Abstract
Purpose
To identify the extent of persistence (period of time of continuous therapy with the drug prescribed) of glaucoma patients treated with prostaglandins (latanoprost, bimatoprost, or travoprost), or β-blocker (timolol) monotherapy.
Methods
An observational retrospective study of a 24-month follow-up in 191 patients (from four centers) was done to identify the time elapsed until patients discontinued their antiglaucomatous treatment. The relevant information was extracted from patients’ medical charts. A descriptive analysis, a Kaplan–Meier survival analysis, and a Cox regression model were used to determine which drug was associated with greater patient persistence and to detect variables significantly influencing persistence.
Results
Descriptive analysis and survival curves showed that after 24 months, latanoprost was associated with a higher persistence in glaucoma treatment than the alternative agents: 81.6% versus 22.9% for bimatoprost, 65.4% for travoprost, and 60.5% for timolol (P < 0.0001). Persistence was significantly influenced by the antiglaucoma agent used as monotherapy (with a six-fold higher risk of treatment discontinuation during the follow-up period due to receiving bimatoprost instead of latanoprost; P < 0.0001) and patient age (P = 0.001). Even though comorbidities could not be directly related to persistence, their occurrence was related to patient age. The main reasons for treatment discontinuation were lack of efficacy, development of intolerance and/or adverse events, which were significant in the bimatoprost group, 28.6% (P < 0.001) and 48.6% (P < 0.001), respectively.
Conclusions
Latanoprost shows higher patient persistence compared with travoprost, bimatoprost, and timolol in routine clinical practice, and could lead to better control of intraocular pressure and lower associated economic costs.
Keywords: first-line monotherapy, glaucoma, treatment, persistence, Spain
Introduction
Glaucoma, a chronic optic neuropathy, is characterized by a progressive loss of the retinal nerve fiber layer and visual field defects, related or not related to an increase of intraocular pressure (IOP) and, at present, is the third cause of irreversible blindness worldwide, with cataracts being the major cause.1
In Spain, the prevalence of glaucoma in the population over 40 years is 1.5%, and increases in individuals over 60 years of age.2 Primary open angle glaucoma, known also as simple chronic glaucoma, is the most frequent type, accounting for up to 60% of glaucoma cases.
IOP, past family history, age, race, male gender, cup-disk ratio, a high standard deviation (SD) on Humphrey’s visual field pattern, cardiovascular disease, and a thin Clinical Ophthalmology 2010:4 central cornea thickness are the primary risk factors for glaucoma (Ocular Hypertension Treatment Study). IOP is the only known risk factor which can be modified to halt disease progression, and all antiglaucomatous treatments are targeted to IOP.
In spite of the American Ophthalmology Academy recommendation to start with medical therapy, laser trabeculoplasty, or filtration surgery for glaucoma, most patients receive initial treatment with a topical hypotensive drug.3 If the prescribed hypotensive drug effectively reduces IOP, it might be assumed that the patient would indefinitely continue using this ocular hypotensive drug with the aim of obtaining clinical improvement.4 Various studies have demonstrated that reduced IOP decreases the risk of loss of visual field5 and slows the process of ocular hypertension (OHT) progression to glaucoma.6 Even so, many patients withdraw from topical hypotensive treatment of their own accord.7–14
Marketed topical prostaglandins, including latanoprost, approved by the European Medicines Agency (EMEA) in 1997, and bimatoprost and travoprost, approved in 2002 and 2001, respectively, effectively reduce IOP levels by increasing aqueous fluid drainage through the uveal-scleral route.
The last version of the European Ophthalmology Society guidelines, published in 2008, states that the objectives of glaucoma treatment are to assure effective IOP management from the outset to prevent development of glaucomatous damage and to concentrate on treatment efficacy, tolerability, and compliance. Collection of data on clinical effectiveness, reflecting the clinical results of drugs used common medical practice conditions, has become increasingly important.
One of the most important contributors to good results in clinical practice is the continuous taking of the prescribed drug by the patient for the time period considered necessary. Thus, it is important to identify the likely duration of persistence (period of time for which the patient takes the prescribed drug) with antiglaucomatous medication. If the period of time of continuous medication is short, the therapeutic benefit will be minor, and lower than the benefit that might have been achieved if the patient had taken the medication for a longer time. This will cause a need for a second- line therapy prescription, thus increasing consumption of potentially unnecessary health care resources.15
Even though a number of factors may affect patient persistence with treatment, one of the most important ones is local tolerability. When tolerability is poor, patients are very likely to discontinue their medication or put pressure on the ophthalmologist to change their treatment. Another important issue is clinical effectiveness of therapy; if IOP levels continue to be high, the ophthalmologist will either opt for a change in medication or will add a second drug to reduce IOP further.16
The objective of this study was to evaluate the extent of patient persistence with the drugs most commonly used as monotherapy for glaucoma in Spain (latanoprost, bimatoprost, travoprost, and timolol).
Materials and methods
An observational, retrospective study of glaucomatous patients was conducted in the ophthalmology departments of the Hospital de Torrevieja (Alicante), Centro de Oftalmología Barraquer (Barcelona), Fundación Hospital Alcorcón (Madrid), and Instituto Universitario de Oftalmobiología Aplicada (Valladolid).
The Ethics Committee for Clinical Research from the Fundación Hospital Alcorcón, Madrid, approved the protocol in December 2006. Due to the observational and retrospective nature of the study, for which data were obtained from review of medical charts, with no intervention or deliberate modification of biologic, physiologic, psychologic, or social variables, patient informed consent was not required.
Using the hospital databases, clinical charts for glaucomatous patients treated with commonly used monotherapy (timolol 0.5% twice daily, latanoprost 0.005%, bimatoprost 0.03%, or travoprost 0.004% once daily in the evening) were reviewed.
Inclusion criteria for the study were age over 18 years of age, a diagnosis of glaucoma or OHT, monotherapy with any of the study drugs between July 2003 and June 2004, and sufficient information in medical charts to monitor follow- up for at least 24 months after initiation of monotherapy. Patients may or may not have received antiglaucomatous treatment before the date of study initiation. Patients who had received concomitant antiglaucomatous treatment during the study period or whose medical charts did not contain reliable data for at least 24 months after monotherapy initiation were excluded.
Details of age, gender, time from diagnosis of glaucoma, last recorded IOP, associated comorbidities, previous glaucoma treatments, and antiglaucomatous monotherapy were collected from medical charts.
Every selected patient had been followed up at three, six, nine, 12, and 24 months, so we were able to identify dates and reasons for treatment discontinuation. Thus, any treatment changes during the 24-month follow-up period were able to be collected.
A persistent patient was defined as one who had taken the prescribed drug as monotherapy continuously during the 24-month study period. For patients who did not persist with antiglaucomatous treatment for 24 months, the days to treatment discontinuation or to a medication change/referral for surgery by the ophthalmologist was recorded. A medication change was defined as any change in monotherapeutic agent used or addition of another ocular hypotensive agent. For patients who discontinued treatment at any time during the study, reasons for discontinuation (intolerance, adverse effects, poor compliance, patient request, or lack of efficacy), as well as any changes in the therapeutic approach of the attending ophthalmologist (treatment change, addition of a new therapy, or referral for surgery) were recorded.
Ophthalmology medical charts from July 2003 to June 2004 were reviewed over a period of six months (from March to September 2007). A descriptive analysis of patient sociodemographic and clinical characteristics was conducted, which enabled a comparison of the extent of patient persistence and clinical variables between patients who initiated monotherapy with a prostaglandin or with a topical β-blocker (timolol). The distribution of each variable was analyzed in order to define possible recoding for both categoric and continuous variables. Variable recoding was based on three criteria, ie, to define clinically significant categories, to reduce as far as possible the number of categories, and to obtain the maximum homogeneity in the number of cases in each category.
To assess patient persistence with the study drug, a Kaplan–Meier survival analysis was done to estimate the persistence curves for patients treated with the different study medications over 24 months. To identify the statistical significance of patient persistence with the different study drugs, Log-Range, Tarone–Ware, and Breslow contrast tests were used. Finally, a Cox regression model was used to identify the predictive effect of variables on the degree of patient persistence, with time to discontinuation as a dependent variable, and age, gender, concomitant drugs, associated comorbidities, IOPs, time from diagnosis of glaucoma, previous glaucoma treatments, and antiglaucomatous monotherapy (latanoprost reference group) as independent variables. For the statistical analysis, the SPSS statistical program was used (version 14.0; SPSS Inc., Chicago, IL).
Results
Of 191 patients who fulfilled our inclusion criteria, 87 had been initiated on treatment with latanoprost, 35 with bimatoprost, 26 with travoprost, and 43 with timolol. Demographic and clinical characteristics of these patients are summarized on Table 1.
Table 1.
Characteristics of study patients
| Latanoprost (n = 87) | Bimatoprost (n = 35) | Travoprost (n = 26) | Timolol (n = 43) | P value | |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 64.8 (12.8) | 68.5 (14.05) | 67.5 (16.8) | 67.5 (14.4) | NS |
| Gender, n (%) | 0.002 | ||||
| Males | 30 (34.5) | 24 (68.6) | 7 (26.9) | 21 (48.8) | |
| Females | 57 (65.5) | 11 (31.4) | 19 (73.1) | 22 (51.2) | |
| Last IOP value (mmHg), mean ± SD | 16.6 (2.7) | 17.0 (2.9) | 17 (2.5) | 17.4 (3.4) | NS |
| Glaucoma (years)*, median ± SD | 2.5 (4.1) | 2.8 (2.9) | 1.6 (1.5) | 1.4 (3.5) | NS |
| Previous glaucoma treatment, n (%) | 40 (46) | 26 (74.3) | 8 (30.8) | 11 (25.6) | 0.00011 |
| Associated comorbidities, n (%) | NS | ||||
| None | 47 (54) | 18 (51.4) | 6 (23.1) | 20 (46.5) | |
| Cardiovascular (includes diabetes) | 31 (35.6) | 12 (34.3) | 16 (61.5) | 21 (48.8) | |
| Ophthalmologic | 8 (9.2) | 5 (14.3) | 3 (11.5) | 1 (2.3) | |
| Neurologic | 1 (1.1) | 0 (0) | 1 (3.8) | 1 (2.3) |
Notes:
Time since glaucoma diagnosis (years).
Including diabetes mellitus.
Abbreviation: NS, not statistically significant.
Approximately 60% of patients were female, and a statistically significant difference in gender ratio was found among the different treatment groups (P = 0.002). Mean patient age was 66.4 years (SD 14.0); the bimatoprost group showed the highest mean age, but age differences between groups were not statistically significant. Overall mean IOP was 16.9 mmHg (SD 2.9); no statistically significant differences by treatment were observed, although it should be noted that IOPs recorded in patients’ medical charts were not always recorded on a date close to the initiation of follow- up. The mean time elapsed from the date patients had become aware of the diagnosis of glaucoma was 3.7 years (SD 3.6).
Forty-four percent of patients had received previous treatment for glaucoma. However, this percentage varied between treatments, the bimatoprost group having the highest percentage (74.3%), followed by latanoprost (46%). Timolol was the drug most frequently prescribed as an initial treatment after diagnosis, followed by brimonidine and carteolol. No significant differences were observed among treatment groups for comorbidities.
Numbers and rates of patient persistence throughout the study with the various prescribed drugs are shown in Table 2. Only 36.1% of patients discontinued their treatment. When comparing persistence with the different study drugs, six possible combinations were analyzed. Latanoprost was statistical significant versus bimatoprost and timolol (P = 0.0001 and P = 0.01, respectively); but did not achieve statistical significance versus travoprost (P = 0.058), with a difference in persistence of 16.2%. Bimatoprost was found to be inferior versus travoprost and timolol (P = 0.003 and P = 0.001, respectively); travoprost and timolol did not show statistical significant (P = 0.8). Percentages and statistical significance of the differences in persistence with the study drugs are shown on Table 3.
Table 2.
Patient persistence with treatment
| N (%) | Latanoprost (n = 87) | Bimatoprost (n = 35) | Travoprost (n = 26) | Timolol (n = 43) | P |
|---|---|---|---|---|---|
| No treatment discontinuation | 71 (81.6) | 8 (22.9) | 17 (65.4) | 26 (60.5) | P < 0.0001 |
| Treatment discontinuation | 16 (18.4) | 27 (77.1) | 9 (34.6) | 17 (39.5) |
Table 3.
Degree of patient persistence with the study drugs
| n | Persistence (%) | Difference | P value | |
|---|---|---|---|---|
| Latanoprost | 87 | 18.6 | 58.7 | 0.0001 |
| Bimatoprost | 35 | 22.9 | ||
| Latanoprost | 87 | 81.6 | 16.2 | 0.058 |
| Travoprost | 26 | 65.4 | ||
| Latanoprost | 87 | 81.6 | 21.1 | 0.01 |
| Timolol | 43 | 60.5 | ||
| Bimatoprost | 35 | 22.9 | −42.5 | 0.003 |
| Travoprost | 26 | 65.4 | ||
| Bimatoprost | 35 | 22.9 | −37.6 | 0.001 |
| Timolol | 43 | 60.5 | ||
| Travoprost | 26 | 65.4 | 4.9 | 0.8 |
| Timolol | 43 | 60.5 |
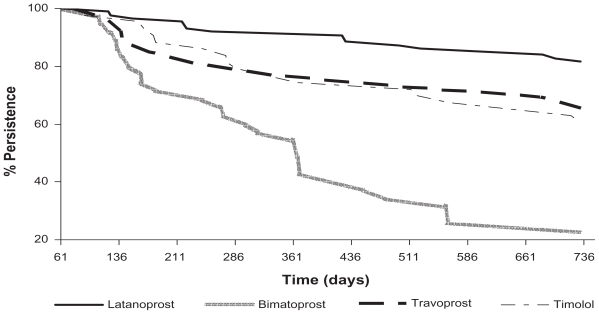
Figure 1 shows a Kaplan–Meier survival plot where patient persistence is assessed over the 24 months. Table 4 shows percentages for treatment discontinuation. Differences were found between the four treatment groups for intolerance and/or adverse effects as well as for lack of efficacy variables (P < 0.001). Fewer latanoprost-treated patients reported intolerance and/or adverse effects and fewer travoprost-treated patients reported lack of efficacy. The bimatoprost group showed higher rates of lack of efficacy compared with latanoprost, travoprost, and timolol.
Figure 1.
Kaplan-Meier survival plot (persistence curve).
Table 4.
Reasons for withdrawal from treatment during the study
| Reason from treatment for withdrawal n (%) | Latanoprost (n = 87) | Bimatoprost (n = 35) | Travoprost (n = 26) | Timolol (n = 43) | P value |
|---|---|---|---|---|---|
| Intolerance and/or adverse effects | 2 (2.3) | 10 (28.6) | 6 (23.1) | 4 (9.3) | P < 0.001 |
| Lack of efficacy | 14 (16.1) | 17 (48.6) | 3 (11.5) | 13 (30.2) | P < 0.001 |
For all possible variables with a predictive and/or modifying effect on patient persistence, only the choice of antiglaucomatous drug used in monotherapy and patient age were statistically significant. The risk of treatment discontinuation during the follow-up period was six-fold with bimatoprost versus latanoprost (P < 0.0001) and 2.4-fold for timolol versus latanoprost (P = 0.01). An increased risk of 3.2% per year was observed with patient age (P = 0.001). Other variables analyzed were not related to duration of persistence.
Discussion
This study indicated greater patient persistence with latanoprost than with other prostaglandins or the β-blocker timolol in routine clinical practice.
This is the first research done in Spain on patient persistence with antiglaucomatous treatment, and the obtained results are similar to those of studies conducted in other countries,12,17–20 indicating greater patient persistence with latanoprost as a topical hypotensive therapy for glaucoma, which might be due to three factors. First, latanoprost has been shown to be more effective in IOP reduction than other therapies;21–24 second, unlike timolol, latanoprost has a more convenient once-daily dosing schedule; and third, it has a lower rate of adverse effects, in particular for hyperemia, when compared with the other topical hypotensive drugs. Reported incidence rates for hyperemia in the clinical trials are 5%–10% for latanoprost,25 15%–45% for bimatoprost,26 and 35%–50% for travoprost.21
In a study conducted by Tingey et al, age, life expectancy, associated comorbidities, and satisfaction with treatment were the factors found to influence persistence with treatment and IOP control.18 The results of preliminary research have suggested that treatment with latanoprost is associated with greater persistence than with bimatoprost or travoprost,10,12,13,17,22–26 and this is possibly due to its better tolerability.10
In three independent, retrospective studies of US cohorts, each including over 1000 patients, and with follow-up periods between 18 and 30 months, patients who initially received latanoprost as a single agent continued treatment for significantly longer than patients treated with β-blockers, carbonic anhydrase inhibitors, or brimonidine.8–10
Recent clinical trials have documented that a low IOP in glaucomatous patients contributes to visual field preservation. 5,27 Thus, low persistence with the ocular hypotensive drug will limit the clinician’s ability to prevent visual loss related to glaucoma. Use of prostaglandin analogs, including latanoprost, bimatoprost, travoprost, and unoprostone, is related to higher persistence compared with other marketed hypotensive agents.4,5
The concept of persistence with treatment allows better assessment of the effectiveness of a treatment, reflecting its use in daily practice and identifying any possible change (eg, treatment failure), regardless of the underlying cause.
The introduction of prostaglandins resulted in a simplified more convenient dosing schedule, enabling more effective IOP reduction. Furthermore, it resulted in surgery becoming a second-line treatment for glaucoma, to be used only when IOP levels cannot be controlled by these potent drugs.28 We now need to identify drugs for glaucoma with as few side effects as possible to ensure continuity of treatment.
In our study, 81.6% of latanoprost-treated patients had continued treatment at the end of the second year, while only 22.9% of bimatoprost-treated patients persisted. Patient persistence with travoprost and timolol was approximately 60%. These results are in agreement with those published by Diestelhorst et al14 who, in an observational and retrospective review conducted in 13 European centers, observed that patients who received initial treatment for primary open angle glaucoma or OHT with latanoprost as monotherapy continued taking their treatment for a time period twice that of those patients who had received a β-blocker, underwent fewer changes of therapy, and achieved a greater mean IOP reduction. All these differences were statistically significant (P < 0.0001).
Discontinuation rates differed among the treatments. Fewer latanoprost-treated patients reported an ocular adverse event compared with those receiving bimatoprost, travoprost or timolol (P < 0.001). These results are consistent with those published by Parrish et al where latanoprost also showed greater ocular tolerability.29 A possible factor influencing the poor efficacy of bimatoprost may be the patient having received previous treatment, which might create a negative comparison for the patient.
The proportion of patients who had received previous treatment for glaucoma varied among the treatment groups, with the bimatoprost group having the highest percentage (74.3%), followed by latanoprost (46%). It would have been helpful to know why the bimatoprost group had the greatest proportion of previously treated patients but, unfortunately, the study did not gather this information.
One strength of this study was that it enabled patient persistence with the study drugs to be quantified, an important factor to bear in mind when deciding between pharmacologic options, which may result in improved disease management.
Regarding variables related to persistence, it should be emphasized that, even though comorbidities did not appear to be directly related to persistence, patient age was found to be significantly related.
One of the study limitations is the absence of efficacy measurements (in terms of IOP reduction), in order to study whether this might be a factor influencing persistence. As previously mentioned, no reliable data on baseline IOP levels were available, from which effectiveness of the treatments could have been calculated.
In conclusion, at present, multiple monotherapy and combination therapy options are available for glaucoma and OHT management. Thus, the strategy selected to maximize the clinical benefit while minimizing associated health expenses will be critical.
Latanoprost has shown greater patient persistence than travoprost, bimatoprost, and timolol in routine clinical practice, resulting in better control of IOP levels, and lower associated costs. This attributes should avoid the occurrence or progression of complications, improve quality of care for glaucomatous patients, and result in a considerable resource saving for the national health system.
Acknowledgment
The authors would like to thank Paloma Gonzalez and Antonio Pardo for their invaluable assistance with editorial review and statistical analysis of the data reported in this paper.
Footnotes
Disclosures
There was no financial support for this study, nor is there any conflict of interest on the part of the authors. None of the authors have any proprietary interest in the products reviewed.
References
- 1.Foster A, Gilbert C, Johnson G. Changing patterns in global blindness: 1988–2008. Community Eye Health. 2008;21:37–39. [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreiro López S, Ruíz Navarro S. Oftalmología en Atención Primaria. Capítulo X: Glaucoma. [Accessed February 10, 2010]. Available at: www.esteve.es/EsteveArchivos/1_8/Ar_1_8_44_APR_9.pdf.
- 3.American Academy of Ophthalmology Preferred Practice Patterns Committee Glaucoma Panel. San Francisco: American Academy of Ophthalmology; 2008. [Accessed on 16 March 2010]. Preferred practice pattern: primary open angle glaucoma, limited revision. Available at : one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid. [Google Scholar]
- 4.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7, the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 6.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 7.Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: Adherence by the elderly. Am J Public Health. 1993;83:711–7116. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta S, Oates V, Bookhart BK, Vaziri B, Schwartz GF, Mozaffari E. Population-based persistency rates for topical glaucoma medications measured with pharmacy claims data. Am J Manag Care. 2002;8(Suppl 10):S255–S261. [PubMed] [Google Scholar]
- 9.Spooner JJ, Bullano MF, Ikeda LI, et al. Rates of discontinuation and change of glaucoma therapy in a managed care setting. Am J Manag Care. 2002;8(Suppl 10):S262–S270. [PubMed] [Google Scholar]
- 10.Shaya FT, Mullins CD, Wong W, Cho J. Discontinuation rates of topical glaucoma medications in a managed care population. Am J Manag Care. 2002;8(Suppl 10):S271–S277. [PubMed] [Google Scholar]
- 11.Reardon G, Schwartz GF, Mozaffari E. Patient persistency with pharmacotherapy in the management of glaucoma. Eur J Ophthalmol. 2003;13(Suppl 4):S44–S52. doi: 10.1177/112067210301304s05. [DOI] [PubMed] [Google Scholar]
- 12.Reardon G, Schwartz GF, Mozaffari E. Patient persistency with topical ocular hypotensive therapy in a managed care population. Am J Ophthalmol. 2004;137(Suppl 1):S3–S12. doi: 10.1016/j.ajo.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GF, Reardon G, Mozaffari E. Persistency with latanoprost or timolol in primary open-angle glaucoma suspects. Am J Ophthalmol. 2004;137(Suppl 1):S13–S16. doi: 10.1016/j.ajo.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Diestelhorst M, Schaefer CP, Beusterien KM, et al. Persistency and clinical outcomes associated with latanoprost and beta-blocker monotherapy: Evidence from a European retrospective cohort study. Eur J Ophthalmol. 2003;13(Suppl 4):S21–S29. doi: 10.1177/112067210301304s03. [DOI] [PubMed] [Google Scholar]
- 15.Jonson ES, Mozaffari E. Measuring patient persistency with drug therapy using methods for the design and analysis of natural history studies. Am J Manag Care. 2002;8:S249–S254. [PubMed] [Google Scholar]
- 16.Schwartz GF. Compliance and persistency in glaucoma follow-up treatment. Curr Opin Ophthalmology. 2005;16:114–121. doi: 10.1097/01.icu.0000156139.05323.26. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman TJ, Hahn SR, Gelb L, Tan H, Kim EE. The impact of ocular adverse effects in patients treated with topical prostaglandin analogs: Changes in prescription patterns and patient persistence. J Ocul Pharmacol Ther. 2009;25:145–152. doi: 10.1089/jop.2008.0072. [DOI] [PubMed] [Google Scholar]
- 18.Tingey D, Bernard LM, Grima DT, et al. Intraocular pressure control and persistence on treatment in glaucoma and ocular hypertension. Can J Ophthalmol. 2005;40:161–169. doi: 10.1016/S0008-4182(05)80027-4. [DOI] [PubMed] [Google Scholar]
- 19.Reardon G, Schwartz GF, Mozaffari E. Patient persistency with ocular prostaglandin therapy: A population-based, retrospective study. Clin Ther. 2003;25:1172–1185. doi: 10.1016/s0149-2918(03)80074-7. [DOI] [PubMed] [Google Scholar]
- 20.Day DG, Schacknow PN, Sharpe ED, et al. A persistency and economic analysis of latanoprost, bimatoprost, or beta-blockers in patients with open-angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2004;2:383–392. doi: 10.1089/jop.2004.20.383. [DOI] [PubMed] [Google Scholar]
- 21.Hedman K, Alm A, Gross RL. Pooled-data analysis of three randomized, double-masked, six-month studies comparing intraocular pressure-reducing effects of latanoprost and timolol in patients with ocular hypertension. J Glaucoma. 2003;12:463–465. doi: 10.1097/00061198-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JS, Gross RL, Cheetham JK, VanDenburgh AM, Bernstein P, Whitcup SM. Two-year double-masked comparison of bimatoprost with timolol in patients with glaucoma or ocular hypertension. Surv Ophthalmol. 2004;49(Suppl 1):S45–S52. doi: 10.1016/j.survophthal.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Thomas R, Parikh R, Muliyil J, George R, Paul P, Abraham LM. Comparison between latanoprost and brimonidine efficacy and safety in Indian eyes. Indian J Ophthalmol. 2003;51:123–128. [PubMed] [Google Scholar]
- 24.Niazi MK, Raja N. Comparison of latanoprost and dorzolamide in the treatment of patients with open angle glaucoma. J Ayub Med Coll Abbottabad. 2004;16:50–53. [PubMed] [Google Scholar]
- 25.Travatan [Package insert] Alcon Laboratories, Inc; Fort Worth, TX: 2001. [Google Scholar]
- 26.Weinreb RN. Compliance with medical treatment of glaucoma. J Glaucoma. 1992;1:134–136. [Google Scholar]
- 27.Anderson DR Normal Tension Glaucoma Study. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14:86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Baudouin C, Rouland JF, Le Pen C. Changes in medical and surgical treatments of glaucoma between 1997 and 2000 in France. Eur J Ophthalmol. 2003;13(Suppl 4):S53–S60. doi: 10.1177/112067210301304s06. [DOI] [PubMed] [Google Scholar]
- 29.Parrish RK, Palmberg P, Sheu WP XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: A 12-week, randomized, masked-evaluator icenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]