, McClure et al. observed activity in dopamine-innervated regions within the limbic system—amygdala, nucleus accumbens, ventral pallidum, and related structures—regions that in the present study were active when pathological gamblers viewed winning outcomes. Bechara (2005) labeled these two systems the “impulsive” and “reflective” systems. It appears that the impulsive system is recruited when pathological gamblers experience winning spins, whereas the reflective system is recruited when nonpathological gamblers are faced with losing spins. Compatible findings with regards to the distinction between the impulsive limbic system and the reflective/executive frontal/parietal system have been reported in several other fMRI studies as well (Ballard & Knutson, 2009; Boettiger et al., 2007; Hariri et al., 2006; Hoffman et al., 2008; Kable & Glimcher, 2007; Wittmann, Leland, & Paulus, 2007).
Besides similar regions of activation, the delayed discounting literature is relevant because previous research has indicated that pathological gamblers tend to discount delayed rewards to a greater extent than nonpathological gamblers. For example, Petry and Casarella (1999) examined delayed discounting in pathological gamblers with and without substance-abuse problems and control subjects. They found that the pathological gamblers without substance abuse problems discounted more than control subjects; however, the pathological gamblers with substance abuse problems discounted significantly more than both the control subjects and the pathological gamblers without substance abuse problems. Similarly, Alessi and Petry (2003) demonstrated that the severity of pathological gambling as measured by the SOGS was positively correlated with delayed discounting: Subjects with more severe pathological gambling behavior (SOGS > 13) discounted more than subjects with less severe pathological gambling behavior (6 < SOGS < 13). Finally, Dixon, Marley, and Jacobs (2003) reported that even moderate pathological gamblers (mean SOGS = 5.85) discounted more than nonpathological gamblers on a delayed discounting procedure. Given the tendency for greater discounting and overlap in activated brain regions, these findings suggest that pathological gambling may be viewed as an impulse control problem.
Differences in activity between pathological and nonpathological gamblers were noted in the left midbrain, near the substantia nigra and ventral tegmental area (SN / VTA). The SN / VTA is the origin of the mesostriatal and mesolimbic pathways (Adinoff, 2004). Dopaminergic neurons of the mesolimbic pathway project primarily to the NA in the ventral striatum (Robbins & Everitt, 1999). We found that in pathological gamblers, activity in the left midbrain correlated with activity in the right nucleus accumbens. The nucleus accumbens, through the neurotransmitter dopamine, has been shown to mediate the experience of natural rewards such as food and sex (Adinoff). In drug addiction, the nucleus accumbens has been linked to the rewarding effects (“high”) of illicit drugs such as amphetamine and cocaine (Robbins & Everitt) as well as the prediction of the occurrence of a reward (Volkow & Li, 2004). It has been hypothesized that a reduction in the sensitivity of the mesolimbic reward pathway to natural reinforcers may lead individuals to seek out illicit drugs in order to activate this reward system (Volkow et al., 2002). Consistent with this hypothesis, the lower level of activity in the midbrain dopaminergic system paired with a positive correlation with the nucleus accumbens suggests that pathological gamblers may also have a hyposensitive reward system (Reuter et al., 2005). In a manner similar to the development of drug addiction, this may lead individuals to seek out gambling as a means of activating the mesolimbic reward system, potentially leading to the development of pathological gambling over time. Two caveats about this set of results should be mentioned, however. First, while we prefer this interpretation of the present data, it should be noted that because a nongambling baseline condition was not included in the study, it is unclear whether the observed differences between pathological and nonpathological gamblers in the SN / VTA are specific to gambling stimuli or whether they are global differences in brain activity. Second, while there is some debate with regards to the ability to localize the BOLD signal within the SN / VTA (cf. D'Ardenne, McClure, Nystrom, & Cohen, 2008; Düzel et al., 2009), the location of the activation and the fact that it correlated with activity in the ventral striatum, the projection site of SN / VTA dopaminergic neurons, suggests to us that indeed the source of the BOLD signal was in the SN / VTA. Future research will be needed to examine both issues in more detail.
Severity of pathological gambling was found to correlate negatively with activity in the right middle frontal gyrus, ventral medial frontal gyrus, and the thalamus (see Figure 3). Thus, as gambling severity increased, activity in these regions declined. The ventromedial frontal cortex is the projection site for a third midbrain dopaminergic tract (Adinoff, 2004), the mesocortical pathway, and has been shown to be hyperactive in drug intoxication while hypoactive during drug withdrawal (Volkow et al., 2002). One putative function for the ventromedial frontal cortex in drug addiction is in inhibitory control (Volkow et al.)—the processes necessary to restrain maladaptive behaviors such as impulsive and compulsive drug administration (Robbins & Everitt, 1999; Volkow et al.). The negative correlation between neural activity in the ventromedial frontal cortex and the severity of pathological gambling may be related to its role in inhibitory processes. This correlation suggests that as the severity of the addiction increases, the ability for these individuals to control their cravings and inhibit their impulsive and compulsive need to gamble may diminish.
In summary, our data show that while behavioral measures of the near-miss effect indicate homogeneity of responding across both pathological and nonpathological gamblers, it appears that the effect is only “skin deep.” As Skinner noted, the world within the skin is important for a comprehensive analysis of behavior, and when we have the tools to explore this world, we should do so. When supplemental dependent measures of neurological activity were added to the analysis, marked differences emerged that were orderly between our two groups of participants. This merging of research traditions (behavioral and neuroscience) has been debated within the behavioral community for some time (see Timberlake, Schaal, & Steinmetz, 2005 for a discussion), and our findings indicate three specific advantages of this translational research approach. First, the behavior we typically measure is not the only measurable activity occurring in the organism that is correlated with environmental events. As we showed, and as Skinner (1974) noted, the world within the skin is worthy of analysis, and should not be a boundary of our science. He stated: “The promise of physiology is of a different sort. New instruments and methods will continue to be devised, and we shall eventually know much more about the kinds of physiological processes, chemical or electrical, which take place when a person behaves.” (p. 214–215). In the current study, observable behavior in response to the near-miss (its rating as similar to a win) did not vary between groups. Nevertheless, the correlated brain events were clearly different for pathological gamblers. Thus, in this context the momentary effects of a near-miss, a potentially powerful event in an extended episode of gambling (Kassinove & Schare, 2001; MacLin et al., 2007; Strickland & Grote, 1967), could only be differentiated at the brain level. We argue that this constitutes strong support for including neuroscience approaches in investigations of human behavior. Second, the collateral collection of supplemental neurological activity of the organism allows the present data to speak to scientists beyond the traditional behavioral community. Although the behavioral scientist may be satisfied with rate, or response allocations as a sufficient measure of organismic activity, those beyond the walls of behavior analysis will find more comfort in contemporary and biologically-based measures of behavior. While we are not advocating an abandoning of rate and other very usual dependent variables, we are suggesting that many such analyses could be supplemented with neurobehavioral markers to increase impact within the scientific community. Third, our data provide an example of how a behavioral analysis can coexist with a neurological analysis, with the latter not needing to be the cause of the former. The cohabitation of levels of analysis, in contrast to the dependency of a behavioral on neurological analysis, is perhaps what Skinner hoped for when he stated “A small part of the universe is contained within the skin of each of us. There is no reason why it should have any special physical status because it lies within this boundary, and eventually we should have a complete account of it from anatomy and physiology” (1974, p. 21). Skinner's “physiologist of the future” may be here today, contributing to a more complete understanding of behavior. In the present study, this was true in understanding the dynamics of the near-miss effect and its impact on various gambler types. When the eventual goal of such research is to treat actual people with actual clinical disorders, the end may appear to justify such translational means.
Acknowledgments
The authors thank Valeria Della Maggiore and Lars Nyberg for comments on an earlier draft. The authors also thank Jessica Gerson, Olga Nikonova, and Holly Bihler for assistance with data collection and Julie Alstat and Gary Etherton for assistance with MRI scanning.
REFERENCES
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard Review of Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi S, Petry N. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behaviour Processes. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Boettiger C, Mitchell J, Tavares V, Robertson M, Joslyn G, D'Esposito M, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. Journal of Neuroscience. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania A.C. Gambling, shaping, and ratio contingencies. Analysis of Gambling Behavior. 2008;2:69–72. [Google Scholar]
- Clark L, Lawrence A.J, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61(3):481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, McClure S, Nystrom L, Cohen J. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- DeLeon I.G. What else might we ask?: Commentary on Fantino and Stolarz-Fantino's “Gambling: Sometimes unseemly; Not what it seems”. Analysis of Gambling Behavior. 2008;2:89–92. [PMC free article] [PubMed] [Google Scholar]
- Dixon M.R. Manipulating the illusion of control : Variations in risk-taking as a function of perceived control over chance outcomes. The Psychological Record. 2000;50:705–720. [Google Scholar]
- Dixon M.R, Nastally B.L, Jackson J.W, Habib R. Altering the “Near-Miss” effect in slot machine gamblers. Journal of Applied Behavior Analysis. in press. [DOI] [PMC free article] [PubMed]
- Dixon M, Marley J, Jacobs E. Delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2003;36:449–458. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M.R, Schreiber J. Near-miss effects on response latencies and win estimations of slot machine players. The Psychological Record. 2004;54:335–348. [Google Scholar]
- Düzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott B, Tobler P. Functional imaging of the human dopaminergic midbrain. Trends in Neurosciences. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Fantino E, Stolarz-Fantino S. Gambling: Sometimes unseemly; Not what it seems. Analysis of Gambling Behavior. 2008;2:61–68. [PMC free article] [PubMed] [Google Scholar]
- Hariri A, Brown S, Williamson D, Flory J, de Wit H, Manuck S. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. Journal of Neuroscience. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W, Schwartz D, Huckans M, McFarland B, Meiri G, Stevens A, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berlin) 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon A, Dymond S, Jackson J.W, Dixon M.R. Contextual control of slot-machine gambling: Replication and extension. Journal of Applied Behavior Analysis. 2008;41:467–470. doi: 10.1901/jaba.2008.41-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J, Glimcher P. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P, Volkow N. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kassinove J.I, Schare M.L. Effects of the ‘near miss’ and the ‘big win’ on persistence at slot machine gambling. Psychology of Addictive Behaviors. 2001;15:155–158. doi: 10.1037//0893-164x.15.2.155. [DOI] [PubMed] [Google Scholar]
- MacLin O.H, Dixon M.R, Daugherty D, Small S.L. Using a computer simulation of three slot machines to investigate a gambler's preference among varying densities of near-miss alternatives. Behavior Research Methods, Instruments, and Computers. 2007;39:237–241. doi: 10.3758/bf03193153. [DOI] [PubMed] [Google Scholar]
- Madden G.J. Discounting within the gambling context. Analysis of Gambling Behavior. 2008;2:93–98. [Google Scholar]
- McClure S, Laibson D, Loewenstein G, Cohen J. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Parke A, Griffiths M. Gambling addiction and the evolution of the ‘near miss’. Addiction Research & Theory. 2004;12:407–411. [Google Scholar]
- Petry N, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug and Alcohol Dependence. 1999;56:25–32. doi: 10.1016/s0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Petry N.M. Pathological gambling: Etiology, comorbidity, and treatment. Washington, DC: American Psychological Association; 2005. [Google Scholar]
- Potenza M.N, Steinberg M.A, Skudlarski P, Fulbright R.K, Lacadie C.M, Wilber M.K, et al. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2003;60:828–836. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Robbins T.W, Everitt B.J. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Skinner B.F. Science and human behavior. Knopf; New York: 1953. [Google Scholar]
- Skinner B.F. About behaviorism. Knopf; New York: 1974. [Google Scholar]
- Strickland L.H, Grote F.W. Temporal presentation of winning symbols and slot-machine playing. Journal of Experimental Psychology. 1967;74:10–13. doi: 10.1037/h0024511. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Timberlake W, Schaal D.W, Steinmetz J.E. Relating behavior and neuroscience: Introduction and synopsis. Journal of the Experimental Analysis of Behavior. 2005;84:305–312. doi: 10.1901/jeab.2005.99-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Li T. Drug addiction: the neurobiology of behaviour gone awry. Nature Reviews Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volkow N.D, Fowler J.S, Wang G.J, Goldstein R.Z. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiololgy of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Weatherly J.N. Slot machine preferences are insensitive to programmed contingencies. Journal of Applied Behavior Analysis. in press.
- Weatherly J.N, Dixon M.R. Toward an integrative behavioral model of gambling. Analysis of Gambling Behavior. 2007;1:4–18. [Google Scholar]
- Wittmann M, Leland D, Paulus M. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Experimental Brain Research. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Zlomke K.R, Dixon M.R. The impact of altering stimulus functions and contextual variables on gambling. Journal of Applied Behavior Analysis. 2006;39:51–361. [Google Scholar]