Abstract
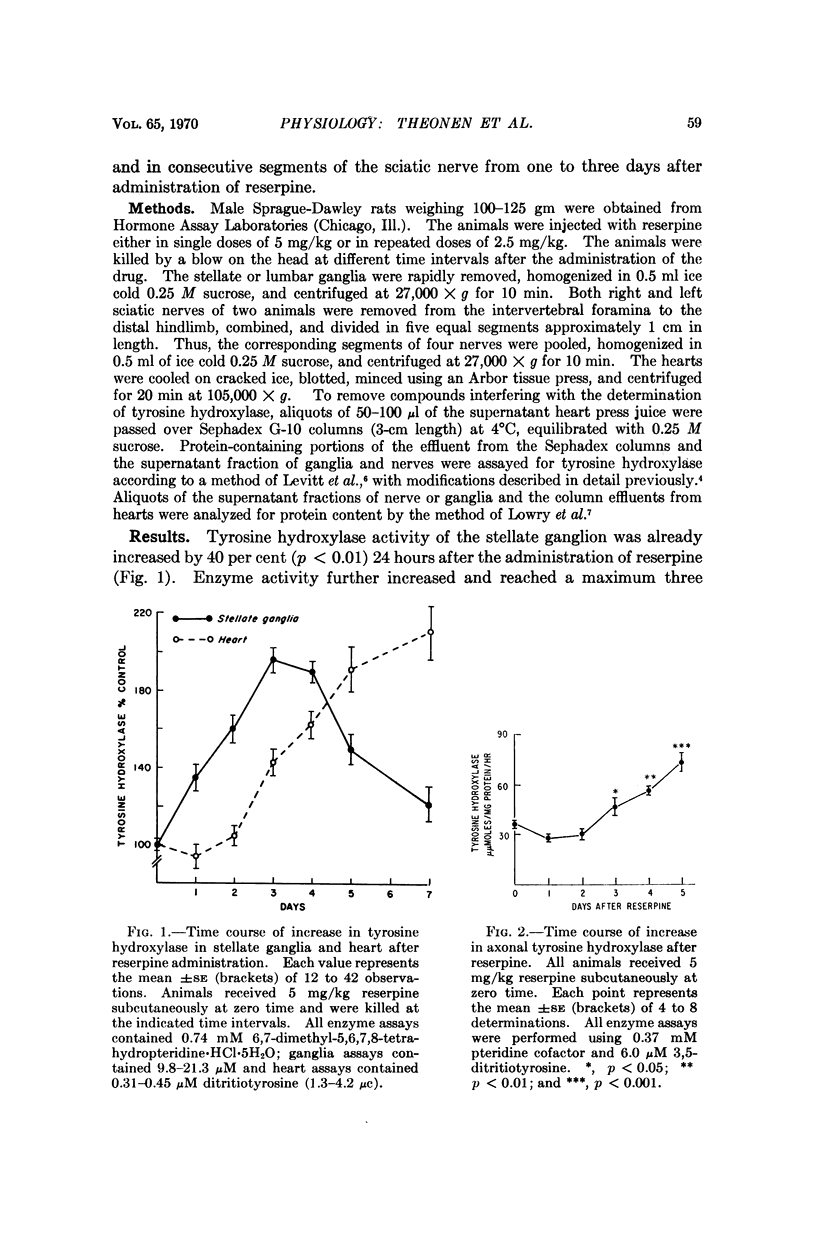
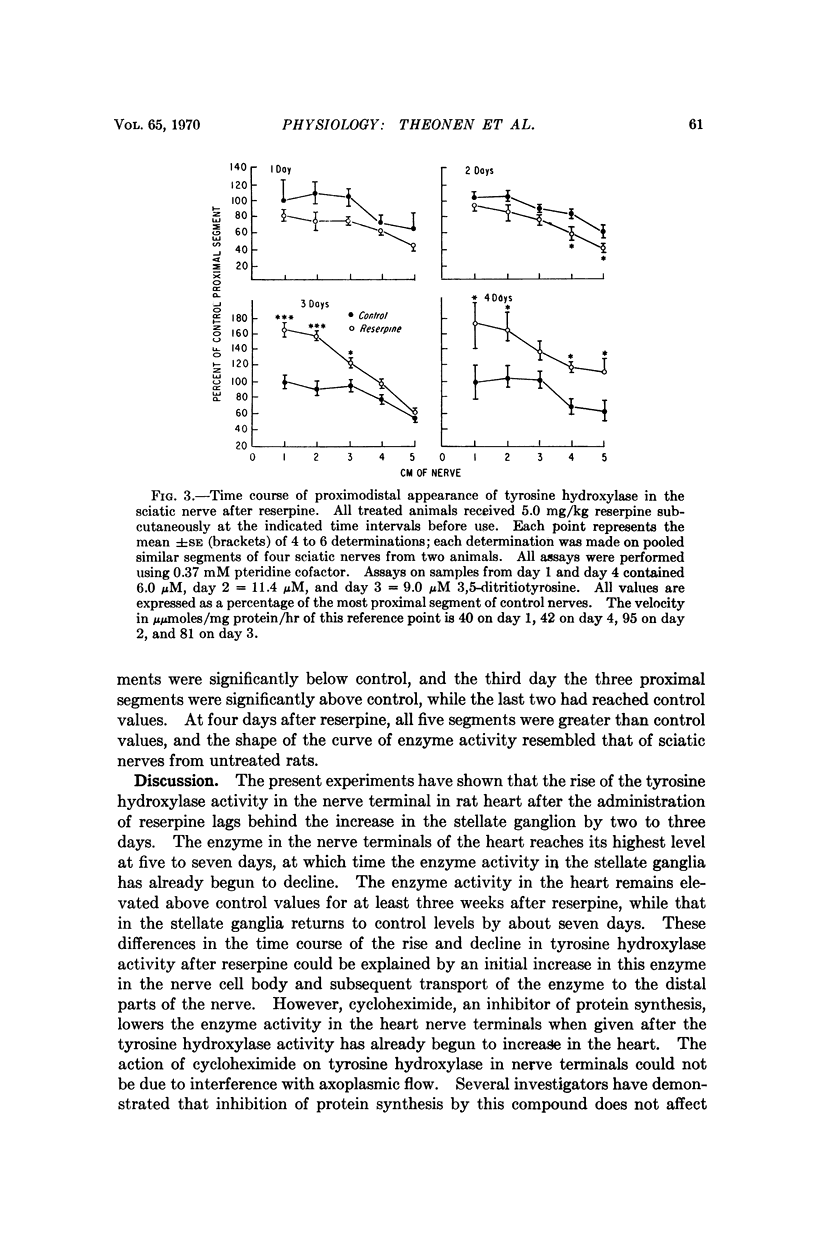
The induction of tyrosine hydroxylase in the nerve terminals of the rat heart by reserpine lags behind that in the stellate ganglion by two to three days. Cycloheximide given three days after reserpine blocks the further rise of the enzyme in the nerve terminals. The increase in tyrosine hydroxylase activity of the lumbar ganglion is as marked as that in the stellate ganglion. The increase of enzyme activity in the sciatic nerve after reserpine administration resembles that found in the heart nerve terminals. Determination of enzyme activity in segments of sciatic nerves indicates a two-day lag and then a proximal-distal transport of enzyme, but the apparent rate is not sufficient to account for the increase in enzyme in the nerve terminals. These findings are compatible with the local synthesis of induced tyrosine hydroxylase in the nerve terminals rather than the peripheral movement of the completed enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H. Further studies of the transport of protein to nerve endings. J Neurochem. 1968 Apr;15(4):343–350. doi: 10.1111/j.1471-4159.1968.tb11619.x. [DOI] [PubMed] [Google Scholar]
- Dahlström A., Häggendal J. Studies on the transport and life-span of amine storage granules in a peripheral adrenergic neuron system. Acta Physiol Scand. 1966 Jul-Aug;67(3):278–288. doi: 10.1111/j.1748-1716.1966.tb03313.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laduron P., Belpaire F. Evidence for an extragranular localization of tyrosine hydroxylase. Nature. 1968 Mar 23;217(5134):1155–1156. doi: 10.1038/2171155a0. [DOI] [PubMed] [Google Scholar]
- Levitt M., Gibb J. W., Daly J. W., Lipton M., Udenfriend S. A new class of tyrosine hydroxylase inhibitors and a simple assay of inhibition in vivo. Biochem Pharmacol. 1967 Jul 7;16(7):1313–1321. doi: 10.1016/0006-2952(67)90162-1. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Geffen L. B., Austin L. Axoplasmic transport of 14C-noradrenaline and protein in splenic nerves. Nature. 1968 Jan 20;217(5125):278–279. doi: 10.1038/217278a0. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Geffen L. B., Rush R. A. Immunohistochemical evidence for the transport of dopamine-B-hydroxylase and a catecholamine binding protein in sympathetic nerves. Biochem Pharmacol. 1969 Apr;18(4):923–924. doi: 10.1016/0006-2952(69)90063-x. [DOI] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Adrenal tyrosine hydroxylase: compensatory increase in activity after chemical sympathectomy. Science. 1969 Jan 31;163(3866):468–469. doi: 10.1126/science.163.3866.468. [DOI] [PubMed] [Google Scholar]
- Peterson R. P., Hurwitz R. M., Lindsay R. Migration of axonal protein: absence of a protein concentration gradient and effect of inhibition of protein synthesis. Exp Brain Res. 1967;4(2):138–145. doi: 10.1007/BF00240359. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Mueller R. A., Axelrod J. Increased tyrosine hydroxylase activity after drug-induced alteration of sympathetic transmission. Nature. 1969 Mar 29;221(5187):1264–1264. doi: 10.1038/2211264a0. [DOI] [PubMed] [Google Scholar]



