“(1) Evolution is a passage from the most to the least organized; that is to say, from the lowest, well-organized centers, up to the highest, least-organized centers; putting this otherwise, the progress is from centers comparatively well organized at birth up to those, the highest centers, which are continually organizing through life. (2) Evolution is a passage from the most simple to the most complex; again from the lowest to the highest centers…[these] being at the same time most complex and least organized. (3) Evolution is a passage from the most automatic to the most voluntary.”
John Hughlings Jackson, ‘Evolution and dissolution of the nervous system’,
Croonian Lectures, Royal College of Physicians, March 1884.
Introduction
The words of Hughlings Jackson in 1884 were inspired by a consideration of what was then the recent work of Darwin on evolution. In the quoted article he applied this perspective to CNS evolution, development and subsequent neurological damage. His overall framework likely remains relevant today. Our paper’s goal is to review current perspectives on motor modularity and its mechanisms, especially at the spinal level (for Jackson ‘well-organized’, ‘simpler’ and ‘more automatic’), but also beyond. Modularity can be defined as the use of designs employing standardized components or units, allowing easy assembly, repair and flexible arrangements of the components. The simplest modules in a system, from which other larger modules might be made, can be termed primitives. This term derives from a combination of the biological definition of primitive as ‘occurring in or characteristic of an early stage of development or evolution’, the definition of primitive as an element assumed as a basis, and the computer science definition of ‘a basic or fundamental unit of machine instruction’. Like Jackson, we will argue that these modules and primitives are in significant part already organized at birth. At the end of this review, we discuss how these issues in spinal modularity and protective reflex structure may relate to trunk control and low back pain mechanisms in humans.
The degrees of freedom problem
Modularity is observed in motor control across a range of tasks, in many contexts and in most species (see Giszter, 2008). However, its origins and basis in the motor system remain controversial. There are two competing perspectives on modularity (see below), but both arise from a consideration of the degrees of freedom (DOF) problem in motor control. The DOF problem was first succinctly described by Bernstein (1967), and remains relevant in modern perspectives (Wolpert et al., 2001; Ting 2007; Todorov et al., 2005). The problem arises from an embarrassment of riches in the motor apparatus. For example, for arm reaching movements there are more degrees of freedom in the limb joint space than are needed to define the hand effector position and orientation in space, and there are more muscles operating about these joints than in principle needed to actuate the joints. This redundancy of joints and musculature necessarily causes ill-posed, or non-unique mappings and transformations to arise in motor control. These define ‘null-spaces’, as recognized and formally analyzed in robotics literature (Asada and Slotine,1986). Within the null-space there are multiple limb configurations and muscle activations that are equivalent, and solutions are non-unique. This poses problems for choosing how to move. Redundancy problems in the motor system extend beyond these arising from the limb design, and arise in equal measure within the CNS. The numbers of ways of activating, controlling and altering the patterned behavior of the limb and redundant muscles, through the interneuronal networks and the limb feedback systems are equally complex in the motor systems of mammals. Spinal circuitry presents a dazzling array of options: descending controls can act directly on muscles’ motor pools, or on the sensitivity of muscle spindles through the gamma motoneuron system, or can act on a number of complex networks of interneuronal feedback and outflow systems, e.g., the well-identified Ia, Ib, Renshaw and FRA systems. This span of options combined with distributed network organization presents numerous control difficulties (see Abbott, 2006 for examples of this issue), as well as possibilities (see Todorov et al. 2005). The complexity and richness available in motor control means there are likely to be many more actions available to an animal, and many more ways of potentially executing these actions, than the actions needed. These options may greatly exceed the total of those that will be actively used by the animal through its lifetime. This richness allows the ‘motor equivalence’ of Bernstein - the execution of the same action in numerous ways with different degrees of freedom engaged. The richness also potentially allows invention of novel motor actions, and extension of action in novel ways. This immense flexibility and its utilization is a large part of what we value in human motor control, e.g., in skilled sports, and the performing arts. However, any individual animal is unable to explore the full richness of these possibilities. Indeed for many animals the problem of selection and optimization of action in this vast search space may present acute and serious issues for its survival.
The tension between the acute need to act quickly when faced with a predator, or a prey opportunity, and the ability to act flexibly is at the heart of the alternative perspectives on modularity.
Perspectives on modularity: flexibility versus rapid response
Modularity is generally proposed to be a means for managing the redundancy and complexity described above (e.g. Callebaut and Raaskin-Gutman, 2005; Ting 2007; Giszter et al. 2007). Modularity can solve the combined problem of allowing the rapid deployment of actions in real-time and the flexible development of new actions on longer time-scales that are needed to extend a repertoire. The idea is that a modular and extensible basis set, or collection of ‘primitives’, can provide a library for the composition of movements and support optimal control and/or simpler control heuristics. Primitives can be rapidly deployed, and may simplify aspects of control if well chosen. The two competing perspectives on modularity each provide different accounts of the ontogeny of these modular basis sets and primitives, and present differing perspectives on how labile the bases and primitives are.
One perspective suggests that a set of primitives might be built into the motor system a priori by evolution, and these are available at birth as suggested in the Jackson quote. Such primitives have co-evolved with, and are matched to, the motor apparatus and matched to the actions commonly needed by every individual of a species in order to survive and thrive. An intuitive support for this perspective can be argued from a Darwinian point of view. Although some animals such as humans and other primates, and various carnivores provide the nurturing necessary for extensive trial and error practice and motor ‘play’, this is a relatively rare luxury. There is an evolutionary need for extremely rapid organization of effective action comparatively early in development in many species such as the turtle, or wildebeest, or chick (Bradley et al. 2005, 2008; and see Bradley 2003). The sea turtle must rapidly locomote immediately after hatching and leave the beach on which its egg incubated or quickly become food for a variety of predators. Built-in modules and pattern generation mechanisms provide a motor infrastructure to support this rapid development (Grillner et al., 1976; Fentress 1973).
The second competing perspective focuses strongly on plasticity and learning. It suggests that the motor system is organized to discover optimal feedback controllers and the matching optimal basis sets for motor tasks (Loeb et al. 1989, 1990; Todorov, 2004). Movement is organized flexibly via an optimal control procedure of some kind, and any resemblance of modules across tasks simply represents the constraints implicit in the chosen biomechanics and task, and not any built-in neural constraints, or the re-use of fixed modules (though re-use of learned solution sets is not excluded). An intuitive support for this perspective arises from the great flexibility of adult humans and of many mammalian behaviors, and the success of optimality principles and approaches in motor control on many levels (see Todorov, 2004).
The two positions can clearly be reconciled in a hybrid framework like that posited by Jackson: Low-level primitives built in by evolution were historically crucial to survival and even in more complex animals they likely remain very useful. They can seed early motor development and provide a rapidly deployed set of basic actions appropriate to survival and motor behaviors like locomotion that are common across all individuals. However, in complex animals such as man, they are subsequently subsumed, suppressed or modified in support of more individualized and novel elaborations of skilled actions, and likely novel basis sets for these skills are constructed de novo (e.g., see Todorov et al. 2005). We will here review the data supporting both perspectives and thus this hybrid framework.
Types of modules
Modularity has been defined in various ways, as functional actions, or as kinematic units within actions (i.e., neglecting movement execution details), or as kinetic execution elements. All definitions provide compositional bases and bootstraps for motor construction, albeit on different time-scales, and these compositions may be nested. These can also be analyzed in the Marr framework as representing modularity at levels of Task, Algorithm or Implementation (Marr 1983). Modules for movement could simply represent ‘task-oriented’ controls that are organized in motor patterns (i.e. learned), or could represent more direct ‘implementation’ features, represented a priori in the biomechanics, anatomical design and the structure and connectivity of the motor circuitry (i.e. built-in).
Modularity of actions: functional units in ethology and physiology
Both physiologists (Sherrington 1961) and ethologists (Hinde, 1970 ) have striven to identify units of functional action. Both the reflex, as conceptualized by Sherrington, the pattern generator as conceptualized by Brown, Wilson and Grillner, (see Marder and Bucher, 2001) and the fixed action pattern of the ethologists represent modular functional units within the larger behavior of the organism. These modules are situated, ecologically relevant and compositional. For ethologists, behavior is frequently described as pre-specified structures, organized through evolution. Such pre-specification combined with some ontogenetic plasticity can anticipate the specific needs and problems encountered in ontogeny, and through the lifecycle. Reflexes are similar: they combine pre-specification with some ontogenetic flexibility (Sherrington 1961, Schouenborg 2004, Wolpaw 2007). ‘Primitive reflexes’ continue to exist in mammals, including man, e.g. routing reflexes for seeking the nipple in the infant (e.g., see Zafeirou, 2004). The approaches of spinal physiologists and ethologists can thus in principle meet at the level of motor execution, and in some instances they have (e.g., Fentress, 1974). However, the complexity of the motor apparatus and the issue of motor equivalence raised by Bernstein (1967) have generally frustrated efforts aimed at a simple completion of this type of programme of strong reductionism at the level of action (e.g. see Hinde, 1970).
Kinematic modularity: strokes and limit cycles
Strokes
A range of unitary kinematic characteristics have been identified in primate and other limb movements, leading to the idea of kinematic primitives. Kinematic strokes (or ‘kinematic primitives’) are kinematic patterns identified in trajectory patterns. These were first introduced as a basis for the analysis and decomposition of multiple and figured movements (Viviani and Terzuolo, 1982). The trajectory of the hand or limb tip in reaching movements tend to be relatively straight and smooth, conforming to a minimum jerk principle (Hogan 1984; Flash and Hogan, 1985) and also several other optimality formulations (e.g., see Todorov 2004; Todorov and Jordan, 2002). Deriving from these observations, an account of movement construction as a series of kinematic strokes has been proposed. Several laboratories have developed careful decompositions of limb motions in terms of such individual trajectory strokes (e.g., Figure 1). For detailed accounts see Flash and Hogan, 1985; Burdet and Milner, 1998; Sanger, 2000; Rohrer et al. 2002, 2004; Sosnik et al., 2004. These provide a strong descriptive account of the planning of motion and trajectory structure. These trajectory organization principles hold across legged vertebrates. Similar characteristics in isochronous trajectories have been shown to occur in the cat (Martin et al., 1995) and frog (Kargo and Giszter, 2000a,b). In a still more comparative approach, Flash has shown that some features of such kinematic primitives may be broadly conserved across phyla, occurring in cephalopods possessing neither an endo- nor an exoskeleton (e.g., see Sumbre et al. 2006). The octopus tentacle tip motions show kinematic similarities to those seen in the jointed limb motions. Do these common principles imply mechanistic similarities?
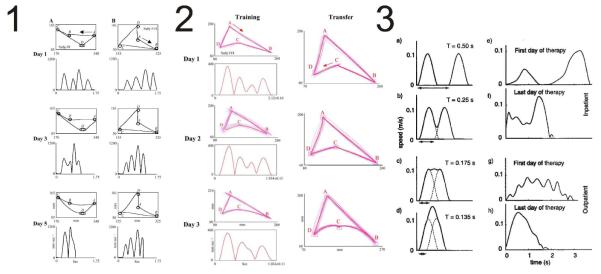
Figure 1.
Kinematic strokes or primitives in man. Panel 1. Elegant skilled movement is expected to arise from a coarticulation of unitary kinematic primitives and depend on the hand motion task. Coarticulation occurs in A in a quadrilateral with an obtuse concave angle (A) and strokes fuse, but this does not occur in one with a more acute angle (b) Panel 2. Learned coarticulation skill is shown to transfer among similar tasks. (both from Sosnik et al. 2004). Panel 3. In victims of stroke, fusion of individual motions and increasing smoothness is observed through rehabilitation (from Rohrer et al. 2002).
Equilibrium models and kinematic planning
It is known that the execution of kinematic strokes requires more complex and less unitary kinetics and muscle patterns. This is true even for single joint motions, and especially for full upper limb motions (Gottlieb 1998; D’Avella et al 2006, 2008). Efforts to unify kinematic modules directly with their execution have been attempted. How do the necessary supporting muscle patterns emerge for a unitary stroke or kinematic primitive? Is a unitary scheme possible? The strongest ideas that offered a possible solution to this control were notions of equilibrium point transitions (Feldman 2009; Feldman and Levin, 2009; Polit and Bizzi, 1979). The idea that posture and movement could both be achieved under a common and uniform execution scheme was very attractive. The notion was that smooth equilibrium transitions in an impedance controlled limb could account for trajectory dynamics, and kinematic stroke organization. Simple planning of motion and external mechanical interactions as series of equilibrium transitions was then possible. However, the generality of these frameworks has since lost significant credibility as a result of the lack of motion equifinality in coriolos fields (Lackner and Dizio, 1994, 2005, 2009; Dizio and Lackner, 1995 ), and cumulative results obtained in the adaptation paradigm first initiated by Shadmehr and Mussa-Ivaldi (1994). Any explicit representations of equilibrium trajectory, postures or strokes per se, have also now been questioned on computational grounds (e.g., see Todorov 2004). Further, it has been shown that there are anticipatory switches in control that occur in the transitions from free-limb motion to contact tasks, well in advance of contact. These occur both in the more automatic feline locomotion (Gorassini et al. 1994; ) and in the more skilled voluntary hand and finger behaviors of man (Venkadesan and Valero-Cuevas, 2008). Thus the unitary account posited by the equilibrium framework for controlling both kinematics and contact properties in a simple fashion is under question, though the importance of impedance control as advanced by Hogan and colleagues is not in doubt.
Acceleration fields and limit cycles
A bipartite scheme of modular kinematics, and subsequent execution and interaction planning, remains interesting, and has been used in robotics. In this scheme, kinematic planning is separated from an execution layer. Explicit trajectory plans are not formulated. Instead, acceleration fields are defined, from which motion emerges. The modular acceleration field framework captures features of both flexible motion planning and imitation learning in a general way (Schaal et al., 2003; Ijspeert et al., 2003). The modules include (1) discrete point-to-point kinematic motion primitives, with static attractor fields (similar to unitary strokes and equilibria described above), and (2) limit-cycle motion primitives. These can be used separately or in combination. Execution emerges from the combinations of these used together with a lower-level kinetic control layer comprised of a PID (proportional, integral, derivative) or other limb/body controller. This hierarchy of rhythmic and discrete kinematic planning over an execution layer has features in common with the hierarchical physiological perspective of MCrea and Rybak (2007). The rhythmic kinematic primitives in the Schaal-Ijspeert framework form compact dynamical representations of multi-joint tasks and/ or planning kinematics that guarantee task behaviors and kinematic stability. They can be used to support flexible imitation learning. The potentially close relations of acceleration fields and the spinal force-field primitives frameworks (described below) has not been carefully explored to date.
Returning to a neuroethological perspective, it should be noted that most functional behaviors involve both free kinematics and interaction phases in which force is exerted on the ground or an object. Terrestrial locomotion, food acquisition, biting, chewing and many other activities of importance to animals involve discrete or rhythmic transitions among phases of this type. Equilibriium point control attempted a unitary description of these phases. Kinematic schemes alone deal with the kinematics but do not specify the forces needed. A plausible modular basis for action construction should readily span both kinematic motion tasks, force application tasks and hybrid tasks. Execution modularity approaches explicitly attempt to do this.
Execution modularity: synergies and primitives
A modular basis that can be used seamlessly to control both kinetic and kinematic tasks, and manage environmental interactions may be ideal. Limb force-fields, and motor or muscle synergies have been proposed as such compositional bases. Modularity based on combining muscle activations and their effects in groups as primitives or synergies would be classified in a Marr scheme of task decomposition as operating at either the algorithmic or the implementation level. Muscle and force-based modular compositions are appealing because, in the limit, the basis of arbitrary force and motion generation must be the fractionated activation of individual muscles and their control of resultant joint torques and properties. Coalescing and constraining these properties into functionally useful groupings is likely to be a computationally natural and parsimonious approach to the degrees of freedom problem.
Force/torque field patterns
Under isometric conditions limb force-fields can be measured following spinal cord stimulation or attempted spinal behaviors. Acceleration fields have been proposed as a basis by Schaal and Ijspeert as described above. Muscle-generated torque-fields, and interaction force-fields combine with the passive viscoeleastic properties of the limb and its inertia to determine the limb acceleration. A force-field modularity and the associated muscle synergy modularity has gradually gained experimental support from a range of experiments (Bizzi et al. 1991; Giszter et al. 1993; Tresch and Bizzi, 1999; Kargo and Giszter, 2000,2008; Kargo, Hart, Ramakrishnan, Rome and Giszter, 2009).
Identified force-field primitives: primitives and synchronous muscle synergies
Force-field modularity makes strong predictions about muscle use and control (see Kargo and Giszter, 2008, Appendix). A synergistic use of muscles is necessary to generate modular force-fields. The muscle synergies generate the joint torques, stiffnesses, and viscosities, that with the limb configuration and velocity determine the end-point or effector force transmission, and the whole limb visco-elastic field. These properties form the basis for movement. A force-field therefore cannot be fully specified outside a consideration of the limb configuration, motion, muscle moment arms and the length and shortening velocity variations of muscles that are activated in muscle synergies. A description of force and torque generation in the limb necessarily depends on biomechanics, and the force-field description combines the muscle synergy activation with these biomechanics.
Synchronous drive to several muscles in combination with features of limb state will specify the properties of a viscoelastic force-field module. Neglecting the short-term stress/strain history of the muscle, the force and torque resulting from the muscle’s activation depend on the length and velocity of stretch of the muscle and the moment arms of the muscles. These derive from limb configuration, and joint velocities. These several transforms can be mapped as a force-field or torque-field description of the synergy muscle effects, across the configuration and velocity space of the limb. At the most fundamental level, the idea of force-field primitives thus implies modular linearly covarying muscle use (Bizzi et al. 1991; Giszter et al. 1993, Mussa-Ivaldi et al., 1994). Each synchronous muscle synergy defines such a mapping, and a set of synchronous drives and synergies thus define a set of visco-elastic force-fields. Such fields can be used as a basis set of motor primitives, in order to build the actuation patterns for a limb (Mussa-Ivaldi 1992, Mussa-Ivaldi and Giszter 1992). If pulsed activation dynamics of the synchronous muscle synergies are used then the non-linear and history-dependent stress/strain effects in muscle may be better predicted. Such pulses have been observed in experimental data (Kargo and Giszter, 2008; Hart and Giszter, 2004; Kargo and Giszter 2000b). In summary, a basis of force-field primitives necessitates a specific structure of synchronous muscle synergies and reflexes, if they are to be compositional elements that are re-usable in a simple fashion. The force-field description, and synchronous synergies, thus imply specific quantitative and testable hypotheses about premotor drive, feedback organization, and the construction of movement(Kargo and Giszter 2008; Giszter et al. 2007).
Many of these features have been experimentally confirmed in muscle synergy analyses across species.
Muscle synergies and muscle level analyses: what features should define a synergy?
Muscle synergies as premotor drive pulses and primitives have been discussed. These are a particular case of muscle-synergy-based modularity. Muscle synergy modularity has also been used much more broadly, often finessing the roles of biomechanics and force-production details, focusing on patterns of use.
Synchronous and Time-varying synergies
Muscle Synergies represent co-occuring or, more strongly, covarying muscle use, on some timescale. Bernstein emphasized this idea of coalescing degrees of freedom into synergies. Today there are sometimes quite different usages of the term ‘synergy’ (e.g., Gottlieb, 1998; D’Avella et al., 2003; Hart and Giszter, 2004; Capellini et al., 2006; Torres-Oviedo et al., 2006; Gorniak et al. 2009). A central reduction in degrees of freedom is inferred in all uses. The term synergy has been used to cover a wide range of motor control possibilities and as a result is a very weak term if not carefully defined and qualified. A ‘muscle synergy’ could represent muscles activated by a common drive, muscles recruited together in time but controlled separately, or muscles controlled in a stereotypic (but not necessarily synchronous) temporal pattern. Each of these three types of descriptions implies different things about motor patterns, underlying circuitry and subsequent force generation. Each generates different motor pattern statistics, and thus different statistical decomposition methods may be best suited to their examination. Each implies different circuitry and thus physiology. For example, synchronous muscle synergies recruited with a common drive will be best separated in electromyographic (EMG) analysis by simpler matrix factorization methods (e.g., Independent Components Analysis, ICA, or non-negative matrix factorization, or Varimax Factor Analysis, see Tresch et al. 2006; Hart and Giszter, 2004; Torres-Oviedo and Ting, 2006,7; Capellini et al. 2006). Muscles with common onsets but independent regulation of amplitudes within the subsequent burst will be best analyzed using timing-based methods of decomposition (e.g., Krouchev et al., 2006). Time varying synergies will need more complex analyses. Time-varying synergies (non-synchronous patterns) analyses require significantly more data, as compared to other analyses, because of the need for use of a joint time-amplitude decomposition to extract the time-varying patterns and to compare fit quality with the other analyses with confidence (e.g., see Discussion in Cheung et al. 2009).
Statistical data fitting techniques support modularity. Not all are ‘doomed to succeed’ as has often been a criticism (e.g. see Hart and Giszter, 2004; Giszter et al. 2007). The extracted modules can be used to test computational ideas (e.g. Ting 2007; Kargo et al. 2009; Berniker et al. 2009). However, rather than data fitting, and modeling with statistically determined bases, the larger hope of modularity research is to identify neural mechanisms. In this effort physiology is likely preferable to statistical methods. In synchronous synergy descriptions at spinal levels we may be closest to identifying such physiological mechanisms.
Physiological testing of synchronous muscle synergies and primitives
We have used synchronous muscle synergies and force-fields to analyze spinal frog behaviors (Figure 2 and Giszter et al. 1993; Kargo and Giszter, 2000b, 2008), and these can be shown to be competent to generate frog behaviors using simple feedback based heuristics (Kargo, Hart, Ramakrishnan, Rome and Giszter, 2009).
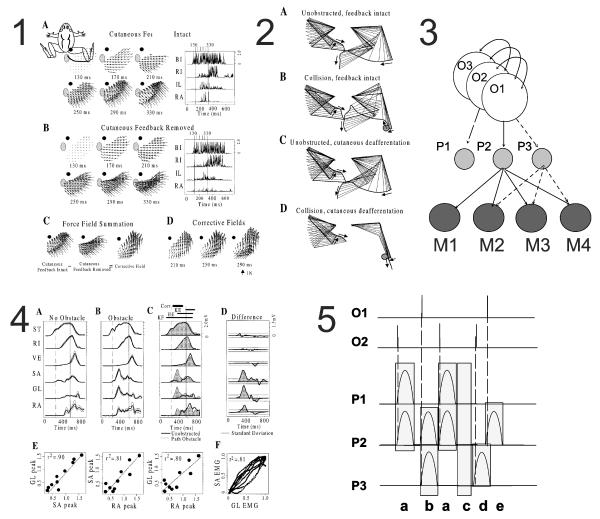
Figure 2.
Synchronous burst synergies and force-field primitives in a spinal behavior. The wiping behavior can be examined in free kinematics (Panel 2 and 4) or measuring isometric force (Panel 1). The manipulations in free kinematics show cutaneous sensing of impact is required to trigger correction (compare Panel 2 C and D). This allows the isometric force generation to be compared with and without corrections (Panel 1 A and B). The correction is a scaled and similar force-field added into the force-fields generated unde isometric conditions for wiping (Panel 1 D). This is compatible with composition using force-field summation. The scheme suggested by this is shown in Panel 3. Rhythm generation systems (O1, O2, O3…) recruit circuits for primitives that project to motor pools for multiple muscles (e.g. P2 to M1, M2, M3, M4 pools). These provide balanced drive to the muscles generating balanced and predictable torque and force. The balanced drive in the correction is shown in Panel 4. The electromyograms are from free kinematics and real obstacle corrections. EMG changes in A and B are overlain in C and subtracted in D. In D a synchronous pulse is clear. In E the correlation of pulse peaks among the three muscles across multiple corrections are shown. In F the evolution of the pulses in two muscles is displayed. The behavior of the muscles in Panel 4 is fully compatible with a synchronous pulsed drive system like that shown in Panel 5. The motor pattern generated at spinal levels is organized as a sequence of co-active and phased pulses of synchronous drive (P1-P3) that are recruited in a hierarchical fashion by a rhythm generator system (O1,2). These can be recruited in combinations (a, b) or individually (d,e). This pattern can then be deconstructed back into component synchronous muscle-synergy pulses P1,P2,P3 and their combinations. The pattern generation scheme supporting this compositionality comprises rhythm generators that project to a pattern shaping system comprised of primitives as in panel 3. This matches schemes proposed independently by McCrea and Rybak, and by Cappelini, Ivanenko, Poppele Lacquaniti and colleagues. (Data in Panels 1, 2, 4 from Kargo and Giszter 2000b). Neurons that could support such a synchronous premotor drive in frogs have now been recorded (Hart and Giszter, accepted).
Force-field primitives have been shown to be recruited in fixed duration or bounded duration bursts at spinal levels (Kargo and Giszter 2000b; Hart and Giszter, 2004). The force-field primitives can be demonstrated to be regulated by feedback systems as units during trajectory generation by frog spinal cord (Kargo and Giszter, 2008). They are regulated, added or deleted independently of other such units comprising the pattern (Kargo and Giszter 2000a; Giszter and Kargo 2000b; Kargo and Giszter, 2008) and combined by vector superposition of forces/torques (Mussa-Ivaldi et al. 1994; Kargo and Giszter, 2000b). This co-regulation of the muscles grouped in a synergy and the consequent behavior of the associated force-fields as units can be seen using classical physiological techniques such as muscle vibration (Kargo and Giszter, 2008). Unitary behavior of primitives holds true at both muscle synergy and force field measurement levels, and in all testing in the spinal frog so far, with durations of pulses conserved. It seems that at isolated spinal levels, modules comprised of pulsed synchronous drives may be the rule. These likely lie in a pattern shaping relationship to rhythm generation and pattern generation (see McCrea and Rybak, 2007).
Competence of Muscle synergies and Force-field primitives in behaviors
Force-field primitives1 and associated motor bursts may underlie the great flexibility of spinal generated behaviors. A trajectory correction response that uses vector summation of a force-field primitive has been identified in the frog (Kargo and Giszter, 2000b). Correction of hindlimb wipes in frogs demonstrates use of primitives in a real behavior (Figure 2). This analysis of correction behaviors has the significant advantage that, like physiological testing, it does not require any statistical decomposition. The correction primitive represents one element from a set of 6 primitives identified by other means. This primitive is added into the motor pattern denovo following a collision as a unitary pulse of about 275ms duration. This pulse duration was also observed in vibration (Kargo and Giszter 2008) and in motor pattern decomposition (Hart and Giszter, 2004). Muscles covary cleanly within the added pulse. This pulse of premotor drive and the resultant viscoelastic force-field addition acts to modify the trajectory generation so as to circumvent the encountered obstacle. In the spinalized frog this adjustment is generated entirely at the spinal level. As a result the limb reaches the target within 50ms of what would have been its unperturbed arrival time. The primitive is only recruited when collision occurs during the period in the motion when its superposition can successfully achieve the correction. For example, it is no longer recruited after the hip extensors’ activation, at which time the hip flexor correction response would be overpowered and ineffective. The spinal cord thus assembles the primitives based on their utility for goal attainment. In some way the cord represents the contingent relationships among the primitives and task. The degree to which this result should be thought of as representing the existence of a predictive model of the task, or an optimal control is currently unclear, but is an interesting issue. The competence of this framework for more general motion has been tested in a reduced planar version of the Kargo and Rome (2002) frog model and is near optimal (Berniker et al. 2009). The capacity of the physiological mechanisms and muscle groups identified to use simple spindle patterns to organized full 3D wiping motions in a 5DOF limb has been tested by Kargo and colleagues (Kargo et al. 2009). The compositional results in frog wiping are broadly similar to blending in turtle scratch (Earhart and Stein, 2000). Deletions of primitives have also been shown (Giszter and Kargo, 2000), and are similar to deletions observed earlier in the turtle (see Stein 2005) and to deletions also seen in fictive cat locomotion (Lafreniere-Roula and McCrea, 2005). A neural underpinning of the primitives in frog has now also been identified in extracellular multielectrode single-unit recordings (Hart and Giszter, 2009), with activity patterns resembling those of Berkowitz (2008) and Stein (2005) in turtle.
These properties of synergies and primitives in lower vertebrates appear to hold in mammals. In cat and human postural responses, it has also been shown that synergy decompositions provide compact descriptions of adjustments. In awake cats, locomotion patterns can also be considered composed of a few burst patterns which can be identified through ICA, Factor Analysis or burst onset analyses (Krouchev et al. 2006). Finally, in both walking and running in human locomotion, and in a range of additional activities, EMG can be statistically decomposed into a series of pulses of synchronous drives (Capellini et al. 2006). These are reorganized in phase and elements added and deleted across activities (Figure 3 rearranged from Capellini et al. 2006). This organization and rearrangement of modules occurs in a manner analogous to the lower vertebrates such as turtle (Stein, 2009) and frog (Giszter et al., 2007) where similar phase changes, deletions and corrections account for spinal movements. However, in contrast to spinal frogs, the burst durations in human locomotion are dilated and scale with step cycle duration.
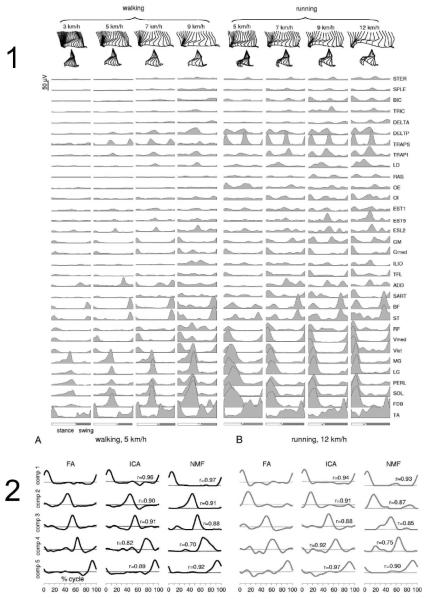
Figure 3.
Burst structure in adult human locomotion. Cappelini et al. have decomposed walking and running patterns in many muscles (Panel 1) using multiple statistical methods. In panel the similar results of factor analysis (FA), Independent Components Analysis (ICA) and Non-Negative matrix factorization are shown. All produce similar pulsed pattern structure and variations in walking (left) and running (right). The pulses shift between walking and running, and have minor alterations in muscle balance composition but are the muscle synergies are mostly preserved across conditions. (from Cappelini et al. 2006)
The origins of Modularity
Taken together, the data reviewed here provide substantial evidence for modular controls across a variety of tasks, and suggest modularity may be used in relatively stereotyped ways across limbed species. A number of important questions arise about the origins of modularity: Is there a common generalizable basis in every individual, or are there many special-purpose bases, perhaps each being unique to individual animals? Are bases and primitives in some way optimal and if so how? Do bases and primitives have a structural basis in the CNS or are they an emergent property? Are modular ‘primitives’ in different species builtin by evolution, or constructed de-novo in development and as learned task solutions? Examining these questions and their implications occupies the remainder of this review.
How does biomechanical design impact modularity: is anatomy destiny?
A key element of any account of modularity is the biomechanics of the controlled body and limbs. Limb structure determines the feasible task sets, modularity, and optimal controls. Bernstein considered system of many independent degrees of freedom in his discussions. However, in reality there are many anatomical constraints in biology. Muscles span multiple joints, aponeuroses couple degrees of freedom and so on. Anatomical constraints that couple degrees of freedom always potentially reduce the range of available actions, and the ranges of end-point force and joint torque relations, as elaborated by Valero-Cuevas (2009). In real-world biomechanics the apparent degrees of freedom in joint configuration and independence of actuation may be lower than it initially appears (Clewley et al., 2008; Valero-Cuevas, 2009). Multi-articular muscles and complex aponeuroses couple degrees of freedom and constrain possible patterns of use in the human hand (Valero Cuevas 2009; Valero-Cuevas et al., 2007). The body is thus not a general purpose mechanism, but is itself mechanically specialized. Significant control may be embedded in the body biomechanics and these specializations. Muscle, aponeuroses, and ligament structure and properties form ‘preflexes’ and organize mechanical ‘computations’ (Full and Koditschek 1999; Dickinson et al. 2000; Valero Cuevas et al. 2007). The feasible torque set and associated feasible force set are reduced in size by any coupling constraints. These mechanical constraints in the anatomy may initially help define robust and important behaviors, and seed learning and motor search. Both neural structure and anatomical design (Valero-Cuevas 2009) in the segmental motor system may play such a role and initially conspire against a flexible and arbitrary utilization of the motor apparatus degrees of freedom. However, to optimally use the motor apparatus there is need to transcend these mechanical and neural constraints.
Valero-Cuevas argues that the problem of the human CNS is actually elaborating flexible and optimal action in the face of the various constraints, more than the degrees of freedom problem and the associated search space issues! The CNS must learn to identify, anticipate and bypass these relatively ‘hard’ anatomical constraints so as to most fully span the capabilities of the motor apparatus. The CNS must eventually incorporate, or circumvent the constraints of anatomical structure. The construction and extension of a motor repertoire must also involve control and management of any low-level neural structures historically built-in by evolution like those in lower vertebrates. Any mechanical or neural structure that is predefined by evolution will both seed motor learning, by constraining initial motor actions, but must be overcome or bypassed for the fullest flexibility (see Wagner et al., 2007). This perspective thus contrasts strongly with that arising from a view of the motor apparatus as a truly general purpose mechanism. It is likely both anatomical and circuit constraints have been incorporated that limit the initial options for use of the motor system. The issue of how to seed motor learning in this way, and how new actions can best be elaborated in the face of these constraints, may determine what kinds of modules should form an initial compositional system for movement construction. Such modules should robustly support expansion to the entire range of learning encountered. Module structures in the initial basis should also be closely matched to limb mechanical properties as suggested by the simulations of Berniker et al. 2009 in the frog.
Only the ability to arbitrarily control muscles, so as to overcome any biomechanical or neural constraints, allows full access to the motor apparatus. This is a prerequisite for optimal controls with arbitrary task and optimality criteria. As suggested by Jackson in his 1887 quote, automaticity, structure and simplicity must eventually give way to complexity and flexibility.
Alternate perspectives: timescales for the optimization and selection of modularity
Computational frameworks enabling discovery and generation of rich optimal controls in the motor system have been proposed and explored in various ways in motor control (Loeb et al., 1989, 1990, 1999; Sanger 1994; and Lohmiller, 2001; Todorov 2004; Scott 2008). Optimal feedback control frameworks may shed light on a number of issues in motor control. To apply optimal feedback control frameworks, it is necessary to specify the family of admissible control laws, the plant structure and model to be controlled, and the quantitative task performance metrics and cost function.
In Darwin’s 200th year, like Hughlings Jackson, it may be useful to re-examine the implications of his work. From the Darwinian perspective there is a natural cost-function defined through selection in evolution. McFarland and Houston suggest the ultimate cost function of all animal behavior is inclusive fitness (as defined by Hamilton 1964): inclusive fitness is the classical fitness of the individual in combination with an adjusted fitness of the closely related individuals of partly shared genotypes which are aided by the individual. However, as they discuss, this restatement of Darwin offers little direct experimental insight into the animal’s goal function (i.e., its methods and ability to estimate and use this evolutionary cost) nor the behavior and motor control needed to implement it. Indeed the structure of this fitness ‘landscape’ may be unusual compared with the needs of conventional engineering formulations (e.g., Stadler et al., 2001). Further, the relationship of selection, fitness and observed morphology and behavior remains controversial (e.g., Dawkins ; Gould and Lewontin, 1979; Pigliuci and Kaplan 2000; Calabretta et al. 2003; Freeling and Thomas 2006). However, as an example of evolutionary construction of important structure and controls, feeding motor patterns and control structures in vertebrates are strongly conserved and/or have been very stable over evolutionary time scales (Wainwright 2002; Nishikawa et al. 1992). It seems clear that near-optimal mechanisms of modularity could be constructed on an evolutionary timescale by selection processes. They could also be constructed on ontogenetic and learned behavior timescales. However, assumptions of near-optimality need to be applied with caution for evolved biological systems. There is a potential danger of falling into the adaptationist fallacies described by Gould and Lewontin (1979) and revisited by Pigliuci and Kaplan (2000). Nonetheless optimal control approaches to motor control offer significant insights.
Optimal control approaches to modularity and selection of bases?
Optimization or constraint satisfaction approaches to motor behavior must currently be attacked in a piece-meal way, and must be focused on more tractable and localized tasks than a whole animal’s fitness. Recent optimal control successes in focused areas include predicting cosine tuning and truncated cosine tuning using a combination of signal dependent noise, accuracy, and energetics in a cost function (Todorov, 2002). Optimization has been used to examine modular postural mechanisms (Lockhart and Ting, 2007), and re-adaptation of trajectories (Izawa et al. 2008). Appropriate optimal controls choices and designs are usually Bayesian, and support the idea of some types of internal models. Optimal control algorithms can develop reduced dimensionality representations (i.e., modular or primitive-like controls) as a part of the on-line solution. Optimization frameworks thus provide both accounts and predictions about the de-novo construction of modules and dimensionality reduction. The framework also provides accounts of uncontrolled manifolds in high DOF movements (Todorov, 2004). Uncontrolled manifolds arise naturally in the models. These represent null-spaces for the task in which control effort is wasted and thus costly. Further, with signal-dependent noise, variance in the uncontrolled manifold cannot be reduced without an incurred cost of increased variance in task dimensions. The framework leads naturally to the minimum intervention principle. The predictions in this area have some experimental verification, most centered on motor control in the primate hand and arm (Liu and Todorov, 2007; Kutch et al. 2008; Valero-Cuevas et al. 2009). Explicit reference trajectories are not necessary in newer optimal control frameworks and a range of on-line correction phenomena are thereby captured. The great successes and importance of these perspectives are indisputable, and they provide a possible ontogeny for primitives. However, these approaches have not yet been able to shed light on the relative importance of historical and ontogenetic timescales (or nature/nurture issues) for the optimization and construction of motor modularity.
Evolution of modularity
A range of different evolutionary pressures can impact motor tasks and modularity and the ‘design’ of a low-level basis. For example, in control of terrestrial locomotion, an animal might at different times be most concerned with mechanical efficiency, with specific patterns of energy delivery and recovery, with motion symmetry, with foot placement, with joint and muscle wear and tear, with balance and stability to perturbation, with terrain tracking, with steering, or with the efficacy of its pursuit or avoidance strategies. Can simple bases of primitives support such a range of needs? Several of these listed task constraints may in practice be handled as much by physical design of the body as by active neural control (e.g., Kuo et al., 2005; and earlier discussion here). Many of the task features desirable in a limb may also be near-optimized simultaneously using appropriate controls matched with the evolutionarily constructed biomechanics (Collins, 1995).
Animals must perform well across a range of significant body size changes, on a range of terrains, with various orientations to gravity, and across a range of speeds. These performance ranges can radically alter the optimal strategies of control and roles of musculature. For example Full has shown the cockroach can run at speeds that preclude the use of feedback operating in the same way as it does at slower speeds (Full and Tu, 1991). Cockroaches can climb vertical walls and crawl upside down with radically altered body loading (Duch and Pfluger, 1995; Larsen et al., 1995). Cats also significantly alter their locomotor patterns on inclines (Smith and Carlson-Kuhta 1995). There is a clear need for flexibility and extensibility, and built-in support for on-line optimization of such behaviors. Any CNS modularity must be able to robustly support near-optimal behavior across the full range of tasks, conditions and the motor adaptations used. Low-level neural structures might be evolved to ‘anticipate’ this range in their structural organization. Modularity supporting construction of simple optimal feedback control strategies across the repertoire is likely needed. The work of Berniker et al. 2009 supports this view that observed primitives may have near-optimal properties of some kind. It remains to be clearly established what the relative contributions of nature or nurture are to construction of such modular primitives.
The idea of incorporation and modularizing of structural innovations and optima by natural selection has been explored by researchers in the evolution of modularity and complexity, and may relate also to investigation of the idea evolvability (see Brookfield, 2009, Draghi and Wagner, 2007). The ideas have first been explored in terms of cellular and anatomical complexity. An account of the evolution of modularity can be drawn from Thomas (2005), Wagner et al. (2007, 2005) and their considerations of skeletal and neural structures. An abbreviated account of the evolution of built-in modularity would go loosely as follows: First, we presume that the units of control, initially muscles and joints spanned, are also units of selection and can undergo several selection-related operations in their low-level control circuits. Examples would be deletion from use, control module duplication, merging of modules with other units, or splitting into sub-component units such as separate muscle compartments. Ab initio, given the definition of muscles as the finest grain of control and selection, we ignore splitting and changes in the skeletal structure. A modular system would evolve due to economies of scale from a system formerly composed of separated circuits and individuated motor controls. Under selection pressure, it would be energetically efficient for circuits supporting particularly useful movements to be merged where feasible (e.g., escape behaviors, mating behaviors etc). Instead of separate control circuits for these behaviors, a new modular basis for these would be built. This modular basis would necessarily provide a degree of generalization across the ethologically important behaviors from which its circuits were derived. The generalized basis could enable new behaviors, and be duplicated, or merged with other such bases and modules. These operations would result in further modularity within the system, while the generalization properties extend the use of these new modules to multiple contexts. The extension of use made possible would likely to occur on both evolutionary and ontogenetic timescales, allowing the modules to “span the space” of possible tasks. The ontogenetic plasticity of tasks enabled by modularity can provides ‘Baldwin effect’ : a selection for generalization learning enhancing the evolutionary plasticity. In such a model of motor evolution, increasing modularity and generalization of motor control becomes much more likely as new environmental contexts place new constraints and demands on the control and the physical plant. Finally, we re-emphasize that the repeated de novo learning of the same predictable optimization solutions over and over in each individual in a population, and in each generation is energetically inefficient. Search for optimal solutions, even if it is possible in real time, expends energy and wastes time and resources that could be devoted to other, more pressing tasks, or to better optimizing a task in the allotted time available if the solution were built-in. The first organism that short-cuts that search with (an appropriately general) hard-wired solution would be at an energetic (and temporal) advantage (see Wagner, 2007). The built-in solution clearly need not be fully optimal, but merely confer some speed up and fitness gain.
Evolution, nested modularity and construction of optimal controllers for novel motor behaviors
Often, the framework of optimality principles and the notion of built-in spinal primitives are characterized as in opposition, especially as modularity may be a control outcome from optimal controls. However, from an evolutionary perspective, mechanisms that improve motor function are unlikely to operate only on the timescale of the individual organism any more than on the timescale of a single movement, but rather on all. The motor system is a structure constructed historically by evolutionary processes. Built-in solutions may have advantages, as just discussed, provided flexibility is not severely compromised. Evolutionary processes layer onto, rescale and modify designs. Such historical continuity places additional structural constraints on how biological motor systems are built and organized on the timescale of the individual lifespan as well as on evolutionary timescales (e.g., see Wagner et al. 2007). On evolutionary time-scales new structures must be compatible with older structures. In contrast to industrial practice, when new evolutionary innovations in anatomical design or neural control principles occur, the biological systems cannot be retooled from the ground up to best implement or utilize the improvements, as an engineer might do with a new technology. Nonetheless, populations subject to selection can clearly search execution spaces much more thoroughly than their component individuals. For an individual organism the time for exploration (or play) in movement development may be severely limited. For example, there are significant selection pressures for rapidly elaborating successful movement in many organisms including mammals: a wildebeest calf must be ready to move with the herd within only a few hours at best if it is to survive. There are also tasks that are needed only briefly and transiently either in early or in later life with little or no practice opportunity available. There are needs for contingency behaviors that are only intermittently employed in individuals but are crucially important to survival (e.g., Tritonia escape behaviors, see Wyeth and Willows, 2006). The extent to which the degrees of freedom problem looms large in selection of the neural controls of these processes is a subject of debate. However, present engineering capabilities fall well short of designing either mechanisms and/or controllers that can rapidly and safely learn to control themselves in real-time and on-line when faced with, say, the high degrees of freedom of a locomoting wildebeest calf.
In summary, from a Darwinian perspective, built-in motor structures and their variability can significantly impact survival and are subject to selection. They are likely to have a structural basis and contain strong structural constraints, which can be both adaptive and/or historical accidents. These constraints can incorporate information related to the prior motor experiences of many generations. The resulting structures can potentially assist plastic motor behavior to operate in near-optimal and robust domains and provide for rare but survival critical eventualities. From an evolutionary perspective, avoiding long-term failure is more crucial than briefly succeeding in a task optimally. Appropriate structures can also potentially seed the motor development process, as they circumvent some problems of degrees of freedom, and thus support the more flexible learned motor behaviors that may be needed on an individual basis.
If evolution builds in prior anatomical and neural control structures, it would make sense that these should likely be modular and satisfy several criteria:
provide a compositional basis, (a set of primitives and other motor infrastructure) adequate and possibly near optimal for the most common evolutionarily tasks.
support survival critical tasks that are not readily or sufficiently quickly learned denovo. These include protective, escape and other emergency reflexes.
provide a robust starting point for learning that can be generalized and optimized.
Given these features, optimal feedback control mechanisms that operate at a more abstract level in the upper motor hierarchy can then elaborate more sophisticated task controls for more arbitrary and specialized tasks. The motor system must learn to work with, through or around the prior built-in structures to construct these more optimal task controllers for novel behaviors.
Optimal feedback research in motor control can shed light on these extensions and expansions of function. Without explicit consideration of a notion of an evolutionary or ‘ecological’ basis set at lowest levels, similar issues have been discussed by Todorov. Hierarchical modularity and can be used as a way to cope with redundancy and to achieve near optimal feedback controls in such systems (see Todorov et al. 2005). Todorov shows how near optimal feedback controls can be constructed in such a hierarchical framework. Intuitively, in his framework the low-level controllers and plant form a new ‘augmented’ plant for the higher-level controller, and when well chosen allow near-optimal control by this higher-level controller (Figure 4, redrawn from Todorov, 2005). Successive nestings of this framework in principle allow a hierarchical control of a redundant plant supporting approximately optimal feedback controls. The characteristics of the low-level controllers in the motor system are likely to be highly significant in enabling such approaches, as discussed above. Low-levels should guarantee stability, e.g., by emulation of passive dynamics (Colgate and Hogan 1988) or design of more general contracting system structures (Wang and Slotine 2005; Richardson et al. 2005; Slotine and Lohmiller, 2001). Appropriate muscle synergy modularity may satisfy these criteria. Low level robustness and optimality would presumably be useful design criteria in the successive augmented plant structures in the hierarchy. Evolution of such a hierarchy may have largely hardwired the lowest levels’ neural components in such a scheme to provide a near optimal generalizable basis. The degree of plasticity and hardwiring ascending through the hierarchy would match that suggested by Jackson.
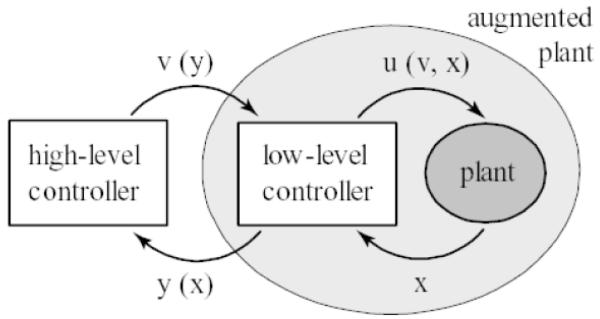
Figure 4.
A hierarchical optimization scheme proposed by Todorov et al. 2005 has the structure shown (from Todorov 2005). The low-level control provides plant control u(v,x) and abstracts and delivers information suited to the high-level control y(x). The high-level controller achieves an optimal feedback control by treating the low-level control and physical plant and an augmented plant through control v(y) which modified low-level control behavior u(v,x). The spinal frog will presumably exhibit u(0,x), or at least a static y(y) effect in this scheme.
New motor behaviors could thus be built in such a hierarchical control (1) using the augmented plant by gradual extensions derived from the prior low level basis of built-in motor primitives, or (2) by adding controls and new sets of task-dependent primitives using the other control options presented by the augmented plant. Indeed to build a repertoire that fully utilizes the possibilities of the anatomical motor apparatus may need both approaches, for the reasons described above. In such a scheme many ‘task-dependent primitives’ might be constructed in the higher centers of the hierarchy. It has been argued that in cortex/cerebellar/basal ganglia systems there may be many more such ‘task-dependent primitives’ than muscles. This matches very well with Hughling Jackson’s perspective. However, presumably, in early learning, using the low level basis will initially be simpler than identifying controls de-novo from within the potentially highly redundant circuitry and biomechanics.
Spinal cord and corticospinal development and plasticity in relation to modularity in mammals
Low-level spinal control systems do show significant plasticity on several different time-scales. Mechanisms to alter spinal pathways fairly permanently as well as transiently exist (see Wolpaw, 2007). Many of these are supervisory descending controls. However, even the isolated spinal cord has plastic mechanisms supporting some local but ill-defined optimality (see Grau et al. 2006). The goals of intrinsic low-level or spinal plasticity may be simply to support motor execution by the evolutionarily defined structures. For example, in invertebrates, in stomatogastric ganglia of crustacean adult pattern generation may operate and self-adjust within a contiguous region of the adjustable neural parameter space (see Prinz 2006; Marder and Goaillard, 2006). However, we do not know if development and adult maintenance of low level motor patterns, in most animals, admit multiple solutions and allow significant parameter wandering. The circuitry could potentially remain in flux in a contiguous ‘null-space’ domain in the neural parameter space where pattern, primitive or task performance is unaffected. This has been suggested by Bizzi and colleagues in the cortex (Rokni et al. 2007) but might partly hold even in low-level spinal circuitry.
We do not know if solutions for movement for limbs and whole body control that arise early in development can seamlessly be modified and carry forward as behavior becomes complex (but see Bradley et al., 2005). In the development of the rat, (see Clarac et al., 2004) crawling skills may transfer continuously to waddling and finally to parasagittal limb use in walking, trotting and galloping. The later behaviors only mature in the context of developing corticospinal and cerebellar controls, and patterns and compensations mature very differently after spinalization (Giszter et al. 1998, 2007, 2008). However, from the outset, the spinal modular bases needed for a development like the rat’s across these variations might also have to be predictive of likely future needs across the task sets.
There is also strong evidence for precise and hardwired ‘scaffolding’ of the lowest elements of the motor system in spinal cord. Motor pools are segregated anatomically, with numbers proportional to their ultimate needs, into columns in stereotypic locations during development (Dasen et al., 2003). A relatively uniform fraction of the cells are then pruned by subsequent cell death. Primary afferent projections to motor pools are also relatively hard-wired. These observations suggest there are quite orderly genetic controls of several spinal cord structures that are ‘built in’ by evolution before any plastic tuning can occur. Projections are precisely ordered (e.g., Cabaj et al. 2006; Lundberg et al., 1987a,b). Some of these circuits may relate to described primitives (e.g., Lundberg 1987b).
Descending mechanisms that control, alter and tune spinal motor systems are likely to work best in the framework of some initial ordered connectivity and structure of their targets as suggested in the scheme of Todorov et al. (2005). Descending systems may first build an appropriate connectivity with the spinal apparatus of pattern generators and primitives constructed by evolution. They otherwise face a more complex problem of where to connect, of how to modulate pathways, and of making credit assignments to the control outcomes (e.g. see Abbott 2006).
Throughout life, further tuning, plasticity, and adaptation to ontogenetic variations and life history mishaps are also clearly needed in spinal cord and are used in most animals (e.g. see Loeb 1999; Wolpaw and Carp, 1993; Schouenborg, 2004; Martin et al., 2004; Rossignol, 2006; Grau, 2006; Wolpaw, 2007). Despite the exquisite plasticity available, and paralleling Hughling Jackson’s quote, for the reasons discussed above we suggest that employing relatively structured hardwiring of modularity at the low levels of motor control is favored by evolution. It initially simplifies learning and plasticity, and creates an appropriate scaffold and bootstrap for building motor function as efficiently as possible. It can also provide protective reflex infrastructure that anticipates the needs in trauma or excess stress. However, how quickly such low-level structures can evolve and alter in vertebrates is unknown.
Speculations on trunk control and low back pain in man from a perspective of evolution and modularity
Human low back pain may be an interesting place to consider these evolutionary, hierarchy, optimality and modularity issues. A set of well-organized modules and protective reflexes for trunk control may exist in man, which were built in by evolution. However, we speculate that these are best suited and organized for quadrupedal task functions. They are needed in developmental processes in infancy and have not yet been subject to enough selective pressure or modified by evolution sufficiently to best meet the needs of bipedalism. We suggest that such a disparity between the biomechanical and control needs of bipedalism and a protective modular motor infrastructure organized for quadrupedal function may conspire to slow or interfere with optimal motor recovery from low back pain and trauma.
Spinal column control and low back pain are areas of motor control in which a combination of many degrees of freedom, neurally ancient mechanisms, and motor learning and adaptation all interact. There has been little exploration of notions of evolution-determined reflex motor primitives, and learned motor primitives in trunk control in quadrupeds and man, and their potential interactions. The trunk is a basis for all limb motion, and trunk motion errors will propagate and are amplified to still larger limb end-point errors as discussed by Scott and Loeb, 1994. Trunk postural control is likely integral to understanding limb control, though it has not been as well explored. For example, the body is often actively turned at speeds of 20-30 rpm. In the Lackner and Dizio rotating room experiment (Lackner and Dizio 1994 ) this induces major coriolis forces and resulting pointing errors and requires considerable on-line adaptation, but the anticipation of these forces in day to day motion is seamless. A gradient of proprioceptive density in muscles reaches peaks in axial musculature and this gradient is consistent with error propagation concerns (Scott and Loeb, 1994). Learned control of spinal column motion is likely crucial in the transition from quadrupedal postures, with the spinal column acting as a catenary, to bipedal postures and locomotion in man, with the spinal column acting as an unstable column. This likely involves the reorganization, and suppression of a range of reflex responses and learning de-novo of a new set of spinal and voluntary responses in the trunk. The strategies and the quality of spine balancing may also vary, with concomitant alterations in muscle use (e.g., Claus et al. 2009). These bipedal controls must be learned on a background of ‘spinal infrastructure’ that evolved for quadrupedal locomotion and/or brachiation for much of primate history.
The proposed framework
Low back pain is one of the most costly disorders in western society. It tends to afflict middle aged humans, who are possibly beyond the evolutionarily usual ‘expiration dates’ of the even recent past. The stabilization of the spine in vertical postures must be active (Panjabi 1992) and motor activity alters in back pain (e.g., van Dieen et al,2003) . There are several proposed models to account for low back pain, motor patterns in low back pain and recurrence patterns. Current models of low back pain include (1) the pain-spasm-pain model (Roland), in which pain increases muscle activity, causing further loading of damaged tissues and increasing pain, (2) the pain adaptation model (Lund) in which agonist activity is reduced, antagonist activity increased so as to slow motions and reduce range to protect tissues. Neither model is unambiguously supported by the literature on motor patterns (van Dieen et al. 2003) or the clinical community. Motor activity may increase or decrease in different studies and instances. The most consistent changes are reduction of activity and anticipatory action in the deepest axial and trunk muscles, specifically the multifidus and tranverse abdominis (Hodges and Moseley 2003, MacDonald et al. 2006). Another alternative model (3) is that all changes are geared to stabilization to cope with problems of instability due to poorer mechanical, muscular coordination, or muscular power generation (van Dieen et al. 2003). A framework which might reconcile these notions and some of the disparities may be obtained by reframing the clinical problems in an evolutionary and comparative framework. Bipedalism likely developed in early hominids as a learned behavior well prior to cortical expansion (Jablonski and Chaplin, 1993). We hypothesize that biomechanical protective mechanisms and reflexes, especially damage related ones, are likely built into the motor system and hardwired by evolution. Moreover, they may have evolved further only very slowly in man following the development of bipedalism. In support of this, many aspects of the basic musculo-skeletal plant structure in man shows remarkable anatomical similarity to that of quadrupeds despite human bipedalism (Schilling et al. 2005). Many anatomical peripheral and central mechanisms seem somewhat conserved in mammalian trunk and proximal limb evolution (Fischer and Witte 2007). Locomotor mechanisms are strongly conserved (see review of Dietz, 2002). A range of ligament and spindle based motor reflexes and protective reflexes clearly exist in the feline (e.g., Stubbs et al. 1998; Holm et al. 2002). Some of the grosser of such elements are seen in early human development or revealed in stroke (e.g. Galant reflex, see Zafeirou, 2004). How much the quadruped system of trunk reflexes is altered or lost in man is unknown. CNS genetic change in man may be slowed compared with other primates (Wang et al. 2007). If we assume there has been significant conservation of many trunk structures and controls as our departure point, this points toward several possibilities
Quadrupedal protective and locomotor control structures remain as a built-in predisposition in the human motor system. Among these are protective reflexes that were evolved as a protection for a spinal column that acted as a loaded catenary between two points of suspension from which the abdomen was suspended. They come into play instead in bipedal humans in a spinal column loaded in compression along its axis and intrinsically unstable.
Quadrupedal locomotor control mechanisms available at birth are gradually coopted or suppressed by a higher level control in support of the learning and development of voluntary control of bipedalism. An example would be the infant Galant or trunk incurvation reflex.
The spinal column protective reflexes (derived by evolution for quadrupedal biomechanics) remain available, but dormant or covert, in the absence of pain and inflammation. They can later be released by physical trauma or degenerative damage. They then act to interfere with voluntary bipedal control: they ‘stabilize’ the spine in a manner best suited to quadrupedal not bipedal locomotion (i.e., adapted for a different mechanical task set), and thereby exacerbate pain and instability conditions.
Protective neural reflex mechanisms never before experienced can re-assert themselves after later-life back trauma. These mechanisms present the motor control system with a unique interference problem - one not readily anticipated during the ontogeny of bipedal control. A new complex high-dimensional dynamics is imposed on the normal trunk controls by the previously dormant protective reflexes well after critical periods and the high plasticity of early development. As a result learning is slower: the newly experienced but evolutionary determined mechanisms need to be identified de-novo by the human motor control system and their management must be learned anew in the traumatized axial neuromechanical plant. This may be a nearly unsolvable motor problem (e.g., see Sanger, 2004).
This framework is diagrammed schematically in Figure 5. How does it impact ideas about low back pain in man?
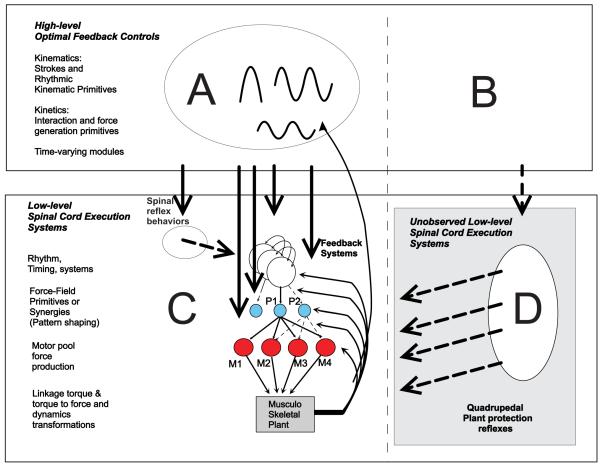
Figure 5.
The synthesis of high-level and optimal feedback controls (A) with low-level built-in compositional units (C). The optimal feedback controller A can suppress spinal reflexes, recruit pattern/rhythm generation, pattern shaping primitives, or motor pools, and alter or drive reflexes feedback systems. Eventually the control might completely suppress or alter any initial set of built-in primitives. In our view, this normal behavioral learning may never visit the states that recruit the quiescent protective reflexes (D). i.e., create plant trauma. The optimal controls of these (B) are never explored or formed progressively in critical periods of motor development. System A meanwhile creates (potentially very many) high level modules/primitives suited to bipedalism and many tasks and skills throughout development and adult life. When released/activated by acute trauma D generates massive interference in the augmented plant C that cannot be predicted a priori. The high-dimensional propagating effects are hard to identify and compensate in the bipedal task, having been historically evolved in the context of a quadrupedal task. Further, every system, module and task has to compensate given the central role of trunk as a central mechanical basis for limb motion.
The biped human spine: anatomical constraints
The spine is a complex biomechanical linkage composed of many joints and muscles. Despite the transition to bipedalism, only minor evolutionary changes of this region are reported: shortening of the lumbar spine; widening of the pelvis; reduction of the cross sectional area of the lumbar extensor musculature; and development of a lumbar lordosis (Schilling et al., 2005). It is well accepted that this complex structure of bones, ligaments and intervertebral discs (passive structures) is inherently unstable in man without the contribution of the spinal musculature (Panjabi 1992; Cholewicki & McGill 1996). Devoid of muscles, the thoracolumbar spine will buckle under a 20N compressive load (Lucas & Bresler 1960). Therefore, trunk muscle activation is necessary to stabilize this complex linkage (Cholewicki & McGill, 1996) However, neuromuscular control of spine stability must also integrate with control of spine movement, respiration and continence functions. Task conflicts can occur among maintenance of spine posture, whole body equilibrium with respect to gravity, coordination of trunk and extremity motion and control of intervertebral motion (linkage stability). The CNS must continually organize controls to overcome predictable and unpredictable challenges to the system using appropriately timed and modulated responses.
Muscle synergies in posture
For ease of understanding the roles of specific trunk muscles a functional separation was proposed by Bergmark (1989). This model differentiates contribution of the musculature to stability and movement roles, based upon their anatomical characteristics. The ‘local’ muscles are those muscles that cross only a few intervertebral segments, have small moment arms and are suited to control intervertebral motion (stability). These include the lumbar multifidus, intertransverse and interspinal muscles along with the transverse abdominis. The ‘global’ muscles are much larger with significant moment arms and torque generating capacity with attachments spanning from the pelvis to the thorax, and thus crossing multiple intervertebral segments. These muscles appear better suited for control of trunk motion and orientation (erector spinae, rectus abdominus and obliquus abdominis muscles). In addition, the quadratus lumborum and psoas major have been described as both stabilizers and prime movers. These former limb muscles in quadrupeds, now serve to stabilize and control frontal and sagittal plane movement (Schilling et al., 2005). While this description is likely an oversimplification of the trunk muscle system it has contributed to an appreciation of why the CNS uses many different control strategies. The CNS has to integrate competing functions to accomplish a defined task, and functional muscle synergies may be constructed to reduce the multiple degrees of freedom and manage muscle and skeletal redundancy. This has been supported in part by Torres-Oviedo and Ting, (Torres-Oviedo and Ting, 2007) who identified several robust muscle synergies that reproduced the reported “hip/trunk” strategy used by subjects during multidirectional platform perturbations and by work in our lab that identified several muscle synergies consistent across subjects and tasks that reconstructed control of trunk forward bending and reaching tasks. (Silfies, Hart, Cannella and Giszter, 2009).
Development of low back pain
So how are muscle synergies developed for bipedalism and balance of a now vertically oriented spine? The development of postural control of the trunk is essential to the development of standing balance and gait. The progression of control in infants moves from the head (mapped to visual and vestibular reflexes), to coordination of head-trunk in sitting (mapping head sensorimotor control to the trunk). As muscle strength increases, infants move to exploration of the environment via a more primitive crawling. They then begin to develop upper extremity reaching, standing and walking (integrating control of the leg muscles and the trunk). Finally this sets the stage for adaptive capabilities that allow us to modify these strategies to changes in the environment, or changes in the task, moving gradually from skills of the toddler to those of the expert soccer player. This progression largely holds for both the reflex-hierarchial and the systems model clinical accounts of balance and gait development. The role of reflex and low-level development may be to provide the framework on which to build the more plastic learned sensory and motor relationships.
In keeping with the suggestions of Todorov, Scott, Wolpert, Shadmehr and colleagues, a range of new optimal feedback mechanisms and new synergies may be constructed through development, to subsume the low level primitives and mechanisms available at birth. Across individuals we likely develop very similar feedback controls and synergies that are used in the most common positional changes (sit to stand, stand to walk), anticipatory and reactive balance, (ankle and hip strategies), and gait activities. We also build more specialized motor synergies used for specific but specialized tasks repeated in our work, sporting and leisure activities. The relative strength of these synergies is likely dependent upon the frequency of their use and the health of the physical plant. However, in some cases the excessive repetition of specific patterns of movement and motor tasks may result in asymmetrical loading to tissues and physical plant injuries.
Starting in the third decade, the passive structures of the spine begin to show degenerative changes. This along with an increasingly sedentary lifestyle can result in gradual muscle deconditioning and alteration in weight distributions that further challenges the controller and plant limits, and this could lead to altered control strategies that eventually result in development of low back pain (Cholewicki, 1997; Panjabi, 1992). If we consider degenerative changes or injury to the physical plant as the primary pathway to development of low back pain, then the pain may recruit and support the emergence of unanticipated and more ancient protective behaviors in the spinal controller or ‘augmented spinal cord trunk plant’. The symptoms, reflex interference and biomechanical alterations or inaccurate afferent information may precipitate maladaption of constructed muscle synergies, or result in new reliance on the noxious protective reflexes to manage control and linkage stability of the complex vertebral column. For example, cyclical or static trunk flexion results in reflex changes to the paraspinal musculature that lasts long after the activity is completed (Granata, 2005; Rogers, 2006). These reflex changes may contribute to an increased risk of injury (via altered mechanoreceptor input to the controller). Repetition of these prolonged trunk flexion task may also result in changes to muscle synergies that place a person at risk for development of low back pain.
However, we should also consider the possibility that the route to low back pain originates a-priori in sub-optimal coordination or dis-coordination. Some individuals may not discover and optimally adapt bipedal controls that are well matched to the built in evolutionary modular trunk controls, and so create less robust controls, vulnerability, increased skeletal stress and set the stage for development of low back pain. Evidence of altered motor strategies are reported for anticipatory postural responses in patients with current (Silfies, 2009) and previous low back pain (Hodges, 2001; 1996; 1998). Balance impairments in patients with low back pain are reported during both standing (Luoto, 1998; Bly & Sinnot, 1991; Henry, 2006) and sitting (Radebold,, 2001) and may indicate changes or differences in the accuracy of the postural control system. Changes in trunk control strategies have also been reported particularly related to altered amplitude modulation between ‘local’ and ‘global’ trunk musculature (O’Sullivan, 1997; Silfies, 2005). It has been hypothesized that the reported excessive ‘global’ muscle activity in patients with low back pain is an adaptation that results from poor passive structure or ‘local’ muscle control of the spine (Cholewicki, 1997).
Both reflex increases (Hodges et al, 2001; Zedka et al, 1999) and inhibition of muscle activity (Hides et al, 1994) have been reported in patients with low back pain. Patterns of co-contraction (protective splinting) have also been reported (Radebold et al, 2000; Lariviere et al, 2000; Lamoth, 2002). This co-contraction strategy has been associated with greater force variability and fatigue of trunk musculature that further impairs neuromuscular performance (Reeves et al. 2008). Inhibition of the lumbar multifidus muscles has been suggested as the reason for rapid atrophy in patients with acute low back pain (Hides et al, 1994; 1996), lumbar radiculopathy (Hyun et al, 2007) and those undergoing prolong muscle tissue retraction during spinal surgery (Gejo et al, 1999). These reflex changes in muscle behavior may force the controller to radically alter its behavior and use previously inhibited and abandoned reflexes, old muscle synergies or to create novel or altered muscle synergies to accomplish a specific task. In any event, it is clear that our optimal controls do not cope well. An example of an adaptation reported during gait is a reduction in axial trunk rotation and an increase in-phase movement of the trunk and lower extremity (Lamoth, 2005). If trunk rotation is a trigger for timing of leg synergies, as suggested by Allum (1995) this adaptation may affect other muscle synergies and controls used in the specific task, and present the optimal feedback controllers for limb and trunk motion with a massive and high-dimensional challenge.
Motor adaptations initiated during the acute pain period of a low back injury may remain in place after injury resolution or elimination of pain and may play a key role in recurrence of symptoms (Hides, 1996 and 2001; Rasmussen-Barr, 2003). These new controls may result in undue stress to spinal structures, and may contribute to a 73% (within 12 months) recurrence rate of low back pain symptoms (Pengle, 2003). A growing body of evidence suggests that poor muscular control of the lumbopelvic region is a significant impairment present in a large number of patients with recurrent low back pain (Cholewicki, 2003; O’Sullivan, 1997; Radebold, 2001; Silfies, 2005). In fact, previous history of low back pain appears to be the best predictor of recurrence (Greene, 2001; Cholewicki, 2005).
Current rehabilitation strategies and proposed directions
Despite data that identify altered trunk neuromuscular control strategies in patients with low back pain, the efficacy of current interventions to improve trunk control and stability are largely unknown. To date, exercise approaches for patients with low back pain attributed to poor trunk control have been developed by combining aspects of Panjabi’s (1992) theory of spinal dysfunction, Bergmark’s (1989) functional anatomical model of the trunk, and the research on spine stability and control (Cholewicki & McGill, 1996; Hodges & Richardson, 1996; Hodges 2001; McGill, 1997) resulting in rehabilitation programs that emphasize use of specific stabilizing muscles (transverse abdominis, internal oblique and lumbar multifidus) to restore active control and stability to the trunk (e.g. core stabilization, dynamic lumbar stabilization) (Jull & Richardson, 2000; Saal & Saal, 1989). The most widely used program emphasizes initial training of these stabilizing muscles using isometric co-contractions (Biely, 2006; Jull & Richardson, 2000; O’Sullivan et al., 2000). Following restored ability to co-contract the transverse abdominis and lumbar multifidus, exercises progress toward control of trunk position during extremity movements and functional activities. This approach serves as an opportunity for the CNS to reorganize its control: to remap and re-weight sensory information, trunk position/orientation and motor actions of the extremities. Giving patients the opportunity to practice this remapping with augmented and random feedback during different tasks (sitting, standing, walking, lifting) within different environments (open, restricted, noisy) and conditions (perturbed or unperturbed) may enhance recovery of efficacious muscle synergies. How long this process takes will depend upon the status of the physical plant, which protective neural reflexes or primitive synergies have taken over and what length of time the patient spent functioning with these suboptimal motor synergies. It will also depend on fundamental motor issues that are as yet unresolved, but reflected in Jackson’s quote. If flexible and near-optimal feedback controls operate at the higher levels of the CNS to support bipedal behaviors, how robustly and optimally can these cope with the high redundancy spinal column when faced with sudden perturbations to this core biomechanical structure and its low-level control? These low level perturbations in the core biomechanical structure will have sequelae that propagate throughout the biomechanical chains of the body.
The theory, methods and data analysis techniques discussed in this paper offer a new approach to characterizing the mechanisms underlying control of the human spine that may potentially benefit the study of low back pain. To date, little work has been completed on characterizing task-specific or task-independent trunk muscle synergies in both healthy individuals and in patients with the low back pain during common functional tasks. This approach may also provide a model from which to study the ability of current therapeutic exercises to change or optimize trunk muscle synergies and patient outcomes.
Conclusions
In summary, significant data across vertebrates support modularity in motor control. Without modules and primitives, the degrees of freedom problem may present a major impediment to motor learning and development. However, it is possible in primates and other mammals that instead, or in addition, there are neural computational mechanisms that can readily cope with the design of optimal high degree of freedom controllers. Optimal feedback controllers lead naturally to reduced degree of freedom representations, i.e., modularity or primitives, and may achieve various types of robustness desirable in biological motor control. However it seems unlikely that all modularity observed in mammals is constructed by optimal control processes acting on the timescale of the individual. The nervous system was historically constructed by Darwinian evolution, with a need for robust survivable behavior at every stage. An optimal and highly flexible construction of modularity may be achieved by historically more recent mechanisms like neocortex. However these strategies were presumably unavailable to historically earlier antecedent forms, and in current species without such computational apparatus. Instead, for these, near optimal modularity and robustness might be built-in on evolutionary timescales and constructed in hardwired structures. These might remain as a historical legacy or remain as a bootstrap even when very flexible motor control was layered over these as it was enabled in evolution by the additional or augmented neural circuitry. The conundrum currently faced is to what extent John Hughlings Jackson’s analysis is correct for human movement. To what extent does evolution-constructed modularity remain, either as a seed for motor learning, or as a submerged infrastructure emerging only in neurological deficits or as a result of trauma-elicited protective mechanisms? Some crucial comparative data needed to address question that are currently missing. However, we suggest here that nonetheless there is plenty of data to suggest that the evolved low-level structures have not disappeared. It is likely that they are available and can be active, for better or worse, in man. They cannot be ignored and may form an important part of any account of motor control. We need to not only explain why we can have exquisite human motor performance, but also explain why, in conditions like low back pain, we can sometimes have massively debilitating interference in motor control after relatively minor degenerative changes or trauma in our physical plants, while the clinical data also show that similar trauma can apparently leave other individuals completely asymptomatic. This may require an understanding of both high-level optimal motor learning, and the history-derived low-level composition of trunk and limb motor control systems.
Footnotes
While muscle synergy, premotor drive burst, and motor primitive have been used as largely synonymous terms, we suggest that the term ‘primitive’ may best indicate the idea of a set of building blocks or a developmental bootstrap elements that is used in a constructive or compositional fashion. Synergy is a significantly broader term, while synchronous premotor drive burst is more specific and potentially narrower.
REFERENCES
- Abbott LF. Where are the switches on this thing? In: van Hemmen JL, Sejnowski TJ, editors. 23 Problems in Systems Neuroscience. Oxford University Press; 2006. pp. 423–431. [Google Scholar]
- Allum JH, Honegger F, Acuna H. Differential control of leg and trunk muscle activity by vestibulo-spinal and proprioceptive signals during human balance corrections. Acta Otolaryngol. 1995;115(2):124–9. doi: 10.3109/00016489509139273. [DOI] [PubMed] [Google Scholar]
- Asada H, Slotine JJ. Robot Analysis and Control. Wiley; NY: 1986. [Google Scholar]
- Baldwin MJ. A New Factor in Evolution. The American Naturalist. 1896;Vol. 30(354):441–451. [Google Scholar]
- Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand Suppl. 1989;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Berkowitz A. Physiology and morphology of shared and specialized spinal interneurons for locomotion and scratching. J Neurophysiol. 2008;99(6):2887–901. doi: 10.1152/jn.90235.2008. Epub 2008 Apr 2. [DOI] [PubMed] [Google Scholar]
- Berniker M, Jarc A, Bizzi E, Tresch MC. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Natl Acad Sci U S A. 2009 May 5;106(18):7601–6. doi: 10.1073/pnas.0901512106. 2009. Epub 2009 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N. The co-ordination and regulation of movements. Pergamon Press; Oxford: 1967. [Google Scholar]
- Biely S, Smith S, Silfies SP. Clinical instability of the lumbar spine: diagnosis and intervention. Ortho Phys Ther Practice. 2006;18(3):11–8. [Google Scholar]
- Bizzi E, Mussa-Ivaldi FA, Giszter S. Computations underlying the execution of movement: a biological perspective. Science. 1991;253(5017):287–291. doi: 10.1126/science.1857964. [DOI] [PubMed] [Google Scholar]
- Bly NN, Sinnott PL. Variations in balance and body sway in middle-aged adults. Subjects with healthy backs compared with subjects with low-back dysfunction. Spine. 1991;16(3):325–30. doi: 10.1097/00007632-199103000-00012. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Solanki D, Zhao D. Limb movements during embryonic development in the chick: evidence for a continuum in limb motor control antecedent to locomotion. J Neurophysiol. 2005;94(6):4401–11. doi: 10.1152/jn.00804.2005. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Ryu YU, Lin J. Fast locomotor burst generation in late stage embryonic motility. J Neurophysiol. 2008;99(4):1733–42. doi: 10.1152/jn.01393.2007. Epub 2008 Feb 13. [DOI] [PubMed] [Google Scholar]
- Bradley NS. Connecting the dots between animal and human studies of locomotion. Focus on “Infants adapt their stepping to repeated trip-inducing stimuli”. J Neurophysiol. 2003;90(4):2088–9. doi: 10.1152/jn.00619.2003. [DOI] [PubMed] [Google Scholar]
- Brookfield JFY. Evolution and evolvability: celebrating Darwin 200. Biol. Lett. 2009;5:44–46. doi: 10.1098/rsbl.2008.0639. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumagne S, Cordo P, Verschueren S. Proprioceptive weighting changes in persons with low back pain and elderly persons during upright standing. Neuroscience Letters. 2004;366(1):63–6. doi: 10.1016/j.neulet.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Janssens L, Janssens E, Goddyn L. Altered postural control in anticipation of postural instability in persons with recurrent low back pain. Gait & posture. 2008 doi: 10.1016/j.gaitpost.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Burdet E, Milner TE. Quantization of human motions and learning of accurate movements. Biological Cybernetics. 1998;78:307–318. doi: 10.1007/s004220050435. [DOI] [PubMed] [Google Scholar]
- Cabaj A, Stecina K, Jankowska E. Same spinal interneurons mediate reflex actions of group Ib and group II afferents and crossed reticulospinal actions. J. Neurophysiol. 2006;95(6):3911–22. doi: 10.1152/jn.01262.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta R, Ferdinando AD, Wagner GP, Parisi D. What does it take to evolve behaviorally complex organisms? Biosystems. 2003 May;69(2-3):245–62. doi: 10.1016/s0303-2647(02)00140-5. 2003. [DOI] [PubMed] [Google Scholar]
- Calabretta R, Nolfi S, Parisi D, Wagner GP. Duplication of modules facilitates the evolution of functional specialization. Artif Life. 2000 Winter;6(1):69–84. doi: 10.1162/106454600568320. 2000. [DOI] [PubMed] [Google Scholar]
- Callebaut W, Rasskin-Gutman, editors. Modularity: Understanding the Development and Evolution of Natural Complex Systems. MIT Press; 2005. [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. Journal of Neurophysiology. 2006;95(6):3426–37. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- Chabra M, Jacobs RA. Properties of Synergies Arising from a Theory of Optimal Motor Behavior. Neural Computation. 2006;18:2320–2342. doi: 10.1162/neco.2006.18.10.2320. [DOI] [PubMed] [Google Scholar]
- Cheung VC, d’Avella A, Bizzi E. Adjustments of motor pattern for load compensation via modulated activations of muscle synergies during natural behaviors. J Neurophysiol. 2009;101(3):1235–57. doi: 10.1152/jn.01387.2007. Epub 2008 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech (Bristol, Avon) 1996;11(1):1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Panjabi MM, Khachatryan A. Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine. 1997;22(19):2207–12. doi: 10.1097/00007632-199710010-00003. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Silfies SP, Shah RA, Greene HS, Reeves NP, Alvi K. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine. 2005;30(23):2614–20. doi: 10.1097/01.brs.0000188273.27463.bc. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, van Dieen JH, Arsenault AB. Muscle function and dysfunction in the spine. J Electromyogr Kinesiol. 2003;13(4):303–4. doi: 10.1016/s1050-6411(03)00038-5. [DOI] [PubMed] [Google Scholar]
- Clarac F, Brocard F, Vinay L. The maturation of locomotor networks. Prog Brain Res. 2004;143:57–66. doi: 10.1016/S0079-6123(03)43006-9. Related. [DOI] [PubMed] [Google Scholar]
- Claus AP, Hides JA, Moseley GL, Hodges PW. Different ways to balance the spine: subtle changes in sagittal spinal curves affect regional muscle activity. Spine. 2009;34(6):E208–14. doi: 10.1097/BRS.0b013e3181908ead. [DOI] [PubMed] [Google Scholar]
- Clewley RH, Guckenheimer JM, Valero-Cuevas FJ. Estimating effective degrees of freedom in motor systems. IEEE Trans Biomed Eng. 2008 Feb;55(2 Pt 1):430–42. doi: 10.1109/TBME.2007.903712. 2008. [DOI] [PubMed] [Google Scholar]
- Colgate JE, Hogan N. Robust Control of Dynamically Interacting Systems. Int. J. Control. 1988;48(1):65–88. [Google Scholar]
- Collins JJ. The redundant nature of locomotor optimization laws. J Biomech. 1995;28:251–267. doi: 10.1016/0021-9290(94)00072-c. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu J-P, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- d’Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. PNAS. 2005;102(8):3076–3081. doi: 10.1073/pnas.0500199102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe R della, Popa T, Ginanneschi F, Spidalieri R, Mazzocchio R, Rossi A. Changes in coordination of postural control during dynamic stance in chronic low back pain patients. Gait & posture. 2006;24(3):349–55. doi: 10.1016/j.gaitpost.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Farley CT, Full RJ, Koehl MA, Kram R, Lehman S. How animals move: an integrative view. Science. 2000 Apr 7;288(5463):100–6. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- Dietz V. Do human bipeds use quadrupedal coordination? TINS. 2002;25(9):462–467. doi: 10.1016/s0166-2236(02)02229-4. [DOI] [PubMed] [Google Scholar]
- Dizio P, Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol. 1995 Oct;74(4):1787–92. doi: 10.1152/jn.1995.74.4.1787. 1995. [DOI] [PubMed] [Google Scholar]
- Draghi J, Wagner GP. Evolution of evolvability in a developmental model. Evolution. 2007;62:301–315. doi: 10.1111/j.1558-5646.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- Duch C, Pflüger H-J. Motor patterns for horizontal and upside walking and vertical climbing in the locust. J. Exp. Biol. 1995;198:1963–1976. doi: 10.1242/jeb.198.9.1963. [DOI] [PubMed] [Google Scholar]
- Ebenbichler GR, Oddsson LI, Kollmitzer J, Erim Z. Sensory-motor control of the lower back: implications for rehabilitation. Med Sci Sports Exerc. 2001 Nov;33(11):1889–98. doi: 10.1097/00005768-200111000-00014. 2001. [DOI] [PubMed] [Google Scholar]
- Feldman AG. Origin and advances of the equilibrium-point hypothesis. Adv Exp Med Biol. 2009;629:637–43. doi: 10.1007/978-0-387-77064-2_34. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Levin MF. The equilibrium-point hypothesis--past, present and future. Adv Exp Med Biol. 2009;629:699–726. doi: 10.1007/978-0-387-77064-2_38. [DOI] [PubMed] [Google Scholar]
- Fentress JC. Development of grooming in mice with amputated forelimbs. Science. 1973;179(74):704–5. doi: 10.1126/science.179.4074.704. [DOI] [PubMed] [Google Scholar]
- Ferreira P, Ferreira M, Maher C, Refshauge K, Herbert R, Hodges P. Changes in recruitment of transversus abdominis correlate with disability in people with chronic low back pain. Br J Sports Med. 2009 doi: 10.1136/bjsm.2009.061515. [DOI] [PubMed] [Google Scholar]
- Fischer MS, Witte H. Legs evolved only at the end! Philos Transact A Math Phys Eng Sci. 2007 Jan 15;365(1850):185–98. doi: 10.1098/rsta.2006.1915. 2007. [DOI] [PubMed] [Google Scholar]
- Flash T, Hochner B. Motor primitives in vertebrates and invertebrates. Current Opinion in Neurobiology. 2005;15(6):660–6. doi: 10.1016/j.conb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci. 1985;5(7):1688–703. doi: 10.1523/JNEUROSCI.05-07-01688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M, Thomas BC. Gene-balanced duplications, like tetraploidy, provide predictable drive to increase morphological complexity. Genome Res. 2006;16:805–814. doi: 10.1101/gr.3681406. 2006. [DOI] [PubMed] [Google Scholar]
- Full RJ, Koditschek DE. Templates and anchors: neuromechanical hypotheses of legged locomotion on land. J Exp Biol. 1999 Dec;202(Pt 23):3325–32. doi: 10.1242/jeb.202.23.3325. 1999. [DOI] [PubMed] [Google Scholar]
- Full RJ, Tu MS. Mechanics of rapid running insects: two-, four- and six-legged locomotion. J. exp Biol. 1991;156:215–231. doi: 10.1242/jeb.156.1.215. [DOI] [PubMed] [Google Scholar]
- Galis F, Wagner GP, Jockusch EL. Why is limb regeneration possible in amphibians but not in reptiles, birds, and mammals? Evol Dev. 2003;5(2):208–20. doi: 10.1046/j.1525-142x.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24(10):1023–8. doi: 10.1097/00007632-199905150-00017. [DOI] [PubMed] [Google Scholar]
- Giszter SF. Motor Primitives. In: Larry R, editor. Squire, Editor-in-Chief, Encyclopedia of Neuroscience. Academic Press; Oxford: 2008. 2008. [Google Scholar]
- Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog spinal cord. Journal of Neuroscience. 1993;13:467–491. doi: 10.1523/JNEUROSCI.13-02-00467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Kargo WJ. Conserved temporal dynamics and vector superposition of primitives in frog wiping reflexes during spontaneous extensor deletions. Neurocomputing. 2000;32-33:775–783. [Google Scholar]
- Giszter SF, Kargo WJ. Separation and estimation of muscle spindle and tension receptor populations by vibration of the biceps muscle in the frog. Ital. Archiv. Biol. 2002;140:283–294. [PubMed] [Google Scholar]
- Giszter SF, Kargo WJ. Modeling of dynamic controls in the frog wiping reflex: force-field level controls. Neurocomputing. 2001;38-40:1239–1247. [Google Scholar]
- Giszter SF, Moxon KA, Rybak I, Chapin JK. A neurobiological perspective on design of humanoid robots and their components IEEE Intelligent Systems. 2000;15(4):64–69. [Google Scholar]
- Giszter SF, Moxon KA, Rybak I, Chapin JK. Neurobiological and neurorobotic approaches to design of a controller for a humanoid motor system Robotics and Autonomous Systems. 2001;37:219–235. [Google Scholar]
- Giszter SF, Davies MR, Graziani VG. Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations. J. Neurophysiol. 2007a;97(4):2663–75. doi: 10.1152/jn.00308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Davies MR, Graziani V. Coordination strategies for limb forces during weight-bearing locomotion in normal rats, and in rats spinalized as neonates. Exp. Brain Research. 2008a;190(1):53–69. doi: 10.1007/s00221-008-1451-4. Epub 2008 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Davies MR, Ramakrishnan A, Udoekwere UI, Kargo WJ. Trunk sensorimotor cortex is essential for hindlimb weight-supported locomotion in adult rats spinalized as P1/P2 neonates. J. Neurophysiology. 2008b;100(2):839–51. doi: 10.1152/jn.00866.2007. Epub 2008 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Patil V, Hart CB. Primitives, premotor drives, and pattern generation: a combined computational and neuroethological perspective. Prog Brain Res. 2007b;165:323–46. doi: 10.1016/S0079-6123(06)65020-6. 2007. [DOI] [PubMed] [Google Scholar]
- Goldby LJ, Moore AP, Doust J, Trew ME. A randomized controlled trial investigating the efficiency of musculoskeletal physiotherapy on chronic low back disorder. Spine. 2006;31(10):1083–93. doi: 10.1097/01.brs.0000216464.37504.64. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJ. Corrective responses to loss of ground support during walking. I. Intact cats. J Neurophysiol. 1994;71(2):603–10. doi: 10.1152/jn.1994.71.2.603. [DOI] [PubMed] [Google Scholar]
- Gorniak SL, Zatsiorsky VM, Latash ML. Hierarchical control of static prehension: II. Multidigit synergies. Exp Brain Res. 2009;194(1):1–15. doi: 10.1007/s00221-008-1663-7. Epub 2008 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb GL. Muscle activation patterns during two types of voluntary single-joint movement. J Neurophysiol. 1998;80:1860–1867. doi: 10.1152/jn.1998.80.4.1860. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Lewontin RC. The Spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. London B Biol. Sci. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Granata KP, Rogers E, Moorhouse K. Effects of static flexion-relaxation on paraspinal reflex behavior. Clin Biomech. 2005;20:16–24. doi: 10.1016/j.clinbiomech.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5(4):191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Greene HS, Cholewicki J, Galloway MT, Nguyen CV, Radebold A. A history of low back injury is a risk factor for recurrent back injuries in varsity athletes. Am J Sport Med. 2001;29(6):795–800. doi: 10.1177/03635465010290062001. [DOI] [PubMed] [Google Scholar]
- Grillner S, Perret C, Zangger P. Central generation of locomotion in the spinal dogfish. Brain Res. 1976;109(2):255–69. doi: 10.1016/0006-8993(76)90529-1. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The Genetical Evolution of Social Behaviour. J.Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. Modular premotor drives and unit bursts as primitives for frog motor behaviors. J Neurosci. 2004 Jun 2;24(22):5269–82. doi: 10.1523/JNEUROSCI.5626-03.2004. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. Neural Underpinnings of Motor Primitives. J Neurosci. 2009 (Accepted) [Google Scholar]
- Hides JA, Jull GA, Richardson CA. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine. 2001;26(11):E243–8. doi: 10.1097/00007632-200106010-00004. [DOI] [PubMed] [Google Scholar]
- Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21(23):2763–9. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19(2):165–72. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- Hodges PW. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain. Experimental brain research Experimentelle Hirnforschung. 2001;141(2):261–6. doi: 10.1007/s002210100873. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21(22):2640–50. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997;77(2):132–42. doi: 10.1093/ptj/77.2.132. discussion 42-4. [DOI] [PubMed] [Google Scholar]
- Hogan N. An organizing principle for a class of voluntary movements. J. Neurosci. 1984;4(11):2745–54. doi: 10.1523/JNEUROSCI.04-11-02745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, Indahl A, Solomonow M. Sensorimotor control of the spine J. Electromyogr. Kinesiol. 2002;12:219–234. doi: 10.1016/s1050-6411(02)00028-7. [DOI] [PubMed] [Google Scholar]
- Hyun JK, Lee JY, Lee SJ, Jeon JY. Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine. 2007;32(21):E598–602. doi: 10.1097/BRS.0b013e318155837b. [DOI] [PubMed] [Google Scholar]
- Ijspeert A, Nakanishi J, Schaal S. Learning attractor landscapes for learning motor primitives. In: Becker S, Thrun S, Obermayer K, editors. Advances in Neural Information Processing Systems. Vol. 15. MIT Press; Cambridge, MA: 2003. pp. 1547–1554. [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56(4):433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Izawa J, Rane T, Donchin O, Shadmehr R. Motor adaptation as a process of reoptimization. J Neurosci. 2008;28(11):2883–91. doi: 10.1523/JNEUROSCI.5359-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski NG, Chaplin G. Origin of habitual terrestrial bipedalism in the ancestor of the Hominidae. J. Hum. Evol. 1993;24:259–280. [Google Scholar]
- Jull GA, Richardson CA. Motor control problems in patients with spinal pain: a new direction for therapeutic exercise. J Manipulat Physiol Therapeutics. 2000;23(2):115–7. [PubMed] [Google Scholar]
- Kargo WJ, Giszter SF. Afferent roles in hindlimb wiping reflex: free limb kinematics and motor patterns. J Neurophysiology. 2000a;83(3):1480–1501. doi: 10.1152/jn.2000.83.3.1480. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Giszter SF. Rapid corrections of aimed movements by combination of force-field primitives. Journal of Neuroscience. 2000b;20:409–426. doi: 10.1523/JNEUROSCI.20-01-00409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Giszter SF. Individual premotor drive pulses, not time-varying synergies, are the units of adjustment for limb trajectories constructed in spinal-cord. J. Neuroscience. 2008;28(10):2409–25. doi: 10.1523/JNEUROSCI.3229-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Ramakrishnan A, Hart CB, Rome L, Giszter SF. A simple experimentally-based model using proprioceptive regulation of motor primitives captures adjusted trajectory formation in spinal frogs. J. Neurophysiology. 2009 doi: 10.1152/jn.01054.2007. (Accepted.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Rome L. Functional morphology of proximal hindlimb muscles in the frog rana pipiens. J Exp Biol. 2002;205(Pt 14):1987–2004. doi: 10.1242/jeb.205.14.1987. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Hounsgard J, Sillar KT. Basic building blocks of vertebrate CPGs. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks and Motor Behavior. MIT press; Cambridge MA: 1997. pp. 47–60. [Google Scholar]
- Koumantakis GA, Watson PJ, Oldham JA. Trunk muscle stabilization training plus general exercise versus general exercise only: randomized controlled trial of patients with recurrent low back pain. Phys Ther. 2005;85(3):209–25. [PubMed] [Google Scholar]
- Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol. 2006;96(4):1991–2010. doi: 10.1152/jn.00241.2006. [DOI] [PubMed] [Google Scholar]
- Kuo AD. The relative roles of feedforward and feedback in the control of rhythmic movements. Motor Control. 2002;6(2):129–45. doi: 10.1123/mcj.6.2.129. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: step-to-step transitions. Exerc Sport Sci Rev. 2005;33(2):88–97. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Kutch JJ, Kuo AD, Bloch AM, Rymer WZ. Endpoint force fluctuations reveal flexible rather than synergistic patterns of muscle cooperation. J Neurophysiol. 2008;100(5):2455–71. doi: 10.1152/jn.90274.2008. Epub 2008 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, Dizio P. Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol. 1994 Jul;72(1):299–313. doi: 10.1152/jn.1994.72.1.299. 1994. [DOI] [PubMed] [Google Scholar]
- Lackner JR, DiZio P. Motor control and learning in altered dynamic environments. Curr Opin Neurobiol. 2005 Dec;15(6):653–9. doi: 10.1016/j.conb.2005.10.012. 2005. Epub 2005 Nov 3. Review. [DOI] [PubMed] [Google Scholar]
- Lackner JR, DiZio P. Control and calibration of multi-segment reaching movements. Adv Exp Med Biol. 2009;629:681–98. doi: 10.1007/978-0-387-77064-2_37. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. Journal of Neurophysiology. 2005;94(2):1120–32. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Lamoth CJCM,OG, Wuisman PIJM, van Dieën JH, Levin MF, Beek PJ. Pelvis-Thorax Coordination in the Transverse Plane During Walking in Persons With Nonspecific Low Back Pain. Spine. 2002;27(4):E92–E9. doi: 10.1097/00007632-200202150-00016. [DOI] [PubMed] [Google Scholar]
- Lamoth CJ, Meijer OG, Daffertshofer A, Wuisman PI, Beek PJ. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: changes in motor control. Eur Spine J. 2005;5:23–40. doi: 10.1007/s00586-004-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere C, Gagnon D, Loisel P. An application of pattern recognition for the comparison of trunk muscles EMG waveforms between subjects with and without chronic low back pain during flexion-extension and lateral bending tasks. J Electromyogr Kinesiol. 2000;10(4):261–73. doi: 10.1016/s1050-6411(00)00013-4. [DOI] [PubMed] [Google Scholar]
- Larsen GS, Frazier SF, Fish SE, Zill SN. Effects of load inversion in cockroach walking. J Comp Physiol A. 1995;176:229–238. doi: 10.1007/BF00239925. [DOI] [PubMed] [Google Scholar]
- Lemay MA, Grill WM. Modularity of motor output evoked by intraspinal microstimulation in cats. Journal of Neurophysiol. 2004;91(1):502–14. doi: 10.1152/jn.00235.2003. [DOI] [PubMed] [Google Scholar]
- Liu D, Todorov E. Evidence for the flexible sensorimotor strategies predicted by optimal feedback control. J Neurosci. 2007;27(35):9354–68. doi: 10.1523/JNEUROSCI.1110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart DB, Ting LH. Optimal sensorimotor transformations for balance. Nat Neurosci. 2007 Oct;10(10):1329–36. doi: 10.1038/nn1986. 2007. Epub 2007 Sep 16. [DOI] [PubMed] [Google Scholar]
- Loeb GE. Asymmetry of hindlimb muscle activity and cutaneous reflexes after tendon transfers in kittens. J Neurophysiol. 1999 Dec;82(6):3392–4. doi: 10.1152/jn.1999.82.6.3392. 1999. [DOI] [PubMed] [Google Scholar]
- Loeb GE. Overcomplete musculature or underspecified tasks? Motor Control. 4:81–83. doi: 10.1123/mcj.4.1.81. discussion 97–116, 2000. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Brown IE, Cheng EJ. A hierarchical foundation for models of sensorimotor control. Exp Brain Res. 1999 May;126(1):1–18. doi: 10.1007/s002210050712. 1999. [DOI] [PubMed] [Google Scholar]
- Loeb GE, He J, Levine WS. Spinal cord circuits: are they mirrors of musculoskeletal mechanics? J Mot Behav. 1989 Dec;21(4):473–91. doi: 10.1080/00222895.1989.10735495. 1989. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Levine WS, He J. Understanding sensorimotor feedback through optimal control. Cold Spring Harb Symp Quant Biol. 1990;55:791–803. doi: 10.1101/sqb.1990.055.01.074. 1990. [DOI] [PubMed] [Google Scholar]
- Lucas DB, Bresler B. Technical Report. Biomechanics Laboratory, University of California, Berkley; San Francisco: 1960. Stabiltiy of the ligamentous spine. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents: 2 Functional characteristics of reflex pathways to a-motoneurones. Exp Brain Res. 1987;65:282–293. doi: 10.1007/BF00236300. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents: 3 Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987b;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Luoto S, Taimela S, Alaranta H, Hurri H. Psychomotor speed in chronic low-back pain patients and healthy controls: construct validity and clinical significance of the measure. Percept Mot Skills. 1998;87(3 Pt 2):1283–96. doi: 10.2466/pms.1998.87.3f.1283. [DOI] [PubMed] [Google Scholar]
- MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther. 2006;11(4):254–63. doi: 10.1016/j.math.2006.02.004. Epub 2006 May 23. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Moseley GL, Hodges PW. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain. 2009;142(3):183–8. doi: 10.1016/j.pain.2008.12.002. Epub 2009 Jan 30. [DOI] [PubMed] [Google Scholar]
- Magnusson ML, Bishop JB, Hasselquist L, Spratt KF, Szpalski M, Pope MH. Range of motion and motion patterns in patients with low back pain before and after rehabilitation. Spine. 1998;23(23):2631–9. doi: 10.1097/00007632-199812010-00019. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic Movements. Current Biology. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7(7):563–74. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Marr D. Vision. A Computational Investigation into the Human Representation and Processing of Visual Information. WH Freeman; 1983. Vision. [Google Scholar]
- Martin JH, Choy M, Pullman S, Meng Z. Corticospinal system development depends on motor experience. J Neurosci. 2004;24(9):2122–2132. doi: 10.1523/JNEUROSCI.4616-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Cooper SE, Ghez C. Kinematic analysis of reaching in the cat. Exp Brain Res. 1995;102(3):379–92. doi: 10.1007/BF00230643. [DOI] [PubMed] [Google Scholar]
- Martin JH, Friel KM, Salimi I, Chakrabarty S. Activity- and use-dependent plasticity of the developing corticospinal system. Neurosci Biobehav Rev. 2007;31(8):1125–35. doi: 10.1016/j.neubiorev.2007.04.017. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Modeling the mammalian locomotor CPG: insights from mistakes and perturbations. Prog Brain Res. 2007;165:235–53. doi: 10.1016/S0079-6123(06)65015-2. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Houston A. Quantitative Ethology: The state space approach. Pitman; 1981. [Google Scholar]
- McGill SM. The biomechanics of low back injury: implications on current practice in industry and the clinic. J Biomech. 1997;30(5):465–75. doi: 10.1016/s0021-9290(96)00172-8. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Nicholas MK, Hodges PW. Does anticipation of back pain predispose to back trouble? Brain. 2004;127(Pt 10):2339–47. doi: 10.1093/brain/awh248. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA. From basis functions to basis fields: Using vector primitives to capture vector patterns. Biol. Cybern. 1992;67:479–489. doi: 10.1007/BF00198755. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Bizzi E. Motor learning through the combination of primitives. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2000;355(1404):1755–69. doi: 10.1098/rstb.2000.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Giszter SF. Vector field approximation: a computational paradigm for motor control and learning. Biol. Cybern. 1992;67:491–500. doi: 10.1007/BF00198756. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Hogan N. Integrable solutions of kinematic redundancy via impedance control. The International Journal of Robotics Research. 1991;10:481–491. [Google Scholar]
- Mussa-Ivaldi FA, Giszter SF, Bizzi E. Linear combination of primitives in vertebrate motor control. Proceedings and National Academy of Sciences. 1994;91:7534–7538. doi: 10.1073/pnas.91.16.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa KC, Anderson CW, Deban SM, O’Reilly JC. The evolution of neural circuits controlling feeding behavior in frogs. Brain Behav Evol. 1992;40(2-3):125–40. doi: 10.1159/000113908. [DOI] [PubMed] [Google Scholar]
- O’Sullivan P, Twomey L, Allison G, Sinclair J, Miller K. Altered patterns of abdominal muscle activation in patients with chronic low back pain. Aust J Physiother. 1997;43(2):91–8. doi: 10.1016/s0004-9514(14)60403-7. [DOI] [PubMed] [Google Scholar]
- O’Sullivan PB. Lumbar segmental ‘instability’: clinical presentation and specific stabilizing exercise management. Man Ther. 2000;5(1):2–12. doi: 10.1054/math.1999.0213. [DOI] [PubMed] [Google Scholar]
- O’Sullivan PB, Twomey L, Allison GT. Altered abdominal muscle recruitment in patients with chronic back pain following a specific exercise intervention. Journal Ortho Sports Phys Ther. 1998;27(2):114–24. doi: 10.2519/jospt.1998.27.2.114. [DOI] [PubMed] [Google Scholar]
- Pang MY, Lam T, Yang JF. Infants adapt their stepping to repeated trip-inducing stimuli. J Neurophysiol. 2003;90(4):2731–40. doi: 10.1152/jn.00407.2003. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5(4):383–9. doi: 10.1097/00002517-199212000-00001. discussion. [DOI] [PubMed] [Google Scholar]
- Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327(7410):323. doi: 10.1136/bmj.327.7410.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M, Kaplan J. The fall and rise of Dr Pangloss: adaptationism and the Spandrels paper 20 years later. TREE. 2000;15(2):66–70. doi: 10.1016/s0169-5347(99)01762-0. [DOI] [PubMed] [Google Scholar]
- Polit A, Bizzi E. Characteristics of motor programs underlying arm movements in monkeys. J Neurophysiol. 1979;42(1 Pt 1):183–94. doi: 10.1152/jn.1979.42.1.183. [DOI] [PubMed] [Google Scholar]
- Poppele R, Bosco G. Sophisticated spinal contributions to motor control. Trends in Neuroscience. 2003;26(5):269–76. doi: 10.1016/S0166-2236(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Prinz AA. Insights from models of rhythmic motor systems. Curr Opin Neurobiol. 2006;16(6):615–20. doi: 10.1016/j.conb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer IL, Lillicrap TP, Scott SH. Temporal evolution of ‘automatic gain-scaling’. J Neurophysiol. 2009 May 13; doi: 10.1152/jn.00085.2009. 2009. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol. 2008 Jul;100(1):224–38. doi: 10.1152/jn.90262.2008. 2008. Epub 2008 May 7. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Stecina K, Gosgnach S, McCrea DA. Stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol. 2005;94(3):2045–52. doi: 10.1152/jn.00175.2005. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine. 2001;26(7):724–30. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25(8):947–54. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- Rasmussen-Barr E, Nilsson-Wikmar L, Arvidsson I. Stabilizing training compared with manual treatment in sub-acute and chronic low-back pain. Man Ther. 2003;8(4):233–41. doi: 10.1016/s1356-689x(03)00053-5. [DOI] [PubMed] [Google Scholar]
- Reeves NP, Cholewicki J, Milner T, Lee AS. Trunk antagonist co-activation is associated with impaired neuromuscular performance. Exp Brain Res. 2008;188(3):457–63. doi: 10.1007/s00221-008-1378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Tresch M, Bizzi E, Slotine JJ. Stability analysis of nonlinear muscle dynamics using contraction theory. Conf Proc IEEE Eng Med Biol Soc. 2005;5:4986–9. doi: 10.1109/IEMBS.2005.1615594. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Loeb GE. Electromyographic studies of neck muscles in the intact cat. II. Reflexes evoked by muscle nerve stimulation. Exp Brain Res. 1992;88(1):59–66. doi: 10.1007/BF02259128. 1992. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Thomson DB, Loeb GE. Electromyographic studies of neck muscles in the intact cat. I. Patterns of recruitment underlying posture and movement during natural behaviors. Exp Brain Res. 1992;88(1):41–58. doi: 10.1007/BF02259127. 1992. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, Stein J, Hogan N. Smoothness during stroke recovery. J. Neurosci. 2002;22(18):8297–8304. 8299. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EL, Granata KP. Disturbed paraspinal reflex following prolonged flexion-relaxation and recovery. Spine. 2006;31(7):839–845. doi: 10.1097/01.brs.0000206361.53451.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni U, Richardson AG, Bizzi E, Seung HS. Motor learning with unstable neural representations. Neuron. 2007 May 24;54(4):653–66. doi: 10.1016/j.neuron.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1647–71. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine. 1989;14(4):431–7. doi: 10.1097/00007632-198904000-00018. [DOI] [PubMed] [Google Scholar]
- Sanger TD. Human arm movements described by a low-dimensional superposition of principal components. J Neurosci. 2000;20(3):1066–72. doi: 10.1523/JNEUROSCI.20-03-01066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD. Failure of motor learning for large initial errors. Neural Comput. 2004;16(9):1873–86. doi: 10.1162/0899766041336431. [DOI] [PubMed] [Google Scholar]
- Sanger TD. Optimal unsupervised motor learning for dimensionality reduction of nonlinear control systems. IEEE Trans Neural Netw. 1994;5(6):965–73. doi: 10.1109/72.329694. 1994. [DOI] [PubMed] [Google Scholar]
- Santos VJ, Bustamante CD, Valero-Cuevas FJ. Improving the fitness of high-dimensional biomechanical models via data-driven stochastic exploration. IEEE Trans Biomed Eng. 2009;56(3):552–64. doi: 10.1109/TBME.2008.2006033. Epub 2008 Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal S, Ijspeert A, Billard A. Computational approaches to motor learning by imitation. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):537–47. doi: 10.1098/rstb.2002.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal S, Schweighofer N. Computational motor control in humans and robots. Current Opinion in Neurobiology. 2005;15(6):675–82. doi: 10.1016/j.conb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Schilling N, Arnold D, Wagner H, Fischer MS. Evolutionary aspects and muscular properties of the trunk--implications for human low back pain. Pathophysiology. 2005;12(4):233–42. doi: 10.1016/j.pathophys.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Holmes P. Mechanical models for insect locomotion: dynamics and stability in the horizontal plane I. Theory. Biol Cybern. 2000;83(6):501–15. doi: 10.1007/s004220000181. Related. [DOI] [PubMed] [Google Scholar]
- Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol. 2008;586(5):1217–24. doi: 10.1113/jphysiol.2007.146068. Epub 2008 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. The computation of position sense from spindles in mono- and multiarticular muscles. J Neurosci. 1994 Dec;14(12):7529–40. doi: 10.1523/JNEUROSCI.14-12-07529.1994. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouenborg J. Learning in sensorimotor circuits. Current Opinion in Neurobiology. 2004;14(6):693–7. doi: 10.1016/j.conb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14(5 Pt 2):3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. The integrative action of the nervous system. Yale University Press; New Haven, CT: 1961. [Google Scholar]
- Silfies SP, Bhattacharya A, Biely S, Smith S, Giszter S. Trunk Control during Standing Reach: A Dynamical System Analysis of Movement Strategies in Patients with Mechanical Low Back Pain. Gait Posture. 2008;29(3):370–6. doi: 10.1016/j.gaitpost.2008.10.053. Epub 2008 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silfies SP, Hart CB, Cannella M, Giszter SF. Task-independent and task-specific trunk muscle synergies are recruited in bending and reaching. Proceedings of the International Society of Posture and Gait Research; Bologna, Italy. 2009. pp. 285–6. [Google Scholar]
- Silfies SP, Mehta R, Smith SS, Karduna AR. Differences in feedforward trunk muscle activity in subgroups of patients with mechanical low back pain. Arch Phys Med Rehabil. 2009;90:1159–69. doi: 10.1016/j.apmr.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Silfies SP, Squillante D, Maurer P, Westcott S, Karduna AR. Trunk muscle recruitment patterns in specific chronic low back pain populations. Clin Biomech. 2005;20(5):465–73. doi: 10.1016/j.clinbiomech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sjölander P, Johansson H, Djupsjöbacka M. Spinal and supraspinal effects of activity in ligament afferents. Electromyogr Kinesiol. 2002;12(3):167–76. doi: 10.1016/s1050-6411(02)00017-2. [DOI] [PubMed] [Google Scholar]
- Slotine JJ, Lohmiller W. Modularity, evolution, and the binding problem: a view from stability theory. Neural Netw. 2001 Mar;14(2):137–45. doi: 10.1016/s0893-6080(00)00089-7. 2001. [DOI] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P. Unexpected motor patterns for hindlimb muscles during slope walking in the cat. J Neurophysiol. 1995;74(5):2211–5. doi: 10.1152/jn.1995.74.5.2211. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine. 1998;23(23):2552–62. doi: 10.1097/00007632-199812010-00010. [DOI] [PubMed] [Google Scholar]
- Sosnik, Hauptmann, Karni, Flash When practice leads to co-articulation: the evolution of geometrically defined movement primitives. Exp Brain Res. 2004;156:422–438. doi: 10.1007/s00221-003-1799-4. (2004) [DOI] [PubMed] [Google Scholar]
- Stadler BM, Stadler PF, Wagner GP, Fontana W. The topology of the possible: formal spaces underlying patterns of evolutionary change. J Theor Biol. 2001 Nov 21;213(2):241–74. doi: 10.1006/jtbi.2001.2423. 2001. [DOI] [PubMed] [Google Scholar]
- Stein PS. Neuronal control of turtle hindlimb motor rhythms. Journal of Comparative Physiology A. Neuroethology, Sensory and Neural Behavior Physiology. 2005;191(3):213–29. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- Stubbs M, Harris M, Solomonow M, Zhou B, Lu Y, Baratta RV. Ligamento-muscular protective reflex in the lumbar spine of the feline. J Electromyogr Kinesiol. 1998;8(4):197–204. doi: 10.1016/s1050-6411(97)00012-6. [DOI] [PubMed] [Google Scholar]
- Sumbre G, Fiorito G, Flash T, Hochner B. Octopuses use a human-like strategy to control precise point-to-point arm movements. Curr Biol. 2006;16(8):767–72. doi: 10.1016/j.cub.2006.02.069. [DOI] [PubMed] [Google Scholar]
- Taimela S, Osterman K, Alaranta H, Soukka A, Kujala UM. Long psychomotor reaction time in patients with chronic low-back pain: preliminary report. Arch Phys Med Rehabil. 1993;74(11):1161–4. [PubMed] [Google Scholar]
- Tin C, Poon C-S. Internal models in sensorimotor integration: perspectives from adaptive control theory. J. Neural Eng. 2005;2:S147–S163. doi: 10.1088/1741-2560/2/3/S01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Blickhan R, Full RJ. Dynamic and static stability in hexapedal runners. J Exp Biol. 1994;197:251–69. doi: 10.1242/jeb.197.1.251. [DOI] [PubMed] [Google Scholar]
- Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res. 2007;165:299–321. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol. 2006;96(3):1530–46. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. Journal of neurophysiology. 2007;98(4):2144–56. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat Neurosci. 2000;3(4):391–8. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]
- Todorov E. Cosine tuning minimizes motor errors. Neural Comput. 2002;14(6):1233–60. doi: 10.1162/089976602753712918. [DOI] [PubMed] [Google Scholar]
- Todorov E. Optimality principles in sensorimotor control. Nat Neurosci. 2004;7(9):907–15. doi: 10.1038/nn1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E. Stochastic optimal control and estimation methods adapted to the noise characteristics of the sensorimotor system. Neural Comput. 2005;17(5):1084–108. doi: 10.1162/0899766053491887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Ghahramani Z. Unsupervised Learning of Sensory-Motor Primitives Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE; Cancun Mexico. 2003. [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5(11):1226–35. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- Todorov E, Li W, Pan X. From task parameters to motor synergies: A hierarchical framework for approximately-optimal control of redundant manipulators. J Robot Syst. 2005;22(11):691–710. doi: 10.1002/rob.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Experimental Brain Research. 1999;129(3):401–16. doi: 10.1007/s002210050908. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Cheung VC, d’Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol. 2006;5(4):2199–212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- Tresch M, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nature Neuroscience. 1999;2:162–167. doi: 10.1038/5721. [DOI] [PubMed] [Google Scholar]
- Tsao H, Hodges PW. Immediate changes in feedforward postural adjustments following voluntary motor training. Exp Brain Res. 2007;181(4):537–46. doi: 10.1007/s00221-007-0950-z. [DOI] [PubMed] [Google Scholar]
- Tsao H, Hodges PW. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J Electromyogr Kinesiol. 2008;18(4):559–67. doi: 10.1016/j.jelekin.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ. A mathematical approach to the mechanical capabilities of limbs and fingers. Adv Exp Med Biol. 2009;629:619–33. doi: 10.1007/978-0-387-77064-2_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. J Neurophysiol. 2009 Apr 15; doi: 10.1152/jn.90324.2008. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Yi JW, Brown D, McNamara RV, 3rd, Paul C, Lipson H. The tendon network of the fingers performs anatomical computation at a macroscopic scale. IEEE Trans Biomed Eng. 2007;54(6 Pt 2):1161–6. doi: 10.1109/TBME.2006.889200. [DOI] [PubMed] [Google Scholar]
- Van Dieen JH, Cholewicki J, Radebold A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine. 2003;28(8):834–41. [PubMed] [Google Scholar]
- Venkadesan M, Valero-Cuevas FJ. Neural control of motion-to-force transitions with the fingertip. J Neurosci. 2008;28(6):1366–73. doi: 10.1523/JNEUROSCI.4993-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani P, Terzuolo C. Trajectory determines movement dynamics. Neuroscience. 1982 Feb;7(2):431–7. doi: 10.1016/0306-4522(82)90277-9. 1982. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Pavlicev M, Cheverud JM. The road to modularity. Nat Rev Genet. 2007;8(12):921–31. doi: 10.1038/nrg2267. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Mezey J, Calabretta R. Natural selection and the origin of modules. In: Callebaut W, Rasskin-Gutman D, editors. Modularity: understanding the development and evolution of natural complex systems. MIT Press; Cambridge, MA: 2005. pp. 33–50. [Google Scholar]
- Wainwright PC. Evolution of feeding motor patterns in vertebrates. Curr Op Neurobiol. 2002;12:691–695. doi: 10.1016/s0959-4388(02)00383-5. [DOI] [PubMed] [Google Scholar]
- Wang W, Slotine JJ. On partial contraction analysis for coupled nonlinear oscillators. Biol Cybern. 2005;92(1):38–53. doi: 10.1007/s00422-004-0527-x. [DOI] [PubMed] [Google Scholar]
- Welch JJ, Waxman D. Modularity and the cost of complexity. Evolution. 2003;57:1723–1734. doi: 10.1111/j.0014-3820.2003.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 2007 Feb;189(2):155–69. doi: 10.1111/j.1748-1716.2006.01656.x. 2007. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Carp JS. Adaptive plasticity in the spinal cord. Adv. in Neurol. 1993;59:163–174. [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends in Cognitive Science. 2001;5(11):487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]
- Wyeth RC, Willows AO. Field behavior of the nudibranch mollusc Tritonia diomedea. Biol Bull. 2006;210(2):81–96. doi: 10.2307/4134598. [DOI] [PubMed] [Google Scholar]
- Zafeiriou DI. Primitive reflexes and postural reactions in the neurodevelopmental examination. Pediatr Neurol. 2004;31(1):1–8. doi: 10.1016/j.pediatrneurol.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Zedka M, Prochazka A, Knight B, Gillard D, Gauthier M. Voluntary and reflex control of human back muscles during induced pain. J Physiol. 1999;520(Pt 2):591–604. doi: 10.1111/j.1469-7793.1999.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]