Abstract
A shift in generalization gradients away from S+ and towards stimuli on the opposite end of the stimulus dimension from S− is a well established phenomenon in the laboratory, occurring with humans and nonhumans and with a wide range of stimuli. The phenomenon of gradient shifts has also been observed to have an analogous relationship to a variety of apparent biases in preference observed in the natural environment. One way to examine the validity of such analogies is by examining whether gradient shifts can be observed with complex and naturalistic stimuli. In the present experiment, undergraduates were trained to discriminate between faces that varied in terms of relative bilateral facial symmetry (a stimulus dimension correlated with health and attractiveness). Comparisons were made within subjects, using two sets of images. For both sets, the faces varied from naturally asymmetrical to symmetrical, and S+ was a face equidistant to the two extremes. With one set, S− was the naturally asymmetrical face, and with the other, S− was the symmetrical face. A peak shift was obtained in both conditions, although the effect was clearer in the aggregate than on the level of the individual. Overall, the results are consistent with the view that the processes responsible for gradient shifts in the lab are relevant to judgments made in the natural environment.
Keywords: discrimination training, stimulus generalization, peak shift, mouse-click, humans
Stimulus generalization describes the production of responses in the presence of stimuli similar to one previously paired with reinforcement (S+). Typically, stimulus generalization is studied using the two-phase Guttman and Kalish (1956) procedure. In a training phase, responding in the presence of S+ is established; in a testing phase, generalization to other stimuli is assessed. The test stimuli (usually including S+) are drawn from various points on a stimulus dimension, and each stimulus is presented repeatedly under extinction. Commonly, stimuli that are relatively similar to S+ evoke more responses than stimuli that are further removed on the dimension. The frequency of responding to each the test stimuli, arranged ordinally along some critical perceptual dimension, makes this generalization gradient visually apparent.
Hanson (1959) employed a variant of the Guttman and Kalish method in a classic experiment involving a light-wavelength stimulus dimension. Performances of pigeons trained to respond in the presence of S+ were compared with those trained to discriminate S+ from a second stimulus paired with extinction (S−). Discrimination training reduced the frequency of responses in the presence of S−, but it did not increase the frequency of responses evoked by S+. Instead, subjects responded most frequently to novel stimuli on the opposite end of the wavelength dimension from S−. Hanson termed this change in the modal response a peak shift; subsequent research made clear that shifts in the gradient away from S− are a robust phenomenon. Gradient shifts (including peak shift) have been demonstrated using such species and stimuli as monkeys and tone frequency (Moody, Stebbins, & Iglauer, 1971), rats and click frequency (Weiss & Schindler, 1981), horses and object size (Dougherty & Lewis, 1991), moths and odor (Daly, Chandra, Durtschi, & Smith, 2001), bumblebees and hue (Lynn, Cnaani, & Papaj, 2005), pigeons and spatial location (Cheng, Spetch, Kelly, & Bingman, 2006), and zebra finches and birdsongs (Verzijden, Etman, van Heijningen, van der Linden, & ten Cate, 2006).
One reason why gradient shifts continue to be a subject of interest is that this laboratory-based phenomenon resembles a number of apparent biases in stimulus preference observed in nonhumans and humans. For example, herring gulls will retrieve to their nest supernormally large eggs instead of their own eggs when the two have equivalent coloration (Baerends, 1982). Ostensibly, such birds treat relatively small eggs as a de facto S− because they are less likely to produce viable offspring. This “S−” is held responsible for the preference for the supernormally large eggs (cf. Ghirlanda, & Enquist, 1999, 2003; Staddon, 1975).
It also has been suggested that aposematic cues (e.g., warning coloration) may evolve in part because of a peak-shift-like process that causes predators to show more aversion to prey displaying stronger aposematic cues than those with which they have had a negative experience (e.g., Gamberale & Tullberg, 1996; Leimar, Enquist, & Sillen-Tullberg, 1986; Yachi & Higashi, 1998). In this regard, domestic chicks disproportionately avoid larvae that are larger (Gamberale & Tullberg, 1996) or redder (Gamberale-Stille & Tullberg, 1999) than one that served as S−. Other examples include the suggestion that peak shift can help explain the evolution of plant defenses and herbivore diet selection (Leimar & Tuomi, 1998), floral characteristics and pollinator selection (Chittka & Raine, 2006), and sexual dimorphism (ten Cate & Rowe, 2007; Weary, Guilford, & Weisman, 1993).
Peak shift has also been invoked to explain the human tendency to find supernormal stimuli (in the form of exaggerated features and caricatures) to be more aesthetically pleasing than natural representations of appearance (Ramachandran & Hirstein, 1999; Zimmer, 2003). Similarly, some textbooks on learning theory have suggested that a man who had a positive relationship with a woman with dark brown hair (S+) and a negative relationship with a woman with light brown hair (S−) should prefer women with very dark hair (Powell, Symbaluk, & McDonald, 2002), or that a man who had a positive relationship with an extrovert and a negative relationship with an introvert should prefer women who are very extroverted (Powell, Symbaluk, & Honey, 2009).
If it is the case that any kind of preference for supernormal stimuli or shift in stimulus preference can be understood in terms of gradient shifts, than surely gradient shifts warrant greater attention then they have received in recent decades from behavior analysts. Consider, for example, the potential connection with certain pathologies. Perceptual distortions are a hallmark of body dysmorphic disorder and anorexia nervosa. Perhaps such distortions are caused by a learning history akin to discrimination training in the lab: The individual has learned to avoid a certain type of “inappropriate” appearance (the presumptive S−) and has difficulty distinguishing this appearance from an appropriate one (S+). In the lab, gradient shifts are most pronounced when S+ and S− are relatively similar (e.g., Baron, 1973; Derenne, 2006; Hanson, 1959). Inappropriate anxiety in obsessive-compulsive disorder likewise can be framed in terms of a shift towards an extreme response due to difficulty discriminating S+ from S−.
Translational analogies about the role of gradient shifts in the natural environment are provocative, but they are also difficult to evaluate, in part because no consensus exists as to why gradient shifts occur in the first place (cf. Kalish, 1969; Lynn et al., 2005; McLaren & Mackintosh, 2002; Thomas, 1993). An additional problem is that laboratory research has favored experimental control over external validity, leaving its relevance to everyday phenomena unclear in several ways. For example, laboratory procedures measure the number of responses in the presence of an isolated stimulus, whereas claims about peak shift in the natural environment generally focus on preference among stimuli. Laboratory stimuli tend to be simple and arbitrary, and therefore of uncertain relevance to the multidimensional stimuli commonly found in nature. Laboratory studies also typically employ stimuli that allow a wide range of variation (e.g., light wavelength), whereas the stimulus dimensions of interest outside the lab may reflect fewer gradations (e.g., hair color). Finally, laboratory-based training and testing procedures usually are protracted, whereas in the natural environment the relevant “training” and “testing” experiences may be comparatively brief.
One way to begin to address the interpretative issues raised by translational analogies is to incorporate into the laboratory stimuli similar to those encountered in the natural environment. This approach reveals, for example, that gradient shifts can occur among categorical stimuli, including categories of status-ranked occupations (Howard, 1979), and categories of human faces (Derenne & Breitstein, 2006). Gradient shifts have also been demonstrated with relatively complex stimuli that permit less stimulus variation than is normally allowed to occur in the lab, including variations in a woman's waist-to-hip ratio (Derenne, Breitstein, & Cicha, 2008), and morphed human faces (Lewis & Johnston, 1999; McLaren & Mackintosh, 2002; Spetch, Cheng, & Clifford, 2004).
The present experiment was conducted with human participants, and, like several earlier studies, used a stimulus dimension based on morphed faces. In previous studies of this kind, the stimulus dimension was created by morphing two or more different faces together. In the present case, the interest was with facial symmetry, and the stimulus dimension was generated by morphing together symmetric and naturally asymmetric versions of the same face. There is considerable evidence that symmetrical faces are perceived as more attractive than asymmetical faces (e.g., Fink, Neave, Manning, & Grammer, 2006; Perrett et al., 1999; Rhodes, Proffitt, Grady, & Sumich, 1998).
The examination of gradient shifts within a facial symmetry dimension is of interest for several reasons. From the standpoint of research on facial attractiveness, the production of gradient shifts within such a dimension suggests that judgments about facial symmetry are susceptible to bias from experiences analogous to discrimination training. From the standpoint of research on stimulus generalization, it should be noted that gradient shifts are more pronounced when there is a wide range of stimuli (cf. Verbeek, Spetch, Cheng, & Clifford, 2006), and morphing one face with a second, slightly modified face (i.e., the symmetric version of a naturally asymmetrical face) permits little variation. Gradient shifts are also less pronounced when S+ and S− can be readily discriminated (cf. Derenne, 2006), as may be expected if humans are especially attuned to facial symmetry. Given these challenges, finding gradient shifts within this dimension would attest to the generality of the phenomenon.
To be impactful in the natural environment, gradient shifts would have to emerge readily rather than depend on the protracted stimulus exposure as is typical of laboratory testing. In recent studies of gradient shifts with humans, for instance, the tests ranged from 66 trials (Bizo & McMahon, 2007) to 180 trials (Spetch et al., 2004) in duration. In studies with nonhumans, which have provided much of the data on gradient shifts, test regimens often have been even more extended. Only rarely have gradient shifts been examined in the context of brief generalization tests, and the findings have been mixed (Howard, 1979; Thomas, Svinicki, & Vogt, 1973). In the present study the generalization test was limited to 21 trials in duration, and an attempt was made to examine generalization upon the first presentation of each test stimulus.
In research involving both humans and nonhumans, it is customary to compare generalization gradients obtained under different conditions on a between-groups basis (e.g., Bernard & Giurfa, 2008; Bizo & McMahon, 2007; Hauf, Prior, & Sarris, 2008; Schneider & Lickliter, 2009; Wearden & Farrar, 2007; Wisniewski, Church, & Mercado, 2009). Between-groups comparisons are often warranted because carryover effects from earlier training may affect performances in later conditions. In the present case, however, data were collected on a within-subjects basis, and the possibility of carryover effects was minimized by training and testing participants with different sets of stimuli in each condition (see Derenne et al., 2008; Spetch et al., 2004, for additional examples of this approach). Each set included images that varied from naturally asymmetrical to symmetrical, but the faces were otherwise distinctive. This procedure permitted assessment of the results both in terms of data averaged across participants, and in terms of individual performances.
METHOD
Participants
Participants were 12 undergraduates (3 male, 9 female) who were enrolled in psychology courses and who received extra course credit for participating. As described below, data from 2 participants (both female) were excluded from the analysis of gradient shifts for failure to meet the training criteria.
Apparatus and Stimuli
Data were collected from either 1 or 2 participants at a time in a room with four adjacent workstations. Each workstation included a 33-cm (13-inch) Samsung DynaFlat color monitor, placed on top of a table. A mouse and keyboard were placed in front of each monitor, and a computer was positioned below the table. The workstations were separated by large wooden dividers.
The sets of facial images were created as follows: Each set was based on a photograph (one of a woman, one of a girl) taken with a Sony Cyber-Shot DSC-w1 5.1 megapixel digital camera. The images were first cropped and then reduced in size, so that they would appear to be 7.5 cm × 8.5 cm on the computer screen. To create a symmetrical image, the images were imported into Microsoft Paint 5.1, and one side of the face (including one eye, one cheek, one side of the nose, and one half of the mouth), was copied, reversed, and transposed onto the opposite side of the face (see Figure 1). The image was saved as a 256-color Bitmap. This procedure simplified the color palette and removed subtle variations in light and tone that otherwise create a visual “seam” in the symmetrical image. Finally, the two images were morphed together using WinMorph 3.0 so as to render five images intermediate to the asymmetrical and symmetrical image. The morphing program uses a linear algorithm, which keeps constant the degree of change between each pair of adjacent stimuli. Figure 2 shows the seven images comprising each of the two image sets. For data analysis purposes, the stimuli were numbered from 1 (naturally asymmetrical) to 7 (symmetrical).
Fig 1.

Procedure for creating a symmetrical face: One half of the face is copied, reversed, and transposed onto the other half of the face.
Fig 2.

The two sets of stimuli.
Procedure
The study consisted of two parts, each involving discrimination training and generalization testing. For all participants, Part 1 involved images of the woman (Figure 2, top), and Part 2 involved images of the girl (Figure 2, bottom). Whether the S− was the asymmetrical image or the symmetrical image with a given stimulus set was counterbalanced across participants (i.e., for 5 participants, the asymmetrical image was S−, and for the other 5, the symmetrical image was S−).
At the beginning of the experiment, the participant was directed to sit at one of the workstations. The computer screen displayed the following instructions:
You will be shown a series of pictures depicting a face. When this picture appears, study it carefully. You will have to remember what this picture looks like. After a short delay, you will be shown a series of pictures of the same person. Some of the images that you see will be the same as the original picture; others will be different. The original picture and the new picture will be only slightly different, but there is a difference in the area of the eyes, nose, and mouth. You will have to indicate whether you think a given image is the original one or not. At first you will be told whether your choices are correct. Later, there will not be any feedback. Try to be as accurate as you can. After you have viewed all of the images of the first person, the procedure will be repeated with pictures of a second person. Click on the “Start” button to start the experiment.
Once the participant clicked on the Start button with the mouse (the button was immediately below the instructions), a screen appeared showing S+. S+ was shown for 10 s, with the message “The original image” displayed above it. A series of training trials then began in which S+ and S− were shown side-by-side. The relative placement of S+ across trials in Part 1 was left (L), right (R), R, L, L, R, L, R, R, L, R, L, L, L, R. During Part 2, the sequence was R, L, R, L, R, R, R, L, L, L, R, R, L, R, L. Each time an image appeared, the message “Which one is the original picture?” appeared at the top of the screen. The participant selected a picture by clicking on a grey button labeled “Left” or “Right” located immediately below the two images. The participant had unlimited time to select a picture. Once a selection was made, the participant was shown, for 3 s, a message indicating that the choice was “Correct” or “Incorrect,” depending, respectively, on whether the participant had selected S+ or S−. The message “Please Wait” was then displayed for 10 s before the next trial began.
After 15 training trials, a 21-trial generalization test began, which included each of the images within a stimulus set. The generalization test was organized into three cycles of images, and within a given cycle, each of the seven images was shown once in a randomized sequence. On each test trial, the message “Is this the same as the original?” appeared at the top of the screen, and two buttons marked “Yes” and “No” appeared immediately below the image. As was the case during training, the participant had unlimited time to click on one of the two buttons using the mouse. Once a response was made, the message “Please Wait” was displayed for 10 s before the next trial began. During the test, feedback about response accuracy was withheld.
RESULTS
Analysis of gradient shifts is customarily based on aggregated data because different groups are assigned to different conditions. In the present case, the data were analyzed both in terms of data averaged across participants and in terms of individual performances. The analysis included participants who, during the discrimination training phase in Parts 1 and 2, made the correct response on 10 of the 15 trials overall and on each of the final 6 trials. Table 1 summarizes training outcomes for the 12 participants (H1–H12). Two participants (H11 and H12) who failed to meet the training criteria were excluded from further consideration. Hereafter, results are presented for 10 participants.
Table 1.
Number of Errors During Discrimination Training.
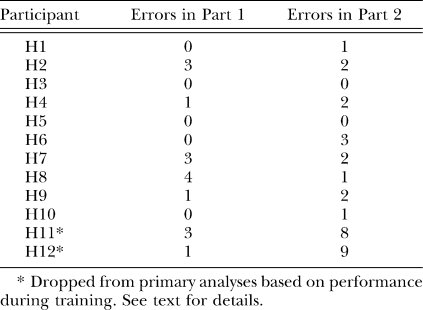
Figure 3 shows the aggregated performances, expressed in terms of the mean number of responses emitted in the presence of each test stimulus (i.e., how many occasions the participant clicked “Yes” to indicate that the stimulus was identical to S+). Participants had three opportunities to select a given stimulus (once for each cycle of test stimuli). The figure shows a peak shift in both conditions, with the modal response occurring in the presence of a stimulus adjacent to S+, in the direction opposite S−.
Fig 3.
Generalization gradients following training with either the asymmetrical (Asymm.) image as S− (open circles) or the symmetrical (Symm.) image as S− (filled circles). Data are averaged; the vertical bars depict the standard error of the mean.
The slopes of both gradients were relatively steep on the asymmetrical end of the dimension and flat on the symmetrical end. To determine whether the difference in the gradients was likely due to chance, a mixed-model ANOVA was performed on the mean response within each test. To calculate the mean response for each participant/condition, each response was assigned a numerical value equivalent to the image present at the time of the response (e.g., 1 for each response to the naturally asymmetrical image, 4 for each response to S+, and 7 for each response to the symmetrical image). The resulting sum was divided by the total number of responses that were emitted. The analysis revealed a significant main effect for the relative position of S−, F (1, 8) = 13.43, p = 0.006, but not for the order in which the conditions occurred (p = 0.616). The interaction was also nonsignificant (p = 0.906).
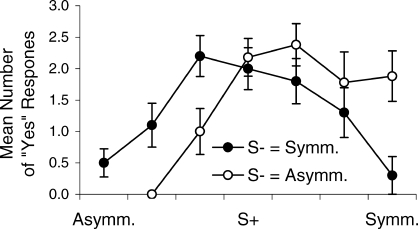
Figure 4 shows aggregated performances during the generalization test when only the first response to each test stimulus is considered. Generalization gradients appeared immediately upon testing that closely resembled those of Figure 3. Peak shift was present within the first cycle when the S− was the symmetrical image, but not when S− was the asymmetrical image. Also, a comparison with overall test performances indicates that there was a slight shift in both gradients toward the symmetrical end of the dimension after the first cycle.
Fig 4.
Aggregated generalization gradients after the initial presentation (cycle) of each test stimulus.
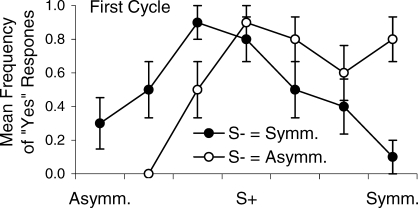
Figure 5 shows the individual performances on which the aggregated data in Figure 3 were based. For some participants, a gradient shift occurred in both conditions (e.g., H1 and H6), while for others, a shift occurred in only one (e.g., H7 and H9). Two participants (H3 and H8) produced no gradient shift, and one participant (H2) produced a marked shift away from S− in one condition, but towards S− in the other. Overall, a shift in the mean response away from S− occurred in 13 of 20 opportunities (each “opportunity” reflects one of the two conditions completed by the 10 participants).
Fig 5.
Generalization gradients for the 10 individual participants following training with either the asymmetrical image (AS.) as S− (open circles) or the symmetrical (S.) image as S− (filled circles). The upper row shows outcomes when the S-s were the asymmetrical image of the woman and the symmetrical image of the girl; the lower row shows outcomes when the S-s were the symmetrical image of the woman and the asymmetrical image of the girl.
DISCUSSION
Gradient shifts have been linked to a wide range of biases in preference and errors in stimulus selection. There has been, however, little empirical basis to support some of the relevant claims. One way to better understand the potential generality of gradient shifts is by examining the phenomenon in a laboratory setting with procedures that incorporate elements of the natural environment (such as relatively complex and naturalistic stimuli). Such research can verify whether the effect can be produced under unconventional conditions, which is a preliminary step toward determining whether a single process operates in all cases inside or outside the lab, as some gradient-shift analogies imply. An added benefit is that this line of inquiry may reinvigorate research on gradient shifts by raising new questions about the phenomenon, by illustrating potential extensions to the natural environment, and by highlighting potentially important variables that are not normally considered (i.e., structural discontinuities between laboratory procedures and natural analogues).
The present experiment addressed the generality of gradient shifts through the use of a procedure that differed from the typical laboratory experiment in terms of the type of stimuli that were used (faces that varied in terms of bilateral symmetry), the manner in which generalization was assessed (a brief test), and the manner in which comparisons were made (both individual and group-aggregate data).
Other studies have shown that it is possible to produce gradient shifts when participants are trained and tested with face stimuli (Lewis & Johnston, 1999; McLaren & Mackintosh, 2002; Spetch et al., 2004). In these cases, the stimulus dimension was created by morphing together two different faces (e.g., a composite face with an individual face). By comparison, in the present experiment, two different versions of the same face (one symmetrical, the other naturally asymmetrical) were morphed together, so as to specifically consider judgments about bilateral facial symmetry. Humans are allegedly sensitive to cues of facial attractiveness, and symmetrical faces are “liked” more than other faces (e.g., Fink et al., 2006). The present procedure did not assess how attractive participants found the faces, but rather addressed preliminary questions concerned with the discrimination of faces varying in the degree of asymmetry. In this regard, the results suggest that individuals can discriminate varying degrees of facial asymmetry, even when there is little variation among the images, and that the choice of S− affects the distribution of responses.
Because data were collected on a within-subjects basis, it was possible to assess whether the results on the level of the individual participant were consistent with the results aggregated across individuals. Sometimes close attention to individual performances yields insights into the individual development of perceptual distortions, but in the present case no systematic relation was found between the number of errors individual participants made during training and the subsequent degree of gradient shift. This finding is notable given that the occurrence of few errors during training is known to mitigate gradient shifts (cf. Rilling, 1977; Terrace, 1964).
A more fruitful comparison concerned differences in the overall gradients (Figure 3) with those for the first presentation of each test stimulus (Figure 4). Here and elsewhere, there are several ways in which gradients can be compared: in terms of the peaks of the gradients, the shape of the gradients (e.g., slope and skew), and the mean response. The visual markers of gradient shape and the means of the gradients suggest a shift in responding occurred between the first cycle and the end of the generalization test. Specifically, regardless of the placement of S−, the gradients shifted from the asymmetrical end of the distribution towards the symmetrical end. This shift increased the departure in responding from S+ when S− was asymmetrical. For this condition, a peak shift was absent in the first cycle, but present in the overall gradients. Similarly, the mean response increased slightly from 5.08 to 5.15. Conversely, this within-test shift decreased the departure in responding from S+ when S− was symmetrical; the mean changed from slightly shifted (3.66) to virtually unshifted (3.93, with S+ = 4.00). In other words, the gradient became more skewed in favor of symmetrical stimuli.
Adaptation-level theory of gradient shifts (Thomas, 1974, 1993) can explain both why shifts in responding may occur within a test and why some conditions may produce more shift than others. In brief, participants are alleged to use the adaptation level (or the average of the stimuli to which they have been exposed), to guide responding during the generalization test when feedback is withheld. The adaptation level is adjusted with each stimulus presentation during training and testing. Because participants learn to judge stimuli on the basis of the adaptation level that was present during training, the gradient will shift to the degree to which the adaptation level changes during the test (i.e., the adaptation level becomes an inaccurate guide).
One reason why both gradients may have shifted towards the symmetrical end of the distribution during the test is that participants had a preexperimental adaptation level for facial symmetry that is close to the naturally asymmetrical end of the dimension. The repeated presentation of symmetrical stimuli in this experiment modified this adaptation level, and continued to change the adaptation level even during the test (cf. Newlin, Rodgers, Dickson, Strub, & Thomas, 1978). However, this interpretation is speculative because adaptation level was not assessed. Also possible is that participants were increasingly drawn to the symmetrical stimuli for other reasons, including, for example, that those faces may have seemed more attractive.
Setting aside questions about underlying mechanisms, the present finding of peak shift supports the relevance of gradient shifts to naturalistic face stimuli. Exactly how relevant depends on something the present study can not answer, namely the extent to which experiences akin to discrimination training affect judgments about faces outside the laboratory. Certainly, simultaneous discrimination training with slightly different versions of the same face is unlikely to occur in the natural environment. Through other types of experiences, however, people may learn to treat a particular facial characteristic as a de facto S−, and this, in turn, may create a shift towards the selection of stimuli quite unlike it. As noted earlier, the preference commonly shown for certain exaggerated representations of form, such as caricatures, can be framed in terms of peak shift (cf. Ramachandran & Hirstein, 1999; Zimmer, 2003). Perhaps a better analogy is the application of makeup to create redder lips than naturally occur, or the use of cosmetic surgery to create fuller lips than naturally occur. Likewise, digital manipulation of photographs is commonly used to enhance the physical attributes of their subjects beyond the limits found in nature (Collins, 2008).
The relation of gradient shifts in the laboratory to naturally occurring shifts in stimulus selection must remain a topic for debate and continued investigation given the many differences between laboratory procedures and analogous natural experiences. Emphasis in the present experiment was placed on one such structural discontinuity: that laboratory testing usually is prolonged whereas possibly homologous experiences in the natural environment may be more limited. The results were encouraging for the validity of translational analogues in that a gradient shift was immediately present (see also Howard, 1979). Future topics for consideration include what minimal duration of discrimination training will allow gradient shifts to occur (e.g., will a single presentation of S+ and S− suffice?), and what role is played by the nature of the consequence (e.g., will social reinforcement, rather than computer-generated feedback, change the effect?). With regard to facial symmetry, specific topics worth consideration include the basis on which participants discriminate between the stimuli (i.e., which cues pertaining to symmetry are most salient) and whether discrimination training affects preference.
REFERENCES
- Baerends G. The herring gull and its egg. Part II. The responsiveness to egg-features. Behaviour. 1982;82:358–363. [Google Scholar]
- Baron A. Postdiscrimination gradients of human subjects on a tone continuum. Journal of Experimental Psychology. 1973;101:337–342. doi: 10.1037/h0035206. [DOI] [PubMed] [Google Scholar]
- Bernard J, Giurfa M. The cognitive implications of asymmetric color generalization in honeybees. Animal Cognition. 2008;11:283–293. doi: 10.1007/s10071-007-0112-5. [DOI] [PubMed] [Google Scholar]
- Bizo L.A, McMahon C.V. Temporal generalization and peak shift in humans. Learning & Behavior. 2007;35:123–130. doi: 10.3758/bf03193047. [DOI] [PubMed] [Google Scholar]
- Cheng K, Spetch M.L, Kelly D.M, Bingman V.P. Small-scale spatial cognition in pigeons. Behavioural Processes. 2006;72:115–127. doi: 10.1016/j.beproc.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Chittka L, Raine N.E. Recognition of flowers by pollinators. Current Opinion in Plant Biology. 2006;9:428–435. doi: 10.1016/j.pbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Collins L. Pixel perfect: Pascal Dangin's virtual reality. The New Yorker. 2008. May 12, pp. 94–103.
- Daly K.C, Chandra S, Durtschi M.L, Smith B.H. The generalization of an olfactory-based conditioned response reveals unique but overlapping odour representations in the moth Manduca sexta. The Journal of Experimental Biology. 2001;204:3085–3095. doi: 10.1242/jeb.204.17.3085. [DOI] [PubMed] [Google Scholar]
- Derenne A. Effects of S+, S− separation on gradient shifts in humans. Journal of General Psychology. 2006;133:163–173. doi: 10.3200/GENP.133.2.163-173. [DOI] [PubMed] [Google Scholar]
- Derenne A, Breitstein R.M. Gradient shifts with naturally occurring human face stimuli. The Psychological Record. 2006;56:365–370. [Google Scholar]
- Derenne A, Breitstein R.M, Cicha R.J. Shifts in postdiscrimination gradients within a stimulus dimension based on female waist-to-hip ratios. The Psychological Record. 2008;58:51–60. [Google Scholar]
- Dougherty D.M, Lewis P. Stimulus generalization, discrimination learning, and peak shift in horses. Journal of the Experimental Analysis of Behavior. 1991;56:97–104. doi: 10.1901/jeab.1991.56-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink B, Neave N, Manning J.T, Grammer K. Facial symmetry and judgements of attractiveness, health and personality. Personality and Individual Differences. 2006;41:491–499. [Google Scholar]
- Gamberale G, Tullberg B.S. Evidence for peak-shift in predator generalization among aposematic prey. Proceedings of the Royal Society of London B, Biological Sciences. 1996;263:1329–1334. doi: 10.1098/rspb.1996.0195. [DOI] [PubMed] [Google Scholar]
- Gamberale-Stille G, Tullberg B.S. Experienced chicks show biased avoidance of stronger signals: An experiment with natural colour variation in live aposematic prey. Evolutionary Ecology. 1999;13:579–589. [Google Scholar]
- Ghirlanda S, Enquist M. The geometry of stimulus control. Animal Behaviour. 1999;58:695–706. doi: 10.1006/anbe.1999.1187. [DOI] [PubMed] [Google Scholar]
- Ghirlanda S, Enquist M. A century of generalization. Animal Behaviour. 2003;66:15–36. [Google Scholar]
- Guttman N, Kalish H.I. Discriminability and stimulus generalization. Journal of Experimental Psychology. 1956;51:79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Hanson H.M. Effects of discrimination training on stimulus generalization. Journal of Experimental Psychology. 1959;58:321–334. doi: 10.1037/h0042606. [DOI] [PubMed] [Google Scholar]
- Hauf P, Prior H, Sarris V. Generalization gradients and representation modes after absolute and relative discrimination learning in young chickens. Behavioural Processes. 2008;78:93–99. doi: 10.1016/j.beproc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Howard R.W. Stimulus generalization along a dimension based on a verbal concept. Journal of the Experimental Analysis of Behavior. 1979;32:199–212. doi: 10.1901/jeab.1979.32-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish H.I. Generalization as an epiphenomenon. In: Marx M, editor. Learning processes. New York: MacMillan; 1969. pp. 219–257. [Google Scholar]
- Leimer O, Enquist M, Sillén-Tullberg B. Evolutionary stability of aposematic coloration and prey unprofitability: A theoretical analysis. American Naturalist. 1986;128:469–490. [Google Scholar]
- Leimar O, Tuomi J. Synergistic selection and graded traits. Evolutionary Ecology. 1998;12:59–71. [Google Scholar]
- Lewis M.B, Johnston R.A. Are caricatures special? Evidence of peak shift in face recognition. European Journal of Cognitive Psychology. 1999;11:105–117. [Google Scholar]
- Lynn S.K, Cnaani J, Papaj D.R. Peak shift discrimination learning as a mechanism of signal evolution. Evolution. 2005;59:1300–1305. [PubMed] [Google Scholar]
- McLaren I.P.L, Mackintosh N.J. Associative learning and elemental representation II: Generalization and discrimination. Animal Learning and Behavior. 2002;30:177–200. doi: 10.3758/bf03192828. [DOI] [PubMed] [Google Scholar]
- Moody D.B, Stebbins W.C, Iglauer C. Auditory generalization for response latency in the monkey. Journal of the Experimental Analysis of Behavior. 1971;16:105–111. doi: 10.1901/jeab.1971.16-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin R.J, Rodgers J.P, Dickson J.F, Strub H, Thomas D.R. The central tendency effect in stimulus generalization: Effects of establishing a “preexperimental” frame of reference. Perception & Psychophysics. 1978;24:161–167. doi: 10.3758/bf03199543. [DOI] [PubMed] [Google Scholar]
- Perrett D.I, Burt M, Penton-Voak I.S, Lee K.J, Rowland D.A, Edwards R. Symmetry and human facial attractiveness. Evolution and Human Behavior. 1999;20:295–307. [Google Scholar]
- Powell R.A, Symbaluk D.G, Honey P.L. Introduction to learning and behavior. 3rd ed. Belmont, CA: Wadsworth; 2009. [Google Scholar]
- Powell R.A, Symbaluk D.G, MacDonald S.E. Introduction to learning and behavior. Belmont, CA: Wadsworth; 2002. [Google Scholar]
- Ramachandran V.S, Hirstein W. The science of art: A neurological theory of aesthetic experience. Journal of Consciousness Studies. 1999;6:15–51. [Google Scholar]
- Rhodes G, Proffitt F, Grady J.M, Sumich A. Facial symmetry and the perception of beauty. Psychonomic Bulletin and Review. 1998;5:659–669. [Google Scholar]
- Rilling M. Stimulus control and inhibitory processes. In: Honig W.K, Staddon J.E.R, editors. Handbook of operant behavior. Englewood Cliffs, NJ: Prentice Hall; 1977. pp. 432–480. [Google Scholar]
- Schneider S.M, Lickliter R. Operant generalization of auditory tempo in quail neonates. Psychonomic Bulletin & Review. 2009;16:145–149. doi: 10.3758/PBR.16.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetch M, Cheng K, Clifford C. Peak shift but not range effects in recognition of faces. Learning and Motivation. 2004;35:221–241. [Google Scholar]
- Staddon J.E.R. A note on the evolutionary significance of “supernormal” stimuli. The American Naturalist. 1975;109:541–545. [Google Scholar]
- ten Cate C, Rowe C. Biases in signal evolution: Learning makes a difference. Trends in Ecology & Evolution. 2007;22:380–387. doi: 10.1016/j.tree.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Terrace H.S. Wavelength generalization after discrimination learning with and without errors. Science. 1964;144:78–80. doi: 10.1126/science.144.3614.78. [DOI] [PubMed] [Google Scholar]
- Thomas D.R. The role of adaptation-level in stimulus generalization. In: Bower G.H, editor. The psychology of learning and motivation. San Diego, CA: Academic Press; 1974. pp. 91–145. [Google Scholar]
- Thomas D.R. A model for adaptation-level effects on stimulus generalization. Psychological Review. 1993;100:658–673. [Google Scholar]
- Thomas D.R, Svinicki M.D, Vogt J. Adaptation level as a factor in human discrimination learning and stimulus generalization. Journal of Experimental Psychology. 1973;97:210–219. [Google Scholar]
- Verbeek E.L, Spetch M.L, Cheng K, Clifford C.W.G. Determinants of range effects in face recognition. Learning & Behavior. 2006;34:229–240. doi: 10.3758/bf03192878. [DOI] [PubMed] [Google Scholar]
- Verzijden M.N, Etman E, van Heijningen C, van der Linden M, ten Cate C. Song discrimination learning in zebra finches induces highly divergent responses to novel songs. Proceedings of the Royal Society B: Biological Sciences. 2006;274:295–301. doi: 10.1098/rspb.2006.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearden J.H, Farrar R. Effects of feedback and calibration on the verbal estimation of the duration of tones. Acta Psychologica. 2007;126:1–17. doi: 10.1016/j.actpsy.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Weary D.M, Guilford T.C, Weisman R.G. A product of discriminative learning may lead to female preferences for elaborate males. Evolution. 1993;47:333–336. doi: 10.1111/j.1558-5646.1993.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Weiss S.J, Schindler C.W. Generalization peak shift in rats under conditions of positive reinforcement and avoidance. Journal of the Experimental Analysis of Behavior. 1981;35:175–185. doi: 10.1901/jeab.1981.35-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski M.G, Church B.A, Mercado E. Learning-related shifts in generalization gradients for complex sounds. Learning & Behavior. 2009;37:325–335. doi: 10.3758/LB.37.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachi S, Higashi M. The evolution of warning signals. Nature. 1998;394:882–884. [Google Scholar]
- Zimmer R. Abstraction in art. Philosophical Transactions of the Royal Society of London B, Biological Sciences. 2003;358:1285–1291. doi: 10.1098/rstb.2003.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]