Abstract
The E7 protein of high-risk human papillomaviruses (HR HPVs) targets pRb family members (pRb, p107 and p130) for degradation; low-risk (LR) HPV E7 only targets p130 for degradation. The effect of HR HPV 16 E7 and LR HPV 6 E7 on p130 intracellular localization and half-life was examined. Nuclear/cytoplasmic fractionation and immunofluorescence showed that, in contrast to control and HPV 6 E7-expressing cells, a greater amount of p130 was present in the cytoplasm in the presence of HPV 16 E7. The half-life of p130, relative to control cells, was decreased in the cytoplasm in the presence of HPV 6 E7 or HPV 16 E7, but only decreased by HPV 6 E7 in the nucleus. Inhibition of proteasomal degradation extended the half-life of p130, regardless of intracellular localization. These results suggest that there may be divergent mechanisms by which LR and HR HPV E7 target p130 for degradation.
Introduction
Cervical cancer is the second most common cancer worldwide and is the leading cause of cancer-related deaths in women in developing countries. Human papillomaviruses (HPVs) are associated with over 99% of all cervical cancer (Hebner and Laimins, 2006; zur Hausen, 2002). HPVs are classified as high-risk (HR) or low-risk (LR) depending on their pathogenicity. HR HPVs (e.g. 16, 18, 31, 33, and 45) are commonly associated with malignancies of the cervix and head and neck. HPVs 6 and 11 are classified as LR, and can cause condyloma acuminata (genital warts) (Longworth and Laimins, 2004).
The HPV life cycle is closely linked to epithelial differentiation. In normal epithelium, proliferation occurs in the basal cell layer; as cells migrate upwards they exit the cell cycle and differentiate. After virus infection, viral DNA is maintained at 50–100 copies in the basal cell layer (Hebner and Laimins, 2006; Longworth and Laimins, 2004). When the infected cells move to the differentiated suprabasal layer viral DNA is amplified and late genes are expressed resulting in mature virions. Several studies indicate that both HR and LR HPVs require the host cell to initiate cellular DNA replication (Hebner and Laimins, 2006; Longworth and Laimins, 2004; Snijders et al., 2006; zur Hausen, 2002). HPV E7 drives suprabasal cells into S phase and causes unscheduled DNA synthesis (Munger et al., 2001; (Collins et al., 2005); however, a recent report suggests that HPVs may initiate viral DNA replication when the cells are in the G2 phase (Wang et al., 2009). The E7 proteins of both HR and LR HPVs are required for viral DNA maintenance and/or amplification (Flores et al., 2000; McLaughlin-Drubin et al., 2005; Oh et al., 2004; Zhang and Roman, unpublished data).
E7 proteins consist of approximately 100 amino acid residues and can be divided into three regions: conserved region 1 (CR1, amino acids 2–15), CR2 (amino acids 16–38), and the C-terminal zinc-binding region (amino acids 39–98) containing two Cys-X-X-Cys motifs (Gage et al., 1990; Jewers et al., 1992; Munger et al., 2001). E7 proteins of HR HPV 16 and LR HPV 6 share 50% amino acid sequence identity and 15% conservative changes (Gage et al., 1990). Both HR and LR HPV E7 proteins bind pRb family members through their LXCXE binding motif (Dyson et al., 1989). In vitro and in vivo studies have revealed that HPV 16 E7, as compared to HPV 6 E7, has a greater affinity for pRb, p107, and p130 (Ciccolini et al., 1994; Gage et al., 1990). Although HPV 6 E7 has a lower affinity for binding p130 than HPV 16 E7, it is as efficient in targeting p130 for degradation (Zhang et al., 2006). Casein kinase II-mediated phosphorylation of HR and LR HPV E7 is necessary for effective binding to and destabilization of p130 (Genovese et al., 2008).
The pRb family of proteins (pRb, p107 and p130) plays important roles in regulating cell cycle control and differentiation (Gonzalez et al., 2001; Munger et al., 2001). pRb family members are homologous in the “pocket” region, composed of subdomains A and B and separated by a spacer region that is highly conserved among each of the proteins. p130 and p107 share more homology than pRb. p130 and p107 contain a region between the A and B subdomains that is responsible for inhibition of cyclin A/Cdk2 and cyclin E/Cdk2 (Classon and Dyson, 2001; Claudio et al., 2002). pRb family members each bind to specific members of the E2F family of transcription factors, which are responsible for the transcription of E2F-responsive genes, and hence S-phase entry (Cam and Dynlacht, 2003; Dimova and Dyson, 2005). E2F1, E2F2, and E2F3 are almost exclusively regulated by pRb, and are referred to as activator E2Fs. p130 and p107 normally associate with E2F4 and E2F5, repressor E2Fs, at the G0/G1 stage of the cell cycle (Dimova and Dyson, 2005; Helin et al., 1993).
p130 levels, like the other pRb family members, are regulated in response to the proliferative state of cells and are controlled by proteolysis in a phosphorylation-dependent manner (Classon and Dyson, 2001); (DeCaprio et al., 1992; Tedesco et al., 2002); (Shirodkar et al., 1992). p130 has been shown to be phosphorylated in cycling cells by cyclin D/Cdk4 or Cdk6, cyclin A/Cdk2 and cyclin E/Cdk2 (Classon and Dyson, 2001; Cobrinik, 2005). Cdk4/Cdk6, not Cdk2, is responsible for targeting p130 for degradation. In cycling cells Cdk4/ Cdk6 phosphorylates p130 on Ser 672, resulting in a hyperphosphorylated form of p130 that is targeted for degradation by an SCF complex (Tedesco et al., 2002). SCF complexes are a class of ubiquitin ligase (E3) enzymes that recognize and polyubiquitylate substrates, usually in a phosphorylation-dependent manner, targeting them for degradation by the 26S proteasome (Deshaies, 1999). In contrast to pRb and p107, p130 is phosphorylated by glycogen synthase kinase 3 (GSK3) in the loop region in the B subdomain and thus stabilized in growth-arrested and terminally differentiated cells (Litovchick et al., 2004).
p130 contains three nuclear localization signals (NLSs), two in the C-terminus and one in the loop region (Chestukhin et al., 2002). In undifferentiated cells, hypophosphorylated p130 is predominantly in the nucleus in the G0/G1 phase of the cell cycle. In S-phase, p130 is typically phosphorylated and transported to the cytoplasm where it is targeted for degradation. Shuttling of p130 between the nucleus and cytoplasm therefore provides a means of regulation (Chestukhin et al., 2002; Tedesco et al., 2002).
HPV 16 E7 has been detected in the cytoplasm and the nucleus and is known to have both cytoplasmic and nuclear targets (Nguyen et al., 2007); (Smotkin and Wettstein, 1987); (Smith-McCune et al., 1999); (Guccione et al., 2002). In support of such observations, it has been reported that HPV 16 E7 has two NLSs and one nuclear export signal (NES) (Knapp et al., 2009). In this study HPV 16 E7, in contrast to HPV 6 E7, was shown to either retain or relocalize p130 to the cytoplasm of HFKs. Leptomycin B, a CRM1/exportin 1 inhibitor, did not have an effect on HPV 16 E7 mediated p130 localization. In addition, p130 was shown to have a shorter half-life in the nucleus of HPV 6 E7 expressing HFKs compared to HPV 16 E7 expressing HFKs or control. These results suggest that HPV 6 E7 and 16 E7 may target p130 for degradation by different mechanisms.
Results
HPV E7-mediated degradation of p130 is independent of cyclin/Cdk activity
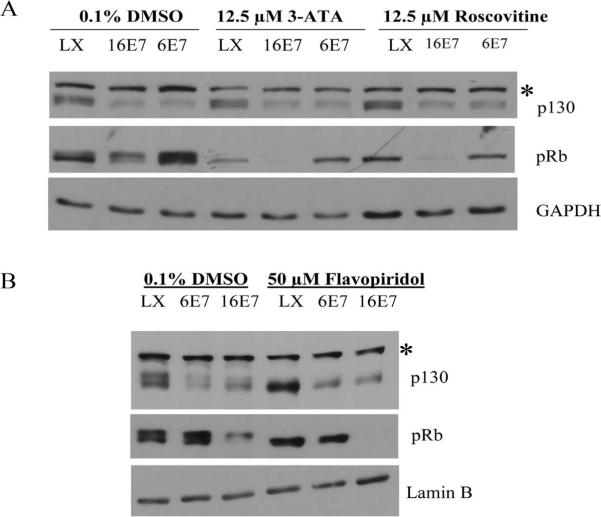
p130 is unique in that it is the only pRb family member that is targeted for degradation by both HPV 6 E7 and HPV 16 E7 (Zhang et al., 2006). In uninfected cells, p130 is phosphorylated by Cdk4/6 and is recognized by an SCF-Skp2 complex for degradation in a cell-cycle dependant manner (Tedesco et al., 2002). To ascertain whether E7-mediated degradation of p130 is dependent on cyclin/CDK activity, HFKs were infected with parental retrovirus LXSN, or retrovirus encoding HPV 16 E7 or HPV 6 E7. Infected cells were grown in keratinocyte serum-free medium (C-K-SFM) supplemented with human recombinant epidermal growth factor and bovine pituitary extract. Cells were then treated with 12.5 μM 3-ATA (an inhibitor of Cdk4/6) (Kubo et al., 1999), 12.5 μM roscovitine (an inhibitor of Cdk1, 2 and 5) (Meijer et al., 1997) or 50 μM flavopiridol (an inhibitor of Cdk 1, 2, 4, 6, and 7) (Sedlacek, 2001). Whole cell extracts were harvested and Western analysis performed. Analysis of the phosphorylation status of pRb validated that the inhibitors were functional. Hypophosphorylated and hyperphosphorylated forms of pRb were present in cells expressing LXSN and treated with vehicle only. However, in the presence of each of the Cdk inhibitors, only the hypophosphorylated form of pRb was detected (Fig. 1). This result was expected as pRb is known to be phosphorylated by cyclin D2-Cdk4, cyclin E-Cdk2, and cyclin A-Cdk2 (Cobrinik, 2005). The hyperphosphorylated band of p130 was lost only upon treatment with flavopiridol, but not 3-ATA nor roscovitine (Fig. 1). The steady-state level of p130 in HPV expressing cells in the presence of each Cdk inhibitor was compared to LXSN expressing cells. In the presence of CDK inhibitors, both HPV 6 E7 and HPV 16 E7 retained the ability to decrease the steady-state level of p130.
Fig 1.
Effect of Cdk inhibitors on p130 stability. HFKs were infected with parental retrovirus LXSN (LX) or retrovirus encoding HPV 16 E7 (16 E7) or HPV 6 E7 (6E7) and grown in C-K-SFM. Cells were then treated with 12.5 μM 3-ATA, 12.5 μM roscovitine, 50 μM flavopiridol or DMSO (vehicle) as a negative control. Whole cell extracts were harvested using EBC buffer and Western analysis performed using mouse monoclonal antibodies to p130, pRb, GAPDH and lamin B. *, unknown protein cross-reacting with anti-p130.
p130 localizes to the cytoplasm in keratinocytes transduced with HPV 16 E7
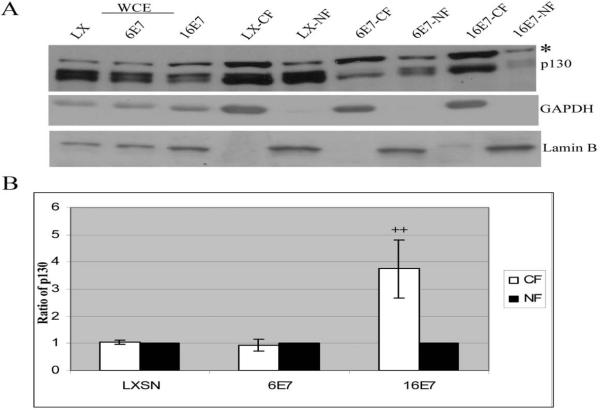
p130 is known to shuttle between the nucleus and cytoplasm (Chestukhin et al., 2002). HPV E7 has been shown to alter the localization of various proteins. Both HPV 6 and 16 E7 relocalize steroid receptor coactivator 1 (SRC1) to the cytoplasm (Baldwin et al., 2006), and HPV 16 E7 reduces the nuclear localization of p21Cip1 (Westbrook et al., 2002). Therefore, the effect of LR and HR HPV E7 on the intracellular localization of p130 was investigated. Sub-cellular fractionation was performed on HFKs transduced with LXSN (control), HPV 6 E7 or HPV 16 E7. Equal-volume fractions of cytoplasmic and nuclear extracts were obtained and Western analysis performed using antibodies to p130, lamin B (marker for nuclear fraction) and tubulin (marker for cytoplasmic fraction). Endogenous p130 was detected at similar levels in the cytoplasmic and nuclear fraction in cells expressing LXSN and HPV 6 E7. In contrast, HFKS expressing HPV 16 E7 had significantly more p130 (three fold) in the cytoplasm (Fig. 2).
Fig. 2.
Determination of p130 localization in nuclear and cytoplasmic fractions. (A) HFKs were infected as in Fig. 1 and grown in C-K-SFM. Cells were harvested using EBC buffer for whole cell extracts [WCEs (left three lanes)], or cytoplasmic and nuclear buffers to harvest cytoplasmic fractions (CF) and nuclear fractions (NF). Equal-volume fractions of nuclear and cytoplasmic protein were analyzed using monoclonal antibodies to p130. *, unknown protein cross-reacting with anti-p130. Antibodies to lamin B (nuclear marker) and GAPDH (cytoplasmic marker) were used to establish that the fractions were enriched. A representative experiment is shown. (B) Quantity one software was used for densitometric analysis. The nuclear fractions were then set to 1 and the ratio of cytoplasmic to nuclear p130 localization is shown. The average of ± standard deviation of 4 independent experiments is shown, (++), p< 0.01.
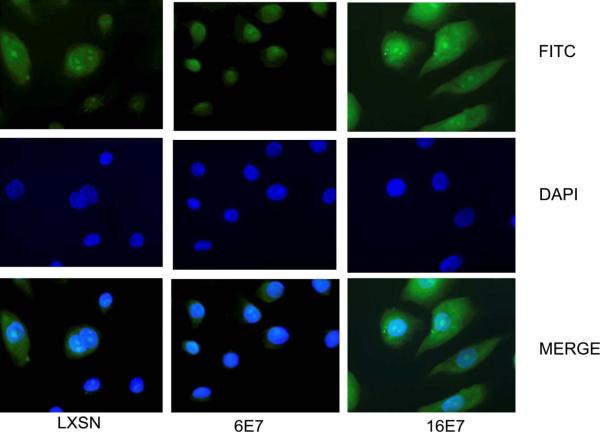
The distribution of p130 in HFKs expressing LXSN, HPV 6 E7 or HPV 16 E7 was also determined by immunofluorescence. Localization of p130 was mostly nuclear in HFKs transduced with LXSN and HPV 6 E7. p130 staining was mostly cytoplasmic in HFKs transduced with HPV 16 E7 (Fig. 3). This difference in the intracellular localization of p130 in the presence of HPV 16 E7 compared to that seen in LXSN or HPV 6 E7 expressing cells is consistent with sub-cellular fractionation results.
Fig. 3.
Determination of p130 localization by immunofluorescence. Immunofluorescence studies were performed on HFKs infected as in Fig.1 and grown on coverslips in C-K-SFM. Cells were incubated with rabbit polyclonal antibody to p130 and visualized by immunofluorescence using FITC-conjugated secondary antibody. A representative experiment is shown.
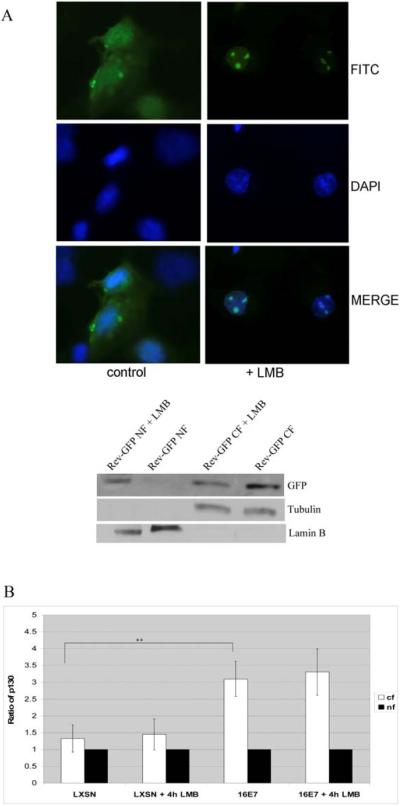
To test whether HPV 16 E7 relocalizes p130 to the cytoplasm via CRM1-dependent nuclear export, HFKs expressing HPV 16 E7 were treated with leptomycin B (LMB), a specific inhibitor of CRM1. LMB inhibits nuclear export of the human immunodeficiency virus Rev protein (Wolff et al., 1997). Therefore, we verified that the concentration of LMB used was sufficient to inhibit CRM1-mediated nuclear export in HFKs by transfecting the cells with a Rev-GFP plasmid. Treatment of transfected cells with LMB resulted in the accumulation of Rev-GFP fusion protein in the nuclei of cells, as detected by immunofluorescence or sub-cellular fractionation (Fig. 4A). However, LMB treatment did not affect cytoplasmic localization of p130 in HPV 16 E7 expressing cells as shown by sub-cellular fractionation (Fig. 4B). Similar results were obtained by immunofluorescence (data not shown).
Fig. 4.
Effect of LMB on the cytoplasmic localization of p130 in HPV 16 E7 expressing cells. (A) HFKs transfected with a plasmid encoding Rev-GFP and grown on coverslips were treated with LMB (1 ng/ml) for 4 h. Cells were fixed with 4% formaldehyde, counterstained with 5 μg/ml of 4'-6-Diamidino-2-phenylindole (DAPI) for 7 min at room temperature to label the nuclei, washed and mounted. Autofluorescence of Rev-GFP was detected by fluorescence microscopy (above). In parallel, whole cell extracts were harvested and Western analysis performed using antibodies to GFP (bottom). (B) HFKs were infected as in Fig. 1 and grown in C-K-SFM to 80% confluence. Cells were then treated with LMB (1 ng/ml) for 4 h and harvested for cytoplasmic and nuclear extracts. Equal-volume fractions of nuclear and cytoplasmic extracts were analyzed using antibodies to p130. Antibodies against lamin B (nuclear marker) and tubulin (cytoplasmic marker) were used as in Fig. 2. Densitometric analysis was performed using Quantity one software and the result normalized as in Fig 2. The averages and standard deviation from 4 experiments are shown, (++), p<0.01.
HPV 6 E7 can target p130 for degradation in the nucleus
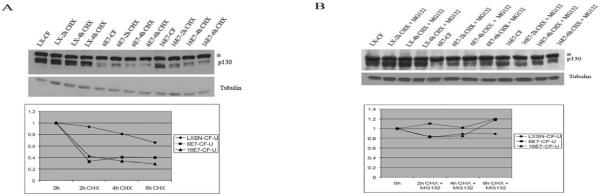
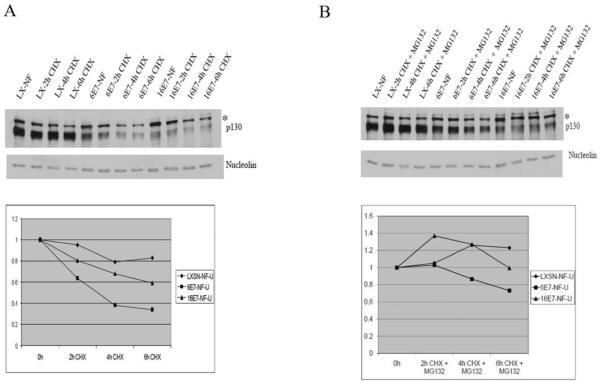
In whole cell extracts, the half-life of p130 in the presence of HPV 6 E7 and HPV 16 E7 is decreased relative to control cells (Zhang et al., 2006). The reduced half-life of p130 in the presence of HPV E7 is restored following treatment with proteasomal inhibitors, implying that E7 targets p130 for degradation through the proteasome pathway (Zhang et al., 2006). To determine the half-life of p130 in the cytoplasmic and nuclear fractions, HPV E7 expressing cells were treated with 0.25 mM cycloheximide, a protein synthesis inhibitor. At 0, 2, 4, and 6 h post-treatment, p130 levels in cytoplasmic and nuclear fractions were determined by Western blot analysis. The half-life of p130 in the cytoplasmic fraction of HPV 6 E7 and 16 E7 expressing cells was ~2 h on average and shorter than control cells (Fig. 5A). In a minority of cases, the half-life of p130 in the cytoplasm was shorter in HPV 16 E7 cells than HPV 6 E7 cells. However in HPV 6 E7 expressing cells, the half-life of nuclear p130 was between 3 and 4 h, less than half as long as in control and HPV 16 E7 expressing cells (Fig. 6A).
Fig. 5.
Decreased half-life of p130 in the cytoplasm in the presence of HPV 6 E7 and HPV 16 E7. (A) HFKs were infected as in Fig. 1. and treated with 0.25mM cycloheximide for the indicated times. Immunoblots of cytoplasmic extracts (top) and densitometry (bottom) from a representative experiment are shown. Cytoplasmic fractions were corrected for tubulin and are relative to LX. (B) Inhibition of p130 degradation by MG132. HFKs were infected as in A and treated with 0.25mM cycloheximide plus 50uM MG132 for the indicated times. Immunoblots of cytoplasmic extracts (top) and densitometry (bottom) from a representative experiment are shown.
Fig. 6.
Decreased half-life of p130 in the nucleus in the presence of HPV 6 E7. (A) HFKs were infected as in Fig. 5A. Immunoblots of nuclear extracts (top) and densitometry (bottom) from a representative experiment are shown. Nuclear fractions were corrected for nucleolin and are relative to LX. (B) MG132 inhibits p130 degradation. Experiments were conducted as in Fig. 5B. Immunoblots of nuclear extracts (top) and densitometry (bottom) from a representative experiment are shown.
To establish whether the decrease in p130 half-life by HPV E7 in the nuclear and cytoplasmic fractions was due to degradation through the proteasome pathway, HFKs were infected as above and treated with 0.25 mM cycloheximide plus 50 μM MG132, a proteasome pathway inhibitor. After treatment with MG132 the ability of either E7 to shorten the half-life of p130 in the cytoplasmic and nuclear fraction was decreased (Figs. 5B and 6B). This suggests that although both HPV 6 E7 and HPV 16 E7 efficiently target p130 for proteasomal degradation in the cytoplasm. HPV 6 E7 is also very efficient at targeting p130 for proteasomal degradation in the nucleus.
Discussion
HPV 16 E7 targets pRb family members for degradation. The ability of HPV 16 E7 to target pRb for degradation in particular, is necessary for malignant transformation (zur Hausen, 2000). HPV 6 E7 does not cause cancer and does not affect the stability of pRb or p107 (Demers et al., 1994; Zhang et al., 2006); however it does target p130 for degradation (Zhang et al., 2006). pRb family members play a key role in regulating progression through the cell cycle. p130 is specifically up-regulated in G0 and is responsible for keeping cells in a differentiated state (Cobrinik, 2005). The fact that both HR and LR E7 target p130 for degradation may indicate that p130 is important for the HPV life cycle. Targeting p130 for degradation may be conducive to creating an “S-phase-like” environment (Banerjee et al., 2006; Collins et al., 2005; Munger et al., 2001). Zhang et al., proposed that the mechanism of degradation of p130 by HPV 16 E7 and HPV 6 E7 might be similar based on mutational analysis (Zhang et al., 2006). However, we have discovered HR and LR E7 may achieve this by different mechanisms.
Experiments were designed to test whether p130 degradation by LR and HR HPV E7 was dependent on cyclin/Cdk activity, since HPV E7 enhances entry of cells into the cell-cycle. An increase in the quantity of hyperphosphorylated p130 may result in enhanced degradation of p130 (Tedesco et al., 2002). Treatment with three Cdk inhibitors, flavopiridol, 3-ATA and roscovitine, showed that both HR and LR HPV E7 retained their ability to target p130 for degradation (Fig. 1). Therefore, destabilization of p130 by either HPV E7 may not simply be a result of entry into the cell cycle induced by E7. Rather, our results demonstrate that degradation of p130 by HPV E7 is independent of cyclin/Cdk activity. Previous studies have shown that, in human fibroblasts, inhibition of Cdk4/6 results in the loss of the hyperphosphorylated form of p130 (Tedesco et al., 2002). However, in E7 expressing HFKs treated with 3-ATA, a Cdk4/6 inhibitor, there was no difference in p130 phosphorylation status as compared to E7 expressing cells treated with DMSO control (Fig. 1). Flavopiridol and 3-ATA are both active against Cdk4/6, and flavopiridol and roscovitine are active against Cdk2. Treatment with flavopiridol resulted in the abolishment of the hyperphosphorylated form of p130. This suggests that in HFKs, hyperphosphorylated p130 may be derived from phosphorylation by both Cdk2 and Cdk4/6.
HPV 16 E7 has been detected in the cytoplasm and in the nucleus. In most experiments where E7 has been localized to the nucleus, E7 was visualized by immunofluorescence (Smith-McCune et al., 1999, Guccione et al., 2002), whereas when E7 has been localized to cytoplasm it has been visualized by western analysis of sub-cellular fractions (Nguyen et al., 2007; Smotkin and Wettstein, 1987). Here we show that more p130 localized to the cytoplasm in the presence of HPV 16 E7 (Figs. 2 and 3). In contrast, the localization of p130 in the presence of HPV 6 E7 was similar to control cells (Figs. 2 and 3). Identical results were also obtained in experiments where cells were grown under differentiated conditions (data not shown). While the current study was in progress, two nuclear localization signals and one nuclear export signal were identified in HPV 16 E7 (Knapp et al., 2009), providing further evidence that HPV 16 E7 shuttles between the cytoplasm and nucleus. Analysis of putative NESs (NESpredictor www.cbs.dtu.dk/) suggests that HPV 6 E7 may have an NES in the N-terminus, in contrast to the identified NES in HPV 16 E7 which is in the C-terminus (Knapp et al., 2009). It is possible that upon binding to p130, a confirmation change occurs in HPV 6 E7 resulting in the NES being concealed. Alternatively, binding of HPV 16 E7 to p130 may result in a conformational change that causes the NLS to be inaccessible. Slight variations in the p130 localization results obtained by subcellular fractionation versus immunofluorescence may be due to differences in the p130 epitopes recognized by the monoclonal and polyclonal antisera, p130 epitopes being masked in the cytoplasm and/or sensitivity issues with the immunofluorescence assay due to p130 being diffuse in the cytoplasm.
In HPV 16 E7 expressing cells, p130 localized mainly to the cytoplasm with or without LMB treatment (Fig. 5). This may be because HPV 16 E7-mediated p130 nuclear export is CRM1-independent; alternatively, HPV 16 E7 may cause p130 to be retained in the cytoplasm. In published reports, p130 cytoplasmic localization has been shown to be insensitive to treatment with LMB (Chestukhin et al., 2002). Additionally, although the C-terminus of HPV 16 E7 fused to GFP was shown to accumulate in the nucleus with LMB treatment (Knapp et al., 2009), no studies have shown that the subcellular localization of full length HPV 16 E7 protein is LMB sensitive.
This is the first study, to our knowledge, to address the capacity of nuclear proteasomes in p130 degradation. It has long been established that proteins can be targeted for degradation in the cytoplasm. There is robust evidence to support that proteins can also be targeted for degradation in the nucleus (von Mikecz, 2006). The data presented here suggest that in the presence of HPV 16 E7, p130 degradation is mainly mediated by cytoplasmic proteasomes. However, for HPV 6 E7, nuclear proteasomes may also play a significant role.
HPV 16 E7 may preferentially target p130 for degradation in the cytoplasm after sequestering it there, whereas HPV 6 E7 may have evolved a mechanism for degrading p130 more efficiently in the nucleus. However, p130 degradation does not occur exclusively in either the nucleus or cytoplasm for HPV 6 E7 or HPV 16 E7. There may be more than one mechanism by which p130 is targeted for degradation by HPV 6 E7 and HPV 16 E7. Zhang et al. proposed that the mechanism of degradation of p130 by HPV 16 E7 and HPV 6 E7 might be similar, because both HPV 16 E7 and HPV 6 E7 N-terminal mutants lose the ability to target p130 for degradation (Zhang et al., 2006). p600 was identified as a cellular protein that binds to both HPV 6 E7 and HPV 16 E7 (Huh et al., 2005). p600 has been speculated to be an E3 ubiquitin ligase (Huh et al., 2005), and has been reported to bind the N-terminus of HPV 16 E7. Therefore, p600 is a candidate regulatory protein needed for efficient p130 degradation by both HPV 16 E7 and HPV 6 E7.
Huh et al. reported that HPV 16 E7 recruits a cullin 2 E3 ubiquitin ligase complex that contributes to degradation of pRb. The authors suggest that this E3 ubiquitin ligase also contributes to the degradation of p130. HPV 6 E7 was not found to bind this cullin 2 complex (Huh et al., 2007). It is possible that HPV 6 E7 facilitates the interaction of another E3 ubiquitin ligase with p130. Additionally, HPV 6 E7, similar to human T-cell leukemia virus type 1 tax, may function as a molecular bridge (Kehn et al., 2005), or as a chaperone, like simian virus large T antigen (Srinivasan et al., 1997; Stubdal et al., 1997), to facilitate interaction of p130 directly with the proteasome.
In conclusion, our data argue that although both LR and HR HPV target p130 for degradation, which may be a critical aspect of their viral life cycles, the mechanism by which the E7 proteins destabilize p130 may be different. HPV 6 E7 seems to have evolved to co-exist better with its host cell, causing a productive infection (a benign condyloma) but not a lesion which is non-productive for the virus (a carcinoma). As such, HPV 6 E7 causes less perturbation of the Rb family circuitry than HPV 16 E7, consequently raising fewer distress signals for the cell to counter. Since HPV 6 E7 distinguishes between the Rb family members but HPV 16 E7 does not, the two E7 proteins may have evolved different mechanisms to deal with p130. By altering the intracellular localization of p130 so that more is found in the cytoplasm, 16 E7 has not only targeted p130 for degradation but also effectively removed p130 from its nuclear targets.
Materials and Methods
Cell culture
Human foreskin keratinocytes (HFKs) were isolated from neonatal foreskins. The foreskin was digested with trypsin-EDTA, and keratinocytes released from the tissue were plated in E medium in the presence of mitomycin C-treated 3T3/J2 fibroblasts. At 60% confluence, cells were subcultured in E medium on the feeder cells. At about 80% confluence, the cells were frozen. The frozen cells were subsequently thawed in complete serum-free medium (C-K-SFM; GIBCO/BRL) supplemented with human recombinant EGF (GIBCO/BRL) and bovine pituitary extract (GIBCO/BRL). Where noted, cells were treated with the following inhibitors: 0.25 mM cycloheximide (Sigma), 50 μM MG132 (Sigma), 12.5 μM 3-ATA (Alexis Biochemicals), 12.5 μM roscovitine (Sigma) or 50 μM flavopiridol (Sigma). Cells treated with 3-ATA were p16 null.
Retroviruses and retroviral infection
Third-passage HFKs were grown to about 40% confluence in a 10-cm-diameter dish and infected with 5 ml of the recombinant retrovirus or parental virus in the presence of 8 μg of polybrene per ml. After 6 h the cells were fed with C-K-SFM and kept at 37°C with 5% CO2 for 48 h. The cells were then transferred to four 10-cm diameter dishes and selected with G418 (200 μg/ml) for 3 days. Selected cells were expanded and used for experiments, generally when at 80% confluence.
Western blotting
For fractionation experiments, cell fractionation buffer (Ambion) was used to resuspend cells and cells were placed on ice for 10 mins. Cells were pelleted at low speed to obtain supernatant, which is the cytoplasmic fraction. 2× Laemmli buffer (4% SDS, 20% glycerol, 0.125M Tris-HCl) was then added to the pellet, and the pellet vigorously resuspended to obtain the nuclear fraction. For whole cell extracts, cells were lysed with extraction buffer C (EBC: 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% NP-40). Protease and phosphatase inhibitors were added before use: protease inhibitor cocktail (Sigma), 125 μM Na3VO4 and 50 mM NaF. Protein concentrations were determined using the Bio-Rad DC protein assay kit. For localization experiments, equal-volume fractions of cytoplasmic and nuclear extracts were used. Proteins were separated by 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE). The proteins were then transferred to nitrocellulose and probed with the following antibodies: monoclonal p130 (BD Biosciences), pRb (BD Biosciences), tubulin (Sigma), lamin B, nucleolin (Santa Cruz Biotechnology) and GAPDH (Chemi-con). For half-life experiments equal quantity of protein was used and samples normalized to tubulin (for cytoplasmic fractions) or nucleolin (for nuclear fractions).
Immunofluorescence
Infected HFKs were grown on coverslips in C-K-SFM. Cells were fixed in 4% paraformaldehyde for 10 min. Cells were then rinsed and permeabilized with 0.1% Triton-X 100 in PBS for 10 min. The cells were immersed in 0.1% BSA/PBS for 30 min to block nonspecific binding. Cells were incubated with polyclonal antibody to p130 (Santa-Cruz Biotechnology) diluted in 0.1% BSA/PBS for 1.5 h at room temperature. Cells were then washed with 0.1% BSA/PBS at room temperature, and incubated with FITC-conjugated secondary antibody (Santa Cruz Biotechnology). Cells were counterstained with 5 μg/ml of 4'-6-Diamidino-2-phenylindole (DAPI) for 7 min at room temperature, washed again and mounted. Fluorescent images were obtained by using Nikon Eclipse microscope. For experiments with Rev-GFP plasmid (Ludwig et al., 1999), HFKs were transfected with FuGENE 6 transfection reagent.
Statistical analysis
Student two tailed t-test was used for statistical analysis. p<0.05 was considered significant.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 AI49294) and the Elwert Award from Indiana University School of Medicine. We thank Johnny He for providing the Rev-GFP plasmid and the fluorescence microscope. We are grateful to Grova Mae Lewis for technical support, Benyue Zhang, Harikrishna Nakshatri, Johnny He, Darron Brown and Maureen Harrington for helpful discussions, and Mark Goebl, Brian Laing and Joanna Walker for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin A, Huh KW, Munger K. Human papillomavirus E7 oncoprotein dysregulates steroid receptor coactivator 1 localization and function. J Virol. 2006;80:6669–6677. doi: 10.1128/JVI.02497-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee NS, Genovese NJ, Noya F, Chien WM, Broker TR, Chow LT. Conditionally activated E7 proteins of high-risk and low-risk human papillomaviruses induce S phase in postmitotic, differentiated human keratinocytes. J Virol. 2006;80:6517–6524. doi: 10.1128/JVI.02499-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam H, Dynlacht BD. Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cell. 2003;3:311–316. doi: 10.1016/s1535-6108(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Chestukhin A, Litovchick L, Rudich K, DeCaprio JA. Nucleocytoplasmic shuttling of p130/RBL2: novel regulatory mechanism. Mol Cell Biol. 2002;22:453–468. doi: 10.1128/MCB.22.2.453-468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccolini F, Di Pasquale G, Carlotti F, Crawford L, Tommasino M. Functional studies of E7 proteins from different HPV types. Oncogene. 1994;9:2633–2638. [PubMed] [Google Scholar]
- Classon M, Dyson N. p107 and p130: versatile proteins with interesting pockets. Exp Cell Res. 2001;264:135–147. doi: 10.1006/excr.2000.5135. [DOI] [PubMed] [Google Scholar]
- Claudio PP, Tonini T, Giordano A. The retinoblastoma family: twins or distant cousins? Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-9-reviews3012. reviews3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- Collins AS, Nakahara T, Do A, Lambert PF. Interactions with pocket proteins contribute to the role of human papillomavirus type 16 E7 in the papillomavirus life cycle. J Virol. 2005;79:14769–14780. doi: 10.1128/JVI.79.23.14769-14780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio JA, Furukawa Y, Ajchenbaum F, Griffin JD, Livingston DM. The retinoblastoma-susceptibility gene product becomes phosphorylated in multiple stages during cell cycle entry and progression. Proc Natl Acad Sci U S A. 1992;89:1795–1798. doi: 10.1073/pnas.89.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers GW, Foster SA, Halbert CL, Galloway DA. Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7. Proc Natl Acad Sci U S A. 1994;91:4382–4386. doi: 10.1073/pnas.91.10.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. doi: 10.1038/sj.onc.1208612. [DOI] [PubMed] [Google Scholar]
- Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Flores ER, Allen-Hoffmann BL, Lee D, Lambert PF. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol. 2000;74:6622–6631. doi: 10.1128/jvi.74.14.6622-6631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage JR, Meyers C, Wettstein FO. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990;64:723–730. doi: 10.1128/jvi.64.2.723-730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese NJ, Banerjee NS, Broker TR, Chow LT. Casein kinase II motif-dependent phosphorylation of human papillomavirus E7 protein promotes p130 degradation and S-phase induction in differentiated human keratinocytes. J Virol. 2008;82:4862–4873. doi: 10.1128/JVI.01202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SL, Stremlau M, He X, Basile JR, Munger K. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J Virol. 2001;75:7583–7591. doi: 10.1128/JVI.75.16.7583-7591.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione E, Massimi P, Bernat A, Banks L. Comparative analysis of the intracellular location of the high- and low-risk human papillomavirus oncoproteins. Virology. 2002;293:20–25. doi: 10.1006/viro.2001.1290. [DOI] [PubMed] [Google Scholar]
- Hebner CM, Laimins LA. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16:83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- Helin K, Wu CL, Fattaey AR, Lees JA, Dynlacht BD, Ngwu C, Harlow E. Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation. Genes Dev. 1993;7:1850–1861. doi: 10.1101/gad.7.10.1850. [DOI] [PubMed] [Google Scholar]
- Huh K, Zhou X, Hayakawa H, Cho JY, Libermann TA, Jin J, Harper JW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol. 2007;81:9737–9747. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102:11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewers RJ, Hildebrandt P, Ludlow JW, Kell B, McCance DJ. Regions of human papillomavirus type 16 E7 oncoprotein required for immortalization of human keratinocytes. J Virol. 1992;66:1329–1335. doi: 10.1128/jvi.66.3.1329-1335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehn K, Fuente Cde L, Strouss K, Berro R, Jiang H, Brady J, Mahieux R, Pumfery A, Bottazzi ME, Kashanchi F. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene. 2005;24:525–540. doi: 10.1038/sj.onc.1208105. [DOI] [PubMed] [Google Scholar]
- Knapp AA, McManus PM, Bockstall K, Moroianu J. Identification of the nuclear localization and export signals of high risk HPV16 E7 oncoprotein. Virology. 2009;383:60–68. doi: 10.1016/j.virol.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Nakagawa K, Varma RK, Conrad NK, Cheng JQ, Lee WC, Testa JR, Johnson BE, Kaye FJ, Kelley MJ. The p16 status of tumor cell lines identifies small molecule inhibitors specific for cyclin-dependent kinase 4. Clin Cancer Res. 1999;5:4279–4286. [PubMed] [Google Scholar]
- Litovchick L, Chestukhin A, DeCaprio JA. Glycogen synthase kinase 3 phosphorylates RBL2/p130 during quiescence. Mol Cell Biol. 2004;24:8970–8980. doi: 10.1128/MCB.24.20.8970-8980.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68:362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig E, Silberstein FC, van Empel J, Erfle V, Neumann M, Brack-Werner R. Diminished rev-mediated stimulation of human immunodeficiency virus type 1 protein synthesis is a hallmark of human astrocytes. J Virol. 1999;73:8279–8289. doi: 10.1128/jvi.73.10.8279-8289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Bromberg-White JL, Meyers C. The role of the human papillomavirus type 18 E7 oncoprotein during the complete viral life cycle. Virology. 2005;338:61–68. doi: 10.1016/j.virol.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- Nguyen CL, Eichwald C, Nibert ML, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin. J Virol. 2007;81:13533–13543. doi: 10.1128/JVI.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ST, Longworth MS, Laimins LA. Roles of the E6 and E7 proteins in the life cycle of low-risk human papillomavirus type 11. J Virol. 2004;78:2620–2626. doi: 10.1128/JVI.78.5.2620-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlacek HH. Mechanisms of action of flavopiridol. Crit Rev Oncol Hematol. 2001;38:139–170. doi: 10.1016/s1040-8428(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Shirodkar S, Ewen M, DeCaprio JA, Morgan J, Livingston DM, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Smith-McCune K, Kalman D, Robbins C, Shivakumar S, Yuschenkoff L, Bishop JM. Intranuclear localization of human papillomavirus 16 E7 during transformation and preferential binding of E7 to the Rb family member p130. Proc Natl Acad Sci U S A. 1999;96:6999–7004. doi: 10.1073/pnas.96.12.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D, Wettstein FO. The major human papillomavirus protein in cervical cancers is a cytoplasmic phosphoprotein. J Virol. 1987;61:1686–1689. doi: 10.1128/jvi.61.5.1686-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208:152–164. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, McClellan AJ, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky JL, Pipas JM. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubdal H, Zalvide J, Campbell KS, Schweitzer C, Roberts TM, DeCaprio JA. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mikecz A. The nuclear ubiquitin-proteasome system. J Cell Sci. 2006;119:1977–1984. doi: 10.1242/jcs.03008. [DOI] [PubMed] [Google Scholar]
- Wang HK, Duffy AA, Broker TR, Chow LT. Robust production and passaging of infectious HPV in squamous epithelium of primary human keratinocytes. Genes Dev. 2009;23:181–194. doi: 10.1101/gad.1735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook TF, Nguyen DX, Thrash BR, McCance DJ. E7 abolishes raf-induced arrest via mislocalization of p21(Cip1) Mol Cell Biol. 2002;22:7041–7052. doi: 10.1128/MCB.22.20.7041-7052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff B, Sanglier JJ, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chen W, Roman A. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc Natl Acad Sci U S A. 2006;103:437–442. doi: 10.1073/pnas.0510012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]