Abstract
The G-protein coupled receptor (GPCR)-kinase interacting proteins 1 and 2 (GIT1 and GIT2) are scaffold proteins with ADP-ribosylating factor GTPase activity. GIT1 and GIT2 control numerous cellular functions and are highly expressed in neurons, endothelial cells and vascular smooth muscle cells (VSMC). GIT1 promotes dendritic spine formation, growth and motility in cultured neurons, but its role in brain in vivo is unknown. By using global GIT1 knockout mice (GIT1 KO), we show that deletion of GIT1 results in markedly reduced dendritic length and spine density in the hippocampus by 36.7 % (p < 0.0106*) and 35.1% (p< 0.0028*) respectively compared to WT controls. This correlated with their poor adaptation to new environments as shown by impaired performance on tasks dependent on learning. We also studied the effect of GIT1 gene deletion on brain microcirculation. In contrast to findings in systemic circulation, GIT1 KO mice had an intact blood-brain barrier and normal regional cerebral blood flow as determined with radiotracers. Thus, our data suggest that GIT1 plays an important role in brain in vivo by regulating spine density involved in synaptic plasticity that is required for processes involved in learning.
Keywords: G-protein coupled receptor kinase interacting protein 1, GIT1; spine formation; cytoskeleton dynamics; brain morphology; blood brain barrier; BBB
1. Introduction
G protein–coupled receptors (GPCRs) are cell surface receptors for a number of neuromodulators including amines (dopamine, noradrenaline), amino acid transmitters (glutamate, GABA), peptides (opioids, neurotensin), and endocrine releasing factors. GPCRs mediate a variety of physiological events including chemosensory recognition (olfaction, taste) and endocrine regulation (Hamm and Gilchrist, 1996). Over 360 nonsensory GPCRs have been characterized (Watson and Arkinstall, 1994) of which more than 90% are expressed in the brain, where they play important roles in numerous neuronal functions (Vassilatis et al., 2003). Aberrant activity of GPCR systems in the brain may contribute to pathological conditions, ranging from hypodopaminergic movement disorders to mania and depression (Wise et al., 2004).
GPCRs are desensitized following activation by phosphorylation mediated by GPCR kinases (GRKs). Phosphorylated receptors are bound by arrestins preventing further stimulation of G proteins and downstream signaling pathways. GIT1 (GPCR kinase interacting protein 1) was originally identified as a protein interacting with GRK2 (Premont et al., 1998). Subsequently another highly related protein GIT2 was also identified (Premont et al., 2000). The main difference between these proteins is that GIT1 exists primarily in its full-length form, while GIT2 is extensively alternatively spliced in a tissue specific manner (Hoefen and Berk, 2006).
A complete expression pattern analysis of GIT genes were provided by (Schmalzigaug et al., 2007) using transgenic lines with β-Gal reporters inserted into the genes. Both GIT1 and GIT2 are found to be coexpressed in all regions of the brain, with the exception being the cerebellar granule cells where GIT1 was absent (Schmalzigaug et al., 2007). In the brain, GIT1 was found to interact directly with Huntingtin protein through its C-terminal regions therby accelerating the aggregation of the mutant protein. Intriguingly, large amounts of C-terminal GIT1 fragments are found in the brains of patients with Huntington’s disease, which could be a significant factor in the pathogenesis (Goehler et al., 2004). In cultured hippocampal neurons, GIT1 localizes to synapses through its synaptic localization domain (SLD) and recruits actin regulators like PAK (p21 activated kinase) and PIX (PAK interacting protein) to these sites thus regulating spine morphogenesis and synapse formation (Zhang et al., 2005). GIT1 Arf-GAP mutants inhibit neurite outgrowth, suggesting an important role for GIT1 in neurite extension through Rac-ARF6 pathway (Albertinazzi et al., 2003). In contrast GIT2 has been shown to play an important role in focal adhesion turnover (Frank et al., 2006) and in neutrophil migration suggesting important functions in the immune system (Mazaki et al., 2006). Recently, Premont’s group showed that GIT1 KO mice exhibit impaired fear response thus implicating a role of GIT1 in learning and memory (Schmalzigaug et al., 2009). Here, in this study using a global GIT1 KO, we show that these mice have defective spine density and dendritic length that correlates with their learning deficits.
2. Results
2.1 Genotyping of GIT1 KO mice
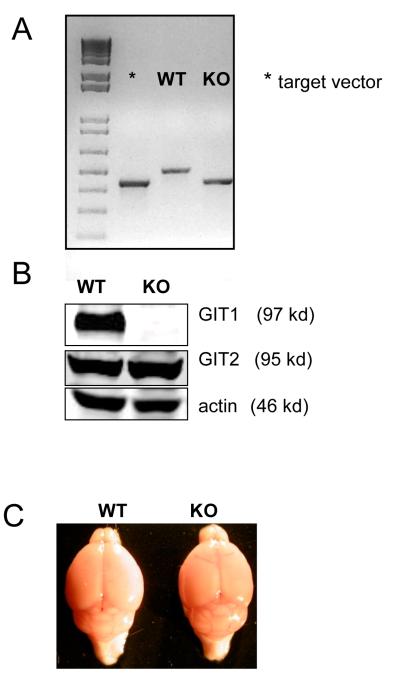
Genotyping of GIT1 WT and KO mice were confirmed using DNA isolated from tail clips (Fig. 1A). The WT allele yielded a 521-bp fragment while the KO allele yielded a 443-bp fragment. Western blot analysis of brain lysates of GIT1 KO mice (3 month old) showed no detectable GIT1 protein expression. Similar GIT2 expression levels were observed in the brain lysates of three month old GIT1 WT and KO mice (Fig. 1B).
Figure. 1. Generation of GIT1 KO mice.
(A) PCR genotyping with primers for GIT1 WT and KO. A 521 bp fragment was generated from the WT allele, and a 443 bp fragment from the KO allele. * = target construct used to generate the knockout cell line.
(B) Expression of GIT1 and GIT2 in whole brain lysate of 3 month-old GIT1 WT and KO brain by Western blot analysis. Actin served as an internal control.
(C) Representative image of the macroscopic analysis of 3-month old brain of GIT1 WT and KO mice revealed no major abnormalities (WT, n=3; KO, n=3)
2.2 Histopathological Analysis of GIT1 KO brain
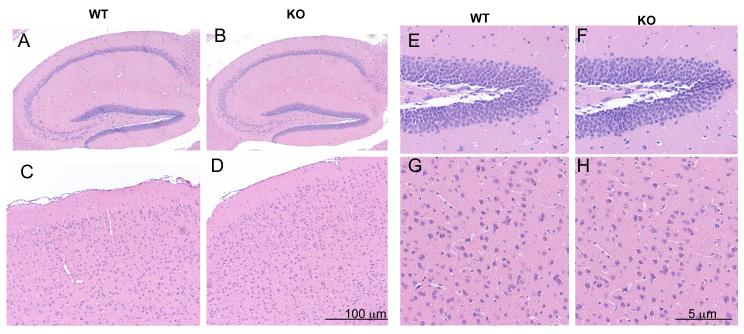
Gross macroscopic analysis of the adult brain revealed no obvious changes in GIT1 KO compared to WT (Fig. 1C). Specific regions of the brain (hippocampus, cortex) revealed no abnormalities in GIT1 KO compared to WT controls (Fig 2). Sagittal sections of the hippocampus revealed that the characteristic cytoarchitecture was unaltered in GIT1 KO mice. The dentate gyrus, CA3 and CA1 were fully formed in GIT1 WT and KO mice. No obvious changes in the hippocampal regions were observed (Fig. 2A, 2B). Similarly, cortical layers in the GIT1 KO appeared normal with no noticeable changes (Fig. 2C, 2D). Higher magnification of these brain regions (Fig. 2E-H) also showed no differences. Hematoxylin and eosin stained sections of the cerebellum from GIT1 KO mice showed that foliation was normal and the molecular layer, Purkinje cell and granule cell layers were present (Supplementary Fig. 1A). Together these results show that GIT1 KO has normal brain morphology compared to GIT1 WT.
Figure. 2. Gross morphology of GIT1 WT and KO brain.
Hematoxylin sections (sagittal) of hippocampus (A, B) and cortex (C, D) showed normal cell patterning between GIT1 WT and KO suggesting that characteristic cytoarchitecture was unaltered in GIT1 KO. High power magnification of hippocampus (E, F) and cortex (G, H) in GIT1 WT and KO showed no differences. (WT, n=3; KO, n=3)
2.3 Dendritic length and spine density are decreased in GIT1 KO
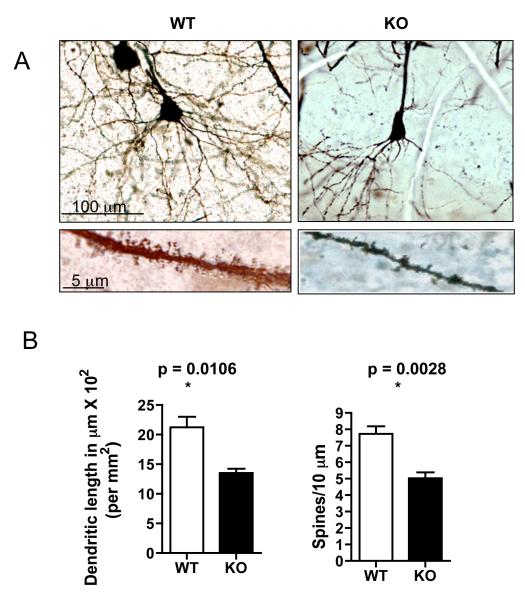
GIT1-βPIX-Cdc42-PAK pathway has shown to play a role in neurite growth (Lieu et al., 2006) and inhibition of recruitment of GIT1 to synapses was shown to impair spine formation in cultured rat hippocampal neurons (Segura et al., 2007). Therefore we analyzed dendritic length and spine density in the hippocampus (CA1 region) using Golgi-Cox impregnation method in GIT1 WT and KO mice (Fig 3A,C). Total dendritic length in micron (normalized per mm2) was decreased by 36.7% [WT= 21.4 ± 1.8 μm × 102; KO = 13.54 ± 0.72 × 102 μm, p=0.0106*] and spines/10 μm was decreased by 35.1 % (WT= 7.74 ± 0.5; KO = 5.02 ± 0.4, p=0.028*) in GIT1 KO compared to WT controls. Similarly decreased dendritic length was observed in the cortex of the GIT1 KO mice (data not shown). No gender differences were observed in GIT1 KO mice.
Figure. 3. Dendritic length and spine density are decreased in the hippocampus of GIT1 KO mice.
(A) Golgi-Cox stain of hippocampal neurons in the CA1 region of GIT1 WT and KO.
(B) Quantitative analysis of total dendritic length in micron (per mm2) and spine density (spines/10 μm) in hippocampal neurons of GIT1 WT and KO. All values are expressed as mean ± SEM. (WT, n=6; KO, n=4).
2.4 GIT1 KO mice adapt poorly to new or changing environments
Since spine density and dendritic length were decreased in GIT1 KO mice, we performed learning and memory tests using operant training and fixed interval fading as described in McKerchar et al., 2005 and Sagare et al., 2007.
(a) Operant Training
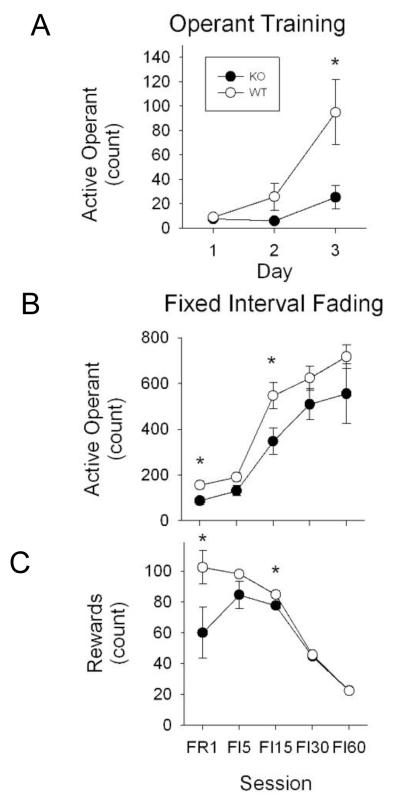
On Day 3 of the operant training GIT1 KO mice made fewer active operant responses compared to WT controls (Fig 4A, p<0.05*). Repeated measures of ANOVA showed a genotype effect (WT vs KO) with fewer active operant responses made by GIT1 KO resulting in fewer dipper presentation and rewards compared to GIT1 WT controls (Supplementary Table 1A).
Figure. 4. GIT1 KO shows reduced ability to adjust to new surroundings suggesting impaired learning.
(A) Operant training consists of 3 sessions in which reward (evaporated milk) is presented on a variable 60 second (s) schedule with a fixed ratio 1 second (FR 1) for snout entries into the hole. Active operant/response is count of the number of hole pokes the mice makes to get the reward during operant training. X-axis indicates the session in days and Y-axis is the active operant measured in counts. Active response showed statistically difference on the final day of training (Day 3, p <0.05*) with the GIT1 KO making less active operant response compared to WT controls. All values are expressed as mean ± SEM. (WT, n=7; KO, n=6).
(B&C) Fixed Interval Fading consists of 5 sessions in which reward schedule was changed from a fixed ratio 1s (FR 1) to a fixed interval of 5s (FI 5) and then the interval was increased to 15s (FI 15), 30s (FI 30) and 60s (FI 60). X-axis indicates the different sessions and Y-axis are the active operant (measured in counts, Fig 6B) and rewards (in counts, Fig 6C). Active responses and rewards were statistically different with GIT1 KO mice making less operant responses and earning fewer rewards compared to WT controls at FR 1 (p=0.02* and p <0.05*, respectively) and FI 15 (p=0.005* and p= 0.026*, respectively). All values are expressed as mean ± SEM (WT, n=7; KO, n=6).
(b) Fixed Interval Fading
Active operant response counts and rewards were lower for the GIT1 KO compared to GIT1 WT at FR 1 (p=0.02* and p <0.05* respectively) and FI 15 (p=0.005*, and p= 0.026*, respectively) (Fig 4B, C). Repeated measures of ANOVA showed differences for the active operant response counts, dipper presentation and rewards (Supplementary Table 2A).
2.5 Blood brain barrier (BBB) and cerebral blood flow are unaltered in GIT1 KO
We hypothesized that GIT1 KO mice may have impaired endothelial cells (EC) function leading to defective BBB and increased vascular permeability and leakage, since we previously showed that GIT1 is an essential mediator for thrombin signaling in EC, including endothelial permeability (van Nieuw Amerongen et al., 2004). Cerebral vascular space (μl/g) and vascular permeability (ml/g/sec × 10−6) were measured by infusion of 99Tc-albumin and 3H-inulin respectively. There were no differences in the vascular space and permeability between the GIT1 WT and KO (frontal cortex: WT = 28.51 ± 3.40 μl/g, 2.23 ± 0.27 ml/g/sec × 10−6; KO = 31.03 ± 3.69 μl/g, 2.54 ± 0.27 ml/g/sec × 10−6,) suggesting that the cerebral vasculature was normal and BBB was intact (Supplementary Fig. 1A, 1B). To confirm further that the BBB was intact, we probed for the expression of the EC tight junction proteins. Expression of ZO-1, claudin-5, and occludin was similar in GIT1 WT and KO (Supplementary Fig. 1C). Expression of the astrocyte marker, GFAP, was also probed because interactions of brain microvascular EC with astrocytes are required for proper brain function. GIT1 WT and KO showed similar GFAP expression (Supplementary Fig. 1C). Microvessel architecture in the brain was examined by fluorescent staining for lectin. Lectin staining in the hippocampus and cortex (Supplementary Fig. 1D) and quantitative analysis of vessel area (per mm2) did not show any significant difference between GIT1 WT and KO (Supplementary Fig. 1E). To measure cerebral blood flow, we assessed the distribution of 14C-iodoantipyrine (14C -IAP) in the cortex and hippocampus regions of the brain (Supplementary Fig. 2). Autoradiographic images generated from GIT1 WT and KO demonstrated a similar distribution pattern in various regions of the brain (Supplementary Fig. 2A). Quantitative analysis revealed similar cerebral blood flow in GIT1 WT and KO mice in the cortex (WT= 1.06 ± 0.18 mg/g/min; KO= 0.89 ± 0.11 mg/g/min) and hippocampus (WT = 0.85 ± 0.03 mg/ml/min; KO = 0.87 ± 0.04 ml/g/min) (Supplementary Fig. 2B). These data show that vascular space, vascular permeability and cerebral blood flow were normal in the GIT1 KO mice.
3. Discussion
The major finding of this study is that in GIT1 KO mice, neurons exhibit decreased dendritic length and spine density in the hippocampus. Our behavioral analysis demonstrates that GIT1 KO mice adapt poorly to new or changing environments as shown by impaired performance in learning tasks. Histological analysis of different regions of the brain in GIT1 KO indicates that these mice exhibit no detectable abnormalities in the gross anatomy. The lack of anatomical deficits in the knockout mice is most likely caused by functional redundancy or developmental compensation by the ortholog protein GIT2. Premont’s group previously showed that with the exception of the cerebellar granule cells where no detectable GIT1 was observed, both GIT genes are co-expressed in all the major areas of the brain including hippocampus, cortex, striatum, and olfactory bulb (Schmalzigaug et al., 2007). Although we did not observe compensation of GIT2 expression in the adult GIT1 KO brain, an increase in GIT2 protein expression at postnatal days 1 and 2 (1.7 and 1.5 fold with respect to WT control respectively) was observed (Supplementary Fig. 5). This suggests that early compensatory mechanisms might be involved during development. Though 60% of the GIT1 KO mice are postnatally lethal due to defective pulmonary vasculature (Pang et al., 2009), the surviving animals develop normally and exhibit normal morphology of different tissues where GIT1 is expressed. However, despite the normal brain morphology, the GIT1 KO mice exhibit deficits in dendritic length and spine density in the hippocampus.
GIT1 is highly expressed in the brain, enriched in synaptic densities and shown to regulate synapse formation by targeting actin modulators in cultured rat hippocampal neurons (Albertinazzi et al., 2003; Kim et al., 2003; Ko et al., 2003; Segura et al., 2007,Webb et al., 2007). By Golgi stain analysis, we observed that dendritic length and spine density were decreased by 36.7 % and 35.1 % respectively in GIT1 KO compared to WT controls. However, deletion of GIT1 did not affect other parameters of neuronal plasticity like number of dendritic branch points and neuronal cells bodies in the hippocampus (Supplementary Fig. 4). Altered synapse formation within the hippocampus is usually associated with difficulty in learning related tasks (Kitabatake et al., 2007; Leuner and Shors, 2004). To correlate the anatomical deficits observed in GIT1 KO to behavior, we subjected the mice to operant learning procedures. Advantages of using operant acquisition procedure compared to other behavioral procedures are (a) that the computer controlled procedure provides quantitative measures of relatively discrete behavior (b) automation minimizes the effect of experimenter intrusion (c) operant acquisition can be established quickly and reliably (McKerchar et al., 2005; Sagare et al., 2007). During operant training, GIT1 KO mice did not emit active operant response as quickly as the WT mice (Fig 4A). During fixed fading interval training, the KO mice did not adjust to the changing reward schedule as quickly as the WT (Fig 4B). Even after the final reward parameters were met, GIT1 KO mice were still missing rewards (reward counts) as shown in Fig 4C. Since impaired motor function can affect operant and fixed interval training, we probed for response duration (time from the start of a response to the end of that same response) and reward reaction (time from end of an operant response to a hopper entry when the reward is presented by the dipper) that are indicators of motor differences. While response duration time, measures only one response (e.g; hopper entry or hole poke), reward reaction time measures the coordinated movements after a mouse has removed its snout from the operant hole, detected that the houselight was turned off and that the dipper has been activated, turns 180 degrees, takes 1 to 3 steps and put its head into the dipper hopper in order to lick the milk from the dipper. If either motor or sensory functions were affected, we would have expected to see consistent differences in these temporal measures. The data shown in Supplementary Table 1B and 2B shows that response duration time for active and inactive operant responses during operant and fading interval training were not statistically different between GIT1 WT and KO. Although there was a statistical significance in the reward reaction time between WT and KO during operant training, continued practice under FI schedule resulted in reward reaction time measures becoming equivalent between GIT1 KO and WT (Supplementary Table 1C and 2C). The change in reward reaction time by the GIT1 KO mice suggests that the initial differences were due to learning and not motor deficits. Additionally, we did not observe any changes in cerebellum (morphology, vessel density, vascular space, vascular permeability, cerebral blood flow) (Supplementary Fig. 3A-E) which mediates motor coordination, further suggesting that the behavioral differences observed in GIT1 KO mice are due to cognitive and not motor defects. It is important to note that despite these significant differences in behavior, GIT1 KO mice appeared to catch up to the WT mice on most measures of performance suggesting that the physiological differences may specifically influence process of adaptation to changes in the environment.
A reduction in spines or alteration in the morphology of the spines is usually associated with Mental retardation (MR) (Luo, 2000; Luo, 2002; Ramakers, 2002). In particular several downstreams mediators of small GTPase signaling are involved in MR syndrome. Examples include (a) Rac GEF-alpha pix (Kutsche et al., 2000), (b) Rac activated PAK3 (Allen et al., 1998) and (c) Rho GAP oligoprhenin (Billuart et al., 1998; Govek et al., 2004), (d) WASP (Wiskott Aldrich syndrome protein) (Wegner et al., 2008) and (e) WAVE proteins (Wiskott Aldrich synrome protein family/Scar (suppressor of cAMP receptor) (Soderling et al., 2003; Soderling et al., 2007). These Rho GTPase modulators have shown to play important roles in learning and memory (Ramakers, 2002). In vitro studies have suggested that GIT1 might be a potential mental retardation gene by regulating spine density forming a GIT1-βPIX complex in association with PAK and thus regulating small Rho GTPases and other downstream targets ( Lieu et al., 2006, Zhang et al., 2003, Zhang et al., 2005, Jones and Katan, 2007; Zhang et al., 2005, Morimura et al., 2009, Saneyoshi et al., 2008). Here we confirm these in vitro studies by in vivo analysis and show that GIT1 KO mice have decreased spine density and dendritic length in the hippocampus. Future studies on the exact signaling pathway(s) as how GIT1 regulates spine density in the brain will be necessary.
An unexpected observation was that GIT1 KO mice showed no abnormalities in BBB function. We have previously shown that depletion of GIT1 increased agonist-stimulated EC rounding and contraction, which caused endothelial hyperpermeability (van Nieuw Amerongen et al., 2004). Stockton et al also showed that PAK-PIX-GIT1 complex was found to be necessary for ERK1/2-mediated myosin phosphorylation, EC contraction and permeability (Stockton et al., 2007). These data suggested that GIT1 deficiency should result in BBB disruption. Surprisingly, vascular space, vascular permeability and cerebral blood flow measured by radioactive isotope infusion were similar in GIT1 WT and KO mice. Evidence for intact BBB was further confirmed by showing that expression of tight junction proteins were unaltered in GIT1 KO mice. Vascularization in the developing vertebrate brain occurs during embryogenesis, capillaries originating in the perineural vascular plexus begin to invade the mouse neuroectoderm as early as embryonic day 10.0 (E 10.0). The microvessels then become associated with perivascular mural cells (pericytes). Once within the neuroectoderm, EC come in close contact with other brain parenchymal cell types (neuroblasts, neuroepithelial cells, radial glia, and astrocytes) (Bass et al., 1992; Janzer and Raff, 1987). These multicellular interactions eventually form the highly selective barrier between blood and brain (McCarty et al., 2002). Embryonic development was normal in the GIT1 KO (Pang et al., 2009), suggesting that these processes do not require GIT1 and/or are compensated by GIT2. These results suggest that in GIT1 KO, BBB and cerebral vasculature is intact, perhaps due to compensation by GIT2 and/or differences in vascular development in brain compared to lung. It is possible that under pathophysiologic conditions such as ischemia that the BBB of the GIT1 KO mice might exhibit greater dysfunction than GIT1 WT.
In conclusion, we provide in vivo evidence that GIT1 is critical for regulating spine density and dendritic length, deficits in which correlates with delayed learning and adaptive behavior in GIT1 KO mice. This study demonstrates that deletion of GIT1 a key scaffold protein that plays an important role in cytoskeleton remodeling events is required for normal cognitive learning processes.
4. Experimental Procedure
4.1 Generation of GIT1 KO mice
GIT1 knockout mice (C57BL/6 background) were generated as described in (Pang et al., 2009). Briefly, linearized targeting vector was electroporated into mouse strain 129 embryonic stem (ES) cells. Drug selection for cells with incorporation of the targeting vector by homologous recombination was performed with G418. Surviving cell colonies were screened for proper recombination by Southern blot. The ES cells were screened by digestion with Xba I, then hybridization of a radiolabeled probe. The Southern blot results were confirmed by PCR screening of the ES cell colonies. Colonies were tested for the presence of the neo cassette at the proper locus by using the primers 5′- GAT CAA TTC TCT AGC TAG AGC TCGCTG A -3′, which binds within the neo cassette, and 5′- ACC TCT GTA CCC TACCAC CTC TTG -3′, which binds to intron 7 near the neo cassette. Chimeric mice generated were backcrossed in C57/BL6 background for greater than 8 generations. Genotyping was done by PCR of DNA isolated from tail snips. All studies were performed according to the National Institute of Health guidelines using an approved institutional protocol.
4.2 Histology
Mice were anesthetized with a cocktail of ketamine (80 mg/kg) and xylazine (5 mg/kg, IM) and perfused with 10% formaldehyde via ventricular cannulation. Whole brain tissues were removed, postfixed overnight, dehydrated and embedded in paraffin. Saggital sections of the brain were cut at 5 μm thickness, mounted on to slides, and stained with hematoxylin-eosin according to standard procedure.
4.3 Immunohistochemistry
Tissue sections were deparaffinized and subjected to antigen retrieval to antigen retrieval using Antigen unmasking solution (Vector laboratories Inc, Burlingame, CA), blocked with 5% swine serum (Dako, Carpentaria, CA, USA) for 1 hr and incubated with fluorescein lycopersicon esculentum lectin (1: 500, Vector laboratories Inc) for 70 mins at 37 °C in a humidified chamber. Images were taken at 20X magnification using Olympus Provis microscope (Olympus, Tokyao, Japan). Vessel area (per mm2) was calculated using National Institutes of Health (NIH) Image J software (1.4 version) (http://rsb.info.nih.gov/ij). WT n=3; KO n=3.
4.4 Western Blot Analysis
For immunoblotting, whole brain tissues were homogenized in a Dounce homogenizer with RIPA buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris-HCl, pH 8.0) containing protease inhibitor cocktail (1:1000, Sigma Chemical Co., St. Louis, MO, USA). Lysates were sonicated for 3 second (s) at 30 Hz and centrifuged for 5 min, 8000 rpm, 4° C to remove tissue and cell debris. Protein concentrations were determined by Bradford protein Assay. The protein samples (30 μg) were boiled for 5 min in 2X SDS sample buffer and separated by SDS-PAGE, transferred to nitrocellulose membranes (Life Sciences, Pall Corporation, NY, USA), blocked and incubated with appropriate primary antibodies. Antibodies for GIT1, GIT2 and actin were from Santa Cruz Biotechlogy, CA, USA. GAPDH was from Millipore, MA, USA. After incubating with fluorescence-conjugated secondary antibodies (Alexa Fluor, Invitrogen CA, USA), immunoreactive proteins were visualized by an Odyssey infrared imaging system (LI-COR Biotechnology, Nebraska, USA). Results were normalized by arbitrarily setting the densitometry of control samples to one. WT n=3; KO n=3.
4.5 Modified Golgi-Cox stain
The brains were subjected to Golgi-Cox staining as described in (Gibb and Kolb, 1998) Brains were immersed in Golgi Cox solution and stored for 10 days in the dark. Tissues were incubated in 30% sucrose for 48 hrs prior to sectioning in vibratome (VT1000S, Leica). 100 μm coronal sections were cut and placed in gelatinized slides, dried for 30 minutes (min), rinsed in water and developed in ammonium hydroxide solution for 30 min. Sections were then rinsed with water and placed in Kodak fixer (1:1) (Eastman Kodak, Rochester, NY, USA) for another 30 min. Sectioned were rinsed in water, dried and cover slipped. Images of the hippocampal (CA1) regions were taken using at 60X resolution using Olympus BX41 microscope (Olympus, Tokyo, Japan). 20 neurons were traced per animal and total of 80-120 neurons were traced for hippocamous per genotype. M=males, F= Females; [WT n=6 (2M, 4F); KO n=4 (2M, 2F)].
4.6 Image Analyses
Images were analyzed as described in (Brodbeck et al., 2008). All neuronal parameters were analyzed with NIH Image J software. The total length of dendritic fragments were measured by tracing their extension using the segmented line selection and normalizing to field area (per mm2). Branch points and cell bodies (per mm2) were counted manually by using the point picker function in Image J software. For spine density calculation, neurons were selected such that most of the dendritic extensions were contained within the 100 _m slice and were not severely covered by blood vessels or dense clusters of dendrites from neighbouring cells. Dendrites of length ≥10 μm were traced and spines counted manually using point picker function and expressed as spines/10 μm.
4.7 Infusion of radioisotopes for vascular permeability and blood flow
Mice were anesthetized with 2-3% isoflurane in 30% oxygen and 70% nitrous oxide, and injected (i.p.) first with a mixture of 99mTc-albumin (10 μCi, Cardinal Health, OH, USA) and 3H-inulin (5 μCi, Amersham BioSciences, NJ, USA), and after 9.5 min with 14C-IAP (15μCi, Amersham). Precisely 30 seconds after 14C-IAP injection mice were sacrificed by cervical dislocation and quickly immersed in liquid nitrogen until frozen. There was a 3s delay (lag time) for isotopes entry into blood after the i.p. injections, as reported by (Maeda et al., 2000) and (Zhong et al., 2008). Frozen brain (frontal cortex) and frozen blood from the left ventricle of the heart were carefully removed in the cold room (0 °C), placed in pre-weighed vials and prepared for radioactivity analysis. Blood samples were decolorized with hydrogen peroxide to reduce quenching of beta radioactivity. Blood and brain samples were first analyzed for 99mTc-albumin radioactivity (Wallac Vizard Gamma Counter, Perkin Elmer, CT, USA), and then for 3H-inulin and 14C-IAP radioactivities (Tri-carb 2100 liquid Scintillation Counter, PerkinElmer) after dissolving the samples with 0.5 ml tissue solubilizer (PerkinElmer) overnight and adding 5 ml scintillation fluid (Ultima Gold, PerkinElmer). Cerebral blood flow (CBF, ml/g/min) was determined as described by (Maeda et al., 2000; Zhong et al., 2008). Frozen brains were carefully removed in the cold room and cryosectioned at 20 μm, mounted on slides, dried on a hot plate at 50°C for 10 min and exposed to Hyperfilm βmax autoradiographic film (Amersham) along with 14C standards. After 6 weeks exposure, the film was developed and the resulting images were analyzed on an MCID image analyzer (Imaging Research) to determine levels of 14C-IAP in frontal cortex and cerebellum by quantitative autoradiography. The local blood flow was calculated with the MCID program. WT n=3; KO n=3.
Calculations
Vascular space (Vs): The cerebral vascular space was determined from the distribution of 99mTc-albumin, using equation (1)
| {Eq.1} |
Blood brain barrier (BBB) permeability : 3H-inulin BBB permeability (PS) was calculated as the PS (permeability surface area) product (ml/g/s) after correction for 99mTc-albumin vascular space distribution, using equation 2.
| {Eq.2} |
Vd is the volume of distribution, T is the experimental time (s), 3H-inulin d.p.m./ml plasma and TCA-precipitable 99mTc-albumin cpm/ml plasma are the integrated plasma concentrations from the lag time after i.p. injection of these tracers to the value measured in the plasma sample from the frozen heart at the end of the experiment (time T), by assuming a linear rise or ramp function over time T (s).
4.8 Behavioral Studies
Subjects
All behavioral studies were carried out with WT (n=7) and KO (n=6) females at 10 to 12 weeks of age. Though concerns are expressed about behavioral variability in female, Voiket et al (Voikar et al., 2001) showed that no overall sex differences were observed in behavioral responses for different mice strains including C57BL/6 on which GIT1 KO mice were backcrossed. All animal studies were performed according to the National Institute of Health guidelines using an approved institutional protocol.
Operant training and Fixed Interval Fading
Apparatus
Custom-made mouse operant chambers were used to test behavioral function in mice as described in (Fowler et al., 2001; Fowler et al., 2003). Mouse chambers (130 × 95 × 130 mm) were equipped with a photobeam over the access hole (25mm) to the dipper to detect responses. Photobeams behind two “response” holes (15 mm) in the opposite wall record pokes by a mouse’s snout, served as the defined operant responses. Each response hole had a light-emitting diode (LED) light. The LEDs were turned on during the session and turned off when the photobeam was broken (i.e. feedback stimulus). The chamber was equipped with a 28-volt incandescent house light that is used to signal the start and end of a session. All outputs and inputs were recorded in real time by a PC computer.
Procedure
Prior to training, mice were exposed to evaporated milk (reward) and their bodyweights were reduced to 85% of their free feeding bodyweight. Mice were then weighed daily and fed to maintain them at 85% of their free feeding bodyweight.
Operant training consisted of 3 sessions (25 min) in which rewards were presented on a variable time (average 60s) schedule concomitantly with a fixed ratio 1-second (FR1) schedule for snout entries into the hole on the right side of the chamber.
Fixed Interval fading consisted of 5 sessions (25 min) in which the reward schedule was changed from a fixed ratio 1 s to a fixed interval 5 s (FI5), and then the interval was increased to 15 (FI15), 30 (FI30) and then 60 (FI60) s.
Fixed Interval 60 s (FI60) testing consists of 5 sessions (25 min) in which rewards could be earned every 60 s. Optimal performance consisted of mice responding late in the 60 s interval, fast enough to earn a reward as soon as it became available.
Measures
A photobeam detected operant responses to both the “active hole” (active operant) and the “inactive hole” (inactive operant). Active response is the count of the number of snout pokes the mice makes to the hole that produces the reward during operant training. Rewards were programmed to occur after a response (operant training) or after a period of time since the last reward and a response (fixed interval). During fixed interval, if a mouse is responding regularly at the end of the interval, a reward will be presented within a second or two of becoming available .The number of rewards (reward count) earned was also used as a measure of performance success. Response duration (defined as the time from the start of a response to the end of that same response) and reward reaction (the time from end of an operant response to a hopper entry when the reward is presented through the dipper) are indicators of motor differences.
4.9 Statistical analysis
For all biochemical assays, experimental values are expressed as mean ± SEM from three independent experiments. The significance of the results was assessed by unpaired two-tailed student’s t-test. For behavioral analysis, all statistical analyses were performed using SYSTAT 10 software (Systat Software Inc, IL, USA). Repeated ANOVA measures followed by post-hoc tests were performed for both operant training and fixed interval. A p value of < 0.05 was considered statistically significant for all biochemical and behavioral analysis.
Supplementary Material
Supplementary Figure. 1. Vascular space, vascular permeability, and vessel number are unaltered in GIT1 KO
Vascular space (A) and permeability (B) were measured in the frontal cortex of GIT1 WT and KO by radioactive infusion. (WT, n = 3; KO, n=3) (C) Expression of tight junction proteins (ZO-1, occludin, claudin 5) and astrocyte marker GFAP in whole cell brain lysates in adult 3 month old GIT1 WT and KO brain. Tubulin served as an internal control (D) Lectin stain for vessels in the cortex and hippocampus and (E) quantitative analysis of vessel area per mm2 in the cortex and hippocampus of GIT1 WT and KO were comparable. All values are expressed as mean ± SEM. (WT, n = 3; KO, n=3)
Supplementary Figure. 2. Cerebral blood flow is unaltered in GIT1 KO
(A) Autoradiography shows normal cerebral blood flow in different regions of the GIT1 WT and KO mice. Images were analyzed on an MCID image analyzer (Imaging Research) (B) Quantitative analysis of blood flow in the frontal cortex and hippocampus was comparable between GIT1 WT and KO. All values are expressed as mean ± SEM. (WT, n = 3; KO, n=3)
Supplementary Figure. 3. Gross morphology, vessel density, vascular space, vascular permeability and cerebral blood flow normal in cerebellum of GIT1 KO
(A) Hematoxylin and eosin stained sections of the cerebellum from GIT1 KO mice showed that foliation was normal compared to WT controls. The molecular layer, Purkinje cell and granule cell layers were all present. Higher magnification of cerebellum in GIT1 WT and KO is shown in lower panel (B) Lectin stain (immunohistochemsitry) of GIT1 WT and KO and quantitative analysis of vessel area per mm2 in the cerebellum showed no statistical difference between of GIT1 WT and KO. (C) Vascular space (WT = 31.68 ± 4.71 μl/g; KO = 37.56 ± 6.71 μl/g) and (D) vascular permeability (WT = 2.30 ± 0.25 ml/g/sec × 10−6, KO = 2.96 ± 0.34 ml/g/sec × 10−6) and (E) cerebral blood flow (WT= 1.01 ± 0.05 ml/g/min; KO = 1.17 ± 0.22 ml/g/min) in the cerebellum measured by radioactive infusion were normal in GIT1 KO compared to WT controls. All values are expressed as mean ± SEM. (WT, n = 3; KO, n=3).
Supplementary Figure. 4. Branch points and neuronal cell bodies in hippocampal neurons are comparable in GIT1 WT and KO
Quantitative analysis of branch points (A) and cell bodies per mm2 (B) in the hippocampus showed no differences between GIT1 WT and KO mice. All values are expressed as mean ± SEM. (WT, n=6; KO, n=4).
Supplementary Figure. 5. GIT2 expression is increased in the GIT1 KO brains at postnatal days 1 (P1) and (P2)
(A) Western blotting of GIT2 in whole brain lysates of GIT1 WT and KO mice at postnatal day P1, P2, P3. GAPDH serves as loading control. (B) Quantitative measurement of fold change in GIT2 expression in GIT1 WT and KO normalized to 1.0 on P1.
Supplementary Table 1- Operant Training
Supplementary Table 2- Fixed interval Fading
Acknowledgements
We would like to thank Dr. James M Powers for his advice. This work was supported by NIH grant HL-63462 to BC Berk, R37 NS34467 to BV Zlokovic and P30 ES001247 to the NeuroBehavioral Facility Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Classification: Research Report
Section: Disease related Neuroscience
References
- Albertinazzi C, Za L, Paris S, de Curtis I. ADP-ribosylation factor 6 and a functional PIX/p95-APP1 complex are required for Rac1B-mediated neurite outgrowth. Mol Biol Cell. 2003;14:1295–307. doi: 10.1091/mbc.E02-07-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- Bass T, Singer G, Slusser J, Liuzzi F. Radial glial interaction with cerebral germinal matrix capillaries in the fetal baboon. Exp Neurol. 1992;118:126–132. doi: 10.1016/0014-4886(92)90029-p. [DOI] [PubMed] [Google Scholar]
- Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, Carrie A, Beldjord C, Kahn A, Moraine C, Chelly J. Oligophrenin 1 encodes a rho-GAP protein involved in X-linked mental retardation. Pathol Biol (Paris) 1998;46:678. [PubMed] [Google Scholar]
- Brodbeck J, Balestra ME, Saunders AM, Roses AD, Mahley RW, Huang Y. Rosiglitazone increases dendritic spine density and rescues spine loss caused by apolipoprotein E4 in primary cortical neurons. Proc Natl Acad Sci U S A. 2008;105:1343–6. doi: 10.1073/pnas.0709906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107:107–24. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand B, Chen R, Vorontsova E, Zarcone T. Behavioral sensitization to amphetamine in rats: changes in the rhythm of head movements during focused stereotypies. Psychopharmacology (Berl) 2003;170:167–77. doi: 10.1007/s00213-003-1528-5. [DOI] [PubMed] [Google Scholar]
- Frank SR, Adelstein MR, Hansen SH. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. Embo J. 2006;25:1848–59. doi: 10.1038/sj.emboj.7601092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Goehler H, Lalowski M, Stelzl U, Waelter S, Stroedicke M, Worm U, Droege A, Lindenberg KS, Knoblich M, Haenig C, Herbst M, Suopanki J, Scherzinger E, Abraham C, Bauer B, Hasenbank R, Fritzsche A, Ludewig AH, Buessow K, Coleman SH, Gutekunst CA, Landwehrmeyer BG, Lehrach H, Wanker EE. A Protein Interaction Network Links GIT1, an Enhancer of Huntingtin Aggregation, to Huntington’s Disease. Mol Cell. 2004;15:853–65. doi: 10.1016/j.molcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Akerman CJ, Cross JR, Van der Veken L, Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat Neurosci. 2004;7:364–72. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- Hamm HE, Gilchrist A. Heterotrimeric G proteins. Curr. Opin. Cell Biol. 1996;8:189–96. doi: 10.1016/s0955-0674(96)80065-2. [DOI] [PubMed] [Google Scholar]
- Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–75. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jones NP, Katan M. Role of phospholipase Cgamma1 in cell spreading requires association with a beta-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol Cell Biol. 2007;27:5790–805. doi: 10.1128/MCB.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ko J, Shin H, Lee JR, Lim C, Han JH, Altrock WD, Garner CC, Gundelfinger ED, Premont RT, Kaang BK, Kim E. The GIT Family of Proteins Forms Multimers and Associates with the Presynaptic Cytomatrix Protein Piccolo. J Biol Chem. 2003;278:6291–6300. doi: 10.1074/jbc.M212287200. [DOI] [PubMed] [Google Scholar]
- Kitabatake Y, Sailor KA, Ming GL, Song H. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg Clin N Am. 2007;18:105–13. x. doi: 10.1016/j.nec.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M, Premont RT, Weinberg RJ, Kim E. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003;23:1667–77. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth U, Boavida MG, David D, Chelly J, Fryns JP, Moraine C, Ropers HH, Hamel BC, van Bokhoven H, Gal A. Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet. 2000;26:247–50. doi: 10.1038/80002. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol. 2004;29:117–30. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu AS, Li JZ, Webb DJ, Hankins GR, Howng SL, Helm GA. Functions of G protein-coupled receptor kinase interacting protein 1 in human neuronal (NT2N) cells. J Neurosurg. 2006;105:103–10. doi: 10.3171/jns.2006.105.1.103. [DOI] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–80. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–35. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Maeda K, Mies G, Olah L, Hossmann KA. Quantitative measurement of local cerebral blood flow in the anesthetized mouse using intraperitoneal [14C]iodoantipyrine injection and final arterial heart blood sampling. J Cereb Blood Flow Metab. 2000;20:10–4. doi: 10.1097/00004647-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Mazaki Y, Hashimoto S, Tsujimura T, Morishige M, Hashimoto A, Aritake K, Yamada A, Nam JM, Kiyonari H, Nakao K, Sabe H. Neutrophil direction sensing and superoxide production linked by the GTPase-activating protein GIT2. Nat Immunol. 2006;7:724–31. doi: 10.1038/ni1349. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvoral AM, Hynes RO. Defective Associations between Blood Vessels and Brain Parenchyma Lead to Cerebral Hemorrhage in Mice Lacking v Integrins. Molecular and Cellular Biology. 2002;22:7667–677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerchar TL, Zarcone TJ, Fowler SC. Differential acquisition of lever pressing in inbred and outbred mice: comparison of one-lever and two-lever procedures and correlation with differences in locomotor activity. J Exp Anal Behav. 2005;84:339–56. doi: 10.1901/jeab.2005.95-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura S, Suzuki K, Takahashi K. betaPIX and GIT1 regulate HGF-induced lamellipodia formation and WAVE2 transport. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Pang J, Hoefen R, Pryhuber GS, Wang J, Yin G, White RJ, Xu X, O’Dell MR, Mohan A, Michaloski H, Massett MP, Yan C, Berk BC. G-Protein-Coupled Receptor Kinase Interacting Protein-1 Is Required for Pulmonary Vascular Development. Circulation. 2009;119:1524–32. doi: 10.1161/CIRCULATIONAHA.108.823997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci U S A. 1998;95:14082–7. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Perry SJ, Lefkowitz RJ. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J Biol Chem. 2000;275:22373–80. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–9. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–31. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, Nozaki N, Natsume T, Soderling TR. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalzigaug R, Phee H, Davidson CE, Weiss A, Premont RT. Differential Expression of the ARF GAP Genes GIT1 and GIT2 in Mouse Tissues. J Histochem Cytochem. 2007 doi: 10.1369/jhc.7A7207.2007. [DOI] [PubMed] [Google Scholar]
- Schmalzigaug R, Rodriguiz RM, Bonner PE, Davidson CE, Wetsel WC, Premont RT. Impaired fear response in mice lacking GIT1. Neurosci Lett. 2009 doi: 10.1016/j.neulet.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 2007 doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci U S A. 2003;100:1723–8. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, Banker G, Raber J, Scott JD. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27:355–65. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of Vascular Permeability: betaPIX and GIT1 Scaffold the Activation of Extracellular Signal-regulated Kinase by PAK. Mol Biol Cell. 2007;18:2346–55. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Natarajan K, Yin G, Hoefen RJ, Osawa M, Haendeler J, Ridley AJ, Fujiwara K, van Hinsbergh V, Berk BC. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res. 2004;94:1041–9. doi: 10.1161/01.RES.0000125627.77235.0C. [DOI] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE. The G protein-coupled receptor repertoires of human and mouse. Proc. Natl. Acad. Sci. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–81. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Watson S, Arkinstall S. The G-Protein Linked Receptor Facts-Book. Academic; San Diago: 1994. [Google Scholar]
- Webb DJ, Zhang H, Majumdar D, Horwitz AF. alpha 5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons. J Biol Chem. 2007 doi: 10.1074/jbc.M610981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Cem. 2008;283:15912–20. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise A, Jupe SC, Rees S. The identification of ligands at orphan G-protein coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004;44:43–66. doi: 10.1146/annurev.pharmtox.44.101802.121419. [DOI] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Horwitz AF. Synapse formation is regulated by signaling adaptor GIT1. J Cell Biol. 2003;161(1):131–42. doi: 10.1083/jcb.200211002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005;25:3379–88. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–2. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. 1. Vascular space, vascular permeability, and vessel number are unaltered in GIT1 KO
Vascular space (A) and permeability (B) were measured in the frontal cortex of GIT1 WT and KO by radioactive infusion. (WT, n = 3; KO, n=3) (C) Expression of tight junction proteins (ZO-1, occludin, claudin 5) and astrocyte marker GFAP in whole cell brain lysates in adult 3 month old GIT1 WT and KO brain. Tubulin served as an internal control (D) Lectin stain for vessels in the cortex and hippocampus and (E) quantitative analysis of vessel area per mm2 in the cortex and hippocampus of GIT1 WT and KO were comparable. All values are expressed as mean ± SEM. (WT, n = 3; KO, n=3)
Supplementary Figure. 2. Cerebral blood flow is unaltered in GIT1 KO
(A) Autoradiography shows normal cerebral blood flow in different regions of the GIT1 WT and KO mice. Images were analyzed on an MCID image analyzer (Imaging Research) (B) Quantitative analysis of blood flow in the frontal cortex and hippocampus was comparable between GIT1 WT and KO. All values are expressed as mean ± SEM. (WT, n = 3; KO, n=3)
Supplementary Figure. 3. Gross morphology, vessel density, vascular space, vascular permeability and cerebral blood flow normal in cerebellum of GIT1 KO
(A) Hematoxylin and eosin stained sections of the cerebellum from GIT1 KO mice showed that foliation was normal compared to WT controls. The molecular layer, Purkinje cell and granule cell layers were all present. Higher magnification of cerebellum in GIT1 WT and KO is shown in lower panel (B) Lectin stain (immunohistochemsitry) of GIT1 WT and KO and quantitative analysis of vessel area per mm2 in the cerebellum showed no statistical difference between of GIT1 WT and KO. (C) Vascular space (WT = 31.68 ± 4.71 μl/g; KO = 37.56 ± 6.71 μl/g) and (D) vascular permeability (WT = 2.30 ± 0.25 ml/g/sec × 10−6, KO = 2.96 ± 0.34 ml/g/sec × 10−6) and (E) cerebral blood flow (WT= 1.01 ± 0.05 ml/g/min; KO = 1.17 ± 0.22 ml/g/min) in the cerebellum measured by radioactive infusion were normal in GIT1 KO compared to WT controls. All values are expressed as mean ± SEM. (WT, n = 3; KO, n=3).
Supplementary Figure. 4. Branch points and neuronal cell bodies in hippocampal neurons are comparable in GIT1 WT and KO
Quantitative analysis of branch points (A) and cell bodies per mm2 (B) in the hippocampus showed no differences between GIT1 WT and KO mice. All values are expressed as mean ± SEM. (WT, n=6; KO, n=4).
Supplementary Figure. 5. GIT2 expression is increased in the GIT1 KO brains at postnatal days 1 (P1) and (P2)
(A) Western blotting of GIT2 in whole brain lysates of GIT1 WT and KO mice at postnatal day P1, P2, P3. GAPDH serves as loading control. (B) Quantitative measurement of fold change in GIT2 expression in GIT1 WT and KO normalized to 1.0 on P1.
Supplementary Table 1- Operant Training
Supplementary Table 2- Fixed interval Fading