Abstract
Purpose
Novel agents are currently combined with radiation and temozolomide (RT+TMZ) in newly diagnosed glioblastoma using overall survival as the primary endpoint. Results of these phase II studies are typically compared to the phase III EORTC survival data that resulted in RT+TMZ becoming standard therapy.
Experimental Design
The New Approaches to Brain Tumor Therapy (NABTT) Consortium accrued 365 patients with glioblastoma to four single-cohort studies with similar eligibility criteria. Patients received RT+TMZ with talampanel (N=72), poly ICLC (N=97), or cilengitide (N=112) or RT+TMZ alone with monitoring of CD4 counts (n=84). Overall survival of those ages 18–70 with glioblastoma were compared to published EORTC data.
Results
NABTT and EORTC patients had comparable performance status and debulking surgery. Median, 12 month, and 24 month survival rates for the EORTC patients (N=287) and the comparable NABTT patients receiving RT+TMZ and novel agents (N=244) are 14.6 months vs 19.6 months, 61% vs 81%, and 27% vs 37%. This represents a 37% reduction in odds of death (P<0.0001) through two years of follow-up. NABTT and EORTC patients receiving only RT+TMZ had similar survival.
Conclusions
Newly diagnosed glioblastoma treated recently with RT+TMZ and talampanel, poly-ICLC, or cilengitide had significantly longer survival than similar patients treated with only RT+TMZ accrued internationally from 2000 to 2002. These differences could result from the novel agents or changing patterns of care. Until the reasons for these different survival rates are clarified, comparisons of outcomes from phase II studies with published RT+TMZ survival data should be interpreted with caution.
Background
Glioblastoma multiforme remains a devastating illness that affects over 17,000 patients in the United States each year. This tumor diffusely infiltrates brain early in its course making complete resections impossible. Post-operative radiation therapy prolongs survival but yields a median survival of less than one year and long term survival is extremely rare. (1) Although the most promising early results with adjuvant chemotherapy contained nitrosoureas, no single trial provided significant survival prolongation and meta-analyses revealed a marginal benefit.(2) In 2005, the European Organisation for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada published a randomized prospective trial comparing radiation therapy with radiation and concurrent temozolomide followed by six months of adjuvant temozolomide (RT+TMZ) in patients with glioblastoma who were age eighteen to seventy.(3) The RT+TMZ arm of the trial was superior, generating an improvement in median survival from 12.1 to 14.6 months and increasing the percent of patients alive at two years from 10% to 26%. Because this was the first study to demonstrate a chemotherapy-related survival benefit in glioblastoma, this regimen was rapidly adopted worldwide as standard therapy for patients with newly diagnosed glioblastoma. Recently updated survival data from this study reveals that 10% of patients receiving combined treatment are alive at 5 years versus 2% with radiation alone (p<0.0001).(4)
Two other agents are approved by the U.S. Food and Drug Administration (FDA) for the treatment of glioblastoma. In September 1996, implantable BCNU containing wafers (Gliadel®) were approved for selected patients with localized malignant gliomas at initial surgery based on a randomized placebo controlled trial.(5) Radiation and BCNU wafers extended the median survival of patients with glioblastoma from 11.4 months to 13.1 months (p=0.08). In May 2009, the FDA approved the use of bevacizumab for patients with recurrent glioblastoma. This approval was based on two phase II trials demonstrating that approximately 25% of patients had clinical and radiologic improvement that lasted for about four months.(6, 7) During the past two decades, the number of clinical trials conducted in patients with high grade gliomas has grown with identification of important signal transduction pathways, relevant targets, and the availability of new agents directed to these pathways and targets. In addition, the continued absence of curative therapies and the maturation of multi-institutional clinical research consortia have rendered glioblastoma an attractive candidate for novel drug development.
In 2005, following the publication of the EORTC's RT+TMZ survival data in newly diagnosed glioblastoma, it became apparent that novel therapies administered to this patient population would need to be combined with radiation and temozolomide. As a result, the New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium developed three trials with novel agents that could be safely combined with this new standard of care. The endpoint of these studies was overall survival as treatment with radiation and temozolomide can increase blood-brain barrier dysfunction leading to increased contrast enhancement, mass effect, and peritumoral edema that may last for many months. (8–11) Other radiologic techniques have been unable to reliably distinguish between tumor growth and treatment related pseudoprogression in this clinical setting. As this presents a currently insurmountable challenge to the accurate determination of tumor response and progression in newly diagnosed glioblastoma, these NABTT trials contained pre-planned comparisons of overall survival with published EORTC results. Surprisingly, the overall survival in each of the NABTT studies was prolonged as judged by this comparison. While the improvements in survival are real and striking, these sequentially positive results are in sharp contrast to decades of disappointing clinical trial outcomes in this patient population. These survival advantages could be attributable to the novel therapies or could reflect changing patterns of care in this patient population. This report reviews these findings and considers if the published EORTC survival data should be routinely used as a comparison group to estimate the efficacy of phase II studies in patients with newly diagnosed glioblastoma.
Methods
The NABTT CNS Consortium was funded by the National Cancer Institute from 1994 to 2009 to conduct early phase clinical trials of novel approaches to the treatment of primary brain tumors.(12) Prior to 2005, eligible patients with newly diagnosed glioblastoma were accrued to individual trials using radiation therapy combined with novel agents. Overall survival was the primary endpoint and the results were compared to the NABTT historical database. After the publication of results documenting improved survival with radiation and concurrent and adjuvant temozolomide in this patient population, the NABTT CNS Consortium initiated studies of novel agents in combination with radiation and temozolomide in single-cohort phase II studies. A comparison of overall survival to EORTC results was formally planned.
Three studies were approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) and the Institutional Review Board (IRB) of each participating institution. Participating institutions included University of Alabama at Birmingham, The Cleveland Clinic, Emory University, Henry Ford Hospital, Johns Hopkins University, Massachusetts General Hospital, H. Lee Moffitt Cancer Center, University of Pennsylvania, and Wake Forest University. The compounds studied were: talampanel (Teva Pharmaceutical Industries, Petach Tikva, Israel), poly ICLC (Oncovir, Washington, DC), and cilengitide (Merck KGaA, Darmstadt, Germany). Talampanel was provided Teva Pharmaceutical Industries, poly ICLC by Oncovir and cilengitide by the NCI. Teva Pharmaceutical Industries also provided additional funding for the trial.
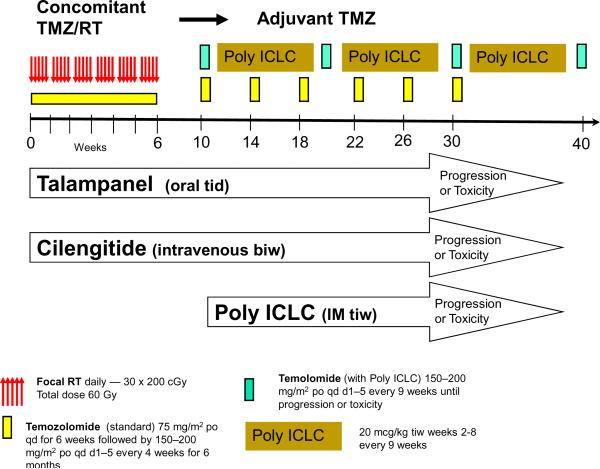
The three trials were designed to enroll similar patients to enable outcome data to eventually be combined in a “post-temozolomide” survival database for the NABTT CNS Consortium. As a result, the eligibility criteria for all trials included age ≥18 years, newly diagnosed supratentorial glioblastoma, no prior treatment with radiation or chemotherapy, stable glucocorticoid requirements, Karnofsky Performance Status (KPS) rating of ≥ 60, normal organ function (as measured by hematologic and chemistry profiles), and the ability to provide informed consent. The design of the studies was also similar. As shown in Figure 1, the basic framework as described by the EORTC for newly diagnosed glioblastoma was utilized. This included 30 fractions (200 cGy/fraction) of focal radiation therapy administered five days per week for 6 weeks to a total dose of 60 Gy. Temozolomide was administered daily beginning on the first day of radiation and ending on the last day of radiation at a dose of 75 mg/m2 per day. Blood counts were checked weekly and temozolomide was held if the platelet count fell below 100,000 or if significant leucopenia developed. Bactrim was administered prophylactically to prevent pneumocystis (jiroveci) pneumonia. After completion of the radiation therapy, the patients were given four weeks to recover and then began receiving temozolomide at doses of 150–200 mg/m2 for five consecutive days each month for a total of 6 months. Patients were followed with magnetic resonance (MR) or computed tomographic (CT) imaging one month after completion of radiation and every two months thereafter. In the talampanel and cilengitide studies the new agents were administered beginning with the initiation of radiation therapy. (13) Talampanel was administered orally every eight hours and cilengitide was given intravenously twice weekly. These agents were continued during the entire period of radiation and concurrent temozolomide, the four week treatment break, the six months of adjuvant temozolomide, and thereafter until there was evidence of progression or toxicity. The poly ICLC study differed in several respects. Although patients were accrued to this study prior to beginning radiation and temozomide, the experimental agent was not initiated until after the radiation and concurrent chemotherapy and the four weeks of recovery had been completed. At that time, adjuvant temozolomide (150–200 mg/m2 daily for 5 days) was begun but the timing and duration of the temozolomide was adjusted to prevent overlapping with the poly ICLC. Thus, it was given for 5 days every 9 weeks rather than the usual every 4 weeks. In an effort to adjust for the reduced temozolomide schedule, this agent was continued on the every 9 weeks schedule until there was evidence of progression or toxicity. The intramuscular administration of poly ICLC (20 mcg/kg) was begun 4 weeks after completion of the radiation and administered three times each week from week 2 to week 8 of each 9 week adjuvant treatment cycle. This was also continued until progression or toxicity was observed. These schedules are presented in Figure 1.
Figure 1.
Treatment strategy: EORTC and Three NABTT Trials
All three trials were designed to detect 25% or larger reduction in the hazard of death per person-year of follow-up (the hazard rate) compared to the EORTC survival data. The numbers of subjects specified in each trial were 72 (talampanel), 94 (cilengitide), and 96 (Poly ICLC) with the required number of death events 49, 63, and 64, respectively to achieve 85% or greater power at a significant level of 0.1 using a one sided test.(13) The NABTT trial designs are conservative in that additional follow-up yields a larger than designed number of events, increasing the precision in the estimate of the hazard rate and increasing the efficiency of comparisons. The survival time was counted from the initial diagnosis of the disease to the time of death. The EORTC trial excluded patients who were over the age of 70, so all NABTT patients ages 70 or younger were included in this comparison. The overall survival for the 244 NABTT patients ages 70 and younger was compared to the 287 patients on the EORTC study, and to the 217 patients with comparable ages in the NABTT pre-temozolomide historical database. The effect of prognostic variables in each NABTT trial was assessed using proportional hazards regression. (14) Cochran-Mantel-Haenszel statistics were used to assess the relative risk of death between the NABTT temozolomide containing trials and the EORTC trial at 6, 12, 18, and 24 months of follow-up. All p-values are reported as two-sided. All analyses were performed using the SAS software (version 9.1, SAS institute, Cary, NC).
In addition, a fourth NABTT study accrued 99 patients with newly diagnosed high grade gliomas who were to receive standard radiation and temozolomide. This protocol was designed to follow monthly CD4 counts for 12 months from the start of their radiation and concurrent temozolomide. Patients were accrued from the participating NABTT institutions during the same time as the talampanel, poly-ICLC, and cilengitide trials. Patients on those trials were permitted to enroll on the CD4 study as well.
Results
This analysis was performed using a total of 797 patients with newly diagnosed glioblastoma who were 70 years of age or younger. The NABTT pre-temozolomide historical database contained 217 patients who received radiation plus a novel agent which has subsequently been found to be ineffective. The EORTC study contained 287 patients treated with radiation and temozolomide. The three therapeutic NABTT trials contained a total of 244 patients treated with radiation and temozolomide combined with talampanel, cilengitide, or poly ICLC. In addition, there were 49 patients on the non-therapeutic NABTT study who had a diagnosis of glioblastoma and who received only radiation and temozolomide. At the time of analysis, the NABTT historical database contained 213 death events. The number of events in the talampanel, cilengitide, poly ICLC, and CD4 trials was 47, 62, 62 and 44 respectively.
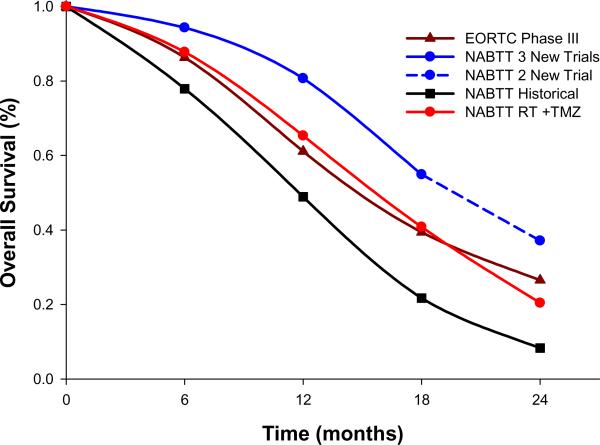
There are no differences in important prognostic variables in the four patient cohorts (Table 1). The recent NABTT trials had proportionately fewer patients undergoing a debulking surgical procedure and more patients with lower performance status than patients in the EORTC trial. The NABTT pretemozolomide historical cohort has a median survival of 12 months with a two year survival of 8% (Table 2 and Figure 2). The EORTC trial reported a median survival of 14.6 months and a 26.5% two year survival (Table 2 and Figure 2). Combined survival data from the three recent therapeutic NABTT studies demonstrates a statistically significant improved median survival compared to the EORTC trial from 14.6 months (95% CI: 13.2–16.8) to 19.6 months (95% CI: 17.8–21.2). In addition, these NABTT studies generated higher overall survival at 6, 12, 18, and 24 months compared to the EORTC trial (p=0.003, p=0.0001, p=0.0003, and p=0.02 respectively). Of note, the 24 month comparison includes data from only two of the NABTT trials; the remaining trial contains mature data to 18 months (Figure 2 dotted line). The estimated common risk ratio was 0.63 (95% CI: 0.51 to 0.78, p<0.0001 by Cochran-Mantel Haenszel statistics) which yields an overall 37% reduction in the risk of death for patients on recent trials compared to those in the TMZ+RT alone trial.
Table 1.
Patient demographics and clinical characteristics for all patients ≤70 years of age at baseline
| Characteristics | NABTT Historical: RT +non-TMZ drug(N=217) | EORTC Phase III: RT +TMZ (N=287) | NABTT RT + TMZ: CD4 (N=49) | NABTT RT + TMZ + new agent(N=244) |
|---|---|---|---|---|
| No. of Patients (%) | No. of Patients (%) | No. of Patients (%) | No. of Patients (%) | |
| Age, years | ||||
| Median (Range) | 54 (21–70) | 56 (19–70) | 58 (29–69) | 55 (21–70) |
| 50–70 | 148 (68%) | 197 (69%) | 38 (78%) | 162 (66%) |
| Sex | ||||
| Male | 140 (65%) | 185 (64%) | 28 (57%) | 144 (59%) |
| KPS** | ||||
| 90–100 | 129 (70%) | 249 (86%) | 39 (80%) | 180(77%) |
| 60–80 | 50 (30%) | 38 (13%) | 10 (20%) | 64 (26%) |
| Extent of surgery | ||||
| Biopsy | 26 (12%) | 48 (17%) | 9 (18%) | 55 (23%) |
| Debulking | 143 (66%) | 239 (83%) | 40 (82%) | 186 (76%) |
| Other or Missing* | 48* (22%) | 3 (1%) | ||
| Weeks from diagnosis to treatment | ||||
| Median (Range) | 3.9 (1.0–9.7) | 5 (1.7–10.7) | 3.1 (2–7.7) | 4.1 (1.9–12.7) |
| MMSE score | ||||
| 27–30 | 138 (64%) | 196 (68%) | 117 (84%) | |
| ≤ 26 | 29 (13%) | 81 (28%) | 39 (16%) | |
| Missing | 50 (23%) | 10 (3%) | ||
| Corticosteroids | ||||
| Yes | 147 (68%) | 193 (67%) | 42 (86%) | 179 (73%) |
| Histological diagnosis | ||||
| Glioblastoma | 165 (98%) | 221 (92%) | 49 (100%) | 239 (98%) |
Table 2.
Survival Comparison
| Patient #'s | Median Survival (months) Median (95%CI) | Overall Survival at 6 months % (95%CI) | Overall Survival at 12 months % (95%CI) | Overall Survival at 18 months % (95%CI) | Overall Survival at 24 months % (95%CI) | |
|---|---|---|---|---|---|---|
| NABTT Historical RT+non-TMZ drug | 217 | 12 (10.3–12.7) | 78 (72–83) | 49 (42–56) | 22 (16–28) | 8 (5–13) |
| EORTC RT+TMZ | 287 | 14.6 (13.2–16.8)* | 86 (82–90)* | 61 (55–67)* | 39 (34–45)* | 27 (21–32)* |
| NABTT RT+TMZ | 49 | 16.2 (12.9–18.9) | 88 (75–95) | 65 (50–78) | 41 (27–56) | 20 (10–34) |
| NABTT RT+TMZ+new agent | 244 | 19.6 (17.8–21.2)* | 94 (91–97)* *P = 0.003 | 81 (75–86)* *P = 0.0001 | 55 (48–61)* *P=0.0003 | 37 (29–46)*+ * P = 0.02 |
As only two trials of the three NABTT trials have mature 24 months survival data so this point contains 143 patients
Figure 2.
Survival data on patients with glioblastoma ≤70 years of age from the NABTT institutions pretemozolomide, NABTT institutions post-temozolomide, EORTC/NCIC institutions post-temozolomide, the NABTT institutions combining radiation, temozolomide, and novel agents
Forty-nine of the 99 patients on the CD4 count trial were between 18 and 70 years of age, had a histological diagnosis of glioblastoma, and did not receive any experimental agents with their standard radiation and temozolomide. These patients were accrued and treated at NABTT institutions. To the date, forty-four of the 49 patients died. There was no statistical significant difference in the baseline patient characteristics and between the patients treated on the therapeutic NABTT trials that included radiation, temozolomide, and an experimental agent and those who received only radiation and temozolomide with serial CD4 measurements with the exception of a shorter time between diagnosis and initiation of treatment in the CD4 patients (median of 3.1 weeks vs. 4.1 weeks, p=0.0001) (Table 1). The unadjusted hazard ratio of death between the NABTT therapeutic trials and the patients on the non-therapeutic CD4 study was 0.64 (95% CI: 0.45–0.89, p=0.008) with the median time of survival 19.6 months (95% CI: 17.8–21.2) and 16.2 months (95% CI:12.9–18.9), respectively. There is a 34.4% reduction in hazard of death (hazard ratio=0.656, 95% CI: 0.46–0.94, p=0.02) after adjusting for age at diagnosis, Karnofsky performance status, extent of surgery, and the time elapsed from diagnosis to the initiation of treatment.
Conclusions
Overall survival of 244 patients with newly diagnosed glioblastoma accrued to three consecutive therapeutic trials conducted by the NABTT CNS Consortium is significantly higher than is reported with radiation and temozolomide alone.(3) The improvement in survival was evident as early as 6 months after study initiation (94% vs 86% survival, p <0.003) and continued over two years (37%vs 27%, p < 0.02). In each of these trials a novel agent was combined with standard radiation and temozolomide. The experimental agents had diverse mechanisms of action and included a glutamate receptor blocker, an immunostimulatory compound, and an integrin inhibitor. Each NABTT study was designed as a single arm phase II study and used overall survival as the primary efficacy outcome measure. The planned comparison was with published EORTC survival data where patients received only radiation and temozolomide.
During the past three decades, thousands of patients have been accrued to clinical trials examining the efficacy of systemically administered adjuvant chemotherapy in this disease.(15, 16) Only one of these trials, the EORTC study using radiation and temozolomide, provided a convincing survival advantage.(3, 4) As a result, the improvement in survival noted in the first three NABTT glioblastoma trials where standard radiation and temozolomide was combined with novel agents was unanticipated and must be thoughtfully considered. One possible explanation is that baseline prognostic factors for patients on the NABTT studies were more favorable than in the EORTC study. However, the baseline characteristics of patients ages 70 or younger in all cohorts were remarkably similar. In addition, the percent of patients with MGMT promoter methylation was substantially lower in the NABTT talampanel trial than in the EORTC study (27% vs 43%), suggesting that this was in fact a higher risk population.(13)
Another potential explanation for these results is that radiation and temozolomide provide a treatment platform that permits improved survival with a variety of novel agents that may not be efficacious as single agents. Other studies that have combined new agents with radiation and temozolomide in this disease have reported varied results. For example, single institution studies combining radiation and temozolomide with erlotinib report improved survival while the addition of cisretinoic acid or thalidomide yielded results more consistent with the EORTC findings.(17, 18)
It is also possible that survival following treatment with standard radiation and temozolomide has improved since 2000 when the original study was initiated. The EORTC study was conducted in 85 hospitals in Europe and Canada when experience with temozolomide was limited. Since then clinicians have recognized that early clinical and radiologic deterioration is often treatment-related (“pseudo-progression”) rather than secondary to true tumor progression. As a result, temozolomide therapy is often continued in this setting rather than aborted early. Clinicians have also become more adept and aggressive at recognizing treatment complications and treating tumor recurrences. Although bevacizumab is associated with radiologic and clinical improvements, it has not yet been shown to prolong survival. (6, 7, 16, 19) In the NABTT talampanel study bevacizumab did not appear to contribute to 42% of patients being alive at two years.(13) The results of the NABTT CD4 trial suggest that therapy with radiation and temozolomide alone administered at the same institutions, during the same time calendar years, and with similar treatments available for recurrent disease generates survival results that are very similar to the EORTC data and are inferior to survival when radiation and temozolomide are combined with talampanel, cilengitide or poly-ICLC.
Although the findings described above are provocative, the available data do not permit us to determine if the improvement in survival in the NABTT studies is secondary to the new agents or to an overall change in the care of patients with newly diagnosed glioblastoma. The control arms of several currently accruing large randomized trials exploring dose intense temozolomide and the early addition of bevacizumab will provide important information to address this issue. A randomized comparison group might have helped to clarify some of the ambiguity in the present circumstance and suggestions have been made that these should be included in every safety and activity (phase II) trial. Aside from the lack of precision in such underpowered comparisons, this would generate other significant inefficiencies. In the past 30 years, there has been only one systemic adjuvant chemotherapy trial has been positive in patients with glioblastoma. There are now more novel agents with different mechanisms of action to test than ever before. Adding an internal control arm would significantly increase the size of all safety and activity trials and would result in fewer agents being tested. In addition, it would likely make these trials much less acceptable to patients who are not anxious to be randomized to a placebo control. Alternative endpoints that might speed decision making have also been proposed. However, accurately determining response and progression in newly diagnosed glioblastomas are exceedingly difficult as standard therapy is known to perturb the integrity of the blood-brain barrier and thus the results of neuroimaging studies.
This report documents significant improvements in the survival of patients with newly diagnosed glioblastoma. As evident from Table 2, the two year survival of patients placed on NABTT CNS Consortium trials was 8% prior to 2005 and now approaches 40% in 244 patients treated at the same participating institutions. However, it remains uncertain if these results are attributable to the novel therapies themselves or reflect evolving patterns of care in this patient population. Until this important issue is clarified, caution should be used in comparing the EORTC survival data to results of phase II studies in newly diagnosed glioblastoma. In the meantime, the priority for clinical investigations in this devastating disease must be to efficiently screen as many novel agents with diverse mechanisms of action as possible for early evidence of activity in an effort to build on the recent progress that has been made in the treatment of this disease.
Statement of Translational Relevance.
For decades, adjuvant chemotherapy provided no significant survival improvement in newly diagnosed glioblastoma. However, in 2005 the EORTC/NCIC documented improved survival from radiation and temozolomide (RT+TMZ) versus radiation alone.3,4 These survival results are now the standard by which single arm phase II trials of novel agents combined RT+TMZ are judged. We now report survival from three phase II studies of the NABTT CNS Consortium where RT+TMZ and a novel agent were given to newly diagnosed glioblastoma. Surprisingly, the survival of patients on each study was significantly better than reported by EORTC/NCIC. Given the paucity of active agents in glioblastoma, finding three consecutive “positive” studies suggests that adding novel agents to RT+TMZ improves the efficacy of multiple novel agents or the EORTC/NCIC study is an appropriate comparison group. These issues are central to the conduct and evaluation of all phase II trials of novel agents in glioblastoma.
Acknowledgments
This project was funded by the NIH CA62475 and NIH CA137443
Footnotes
None of the authors has any potential financial conflict of interest.
References
- 1.Laperriere N, Zuraw L, Cairncross G, Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology Disease Site Group Radiotherapy for newly diagnosed malignant glioma in adults: A systematic review. Radiother Oncol. 2002;64:259–73. doi: 10.1016/s0167-8140(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 2.Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–8. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 7.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5:610–20. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 8.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–61. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 9.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009 doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- 10.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–7. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 11.Roldan GB, Scott JN, McIntyre JB, et al. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci. 2009;36:617–22. doi: 10.1017/s0317167100008131. [DOI] [PubMed] [Google Scholar]
- 12.Grossman SA, Fisher JD, Piantadosi S, Brem H. The new approaches to brain tumor therapy (NABTT) CNS consortium: Organization, objectives, and activities. Cancer Control. 1998;5:107–14. doi: 10.1177/107327489800500201. [DOI] [PubMed] [Google Scholar]
- 13.Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: A multicenter phase II trial. J Clin Oncol. 2009;27:4155–61. doi: 10.1200/JCO.2008.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox DR, Oakes D. Analysis of survival data. Chapman & Hall; New York: 1984. [Google Scholar]
- 15.Grossman SA. Arguments against the routine use of currently available adjuvant chemotherapy in high-grade gliomas. Semin Oncol. 2003;30:19–22. doi: 10.1053/j.seminoncol.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 17.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–84. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesari S, Schiff D, Henson JW, et al. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. Neuro Oncol. 2008;10:300–8. doi: 10.1215/15228517-2008-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–6. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]