Abstract
Introduction:
Nicotine and tobacco smoking administration have demonstrated antinociceptive effects that are mediated by the nicotinic acetylcholine receptor containing the beta2* subunit (β2*-nAChR). In this study, we examined the relationship between β2*-nAChR availability and nociception during acute withdrawal in human tobacco smokers using [123I]5-IA-85380 ([123I]5-IA) and single photon emission computed tomography (SPECT) brain imaging.
Methods:
Tobacco smokers (n = 24, aged 34 ± 11 years) participated in the cold pressor task during acute withdrawal (up to 3 hr) and a second cold pressor task following 7–13 days of smoking abstinence on the day they were imaged with [123I]5-IA SPECT. The cold pressor task is used to measure pain sensitivity (when subjects first feel pain) and pain tolerance (when subjects cannot withstand pain).
Results:
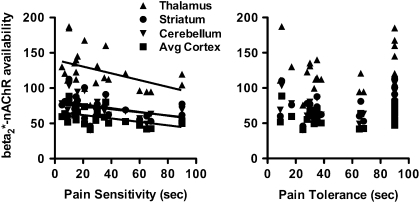
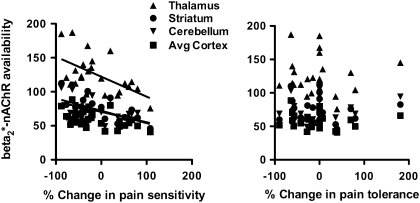
Following 7–13 days of tobacco smoking abstinence, increased pain sensitivity, for example, shorter time to first feel pain, was significantly associated with higher β2*-nAChR availability in the thalamus (r = −.43), parietal (r = −.50), frontal (r = −.55), anterior cingulate (r = −.44), temporal (r = −.43), and occipital (r = −.48) cortices. The percent change in pain sensitivity from the first to second cold pressor task was significantly correlated with β2*-nAChR availability in the thalamus (r = −.57), cerebellum (r = −.50), striatum (r = −.057), parietal (r = −.46), anterior cingulate (r = −.48), temporal (r = −.55), and occipital (r = −.57) cortices. Similar associations were not observed with pain tolerance.
Discussion:
This suggests that β2*-nAChRs play a role in pain sensitivity but not pain tolerance during tobacco smoking withdrawal. If individuals are more likely to relapse in response to painful stimuli, lower β2*-nAChR availability during acute abstinence may be protective.
Introduction
Nicotine and tobacco smoking administration studies have demonstrated antinociceptive effects (Anderson et al., 2004; Jamner, Girdler, Shapiro, & Jarvik, 1998; Pauli, Rau, Zhuang, Brody, & Birbaumer, 1993) that are mediated by nicotinic receptors (Simons et al., 2005). Specifically, the nicotinic acetylcholine receptor containing the beta2* subunit (β2*-nAChR, the * represents other subunits that may be part of the receptor), which is critical for the reinforcing effects of nicotine (Picciotto et al., 1998), is also necessary for nicotine-induced analgesia (Damaj et al., 2007; Marubio et al., 1999). A preclinical study demonstrated that the number of β2*-nAChRs in mouse brain is positively associated with responsivity to nicotine-induced analgesia (Damaj et al., 2007); however, the relationship between β2*-nAChRs in human brain and nociception is not known. During withdrawal from tobacco smoking, smokers commonly report feelings of anxiety, depression and restlessness (Hughes, 2007), and an increased sensitivity to pain (Pomerleau, Turk, & Fertig, 1984), all of which may contribute to the high rates of relapse. It has previously been demonstrated that β2*-nAChR availability is higher in recently abstinent smokers compared with nonsmokers (Cosgrove et al., 2009; Staley et al., 2006), for example, the tobacco smoking–induced upregulation of β2*-nAChRs is measurable in vivo with the radiotracer [123I]5-IA-85380 ([123I]5-IA) and single photon emission computed tomography (SPECT) brain imaging. In this preliminary study, we examined the relationship between β2*-nAChR availability and nociception (determined using the cold pressor task; Walsh, Schoenfeld, Ramamurthy, & Hoffman, 1989), during tobacco smoke withdrawal in human tobacco smokers using [123I]5-IA and SPECT.
Methods
Twenty-four healthy treatment-seeking smokers (13 men, 11 women, age range 18–51 years, and mean ± SD, 34.6 ± 11.4 years) signed informed consent to participate in the study. Subjects had smoked 18.0 ± 6.5 cigarettes/day for 14.6 ± 9.0 years at intake. Smoking status was confirmed by plasma cotinine levels >150 ng/ml, urine cotinine levels >100 ng/ml, and carbon monoxide levels >11 ppm on the day of intake. Smokers were helped to quit smoking using cognitive–behavioral therapy and contingency management (Krishnan-Sarin et al., 2006; Stitzer, Rand, Bigelow, & Mead, 1986). Abstinence from tobacco smoking and other nicotine products was confirmed twice daily for the first 8 days of smoking cessation and a minimum of thrice weekly thereafter by monitoring breath carbon monoxide (<11 ppm) and urine cotinine (<100 ng/ml) levels. Subjects had no history of psychiatric, neurological, or medical disease and no history of drug or alcohol dependence except for nicotine dependence. They could not have regular use of psychotropic drugs in the previous 6 months and no use within the previous month. Subjects were instructed that they could not use any form of nicotine replacement therapy or medication throughout the study. All women were required to have a negative pregnancy test during the screening process and prior to radiotracer injection on scan day. This study was approved by the Yale University School of Medicine Human Investigation Committee, the West Haven Veterans Administration Human Subjects Subcommittee, and the Radiation Safety Committee. The use of the radiotracer, [123I]5-IA, was approved by the Food and Drug Administration.
[123I]5-IA was synthesized as previously described (Zoghbi et al., 2001) and administered as a bolus to constant infusion at a ratio of 7.0 for 8 hr. All subjects received a 0.6-g saturated solution of potassium iodide, to protect their thyroid from possible exposure to radioactive iodide, in the hour prior to radiotracer administration. Subjects were injected with equivalent doses of a bolus (mean ± SD, 149.7 ± 20.0 MBq) and constant infusion (mean ± SD, 21.8 ± 3.0 MBq/hr). Three consecutive 30-min emission scans and one 15-min simultaneous transmission and emission protocol scan were obtained between hours 6 and 8 of the [123I]5-IA infusion on a Picker PRISM 3000 XP (Cleveland, OH) SPECT camera. The PRISM 3000 XP is a three-headed camera equipped with a low energy ultra-high resolution fan beam collimator (photopeak window, 159 keV ± 10%; matrix 128 × 128) with a uniform sensitivity across the field of view. A 57Co-distributed source was measured with each experiment to control for day-to-day variation in camera sensitivity. The axial resolution (full width at half maximum) is 12.2 mm, measured with a 123I line source in water in a cylindrical phantom. Blood was drawn prior to injection and at the beginning and end of the emission scans for analysis of plasma total parent and free fraction of parent tracer in plasma (fP, free fraction). The chemical fate of [123I]5-IA postinjection was assessed in plasma as previously described (Zoghbi et al.).
All subjects also underwent magnetic resonance imaging (MRI) studies, which were obtained on a 1.5 T Siemens camera in a standard orientation (echo time = 5–7 ms; repetition time = 24 ms; 256 × 192 matrix; number of signals acquired = 1; field of view = 30 cm; 124 contiguous slices with 1.2-mm thickness) and were used for coregistration to the SPECT images.
The cold pressor task was administered to all subjects at two timepoints: (a) 3–4 hr after their last cigarette and (b) 7–13 days after their last cigarette on the day they were imaged with [123I]5-IA SPECT. We used the cold pressor task to measure pain sensitivity and pain tolerance during tobacco smoking withdrawal. The task is the same as previously reported (Esterlis et al., 2009) and required subjects to immerse their hand in a circulating cold water bath maintained at 0 °C and to report when they first are aware of pain (pain sensitivity) and then to remove their hand when they could no longer withstand the pain (pain tolerance). Both were measured in seconds, and the task was discontinued at 90 s. Depression, anxiety, and nicotine withdrawal symptoms were assessed with the Center for Epidemiological Studies Depression Scale (Radloff, 1977), Spielberger’s State-Trait Anxiety Inventory (Spielberger, Corsuch, & editors, 1983), and the Minnesota Nicotine Withdrawal Scale (Hatsukami, Hughes, Pickens, & Svikis, 1984), respectively, on each day they completed the cold pressor task.
Images were reconstructed and analyzed as previously described, including a nonuniform attenuation correction (Staley et al., 2005). MRIs were coregistered to the SPECT images to provide an anatomical guide for placement of the regions of interest using Medx (version 3.4) software (Medical Numerics, Inc., Germantown, MD). Regions of interest chosen were those known to contain β2-nAChRs and included frontal, parietal, anterior cingulate, temporal and occipital cortices, thalamus, striatum (an average of caudate and putamen), and cerebellum. Regions of interest are corrected to account for differences in size. Two raters conducted the analysis. Variability between raters was <12% across regions of interest, and the mean of the two raters is reported. The outcome measure VT/fP (regional activity divided by free plasma parent between 6 and 8 hr) was used. VT/fP equals [123I]5-IA uptake in a region of interest (kBq/cc)/free plasma parent (kBq/ml; Innis et al., 2007). VT/fP represents β2*-nAChR availability because we are measuring receptors that are “available” to be bound by radiotracer. Receptors that are already occupied, for example, by residual nicotine or by endogenous neurotransmitter (acetylcholine) are not available.
Differences in pain sensitivity and pain tolerance between the first and second cold pressor task were assessed with paired t tests. Correlational analyses for the associations between receptor availability and the cold pressor task were conducted using SPSS version 16.0 (SPSS Inc. Headquarters, Chicago, IL). Correlations between β2*-nAChR availability, VT/fP, and pain sensitivity and pain tolerance were assessed with Pearson’s correlation coefficients. Correlations between the change in pain sensitivity and pain tolerance between the first and second cold pressor task and β2*-nAChR availability were assessed. Correlations between the change in withdrawal, depression, and anxiety scores between the first and second cold pressor task and the change in pain sensitivity and pain tolerance measures from the same days were also conducted. The percent change score was conducted as ([Task2 − Task1]/Task 1 × 100. Due to the preliminary nature of this report, all p ≤ .05 are reported as significant.
Results
Pain sensitivity scores were higher at Time 1 (Mean ± SD; 39.5 ± 26.4 seconds) compared with Time 2 (32.3 ± 25.4 s); however, they were not significantly different (t = 1.54, df = 23, p = .14). Pain tolerance scores were also higher at Time 1 (66.2 ± 29.0 s) compared with Time 2 (55.5 ± 30.8) but were not significantly different (t = 1.69, df = 23, p = .10). Higher scores at Time 1 versus Time 2 indicate lower pain sensitivity and pain tolerance at Time 1, although this difference was not significant. There were significant correlations between regional β2*-nAChR availability and the pain sensitivity score but not the pain tolerance score. The score (measured in seconds) is inversely related to sensitivity; thus, a lower score indicates greater sensitivity. Specifically, pain sensitivity on the day of the [123I]5-IA scan correlated negatively with β2*-nAChR availability in the thalamus (r = −.43, p = .035), parietal (r = −.50, p = .013), frontal r = −.55, p = .005), anterior cingulate (r = −0.44, p = .032), temporal (r = −.43, p = .037), and occipital (r = −0.48, p = .018) cortices (Figure 1). There were also significant correlations between regional β2*-nAChR availability and the change in pain sensitivity but not pain tolerance between the first and second cold pressor task, for example, from Day 1 of abstinence to Days 7–13 of abstinence. Specifically, the percent change in pain sensitivity from the first to second cold pressor task correlated negatively with β2*-nAChR availability in the thalamus (r = −.57, p = .004), cerebellum (r = −.50, p = .013), striatum (r = −.57, p = .004), parietal (r = −.46, p = .025), anterior cingulate (r = −.48, p = .017), temporal (r = −.55, p = .005), and occipital (r = −.57, p = .004) cortices (Figure 2). There were no significant correlations between the change in depression, anxiety, or withdrawal scores and the change in pain sensitivity or pain tolerance between the first and second cold pressor task days (data not shown). There were no significant correlations between smoking-related behaviors, such as cigarettes smoked per day, number of years smoked, or FTND score with pain sensitivity or pain tolerance scores (data not shown).
Figure 1.
Scatterplots illustrate individual β2*-nAChR availability (VT/fP) in recently abstinent tobacco smokers (n = 24) in the thalamus (filled triangle), striatum (filled circle), cerebellum (filled inverted triangle), and cortex (average of parietal, frontal, anterior cingulate, temporal, and occipital regions; filled square) as a function of pain sensitivity and pain tolerance, measured in seconds.
Figure 2.
Scatterplots illustrate individual β2*-nAChR availability (VT/fP) in recently abstinent tobacco smokers (n = 24) in the thalamus (filled triangle), striatum (filled circle), cerebellum (filled inverted triangle), and cortex (average of parietal, frontal, anterior cingulate, temporal, and occipital regions; filled square) as a function of percent change in pain sensitivity and pain tolerance measured from the first to second cold pressor task.
Discussion
In this study, we found that higher β2*-nAChR availability in recently abstinent tobacco smokers was associated with increased pain sensitivity but not pain tolerance, such that individuals with more available β2*-nAChRs became aware of pain in a shorter time. Additionally, higher β2*-nAChR availability was associated with an increase in pain sensitivity but not pain tolerance over the first week of abstinence. Specifically, individuals with more available β2*-nAChRs reported a shorter time to feel pain between the first and second cold pressor task.
In our previous study, with recently abstinent tobacco smokers, higher β2*-nAChR availability was associated with more craving, specifically the urge to smoke to relieve withdrawal (Staley et al., 2006). We also demonstrated that at 4 weeks of abstinence, higher cerebellar β2*-nAChR availability was associated with more craving (Cosgrove et al., 2009). Taken together, these studies suggest that higher β2*-nAChR availability during acute abstinence may confer a vulnerability, for example, there is more craving and increased pain sensitivity compared with individuals with lower β2*-nAChR availability. Importantly, the change in pain sensitivity over abstinence was not correlated with changes in depression, anxiety, or withdrawal scores, suggesting that these changes are attributable to pain per se and not a worsening of mood or withdrawal effects. This is consistent with a previous study reporting that the antinociceptive effects of nicotine were independent of nicotine’s effects on mood (Jamner et al., 1998).
The β2 subunit of the nAChR appears to be critical for nicotine-induced antinociception. Mice lacking the β2 subunit demonstrated a reduced sensitivity to nicotine-induced antinociception (Marubio et al., 1999), and mice not previously exposed to nicotine expressing higher β2*-nAChRs had increased responsivity to nicotine-induced antinociception (Damaj et al., 2007). A lower affinity nAChR, the α7 subunit, has also been postulated to contribute to nicotine-induced antinociception (Damaj, Meyer, & Martin, 2000). Thus, while the β2-nAChR is necessary for nicotine-induced antinociception, other subunits likely play a role. The current study demonstrates that higher β2*-nAChR availability is associated with increased sensitivity to pain during nicotine withdrawal in tobacco smokers. While higher numbers of β2*-nAChRs may promote responsiveness to nicotine-induced analgesia as in Damaj et al. (2007), during withdrawal from nicotine, the higher β2*-nAChR availability becomes a liability and promotes increased sensitivity to pain. We hypothesize that during acute tobacco smoking withdrawal, the increased pool of β2*-nAChRs that remain upregulated and possibly desensitized for up to 1 week or more in the absence of nicotine is driving some of the withdrawal symptoms, such as craving and pain sensitivity, which resolve as the receptors normalize over time.
A limitation of this study is that we did not obtain the cold pressor task in a control sample of nonsmokers. Therefore, we do not know if there is a similar relationship between nociception and β2*-nAChR availability in nonsmoking humans. Additionally, the cold pressor task was terminated at 90 s. There were a subset of subjects (n = 9) who kept their hand in the water for the duration of the task on scan day; thus, we censored their values at 90 s. This may have affected our finding for pain tolerance. Specifically, if there were not an experimenter-induced ceiling to the task, we may have found a relationship between pain tolerance and β2*-nAChR availability. However, there is likely a divergence of mechanisms underlying pain sensitivity and tolerance, which is why they are separate measures. While one study showed a high degree of concordance within subject in the two measures (Fertig, Pomerleau, & Sanders, 1986), they do not always similarly correlate with other measures (Girdler et al., 2005; Pud, Golan, & Pesta, 2009). Because it is difficult to examine differences between pain sensitivity and tolerance in a preclinical model, disentangling these two measures and their relationship to tobacco smoking needs to be conducted in human subjects. We plan to address these limitations in a larger study.
In summary, these findings highlight a relationship between β2*-nAChR availability and nociception during acute withdrawal in tobacco smokers. Individuals with higher β2*-nAChR availability during acute withdrawal may be at increased risk for relapse if they relapse in response to craving or painful stimuli. This suggests that, in these individuals, treatment drugs targeted at increasing the rate of receptor normalization—for example, the return to nonsmoker control levels—may help prevent relapse.
Funding
Funded by National Institutes of Health (RO1DA015577, P50AA15632, KO1D020651, and KO2DA021863) and VA Connecticut Healthcare System (VA CDA-1 and T32 DA07238-16).
Declaration of Interests
None declared.
Acknowledgments
The authors would like to thank Dr. Marina Picciotto for helpful comments on the manuscript.
References
- Anderson KL, Pinkerton KE, Uyeminami D, Simons CT, Carstens MI, Carstens E. Antinociception induced by chronic exposure of rats to cigarette smoke. Neuroscience Letters. 2004;366:86–91. doi: 10.1016/j.neulet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, et al. beta2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives of General Psychiatry. 2009;66:666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, et al. Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. Journal of Pharmacology and Experimental Therapeutics. 2007;321:1161–1169. doi: 10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Cosgrove KP, Batis JC, Bois F, Kloczynski TA, Stiklus SM, et al. GABA(A)-benzodiazepine receptor availability in smokers and nonsmokers: Relationship to subsyndromal anxiety and depression. Synapse. 2009;63:1089–1099. doi: 10.1002/syn.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertig JB, Pomerleau OF, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addictive Behaviors. 1986;11:239–248. doi: 10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Hughes J, Pickens R, Svikis D. Tobacco withdrawal symptoms: An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine and Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jamner LD, Girdler SS, Shapiro D, Jarvik ME. Pain inhibition, nicotine, and gender. Experimental and Clinical Psychopharmacology. 1998;6:96–106. doi: 10.1037//1064-1297.6.1.96. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, et al. Contingency management for smoking cessation in adolescent smokers. Experimental and Clinical Psychopharmacology. 2006;14:306–310. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d’Exaerde A, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Pauli P, Rau H, Zhuang P, Brody S, Birbaumer N. Effects of smoking on thermal pain threshold in deprived and minimally-deprived habitual smokers. Psychopharmacology (Berlin) 1993;111:472–476. doi: 10.1007/BF02253538. [DOI] [PubMed] [Google Scholar]
- Picciotto M, Zoli M, Rimondin R, Lena C, Marubio L, Pich E, et al. Acetycholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addictive Behaviors. 1984;9:265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Pud D, Golan Y, Pesta R. Hand dominancy–A feature affecting sensitivity to pain. Neuroscience Letters. 2009;467:237–240. doi: 10.1016/j.neulet.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Simons CT, Cuellar JM, Moore JA, Pinkerton KE, Uyeminami D, Carstens MI, et al. Nicotinic receptor involvement in antinociception induced by exposure to cigarette smoke. Neuroscience Letters. 2005;389:71–76. doi: 10.1016/j.neulet.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Corsuch R, Lushene R, Vagg P, Jacobs C. State-trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. Journal of Neuroscience. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, van Dyck CH, Weinzimmer D, Brenner E, Baldwin RM, Tamagnan GD, et al. 123I-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: Feasibility and reproducibility. Journal of Nuclear Medicine. 2005;46:1466–1472. [PubMed] [Google Scholar]
- Stitzer M, Rand C, Bigelow G, Mead A. Contingent payment procedures for smoking reduction and cessation. Journal of Applied Behavior Analysis. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NE, Schoenfeld L, Ramamurthy S, Hoffman J. Normative model for cold pressor test. American Journal of Physical Medicine and Rehabilitation. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- Zoghbi S, Tamagnan G, Fujita M, Baldwin R, Al-Tikriti M, Amici L, et al. Measurement of plasma metabolites of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy) pyridine (5-IA-85380), a nicotinic acetylcholine receptor imaging agent, in non-human primates. Nuclear Medicine and Biology. 2001;28:91–96. doi: 10.1016/S0969-8051(00)00188-8. [DOI] [PubMed] [Google Scholar]