Abstract
Currently, there is no cure and no preventive vaccine for HIV/AIDS. Combination antiretroviral therapy has dramatically improved treatment, but it has to be taken for a lifetime, has major side effects and is ineffective in patients in whom the virus develops resistance. Nanotechnology is an emerging multidisciplinary field that is revolutionizing medicine in the 21st century. It has a vast potential to radically advance the treatment and prevention of HIV/AIDS. In this review, we discuss the challenges with the current treatment of the disease and shed light on the remarkable potential of nanotechnology to provide more effective treatment and prevention for HIV/AIDS by advancing antiretroviral therapy, gene therapy, immunotherapy, vaccinology and microbicides.
Keywords: AIDS, antiretroviral therapy, gene therapy, HIV, immunotherapy, microbicides, nanomedicine, nanoparticles, nanotechnology, vaccines
The emergence of AIDS was first reported in 1981 followed by the identification of HIV as the cause of the disease in 1983 [1–4]. HIV/AIDS is now a global pandemic that has become the leading infectious killer of adults worldwide [5]. By 2006, more than 65 million people had been infected with the HIV virus worldwide and 25 million had died of AIDS [6]. At the end of 2007, around 33 million people were living with the virus, with 2.7 million new infections and 2 million deaths each year [7]. This has caused tremendous social and economic damage worldwide, with developing countries, particularly Sub-Saharan Africa, heavily affected.
A cure for HIV/AIDS has been elusive in almost 30 years of research. Early treatments focused on antiretroviral drugs that were effective only to a certain degree. The first drug, zidovudine, was approved by the US FDA in 1987, leading to the approval of a total of 25 drugs to date, many of which are also available in fixed-dose combinations and generic formulations for use in resource-limited settings (to date, only zidovudine and didanosine are available as true generics in the USA) [8,9]. However, it was the advent of a class of drugs known as protease inhibitors and the introduction of triple-drug therapy in the mid-1990s that revolutionized HIV/AIDS treatment [10,11]. This launched the era of highly active antiretroviral therapy (HAART), where a combination of three or more different classes of drugs are administered simultaneously [11]. The use of the HAART regimen, particularly in the developed world, has resulted in tremendous success in improving the expectancy and quality of lives for patients [12]. However, some HAART regimens have serious side effects and, in all cases, HAART has to be taken for a lifetime, with daily dosing of one or more pills. Some patients also develop resistance to certain combinations of drugs, resulting in failure of the treatment. The absence of complete cure under current treatment underscores the great need for continued efforts in seeking innovative approaches for treatment of HIV/AIDS.
In addition to treatment, the best way to fight global infections is through preventive strategies, vaccines being the most effective agents. Vaccines have historically been very effective at controlling other major infectious diseases such as measles, mumps, rubella and polio, with smallpox completely eradicated. There have been enormous efforts to develop a safe and effective vaccine for HIV/AIDS. However, the pursuit has been very daunting so far, with recent failures of clinical trials for major candidate vaccines [13–15]. This has raised a debate over which path to take in HIV/AIDS vaccine research. Despite this debate, it is clear that novel approaches for identifying new antigens and adjuvants as well as better delivery systems are necessary. Another preventive strategy that has been under investigation is the development of effective intravaginal microbicides that can be used by women. There has been remarkable progress in the understanding and design of technologies for microbicide development. However, recent clinical trials failed to show efficacy, indicating the need for more research and development to design better systems [16,17].
Nanotechnology is a new discipline of science and engineering that is advancing many areas of medicine. It involves the understanding, design, engineering and fabrication of materials at the atomic and molecular level. The National Nanotechnology Initiative defines nanotechnology as the study of structures with roughly 1–100 nm in size in at least one dimension but structures up to several hundred nanometers are also considered under nanotechnology applications [18]. The application of nanotechnology to medicine, commonly referred to as nanomedicine, involves the use of nanoscale materials for preventive, therapeutic and diagnostic purposes [19]. There have been major advances in nanomedicine over the last few decades, particularly in cancer diagnosis and therapy [20–22]. Although at an earlier stage, applications of nanotechnology for prevention and treatment of HIV/AIDS have also gained attention in recent years. There are emerging novel approaches in which nanotechnology can enhance current treatment as well as advance new therapeutic strategies, such as gene therapy and immunotherapy. Moreover, some nanomaterials have therapeutic effects by themselves. Nanotechnology can also play a major role in preventive strategies for developing vaccines and microbicides. In this review, we discuss the potential of nanotechnology in improving the current treatment, advancing new therapeutic strategies as well as providing alternatives in the quest for vaccine and microbicide developments for HIV/AIDS.
Current HIV/AIDS treatment
The current state-of-the-art treatment modality for HIV/AIDS is HAART, where three or more antiretroviral drugs are given to patients simultaneously. The drugs used in combination are in most cases from different classes that work based on different mechanisms. Despite the remarkable successes with the current HAART treatment for HIV/AIDS, there are still various challenges remaining. The major difficulty has been the failure of the treatment, typically due to poor patient compliance [23]. Due to the need to take the medication daily for a lifetime, patients fail to adhere to the treatment schedule, leading to ineffective drug levels in the body and rebound of viral replication [11,24,25].
Moreover, in some patients, the virus develops resistance to particular combinations of drugs even with good adherence. Drug resistance is mainly caused by the high genetic diversity of HIV-1 and the continuous mutation it undergoes [26]. This problem is being addressed with individualized therapy, whereby resistance testing is performed to select a combination of drugs that is most effective for each patient [26]. In addition, side effects due to toxicities of the drugs are also a concern. There are reports that patients taking HAART experience increased rates of heart disease, diabetes, liver disease, cancer and accelerated aging [11]. Most experts agree that these effects could be due to the HIV infection itself or co-infection with another virus, such as co-infection with hepatitis C virus resulting in liver disease. However, the toxicities resulting from the drugs used in HAART could also contribute to these effects.
Under current treatment, complete eradication of the virus from the body has not been possible. The major cause for this is that the virus resides in ‘latent reservoirs’ within memory CD4+ T cells and cells of the macrophage–monocyte lineage [11,25]. A major study recently found that, in addition to acting as latent reservoirs, macrophages significantly contribute to the generation of elusive mutant viral genotypes by serving as the host for viral genetic recombination [27]. The cells that harbor latent HIV are typically concentrated in specific anatomic sites, such as secondary lymphoid tissue, testes, liver, kidney, lungs, gut and the CNS [11,28–31]. The eradication of the virus from such reservoirs is critical to the effective long-term treatment of HIV/AIDS patients. Therefore, there is a great need to explore new approaches for developing nontoxic, lower-dosage treatment modalities that provide more sustained dosing coverage and effectively eradicate the virus from the reservoirs, avoiding the need for lifetime treatments.
Nanotechnology for HIV/AIDS treatment
Nanotechnology for antiretroviral drug delivery
The use of nanotechnology platforms for delivery of drugs is revolutionizing medicine in many areas of disease treatment [32]. Cancer patients have been the biggest beneficiaries of this revolution so far, with significant advances in the last few decades. Many nanoscale systems for systemic cancer therapy are either FDA approved or in clinical trials [19,33]. This tremendous success has been due to the unique features that nanotechnology imparts on drug delivery systems. Using nanotechnology, it has become possible to achieve improved delivery of poorly water-soluble drugs, targeted delivery of drugs to specific cells or tissues and intracellular delivery of macromolecules [18,32].
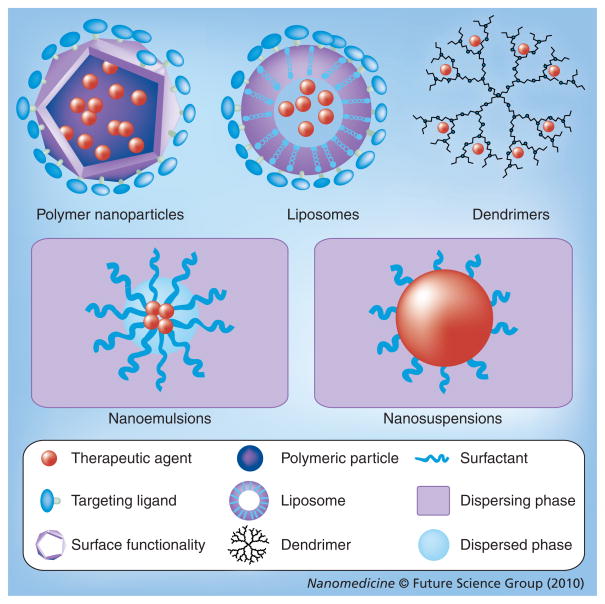
Nanotechnology-based platforms for systemic delivery of antiretroviral drugs could have similar advantages. Controlled-release delivery systems can enhance their half-lives, keeping them in circulation at therapeutic concentrations for longer periods of time. This could have major implications in improving adherence to the drugs. Nanoscale delivery systems also enhance and modulate the distribution of hydrophobic and hydrophilic drugs into and within different tissues due to their small size. This particular feature of nanoscale delivery systems appears to hold the most promise for their use in clinical treatment and prevention of HIV. Specifically, targeted delivery of antiretroviral drugs to CD4+ T cells and macrophages as well as delivery to the brain and other organ systems could ensure that drugs reach latent reservoirs [29,34]. Moreover, by controlling the release profiles of the delivery systems, drugs could be released over a longer time and at higher effective doses to the specific targets. Various nanoscale drug delivery systems shown in Figure 1 could be explored for these purposes. The use of nanotechnology systems for delivery of antiretroviral drugs has been extensively reviewed by Nowacek et al. and Amiji et al. [29,31,34]. In this section, we only highlight a few of the most recent and significant examples of nanotechnology-based drug delivery.
Figure 1.
Schematic representation of various nanotechnology platforms that can be used in HIV/AIDS treatment and prevention.
In a recent study based on polymeric systems, nanosuspensions (200 nm) of the drug rilpivirine (TMC278) stabilized by polyethylene-polypropylene glycol (poloxamer 338) and PEGylated tocopheryl succinate ester (TPGS 1000) were studied in dogs and mice [35]. A single-dose administration of the drug in nanosuspensions resulted in sustained release over 3 months in dogs and 3 weeks in mice, compared with a half-life of 38 h for free drug. These results serve as a proof-of-concept that nanoscale drug delivery may potentially lower dosing frequency and improve adherence.
A series of experiments by Dou et al. showed that nanosuspension of the drug indinavir can be stabilized by a surfactant system comprised of Lipoid E80 for effective delivery to various tissues [36–38]. The indinavir nanosuspensions were loaded into macrophages and their uptake was investigated. Macrophages loaded with indinavir nanosuspensions were then injected intravenously into mice, resulting in a high distribution in the lungs, liver and spleen. More significantly, the intravenous administration of a single dose of the nanoparticle-loaded macrophages in a rodent mouse model of HIV brain infection resulted in significant antiviral activity in the brain and produced measureable drug levels in the blood up to 14 days post-treatment [38].
These studies serve as a proof of concept for indinavir delivery to the brain and the sustained drug levels for up to 14 days, which is important when considering that the half-life of indinavir in its conventional dosage form is 2 h. The demonstration that macrophages could be used to target drugs to the brain could be utilized for in vivo nanoparticle-targeted delivery of other drugs to the brain in the future.
Active targeting strategies have also been employed for antiretroviral drug delivery. Macrophages, which are the major HIV reservoir cells, have various receptors on their surface such as formyl peptide, mannose, galactose and Fc receptors, which could be utilized for receptor-mediated internalization. The drug stavudine was encapsulated using various liposomes (120–200 nm) conjugated with mannose and galactose, resulting in increased cellular uptake compared with free drug or plain liposomes, and generating significant level of the drug in liver, spleen and lungs [39–41]. Stavudine is a water-soluble drug with a very short serum half-life (1 h). Hence, the increased cellular uptake and sustained release in the tissues afforded by targeted liposomes is a major improvement compared with free drug. The drug zidovudine, with half-life of 1 h and low solubility, was also encapsulated in a mannose-targeted liposome made from stearylamine, showing increased localization in lymph node and spleen [42]. An important factor to consider here is that although most of the nucleoside drugs such as stavudine and zidovudine have short serum half-lives, the clinically relevant half-life is that of the intracellular triphosphate form of the drug. For example, despite zidovudine’s 1 h half-life in plasma, it is dosed twice daily based on intracellular pharmacokinetic and clinical efficacy data. Therefore, future nanotechnology-based delivery systems will have to focus in showing significant increase of the half-lives of the encapsulated drugs to achieve a less frequent dosing such as once weekly, once-monthly or even less.
In separate work, a mannose-targeted poly (propyleneimine) dendrimer nanocarrier was used to deliver the drug efavirenz to human monocytes/macrophages in vitro [43]. The targeted nanocarrier resulted in 12-fold increase in cellular uptake compared with free drug. A similar system was used to deliver the drug lamivudine in vitro, resulting in significantly higher anti-HIV activity for the targeted and nontargeted dendrimer systems compared with free drugs [44]. In a more recent study, the tetra-peptide tuftsin (Thr-Lys-Pro-Arg) was conjugated to the same dendrimer to target the drug efavirenz to macrophages in vitro [45]. The targeted dendrimer system resulted in sixfold prolonged release, 34-fold increased cellular uptake and sevenfold increase in anti-HIV activity compared with free drug.
In a new approach to target macrophage HIV reservoirs, a peptide nanocarrier was proposed as a model where a drug is conjugated to the backbone of peptide-PEG and N-formyl-methionyl-leucyl-phenylalanine (fMLF), a bacterial peptide sequence for which macrophages express a receptor, is attached to the PEG for targeting [46]. The study found that fMLF-targeted peptide-PEG nanocarriers show increased cellular uptake and increased accumulation in macrophages of liver, kidney and spleen compared with those which are nontargeted [30,46].
All the aforementioned efforts are examples of the potential nanotechnology platforms hold for improving targeted delivery of antiretroviral drugs to the cellular and anatomical reservoirs of HIV. These early efforts provide evidence for the potential of nanotechnology to improve delivery of antiretroviral therapy and support ongoing efforts to initiate clinical trials. Although the early efforts have not reached clinical trials yet, the works so far provide encouraging evidence that a subset of these preclinical technologies may enter clinical evaluation in the future.
Nanomaterials as therapeutic agents
In addition to being used as delivery agents, nanomaterials have also been shown to have therapeutic effects of their own. Studies have shown that the capsid of HIV could be a target for structure-based drug design for inhibiting viral replication [47,48]. As a result, both computational and experimental studies have identified compounds that could inhibit the assembly of the HIV capsid. Various nanomaterials have been found to inhibit viral replication in vitro and it is suggested that these effects are based on structural interference with viral assembly.
Various fullerene (C-60)-based structures, dendrimers and inorganic nanoparticles, such as gold and silver, have been shown to have anti-HIV activity in vitro [49–60]. While these efforts have not yet progressed beyond in vitro studies, they illustrate the potential of therapeutic nanomaterials to inhibit HIV replication.
Gene therapy for HIV/AIDS
In addition to improving existing antiretroviral therapy, there are ongoing efforts to discover alternative approaches for treatment of HIV/AIDS [25,61]. One promising alternative approach is gene therapy, in which a gene is inserted into a cell to interfere with viral infection or replication. Other nucleic acid-based compounds, such as DNA, siRNA, RNA decoys, ribozymes and aptamers or protein-based agents such as fusion inhibitors and zinc-finger nucleases can also be used to interfere with viral replication [61,62].
Early efforts in gene therapy for HIV/AIDS have been focused on viral vectors as the delivery agents with various clinical trials in progress [61,63–68]. In one of these studies, Benitec Ltd and City of Hope are collaborating in an ongoing clinical trial to study the safety and feasibility of a gene therapy strategy based on the combination of three different inhibitory genes in a single lentiviral vector that utilizes stem cells in the delivery process [65]. Recently, scientists from UCLA reported that a Phase II gene therapy clinical trial showed that cell-derived gene transfer is safe and biologically active in HIV-infected individuals [69]. These efforts are encouraging and support the growing excitement around gene therapy for the treatment of HIV/AIDS. However, lessons learned over the past two decades indicate that the use of viral vectors for gene delivery poses fundamental problems such as toxicity, immunogenicity, insertion mutagenesis and limitations with scale-up procedures [70,71]. These problems have encouraged the investigation of nonviral vectors for gene delivery, where nanotechnology platforms are showing great promise [70–72].
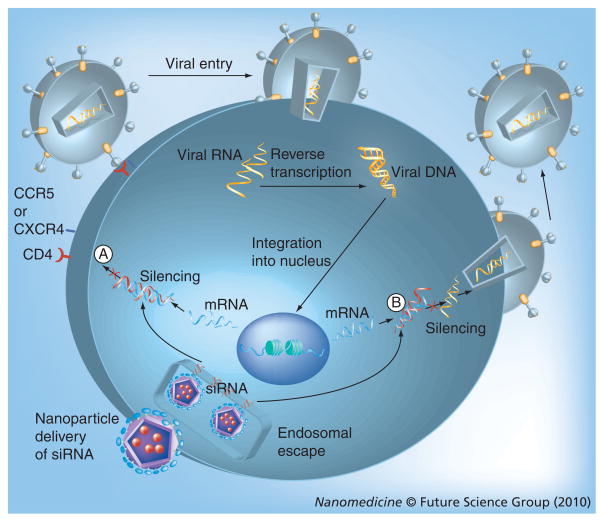
In recent years, the Nobel prize-winning discovery of RNA interference (RNAi) in 1998 by Fire, Mello and colleagues has gained much attention in the clinical therapeutics field and is generating billion dollar investments in therapeutic applications [73,74]. Ongoing clinical trials for the treatment of age-related macular degeneration and respiratory syncytial virus have provided data that are creating tremendous excitement in the field [74]. RNAi is also considered to have therapeutic potential for HIV/AIDS [61,62,75]. Gene silencing is induced by double stranded siRNA, which targets for destruction the mRNA of the gene of interest. For HIV/AIDS, RNAi can either target the various stages of the viral replication cycle or various cellular targets involved in viral infection such as CD4, CCR5, and/or CXCR4, the major cell surface co-receptors responsible for viral entry. HIV replicates by reverse transcription to form DNA and uses the DNA to produce copies of its mRNA for protein synthesis; siRNA therapy could be used to knock down this viral mRNA. These two mechanisms are shown in Figure 2.
Figure 2. Mechanism for siRNA-based gene therapy of HIV/AIDS.
The siRNA acts by degrading mRNA in at least two major ways: (A) Inhibit entry and fusion by interfering with production of receptors or co-receptors and (B) interfere with translation and transcription of viral genes preventing production of proteins and genomic RNA. (The viral entry and replication stages shown here are also the targets for the antiretroviral drugs discussed above [29]).
As with other gene therapy techniques, delivery of siRNA to specific cells and tissues has been the major challenge in realizing the potential of RNAi [74]. New nanotechnology platforms are tackling this problem by providing nonviral alternatives for effective and safe delivery. The first nontargeted delivery of siRNA in humans via self-assembling, cyclodextrin polymer-based nanoparticles for cancer treatment have recently entered Phase I clinical trials [76].
Although at an early stage, nonviral delivery of siRNA for treatment of HIV infection is also gaining ground. A fusion protein, with a peptide transduction domain and a double stranded RNA-binding domain, was used to encapsulate and deliver siRNA to T cells in vivo [77]. CD4- and CD8-specific siRNA delivery caused RNAi responses with no adverse effects such as cyto-toxicity or immune stimulation. Similarly, a protamine-antibody fusion protein-based siRNA delivery demonstrated that siRNA knockdown of the gag gene can inhibit HIV replication in primary T cells [78].
Single-walled nanotubes were shown to deliver CXCR4 and CD4 specific siRNA to human T cells and peripheral blood mononuclear cells [79]. Up to 90% knockdown of CXCR4 receptors and up to 60% knockdown of CD4 expression on T cells was observed while the knockdown of CXCR4 receptors on peripheral blood mononuclear cells was as high as 60%. In a separate study, amino-terminated carbosilane dendrimers (with interior carbon-silicon bonds) were used for delivery of siRNA to HIV-infected lymphocytes [80].
In a recent study, peptide conjugated to an antibody was used for targeted delivery of siRNA to T cells and to suppress HIV infection in humanized mice [81]. The antibody (scFvCD7)-specific to CD7 receptor on T cells was conjugated to the oligo-9-arginine peptide resulting in the delivery system designated scFvCD7–oligo-9-arginine. CD7 is an antigen found on the majority of human T cells that is rapidly internalized on antibody binding [81]. The oligo-9-arginine peptide is a short, positively charged cell-penetrating peptide that has been shown to be highly effective in facilitating cellular uptake of nucleic acids [82]. The peptide binds with the negatively charged siRNA, enhancing delivery to the target cells. In this study, anti-CCR5 and antiviral siRNA delivered with scFvCD7–oligo-9-arginine controlled viral replication and prevented CD4 T cell loss. Moreover, the treatment suppressed viral titers and restored CD4 T cell counts while effectively suppressing viremia in HIV-infected humanized mice. This work opens new avenues for future use of antibody-targeted nanoparticles in the delivery of siRNA to HIV-specific cells.
These pioneering studies demonstrate that nonviral siRNA delivery is possible for HIV/AIDS treatment. However, more work needs to be done in optimizing the delivery systems and utilizing designs for efficient targeting and intracellular delivery. The recent developments in polymer- and liposome-based siRNA delivery systems could be optimized for targeting cells that are infected with HIV, such as T cells and macrophages. Moreover, since HIV mutates and has multiple strains with different genetic sequences, combination siRNA therapy targeting multiple genes should be pursued. For these applications, nanotechnology platforms with capability for co-delivery and targeting need to be developed specifically for HIV-susceptible cells. A macrophage and T-cell-targeted and nanotechnology-based combination gene therapy may be a promising platform for efficient HIV/AIDS treatment.
Immunotherapy for HIV/AIDS
The various treatment approaches described above focus on treating HIV/AIDS by directly targeting HIV at the level of the host cell or the virus itself. An alternative approach is immunotherapy aimed at modulating the immune response against HIV. CD8+ cytotoxic T-cell responses to acute HIV infection appear to be relatively normal, while neutralizing antibody production by B cells is delayed or even absent [83]. Over time, viral mutation leads to loss of the CD8+ T cell cytotoxic function. However, the major effect of an infection by HIV is the loss of CD4+ T cells. These ‘helper’ T cells are responsible for a number of supportive functions for other immune populations and their loss leads to profound immunosuppression, manifested by the presence of dysfunctional B-cells, natural killer cells and the macrophages in chronically HIV-infected patients [84]. In recent years, there has been increasing interest in the therapeutic use of immune responses to restore the regular function of the immune system as an effective way to treat HIV/AIDS [85–87]. There has been increasing evidence that the immune system is capable of controlling HIV in certain individuals [87]. Hence, strategies to rebuild or allow the reconstitution of immune function could be one of the best approaches for effective treatment.
Immunotherapy is a treatment approach involving the use of immunomodulatory agents to modulate the immune response against a disease. Similar to vaccines, it is based on immunization of individuals with various immunologic formulations; however, the purpose is to treat HIV-infected patients as opposed to protect healthy individuals (preventive vaccines will be discussed in an upcoming section). The various immunotherapy approaches for HIV/AIDS could be based on delivering cytokines (such as IL-2, IL-7 and IL-15) or antigens [84,85]. The development of cellular immunity, and to a large degree humoral immunity, requires antigen-presenting cells (APCs) to process and present antigens to CD4+ and CD8+ T cells. Dendritic cells (DCs) are the quintessential professional APCs responsible for initiating and orchestrating the development of cellular and humoral (antibody) immunity [88,89]. Protein/peptide antigens or DNA immunogens (which lead to endogenous protein expression) could then be delivered through viral vectors to endogenous or ex vivo-generated DCs.
Since the first immunotherapeutic clinical trial for HIV/AIDS in 1983, various trials have been carried out and are still ongoing [90–93]. Unfortunately, despite the preclinical studies that showed enhanced immune responses, most of the clinical studies have consistently failed to provide clinical improvements for patients [90]. Most of these clinical trials have been based on the delivery of the immunogenic factors through viruses or ex vivo DCs. As discussed under the gene therapy section above, delivery through viral vectors involves various risks. In addition, ex vivo generation of autologous DCs is a difficult therapeutic strategy to utilize widely as it involves a very labor-intensive procedure with high costs and multiple procedures for product control at different sites [94]. Hence, new approaches using targeted nanotechnology platforms for delivery of immunomodulatory factors and targeting antigens to DC surface receptors in vivo provide immense opportunities [87]. The rationale for nanotechnology-based DC targeting and vaccine delivery will be discussed in more detail in the upcoming section. Here, the most important advances of nanotechnology-based immunotherapy will be described.
Various polymeric systems have been explored for in vivo targeting of DCs and delivery of small molecules, proteins or DNAs showing potential for immunotherapy. Poly(ethylene glycol) (PEG) stabilized poly(propylene sulfide) polymer nanoparticles accumulated in DCs in lymph nodes [95]. Following nanoparticle injection, DCs containing nanoparticles accumulated in lymph nodes, peaking at 4 days with 40–50% of DCs and other APCs having internalized nanoparticles. In a separate study, cross-linked polymer nanoparticles with a pH-responsive core and hydrophilic charged shell were used for delivery of proteins and small molecules to DCs [96]. The cross-linked polymers were composed of 2-diethylamino ethyl methacrylate, PEG dimethacrylate and 2-aminoethyl methacrylate. Fluorescence microscopy showed delivery of the model protein antigen ovalbumin to the cytosol of bone marrow-derived DCs, where antigens can potentially be processed for cross-presentation to CD8+ T cells, in addition to more classical exogenous antigen presentation to CD4+ T cells.
In another study, nanoparticles of the copolymer poly(D,L-lacticide-co-glycolide) (PLGA) showed efficient delivery of antigens to murine bone marrow-derived DCs in vitro, suggesting their potential use in immunotherapy [97]. More recently, a very interesting work showed that HIV p24 protein adsorbed on the surface of surfactant-free anionic poly(D,L-lactide) (PLA) nanoparticles were efficiently taken-up by mouse DCs, inducing DC maturation [98]. The p24-nanoparticles induced enhanced cellular and mucosal immune responses in mice. Although this targeting is seen in ex vivo-generated DCs and not in vivo DCs, the efficient delivery of the antigen to DCs through the nanoparticles is an important demonstration that may eventually be applied to in vivo DC targeting.
The most clinically advanced application of nanotechnology for immunotherapy of HIV/AIDS is the DermaVir patch that has reached Phase II clinical trials [99]. DermaVir is a targeted nanoparticle system based on polyethyleimine mannose (PEIm), glucose and HIV antigen coding DNA plasmid formulated into nanoparticles (~100 nm) and administered under a patch after a skin preparation. The nanoparticles are delivered to epidermal Langerhans cells that trap the nanoparticles and mature to become highly immunogenic on their way to the lymph nodes. Mature DCs containing the nanoparticles present antigens to T cells inducing cellular immunity. Preclinical studies and Phase I clinical trials showed safety and tolerability of the DermaVir patch, which led the progression to Phase II trials. This is the first nanotechnology-based immunotherapy for HIV/AIDS that has reached the clinic and encourages further work in this area. A selection of nanotechnology platforms used for antiretroviral drug delivery, gene therapy and immunotherapy are listed in Table 1.
Table 1.
Summary of nanotechnology-based treatment approaches for HIV/AIDS.
| Type of therapy | Therapeutic agent (drug or gene) | Nanotechnology delivery platform | Development stage | Refs. |
|---|---|---|---|---|
| Antiretroviral therapy | Rilpivirine (TMC278) | Poloxamer 338/TPGS 1000 | Preclinical | [35] |
| Indinavir | Liposome-laden macrophages | Preclinical | [36–38] | |
| Stavudine | Mannose- and galactose-targeted liposome | Preclinical | [39–41] | |
| Zidovudine | Mannose-targeted liposome | Preclinical | [42] | |
| Efavirenz | Mannose-targeted dendrimer | Preclinical | [43,45] | |
| Lamivudine | Mannose-targeted dendrimer | Preclinical | [46] | |
| Nanomaterials | Fullerene derivatives | – | Preclinical | [49–55] |
| Dendrimers | – | Preclinical | [56,57] | |
| Silver nanoparticles | – | Preclinical | [58,59] | |
| SDC-1721/gold nanoparticles | Gold nanoparticles | Preclinical | [60] | |
| Gene therapy | siRNA | Peptide fusion proteins, protamine–antibody fusion proteins, dendrimers, single walled carbon nanotubes, peptide–antibody conjugates | Preclinical | [77–81] |
| Immunotherapy | P24 protein | Poly (D,L-lactide) nanoparticles/dendritic cells | Preclinical | [98] |
| Plasmid DNA | Mannose-targeted polyethyleimine polymers | Phase II clinical trials | [99] |
Nanotechnology for HIV/AIDS prevention
Vaccine delivery
The search for a safe and effective HIV/AIDS vaccine has been challenging in the almost three decades since the discovery of the disease. Recently, high-profile clinical trial failures have prompted great debate over the vaccine research, with some suggesting the need for a major focus on fundamental research, with fewer efforts on clinical trials [13–15,100,101].
The major challenges in the development of a preventive HIV/AIDS vaccine have been the extensive viral strain and sequence diversity, viral evasion of humoral and cellular immune responses, coupled with the lack of methods to elicit broadly reactive neutralizing antibodies and cytotoxic T cells [102]. In order to generate T cell responses, protein antigens must enter APCs (such as DCs) where peptides are processed and loaded into MHC molecules for presentation to CD4+ T cells (extracellular antigen in MHC class II) and CD8+ T cells (intracellular antigen in MHC class I) [103]. The challenge associated with delivery of any exogenous antigen (such as nanoparticles) to APCs, is that exogenous antigens require specialized ‘cross-presentation’ in order to be presented by MHC class I and activate CD8+ cytotoxic T cells [104]. This requirement for cytosolic delivery of antigens and cross-presentation represents yet another hurdle for HIV intracellular antigen vaccine, but potentially an advantage of nanodelivery. Humoral responses (neutralizing antibodies produced by B cells) are generated to intact antigen presented on the surface for the virus, or nanoparticles, but these humoral responses typically require ‘help’ from CD4+ T cells [105], and thus the challenge is not to achieve either a single cellular or humoral response, but rather both.
Nanoparticles have potential as adjuvants and delivery systems for vaccines. Over the past few decades, controlled-release systems have been used for sustained release of various agents. This has great advantages for vaccine delivery since the release of antigens in a controlled manner could lead to a prolonged and stronger initiation of the immune response. By protecting the delivered antigen from body fluids (e.g., lymph, serum and mucus) nanoparticle antigen encapsulation can increase the half-life of an immunizing antigen. Nanoparticles can also be designed to effectively target APCs [106]. Targeting antigen delivery to DCs with surface-functionalized nanoparticles presents a major opportunity for delivery of antigen and initiation of immune responses. Another major benefit of nanoparticle vaccines is that they can be optimized for various routes of administration. Conventional vaccines are mostly administered intramuscularly, but nanoparticles provide expanded opportunity for oral and nasal vaccinations where mucosal immunity could be induced [107].
To deliver antigens, nanoparticles can be used either to encapsulate antigens in their core or absorb the antigens on their surfaces. Both strategies present advantages depending on whether the humoral or cellular immune response is desired. For efficient antigen presentation using DCs, encapsulating antigens can lead to the efficient delivery of an extracellular antigen (nanoparticle) into the cytoplasm [106]. Recently, there has been considerable progress in the field of nanoparticle design for efficient intracellular delivery of various agents. On the other hand, the ability to functionalize the surface of nanoparticles provides the capability to put antigens on the surface of the particles for direct presentation to B cells for antibody-specific immune responses. In order to achieve the coordinated effort of humoral and cellular responses that are essential for the prevention of HIV/AIDS, rationally designed nanoparticles will have the capacity to present antigens to both DCs (encapsulated) and B cells (surface absorbed).
Although the development of nanoparticle-based vaccines for HIV/AIDS is at an early stage, there has been recent progress. Here, we discuss the early developments of nanoparticles based on polymers and liposomes for HIV/AIDS vaccine.
Various lipid-based systems have been investigated for HIV/AIDS vaccine delivery. In an earlier study, nasal immunization of mice with the HIV gp160 protein encapsulated in a liposome induced high titers of gp160-specific neutralizing antibody responses [108]. The liposomes were made from a mixture of cholesterol, sphingomyelin, phosphatidylethanolamine, phosphatidylcholine and phosphatidylserine. The HIV gp 41 protein was also delivered through a variety of liposomes (110–400 nm) eliciting strong antibody responses in mice and rabbits [109–111]. To date, there have been no vaccines that can elicit a broadly neutralizing antibody response to HIV; therefore, it is doubtful that an antibody response to a monovalent encapsulated antigen alone will be enough for a sterilizing HIV vaccine [112,113]. These nanodelivery systems therefore need to be improved to include a variety of HIV epitopes and potentially even epitopes engineered to enhance access to and generation of antibody responses to areas of the HIV glycoproteins not elicited by acute HIV infection [112,113].
Dendritic cells express a variety of microbial pattern recognition receptors such as Toll-like receptors, one of which, TLR9, binds CpG oligonucleotides (CpG ODN) [114]. CpG ODNs are under intense investigation for DC-targeted vaccines, as CpG stimulation leads to activation and maturation of DCs into potent initiators of cellular immune responses. Immunization with CpG ODN delivered through liposomes (made of 1-(2-[oleoyloxy] ethyl)-2-oleyl-3-(2-hydroxy-ethyl) imidazolinium chloride (DOTIM) and cholesterol) led to stronger simian immunodeficiency virus (SIV)-specific T and B cell responses in rhesus macaques compared with control immunization [115]. Although cationic lipids such as DOTIM are not preferred in drug delivery, they present an opportunity in vaccine application because their immunogenic properties can be utilized for increased adjuvanticity.
MF59 is a nanosized (<250 nm) oil-in-water emulsion composed of squalene and the surfactants polysorbate 80 and sorbitan trioleate [116,117]. It has been shown to be safe in human trials and approved for use in over 20 countries as an adjuvant for an influenza vaccine [118]. Immunization of baboons with the HIV env and gag DNA followed by booster immunizations with oligomeric Env protein in MF59 elicited stronger antibodies and cellular responses compared with immunizations with DNA alone [119]. Another study found that priming rabbits with modified HIV env DNA, followed by boosting with oligomeric protein in MF59, elicited higher titers of env-binding and autologous neutralizing antibodies than priming with DNA alone. In a more recent study, immunization of mice with DNA plasmids encoding for the proteins gp140, Gag and Tat formulated in MF59 resulted in complete protection against challenge with HIV-infected murine cells [120]. Moreover, immunization with env proteins in MF59 containing CpG ODN also elicited higher titers of binding and neutralizing antibodies compared with immunization with MF59 alone [121].
Another oil-in-water nanoemulsion (~350 nm) prepared from cetylpyridinium chloride, a nonionic surfactant and soybean oil was used for delivering gp120 protein through the nasal route, eliciting antibody and cellular immune responses in mice and guinea pigs [122]. The potential for nasal administration of vaccine represents an alternative to conventional intramuscular delivery, especially as it regards generation of mucosal immunity.
Nanoparticles based on the polymers PLA and PLGA have also shown potential for delivery of protein- and DNA-based HIV/AIDS vaccines. Surfactant-free PLA nanoparticles (300–600 nm) coated with HIV p24 protein induced high antibody titers in mice, rabbits and macaques, and elicited strong cytotoxic T cell responses in mice [123]. Similarly, co-adsorption of p24 and gp120 proteins onto PLA nanoparticles (185–250 nm) elicited high antibody titers in mice, illustrating the potential for PLA nanoparticle use as multivalent vaccines [124]. Moreover, immunization of rabbits with any of the three HIV antigens (p24, WT Tat and mutated, detoxified Tat) mixed with either PLA nanoparticles or the emulsion MF59 led to the induction of similar level of antibody titers [125]. The ability of PLA to surface adsorb antigens and induce both antibody and cellular responses appears to be an asset in its continued use in nanoparticle vaccines.
Polystyrene nanospheres (~350 nm) coated with inactivated HIV particles were used for nasal immunization, effectively inducing mucosal antibody responses, detected as the HIV-specific vaginal antibody response in mice [126]. The nanospheres contain poly(methacrylic acid) branches with concanavalin A immobilized on their surface. In a more promising study, immunization with the polystyrene nanospheres induced DC-mediated immune responses in mice, characterized by production of efficient antibodies against HIV detectable in the genital tract as well as specific cytotoxic T cells in the spleen [127]. This study is unique because it introduces a novel approach that combines both the older technique of using inactivated viral particles as well as the newer approaches utilizing polymeric nanoparticles for delivery of vaccines.
Nanoparticles (200 nm) composed of hydrophobically modified poly (gamma-glutamic acid) (gamma-hPGA) encapsulating gp120 protein strongly induced antigen-specific cellular immunity when targeted to DCs in Balb/c mice [128]. These nanoparticles were also shown to induce significant CD8+ T cell responses in mice compared with gp120 alone [129]. In another study, immunization of mice with the gamma-hPGA nanoparticles encapsulating the p24 protein activated antigen-specific CD8+ T cells in spleen and induced p24-specific serum antibodies, as compared with immunization with p24 alone [130]. The nanoparticles induced comparable p24-specific serum antibody to that of the complete Freund’s adjuvant, a potent immunomodulator.
The above works indicate some of the major efforts and progress made using nanoparticle systems for HIV/AIDS vaccine delivery. A summary of the major antigen candidates, the nanotechnology platforms used and the development stage for each system is provided in Table 2. Although the results so far are encouraging, future works need to focus on design and specific targeting of the necessary cells of the immune system. Optimization of different parameters such as the size, targeting ligand, surface density, encapsulation efficiency and route of administration would lead to better and more efficient vaccine delivery systems.
Table 2.
Summary of nanotechnology developments for prevention of HIV/AIDS.
| Type of preventive agent | Antigen/adjuvant or drug | Nanotechnology platform | Development stage | Refs. |
|---|---|---|---|---|
| Protein or peptide vaccine | gp41, gp120, gp160, p24, Env, Gag, Tat | Liposomes, nanoemulsion, MF59, PLA nanoparticles, poly(γ-glutamic acid) nanoparticles | Preclinical | [108–111] [119–120] [122–125] [128–130] |
| DNA vaccine | env, rev, gag, tat, CpG ODN | Liposomes, nanoemulsion, PLA nanoparticles | Preclinical | [115,121] |
| Inactivated viral particle | Inactivated HIV viral particle | Polystyrene nanospheres | Preclinical | [126–127] |
| Microbicides | L-lysine dendrimer | L-lysine dendrimer | Phase I/II | [136–138] |
| PLGA nanoparticles | ||||
| PSC-RANTES | PLGA | Preclinical | [139] | |
| siRNA | Nanoparticles, lipids, cholesterol conjugation | Preclinical | [141–144] |
ODN: Oligonucleotides; PLA: Poly(D,L-lactide); PLGA: Poly(D,L-lacticide-co-glycolide).
Intravaginal microbicides
Although vaccines that induce sterilizing immunity are the most ideal way to prevent the spread of HIV/AIDS, other approaches are also being pursued until a safe and effective vaccine is developed. Since sexual transmission is the major route of infection, prevention methods aimed at behavioral changes as well as the use of personal protection such as condoms have helped in reducing the spread of the disease in some countries [131]. However, effective protection methods that can be utilized by women have not been readily available, making women more vulnerable to the disease. Among people infected with HIV/AIDS, women account for nearly 50% of infections worldwide and 60% in Sub-Saharan Africa [132,133]. As a result, there are major efforts focused on developing effective microbicides for HIV/AIDS prevention. Microbicides are preventive agents that are topically applied into the vagina to prevent the transmission of HIV/AIDS or other sexually transmitted diseases.
There are currently over 50 drug candidates in preclinical development and 12 candidates in clinical trials for use as microbicides [132]. These microbicide candidates work by different mechanisms that either target the virus or inhibit viral binding to the target cell. Most of the current microbicides in development are based on gels formed from anionic polymers and polysaccharides, whereas microbicides based on antiretroviral drugs have also gained attention [132,134,135]. However, similar to the difficulties faced in vaccine development, major candidate microbicides for HIV/AIDS failed in recent efficacy clinical trials suggesting the need for further studies and new approaches [16,17]. Here, we discuss the most recent nanotechnology-based approaches that focus on using dendrimers, siRNA and nanoparticles in microbicides for HIV/AIDS.
VivaGel is a microbicide gel formed from the L-lysine dendrimer that has a polyanionic outer surface developed by the company Starpharma [136]. After comparing the antiviral effects of various dendrimers based on L-lysine, poly(amido amine) and poly(propylene imine), the product SPL7013, based on the dendrimer L-lysine, was identified as the best microbicide candidate [137]. In earlier works, SPL7013 applied as a topical microbicide in female pigtailed macaques showed a dose-dependent resistance to viral challenge [138]. Recently, it was shown in a Phase I safety trial that the dendrimer solution is safe in humans [136].
Polymer-based nanoparticles are also being considered in the development of microbicides. PLGA nanoparticles (~260 nm), in which the protein PSC-RANTES (an amino terminus-modified analog of the chemokine RANTES, a ligand for HIV co-receptor CCR5) was encapsulated, maintained comparable anti-HIV activity compared with unformulated PSC-RANTES in HeLa cells [139]. In an ex vivo cervical tissue model, the PSC-RANTES-encapsulated nanoparticles displayed a fivefold increase in tissue uptake, enhanced tissue permeation, and significant localization at the basal layers of the epithelium over unformulated PSC-RANTES. A major study has shown that PSC-RANTES provides potent protection against vaginal challenge in rhesus macaques, indicating its potential for use in microbicides [140]. This protection is based on the inhibition of the CCR5 co-receptor, which prevents HIV entry into cells.
Another novel approach is the use of siRNA as an intravaginal viral microbicide. As discussed above, siRNA are small single-stranded RNA molecules that are complementary to the gene targeted for knockdown [74]. The first study reporting the use of siRNA for intravaginal microbicide-delivered siRNA using lipids and protected mice from lethal Herpes simplex virus 2 infection [141,142]. The efficacy of siRNA gene knockdown was further improved by using cholesterol-conjugated siRNAs to silence gene expression in the vagina without causing inflammation or inducing interferons [143]. More recently, it was reported that biodegradable polymer nanoparticles are effective delivery vehicles for siRNA to the vaginal mucosa [144]. PLGA nanoparticles (<200 nm) mixed with the polyamines spermidine or putrescine delivered siRNA to the vaginal mucosa, leading to sustained gene silencing for up to 14 days. In vivo studies in mice suggest that knockdown of gene expression was proximal (in the vaginal lumen) and distal (in the uterine horns) to the site of topical delivery and the nanoparticles also penetrated deep into the epithelial tissue. Although this work is at an early stage, it shows that siRNA-based microbicides may be a tool for HIV/AIDS prevention in the near future.
Conclusion & future perspective
Nanotechnology can impact the treatment and prevention of HIV/AIDS with various innovative approaches. Treatment options may be improved using nanotechnology platforms for delivery of antiretroviral drugs. Controlled and sustained release of the drugs could improve patient adherence to drug regimens, increasing treatment effectiveness. Targeted nanoparticles utilizing ligands such as mannose, galactose, tuftsin and fMLF peptides have been used to target macrophages, major HIV viral reservoirs. In the future, targeted co-delivery of two or more antiviral drugs in a nanoparticle system could radically improve treatment of viral reservoirs. Our group and other investigators have developed nanoparticles with the potential to co-deliver both hydrophobic and hydrophilic drugs or genes and these may provide versatility for codelivery of antiviral drugs [145–148]. In addition to delivering antiviral drugs, nanomaterials have shown their ability to inhibit viral replication by themselves. Fullerenes, dendrimers and inorganic nanoparticles such as silver have antiviral effects or improve antiviral effects of other molecules, as in the case of gold nanoparticles.
Newer treatment approaches, such as gene therapy and immunotherapy, can be enhanced with nanotechnology. Nonviral delivery of siRNA is one of the most active areas of research in nanotechnology. Delivery of siRNA to HIV-specific cells has been demonstrated; however, work remains in this area since safe and effective nanotechnology for RNAi has yet to be developed for HIV/AIDS applications. Immunotherapy is another major area where nanotechnology could play a significant role. The movement of the DermaVir Patch into Phase II trials is an indication that nanoimmunotherapy may be the earliest form of nanotechnology-based treatment that will be available for HIV/AIDS.
Nanotechnology-based vaccines for HIV/AIDS are also showing potential in early studies. Their ability to target specific cells and release antigens in a controlled and sustained manner makes nanoparticles a great alternative to viral vectors. Lipid- and polymer-based nanoparticles have been shown to induce HIV-specific antibody and cellular immune responses in animal studies. Despite the progress so far, preclinical studies remain important to ensure a clear understanding and optimization of mechanisms involved in the nanoparticle induction of strong humoral and cellular immunity. In addition to continued efforts in vaccine development, research into microbicide development remains important. Nanotechnology can play a major role in microbicide development by providing innovative strategies for nanoparticle-based delivery of therapeutic molecules or RNAi.
While there is exciting potential for nanomedicine in the treatment of HIV/AIDS, challenges remain to be overcome before the potential is realized. These include toxicity of nanomaterials, stability of nanoparticles in physiological conditions and their scalability for large-scale production. These are challenges general to all areas of nanomedicine and various works are underway to tackle them. Moreover, most of the nanotechnology-based studies undertaken so far for the treatment and prevention of HIV/AIDS are in the preclinical stages. This implies that many hurdles remain before there is widespread use of these technologies in the clinic. The in vitro and in vivo studies performed so far are preliminary in most cases and more work needs to be done with clear therapeutic end points that will indicate the efficacy of the nanotechnology-based systems. One of the major challenges to animal studies for HIV/AIDS treatment is the lack of a suitable animal model. Nonhuman primate models utilize the HIV relative SIV, which has similarities but ultimately differs from human HIV pathogenesis. The development of humanized mice, mice that possess a human immune compartment, and therefore can support HIV infection, has allowed the study of human T cells in vivo [149,150]. This has allowed the study and manipulation of HIV interaction with human T cells in vivo. A study that investigated the targeted delivery of siRNA to T-cells was able to use humanized mice to suppress endogenous viral replication and restore CD4+ T cell counts, while effectively suppressing viremia in HIV-infected mice [81]. The use of humanized mice will provide the means for direct experimentation with human cells, and hasten the pace of translational research.
Another important aspect to consider in the application of nanotechnology-based delivery systems for HIV/AIDS treatment and prevention is the route of administration. Most of the studies illustrated so far were based on parenteral administration of the formulations. Recently, a lot of effort has been directed towards needle-free delivery methods, mainly oral, transdermal, nasal and pulmonary routes, in addition to injectable administration [151–154]. In the context of drug delivery for HIV/AIDS, nonparenteral routes could be preferred considering the ease of administration and user-friendliness (e.g., oral vaccine delivery). These also have great advantages in improving patient adherence to the regimens. However, nonparenteral routes have limitations in their efficiency and bioavailability, and their long-term application could have negative effects (e.g., potential irritation by transdermal or pulmonary route). To overcome these obstacles, further research efforts would be required to investigate highly efficient nanoparticle delivery systems for the replacement of injection/infusion of HIV/AIDS drugs.
Another important consideration in investigating nanotechnology-based systems for HIV/AIDS is the economic aspect, as the hardest hit and most vulnerable populations reside in underdeveloped and economically poor countries. In the case of antiretroviral therapy, nanotherapeutics may increase the overall cost of treatment, reducing the overall value. However, if the nanotherapeutics could improve patient adherence by reducing dosing frequency as expected, and furthermore, if they can eradicate viral reservoirs leading to a sterile immunity, these advantages may effectively offset the added cost. In the case of new treatment approaches such as gene therapy and immunotherapy, the use of nanotheraepeutics could even reduce the cost when compared with the other alternative approaches being considered. Although no data exist for such comparisons, it can be assumed that nanotherapeutics might have advantages for large-scale productions when compared with viral or ex vivo DC-based gene therapy or immunotherapy. Similarly, in the case of the prevention methods, the nanotherapeutics should not be more costly compared with existing systems. Since bulk quantities of vaccines are widely distributed through government agencies, development of nanotechnology-enabled vaccines would be one of the most cost effective approaches to address the HIV/AIDS epidemic worldwide. Nanotechnology-enabled microbicides may also need to rely on support from governmental or nongovernmental organizations for effective distribution to economically disadvantaged countries.
Overall, these are exciting times for nanotechnology research and the pace of scientific discovery is beginning to gain momentum. It is widely accepted that with continued support, medicine and the field of HIV/AIDS will be important beneficiaries of nanotechnology for years to come.
Executive summary
Challenges of HIV/AIDS treatment
HIV resides in latent cellular and anatomical reservoirs where current drugs are unable to completely eradicate the virus.
Macrophages are major cellular reservoirs, which also contribute to the generation of elusive mutant viral genotypes by serving as the host for viral genetic recombination.
Anatomical latent reservoirs include secondary lymphoid tissue, testes, liver, kidney, lungs, the gut and the brain.
The major challenge facing current drug regimens is that they do not fully eradicate the virus from these reservoirs; requiring patients take medications for life. Under current treatment, pills are taken daily, resulting in problems of patient adherence. The drugs also have side effects and in some people the virus develops resistance against certain drugs.
Nanotechnology-based drug delivery for HIV/AIDS treatment
Nanotechnology-based targeted delivery of antiretroviral drugs to CD4+ T cells and macrophages, as well as delivery to the brain and lymphocytes, could ensure that drugs reach latent reservoir.
Various nanotechnology-based drug delivery systems have been successfully used to deliver various antiretroviral drugs in vitro and in vivo.
Nanosuspensions that were used to deliver the drug rilpivirine (TMC278) resulted in sustained release over 3 months in dogs and 3 weeks in mice. This result is a major indication of how nanotechnology-based drug delivery could improve antiviral treatment.
Nanomaterials as therapeutic agents
Various nanomaterials such as fullerenes, dendrimers, silver and gold nanoparticles have shown anti-HIV effects in vitro.
Silver nanoparticles showed size-dependent interaction with HIV, inhibiting the virus from binding to CD4+ T cells while gold nanoparticles conjugated to the molecule SDC-1721 (a segment of the CCR5 inhibitor TAK-779) showed strong anti-HIV activity compared with free SDC-1721.
Gene therapy for HIV/AIDS treatment
Gene therapy based on siRNA has shown promise for HIV/AIDS treatment. Nanotechnology platforms for delivery of siRNA for HIV/AIDS treatment are in their early stages but recent work has been met with optimism.
Single-walled nanotubes, dendrimers, fusion proteins, peptide–antibody conjugates have all been used for delivery of siRNA to HIV-specific cells.
Immunotherapy for HIV/AIDS
Immunotherapy for HIV/AIDS based on viral agents and administration of ex vivo-generated autologous dendritic cells involves risks due to the viral agents and the complicated procedures used in ex vivo dendritic cell generation and manipulation.
Nanotechnology-based immunotherapy that uses DNA plasmid delivered through mannose targeted polyethyleimine has reached Phase II clinical trials.
Nanotechnology-based preventive HIV/AIDS vaccine
Generation of an effective HIV/AIDS vaccine has been notoriously difficult and new approaches are always sought.
Nanoparticles have various advantages in improving delivery of antigens to enhance the immune response. They can be used both for encapsulating antigens in their core from which antigen presenting cells can process and present and cross-present antigen to CD4+ and CD8+ T cells respectively, or absorbing the antigens on their surfaces, allowing B cells to generate humoral responses. Nanoparticle vaccines can also be optimized for various routes of administration.
Various polymeric and lipid-based nanoparticles have been used to deliver DNA-, protein- or peptide-based antigens in vivo, eliciting strong cellular, humoral and mucosal immune responses.
Nanotechnology-based intravaginal microbicides
Intravaginal microbicides are preventive agents that are topically applied to the vagina to prevent the transmission of HIV/AIDS or other sexually transmitted diseases.
Nanotechnology-based approaches are being developed to use dendrimers, siRNA and nanoparticles for microbicidal functions.
VivaGel is a dendrimer-based microbicide gel that has been shown to be safe in humans in Phase I clinical trials.
Polymeric nanoparticles have been used to deliver the CCR5 inhibitor PSC-RANTES and HIV-specific siRNA as microbicides.
Acknowledgments
The authors would also like to thank Paul Sax for helpful discussions.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Daniel R Kuritzkes consults for pharmaceutical companies involved in HIV/AIDS drug development. The authors are supported in-part with funding from NIH EB003647 (Omid C Farokhzad), NIH U54-CA119349 (Robert Langer, Omid C Farokhzad), and Koch-PCF Program in Cancer Nanotherapeutics (Omid C Farokhzad, Robert Langer). E Ashley Moseman is recipient of an NIH training grant (T32 HL07623) in Hematology. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Blattner W, Gallo RC, Temin HM. HIV causes AIDS. Science. 1988;241(4865):515–516. doi: 10.1126/science.3399881. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RC. Historical essay. The early years of HIV/AIDS. Science. 2002;298(5599):1728–1730. doi: 10.1126/science.1078050. [DOI] [PubMed] [Google Scholar]
- 3.Gallo RC, Montagnier L. The discovery of HIV as the cause of AIDS. N Engl J Med. 2003;349(24):2283–2285. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- 4.Montagnier L. Historical essay. A history of HIV discovery. Science. 2002;298(5599):1727–1728. doi: 10.1126/science.1079027. [DOI] [PubMed] [Google Scholar]
- 5.Furin JJ, Behforouz HL, Shin SS, et al. Expanding global HIV treatment: Case studies from the field. Ann NY Acad Sci. 2008;1136:12–20. doi: 10.1196/annals.1425.004. [DOI] [PubMed] [Google Scholar]
- 6.Merson MH. The HIV-AIDS pandemic at 25 – the global response. N Engl J Med. 2006;354(23):2414–2417. doi: 10.1056/NEJMp068074. [DOI] [PubMed] [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS. Joint United Nations Programme on HIV/AIDS. Geneva, Switzerland: 2008. Report on the global HIV/AIDS epidemic. [Google Scholar]
- 8.Rodriguez-Monguio R, Seoane-Vazquez E. Patent life of antiretroviral drugs approved in the US from 1987 to 2007. AIDS Care. 2009:1–9. doi: 10.1080/09540120802511950. [DOI] [PubMed] [Google Scholar]
- 9.Lang L. FDA grants tentative approval for 75th generic antiretroviral drug. Gastroenterology. 2009;136(1):5. doi: 10.1053/j.gastro.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 11.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 12.Richman DD. HIV chemotherapy. Nature. 2001;410(6831):995–1001. doi: 10.1038/35073673. [DOI] [PubMed] [Google Scholar]
- 13.Ledford H. Merck’s HIV vaccine flop brings vectors under closer scrutiny. Nat Biotechnol. 2008;26(1):3–4. doi: 10.1038/nbt0108-3. [DOI] [PubMed] [Google Scholar]
- 14.Ledford H. HIV vaccine developers battle on, despite high-profile failures. Nat Biotechnol. 2008;26(6):591–592. doi: 10.1038/nbt0608-591. [DOI] [PubMed] [Google Scholar]
- 15.Uberla K. HIV vaccine development in the aftermath of the step study: Re-focus on occult HIV infection? PLoS Pathog. 2008;4(8):e1000114. doi: 10.1371/journal.ppat.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Aids research. Microbicide fails to protect against HIV. Science. 2008;319(5866):1026–1027. doi: 10.1126/science.319.5866.1026b. [DOI] [PubMed] [Google Scholar]
- 17.Grant RM, Hamer D, Hope T, et al. Whither or wither microbicides? Science. 2008;321(5888):532–534. doi: 10.1126/science.1160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farokhzad OC. Nanotechnology for drug delivery: The perfect partnership. Expert Opin Drug Deliv. 2008;5(9):927–929. doi: 10.1517/17425247.5.9.927. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 21.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 22.Heath JR, Davis ME. Nanotechnology and cancer. Ann Rev Med. 2008;59:251–265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 24.Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401(6756):874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 25.Marsden MD, Zack JA. Eradication of HIV: Current challenges and new directions. J Antimicrob Chemother. 2009;63(1):7–10. doi: 10.1093/jac/dkn455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sax PE, Cohen CJ, Kuritzkes DR. HIV Essentials. Physicians’ Press; Royal Oak, MI, USA: 2007. [Google Scholar]
- 27.Lamers SL, Salemi M, Galligan DC, et al. Extensive HIV-1 intra-host recombination is common in tissues with abnormal histopathology. PLoS One. 2009;4(3):E5065. doi: 10.1371/journal.pone.0005065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGee B, Smith N, Aweeka F. HIV pharmacology: Barriers to the eradication of HIV from the CNS. HIV Clin Trials. 2006;7(3):142–153. doi: 10.1310/AW2H-TP5C-NP43-K6BY. [DOI] [PubMed] [Google Scholar]
- 29.Vyas TK, Shah L, Amiji MM. Nanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sites. Expert Opin Drug Deliv. 2006;3(5):613–628. doi: 10.1517/17425247.3.5.613. [DOI] [PubMed] [Google Scholar]
- 30.Wan L, Pooyan S, Hu P, Leibowitz MJ, Stein S, Sinko PJ. Peritoneal macrophage uptake, pharmacokinetics and biodistribution of macrophage-targeted peg-fmlf (n-formyl-methionyl-leucyl-phenylalanine) nanocarriers for improving HIV drug delivery. Pharm Res. 2007;24(11):2110–2119. doi: 10.1007/s11095-007-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowacek A, Gendelman HE. Nanoart, neuroAIDS and CNS drug delivery. Nanomed. 2009;4(5):557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 33.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 34.Amiji MM, Vyas TK, Shah LK. Role of nanotechnology in HIV/AIDS treatment: Potential to overcome the viral reservoir challenge. Discov Med. 2006;6(34):157–162. [PubMed] [Google Scholar]
- 35.Baert L, van’t Klooster G, Dries W, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (tmc278) for HIV treatment. Eur J Pharm Biopharm. 2009;72(3):502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Dou H, Destache CJ, Morehead JR, et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–2835. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dou H, Morehead J, Destache CJ, et al. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages. Virology. 2007;358(1):148–158. doi: 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Dou H, Grotepas CB, McMillan JM, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183(1):661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg M, Asthana A, Agashe HB, Agrawal GP, Jain NK. Stavudine-loaded mannosylated liposomes: In-vitro anti-HIV-i activity, tissue distribution and pharmacokinetics. J Pharm Pharmacol. 2006;58(5):605–616. doi: 10.1211/jpp.58.5.0005. [DOI] [PubMed] [Google Scholar]
- 40.Garg M, Dutta T, Jain NK. Reduced hepatic toxicity, enhanced cellular uptake and altered pharmacokinetics of stavudine loaded galactosylated liposomes. Eur J Pharm Biopharm. 2007;67(1):76–85. doi: 10.1016/j.ejpb.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Garg M, Garg BR, Jain S, et al. Radiolabeling, pharmacoscintigraphic evaluation and antiretroviral efficacy of stavudine loaded 99mtc labeled galactosylated liposomes. Eur J Pharm Sci. 2008;33(3):271–281. doi: 10.1016/j.ejps.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Kaur CD, Nahar M, Jain NK. Lymphatic targeting of zidovudine using surface-engineered liposomes. J Drug Target. 2008;16(10):798–805. doi: 10.1080/10611860802475688. [DOI] [PubMed] [Google Scholar]
- 43.Dutta T, Agashe HB, Garg M, Balakrishnan P, Kabra M, Jain NK. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. J Drug Target. 2007;15(1):89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 44.Dutta T, Jain NK. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta. 2007;1770(4):681–686. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur J Pharm Sci. 2008;34(2–3):181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Wan L, Zhang X, Pooyan S, et al. Optimizing size and copy number for PEG-FMLF (n-formyl-methionyl-leucyl-phenylalanine) nanocarrier uptake by macrophages. Bioconjug Chem. 2008;19(1):28–38. doi: 10.1021/bc070066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganser-Pornillos BK, Yeager M, Sundquist WI. The structural biology of HIV assembly. Curr Opin Struct Biol. 2008;18(2):203–217. doi: 10.1016/j.sbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pornillos O, Ganser-Pornillos BK, Kelly BN, et al. X-ray structures of the hexameric building block of the HIV capsid. Cell. 2009;137(7):1282–1292. doi: 10.1016/j.cell.2009.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedman SH, Decamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. Inhibition of the HIV-1 protease by fullerene derivatives – model-building studies and experimental-verification. J Am Chem Soc. 1993;115(15):6506–6509. [Google Scholar]
- 50.Bosi S, Da Ros T, Spalluto G, Balzarini J, Prato M. Synthesis and anti-HIV properties of new water-soluble bis-functionalized[60] fullerene derivatives. Bioorg Med Chem Lett. 2003;13(24):4437–4440. doi: 10.1016/j.bmcl.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Kotelnikova RA, Bogdanov GN, Frog EC, et al. Nanobionics of pharmacologically active derivatives of fullerene c-60. J Nanopart Res. 2003;5(5–6):561–566. [Google Scholar]
- 52.Marchesan S, Da Ros T, Spalluto G, Balzarini J, Prato M. Anti-HIV properties of cationic fullerene derivatives. Bioorg Med Chem Lett. 2005;15(15):3615–3618. doi: 10.1016/j.bmcl.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 53.Troshina OA, Troshin PA, Peregudov AS, Kozlovskiy VI, Balzarini J, Lyubovskaya RN. Chlorofullerene c60cl6: A precursor for straightforward preparation of highly water-soluble polycarboxylic fullerene derivatives active against HIV. Org Biomol Chem. 2007;5(17):2783–2791. doi: 10.1039/b705331b. [DOI] [PubMed] [Google Scholar]
- 54.Durdagi S, Supuran CT, Strom TA, et al. In silico drug screening approach for the design of magic bullets: A successful example with anti-HIV fullerene derivatized amino acids. J Chem Inf Model. 2009;49(5):1139–1143. doi: 10.1021/ci900047s. [DOI] [PubMed] [Google Scholar]
- 55.Tanimoto S, Sakai S, Matsumura S, Takahashi D, Toshima K. Chem Commun. 44. 2008. Target-selective photo-degradation of HIV-1 protease by a fullerene–sugar hybrid; pp. 5767–5769. [DOI] [PubMed] [Google Scholar]
- 56.Blanzat M, Turrin CO, Aubertin AM, et al. Dendritic catanionic assemblies: In vitro anti-HIV activity of phosphorus-containing dendrimers bearing gal beta(1)cer analogues. Chembiochem. 2005;6(12):2207–2213. doi: 10.1002/cbic.200500203. [DOI] [PubMed] [Google Scholar]
- 57.Wang W, Guo ZP, Chen Y, Liu T, Jiang L. Influence of generation 2–5 of pamam dendrimer on the inhibition of tat peptide/tar rna binding in HIV-1 transcription. Chem Biol Drug Des. 2006;68(6):314–318. doi: 10.1111/j.1747-0285.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 58.Elechiguerra JL, Burt JL, Morones JR, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnology. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun RW, Chen R, Chung NP, Ho CM, Lin CL, Che CM. Silver nanoparticles fabricated in hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem Commun. 2005;(40):5059–5061. doi: 10.1039/b510984a. [DOI] [PubMed] [Google Scholar]
- 60.Bowman MC, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J Am Chem Soc. 2008;130(22):6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossi JJ, June CH, Kohn DB. Genetic therapies against HIV. Nat Biotechnol. 2007;25(12):1444–1454. doi: 10.1038/nbt1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haasnoot J, Westerhout EM, Berkhout B. Rna interference against viruses: strike and counterstrike. Nat Biotechnol. 2007;25(12):1435–1443. doi: 10.1038/nbt1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li M, Rossi JJ. Lentiviral vector delivery of siRNA and shrna encoding genes into cultured and primary hematopoietic cells. Methods Mol Biol. 2008;433:287–299. doi: 10.1007/978-1-59745-237-3_18. [DOI] [PubMed] [Google Scholar]
- 64.Morris KV, Rossi JJ. Lentiviral-mediated delivery of siRNAs for antiviral therapy. Gene Ther. 2006;13(6):553–558. doi: 10.1038/sj.gt.3302688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M, Li H, Rossi JJ. RNAI in combination with a ribozyme and tar decoy for treatment of HIV infection in hematopoietic cell gene therapy. Ann NY Acad Sci. 2006;1082:172–179. doi: 10.1196/annals.1348.006. [DOI] [PubMed] [Google Scholar]
- 66.Li MJ, Kim J, Li S, et al. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shrna, anti-ccr5 ribozyme, and a nucleolar-localizing tar decoy. Mol Ther. 2005;12(5):900–909. doi: 10.1016/j.ymthe.2005.07.524. [DOI] [PubMed] [Google Scholar]
- 67.Lee SK, Dykxhoorn DM, Kumar P, et al. Lentiviral delivery of short hairpin RNAs protects CD4 T cells from multiple clades and primary isolates of HIV. Blood. 2005;106(3):818–826. doi: 10.1182/blood-2004-10-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnor JS, Miyano-Kurosaki N, Yamaguchi K, Abumi Y, Ishikawa K, Yamamoto N. Lentiviral-mediated delivery of combined HIV-1 decoy tar and VIF siRNA as a single rna molecule that cleaves to inhibit HIV-1 in transduced cells. Nucleosides Nucleotides Nucleic Acids. 2005;24(5–7):431–434. doi: 10.1081/ncn-200059981. [DOI] [PubMed] [Google Scholar]
- 69.Mitsuyasu RT, Merigan TC, Carr A, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15(3):285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109(2):259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 71.Lundin KE, Simonson OE, Moreno PM, et al. Nanotechnology approaches for gene transfer. Genetica. 2009;137(1):47–56. doi: 10.1007/s10709-009-9372-0. [DOI] [PubMed] [Google Scholar]
- 72.Gao X, Kim KS, Liu DX. Nonviral gene delivery: What we know and what is next. AAPS J. 2007;9(1):E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 74.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berkhout B, ter Brake O. Towards a durable rnai gene therapy for HIV-AIDS. Expert Opin Biol Ther. 2009;9(2):161–170. doi: 10.1517/14712590802653619. [DOI] [PubMed] [Google Scholar]
- 76.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 77.Eguchi A, Meade BR, Chang YC, et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsrna binding domain fusion protein. Nat Biotechnol. 2009;27(6):567–571. doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song E, Zhu P, Lee SK, et al. Antibody mediated in vivo delivery of small interfering rnas via cell-surface receptors. Nat Biotechnol. 2005;23(6):709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 79.Liu Z, Winters M, Holodniy M, Dai H. Sirna delivery into human t cells and primary cells with carbon-nanotube transporters. Angew Chem Int Ed. 2007;46(12):2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- 80.Weber N, Ortega P, Clemente MI, et al. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J Control Release. 2008;132(1):55–64. doi: 10.1016/j.jconrel.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 81.Kumar P, Ban HS, Kim SS, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134(4):577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar P, Wu HQ, McBride JL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 83.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes B. The immune response during acute HIV78-1 infection: Clues for vaccine development. Nat Rev Immunol. 2009 doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pett SL. Immunotherapies in HIV-1 infection. Curr Opin HIV AIDS. 2009;4(3):188–193. doi: 10.1097/COH.0b013e328329d090. [DOI] [PubMed] [Google Scholar]
- 85.Gandhi RT, Walker BD. Immunologic control of HIV-1. Ann Rev Med. 2002;53:149–172. doi: 10.1146/annurev.med.53.082901.104011. [DOI] [PubMed] [Google Scholar]
- 86.Cohen J. Building an HIV-proof immune system. Science. 2007;317(5838):612–614. doi: 10.1126/science.317.5838.612. [DOI] [PubMed] [Google Scholar]
- 87.Rinaldo CR. Dendritic cell-based human immunodeficiency virus vaccine. J Intern Med. 2009;265(1):138–158. doi: 10.1111/j.1365-2796.2008.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 89.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 90.Bourinbaiar AS, Root-Bernstein RS, Abulafia-Lapid R, et al. Therapeutic AIDS vaccines. Curr Pharm Des. 2006;12(16):2017–2030. doi: 10.2174/138161206777442119. [DOI] [PubMed] [Google Scholar]
- 91.Dorrell L, Williams P, Suttill A, et al. Safety and tolerability of recombinant modified vaccinia virus ankara expressing an HIV-1 gag/multiepitope immunogen (MVA. HIVA) in HIV-1-infected persons receiving combination antiretroviral therapy. Vaccine. 2007;25(17):3277–3283. doi: 10.1016/j.vaccine.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 92.Gandhi RT, O’Neill D, Bosch RJ, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. Canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27(43):6088–6094. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whiteside TL, Piazza P, Reiter A, et al. Production of a dendritic cell-based vaccine containing inactivated autologous virus for therapy of patients with chronic human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2009;16(2):233–240. doi: 10.1128/CVI.00066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tacken PJ, de Vries IJM, Torensma R, Figdor CG. Dendritic-cell immunotherapy: From ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7(10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 95.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release. 2006;112(1):26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 96.Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials. 2008;29(27):3671–3682. doi: 10.1016/j.biomaterials.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 97.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(d,l-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22(19):2406–2412. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 98.Aline F, Brand D, Pierre J, et al. Dendritic cells loaded with HIV-1 p24 proteins adsorbed on surfactant-free anionic pla nanoparticles induce enhanced cellular immune responses against HIV-1 after vaccination. Vaccine. 2009;27(38):5284–5291. doi: 10.1016/j.vaccine.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 99.Lori F, Calarota SA, Lisziewicz J. Nanochemistry-based immunotherapy for HIV-1. Curr Med Chem. 2007;14(18):1911–1919. doi: 10.2174/092986707781368513. [DOI] [PubMed] [Google Scholar]
- 100.Bass E, Feuer C, Warren M. Aids vaccine research and advocacy: An update. BETA. 2009;21(2):24–30. [PubMed] [Google Scholar]
- 101.Jefferys R. Vaccine failure is not a ‘crisis’ for HIV research. Nature. 2008;453(7196):719–720. doi: 10.1038/453719d. [DOI] [PubMed] [Google Scholar]
- 102.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455(7213):613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and t cell stimulation by dendritic cells. Ann Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 104.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Ann Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 105.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory b cell development. Ann Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 106.Fahmy TM, Demento SL, Caplan MJ, Mellman I, Saltzman WM. Design opportunities for actively targeted nanoparticle vaccines. Nanomed. 2008;3(3):343–355. doi: 10.2217/17435889.3.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles for nasal vaccination. Adv Drug Deliv Rev. 2009;61(2):140–157. doi: 10.1016/j.addr.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 108.Sakaue G, Hiroi T, Nakagawa Y, et al. HIV mucosal vaccine: Nasal immunization with gp160-encapsulated hemagglutinating virus of japan-liposome induces antigen-specific ctls and neutralizing antibody responses. J Immunol. 2003;170(1):495–502. doi: 10.4049/jimmunol.170.1.495. [DOI] [PubMed] [Google Scholar]
- 109.Singh SK, Bisen PS. Adjuvanticity of stealth liposomes on the immunogenicity of synthetic gp41 epitope of HIV-1. Vaccine. 2006;24(19):4161–4166. doi: 10.1016/j.vaccine.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 110.Wagner A, Stiegler G, Vorauer-Uhl K, et al. One step membrane incorporation of viral antigens as a vaccine candidate against HIV. J Liposome Res. 2007;17(3–4):139–154. doi: 10.1080/08982100701530159. [DOI] [PubMed] [Google Scholar]
- 111.Watson DS, Huang Z, Szoka FC., Jr All-trans retinoic acid potentiates the antibody response in mice to a lipopeptide antigen adjuvanted with liposomal lipid a. Immunol Cell Biol. 2009 doi: 10.1038/icb.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6(12):930–939. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 113.Burton DR, Desrosiers RC, Doms RW, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5(3):233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 114.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 115.Fairman J, Moore J, Lemieux M, et al. Enhanced in vivo immunogenicity of SIV vaccine candidates with cationic liposome-DNA complexes in a rhesus macaque pilot study. Hum Vaccin. 2008;5(2) doi: 10.4161/hv.5.3.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. Mf59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 117.Copland MJ, Rades T, Davies NM, Baird MA. Lipid based particulate formulations for the delivery of antigen. Immunol Cell Biol. 2005;83(2):97–105. doi: 10.1111/j.1440-1711.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- 118.Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27(25–26):3331–3334. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 119.Leung L, Srivastava IK, Kan E, et al. Immunogenicity of HIV-1 env and gag in baboons using a DNA prime/protein boost regimen. AIDS. 2004;18(7):991–1001. doi: 10.1097/00002030-200404300-00006. [DOI] [PubMed] [Google Scholar]
- 120.Brave A, Hinkula J, Cafaro A, et al. Candidate HIV-1 gp140δv2, gag and tat vaccines protect against experimental HIV-1/MULV challenge. Vaccine. 2007;25(39–40):6882–6890. doi: 10.1016/j.vaccine.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 121.Burke B, Gomez-Roman VR, Lian Y, et al. Neutralizing antibody responses to subtype b and c adjuvanted HIV envelope protein vaccination in rabbits. Virology. 2009;387(1):147–156. doi: 10.1016/j.virol.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bielinska AU, Janczak KW, Landers JJ, Markovitz DM, Montefiori DC, Baker JR., Jr Nasal immunization with a recombinant HIV gp120 and nanoemulsion adjuvant produces th1 polarized responses and neutralizing antibodies to primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2008;24(2):271–281. doi: 10.1089/aid.2007.0148. [DOI] [PubMed] [Google Scholar]
- 123.Ataman-Onal Y, Munier S, Ganee A, et al. Surfactant-free anionic pla nanoparticles coated with HIV-1 p24 protein induced enhanced cellular and humoral immune responses in various animal models. J Control Release. 2006;112(2):175–185. doi: 10.1016/j.jconrel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 124.Lamalle-Bernard D, Munier S, Compagnon C, et al. Coadsorption of HIV-1 p24 and gp120 proteins to surfactant-free anionic pla nanoparticles preserves antigenicity and immunogenicity. J Control Release. 2006;115(1):57–67. doi: 10.1016/j.jconrel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 125.Guillon C, Mayol K, Terrat C, et al. Formulation of HIV-1 tat and p24 antigens by pla nanoparticles or mf59 impacts the breadth, but not the magnitude, of serum and faecal antibody responses in rabbits. Vaccine. 2007;25(43):7491–7501. doi: 10.1016/j.vaccine.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 126.Miyake A, Akagi T, Enose Y, et al. Induction of HIV-specific antibody response and protection against vaginal sHIV transmission by intranasal immunization with inactivated sHIV-capturing nanospheres in macaques. J Med Virol. 2004;73(3):368–377. doi: 10.1002/jmv.20100. [DOI] [PubMed] [Google Scholar]
- 127.Kawamura M, Wang X, Uto T, et al. Induction of dendritic cell-mediated immune responses against HIV-1 by antigen-capturing nanospheres in mice. J Med Virol. 2005;76(1):7–15. doi: 10.1002/jmv.20317. [DOI] [PubMed] [Google Scholar]
- 128.Akagi T, Wang X, Uto T, Baba M, Akashi M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly(amino acid) derivatives. Biomaterials. 2007;28(23):3427–3436. doi: 10.1016/j.biomaterials.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 129.Wang X, Uto T, Akagi T, Akashi M, Baba M. Induction of potent CD8+ T-cell responses by novel biodegradable nanoparticles carrying human immunodeficiency virus type 1 gp120. J Virol. 2007;81(18):10009–10016. doi: 10.1128/JVI.00489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang X, Uto T, Akagi T, Akashi M, Baba M. Poly(gamma-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: Potential for an AIDS vaccine. J Med Virol. 2008;80(1):11–19. doi: 10.1002/jmv.21029. [DOI] [PubMed] [Google Scholar]
- 131.Stoneburner RL, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in uganda. Science. 2004;304(5671):714–718. doi: 10.1126/science.1093166. [DOI] [PubMed] [Google Scholar]
- 132.Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009;11(1):78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saidi H. Microbicides: An emerging science of HIV-1 prevention in women-15th conference on retroviruses and opportunistic infections, boston, USA, 3–6 February 2008. Rev Med Virol. 2009;19(2):69–76. doi: 10.1002/rmv.601. [DOI] [PubMed] [Google Scholar]
- 134.das Neves J, Bahia MF. Gels as vaginal drug delivery systems. Int J Pharm. 2006;318(1–2):1–14. doi: 10.1016/j.ijpharm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 135.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Ann Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 136.Rupp R, Rosenthal SL, Stanberry LR. Vivagel (spl7013 gel): A candidate dendrimer – microbicide for the prevention of HIV and hsv infection. Int J Nanomedicine. 2007;2(4):561–566. [PMC free article] [PubMed] [Google Scholar]
- 137.McCarthy TD, Karellas P, Henderson SA, et al. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharm. 2005;2(4):312–318. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
- 138.Jiang YH, Emau P, Cairns JS, et al. Spl7013 gel as a topical microbicide for prevention of vaginal transmission of sHIV89.6p in macaques. AIDS Res Hum Retroviruses. 2005;21(3):207–213. doi: 10.1089/aid.2005.21.207. [DOI] [PubMed] [Google Scholar]
- 139.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of psc-rantes for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26(3):502–511. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lederman MM, Veazey RS, Offord R, et al. Prevention of vaginal sHIV transmission in rhesus macaques through inhibition of ccr5. Science. 2004;306(5695):485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 141.Palliser D, Chowdhury D, Wang QY, et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439(7072):89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 142.Rossi JJ. Topical antiviral siRNA: A practical siRNA microbicide? Gene Ther. 2006;13(21):1493–1494. doi: 10.1038/sj.gt.3302764. [DOI] [PubMed] [Google Scholar]
- 143.Wu Y, Navarro F, Lal A, et al. Durable protection from herpes simplex virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell Host Microbe. 2009;5(1):84–94. doi: 10.1016/j.chom.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8(6):526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang LF, Chan JM, Gu FX, et al. Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano. 2008;2(8):1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang LF, Radovic-Moreno AF, Alexis F, et al. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle-aptamer bioconjugates. Chem Med Chem. 2007;2(9):1268–1271. doi: 10.1002/cmdc.200700121. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Gao SJ, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5(10):791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 148.Lee ALZ, Wang Y, Cheng HY, Pervaiz S, Yang YY. The co-delivery of paclitaxel and herceptin using cationic micellar nanoparticles. Biomaterials. 2009;30(5):919–927. doi: 10.1016/j.biomaterials.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 149.Shacklett BL. Can the new humanized mouse model give HIV research a boost. PLoS Med. 2008;5(1):e13. doi: 10.1371/journal.pmed.0050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 151.Damge C, Reis CP, Maincent P. Nanoparticle strategies for the oral delivery of insulin. Expert Opin Drug Del. 2008;5(1):45–68. doi: 10.1517/17425247.5.1.45. [DOI] [PubMed] [Google Scholar]
- 152.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Del Rev. 2009;61(2):158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tang BC, Dawson M, Lai SK, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci USA. 2009;106(46):19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xing JF, Deng LD, Li J, Dong AJ. Amphiphilic poly{[α-maleic anhydride-omega-methoxy-poly(ethylene glycol)]-co-(ethyl cyanoacrylate)} graft copolymer nanoparticles as carriers for transdermal drug delivery. Int J Nanomedicine. 2009;4:227–232. doi: 10.2147/ijn.s7814. [DOI] [PMC free article] [PubMed] [Google Scholar]