Abstract
Objective
The aim of this study was to describe prescribing practices in the treatment of pediatric bipolar disorder in a university practice setting.
Method
A retrospective chart review was performed on 53 youths diagnosed using Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), criteria with bipolar spectrum disorder under the active care of child psychiatrists practicing in a pediatric psychopharmacology specialty clinic. Current medications, doses, and related adverse events were recorded. Clinicians were asked to provide a target disorder (bipolar mania/mixed state, depression, attention deficit hyperactivity disorder [ADHD], or anxiety) for each medication to the best of their ability. The Clinical Global Impressions–Severity (CGI-S) scale was used to measure severity of each disorder before treatment and the Clinical Global Impressions–Improvement (CGI-I) was used to quantify the magnitude of improvement with treatment. Meaningful improvement of the disorder was defined by CGI-I score of 1 or 2.
Results
The mean number of psychotropic medications per patient was 3.0 ± 1.6. A total of 68% of patients were treated for co-morbid disorders; 23% of patients were treated with monotherapy, primarily with second-generation antipsychotics. Mania improved in 80% of cases, mixed state improved in 57% of cases, ADHD improved in 56% of cases, anxiety improved in 61% of cases, and depression improved in 90% of cases.
Conclusion
The management of pediatric bipolar disorder often requires multiple medications. For the treatment of mania/mixed states, clinicians prescribed second-generation antipsychotics more frequently than mood stabilizers, especially in the context of monotherapy. Co-morbidity was a frequent problem with moderate success obtained with combined pharmacotherapy approaches. Further psychosocial strategies to augment pharmacotherapy may improve outcome while reducing the medication burden in pediatric bipolar disorder.
Introduction
Despite controversy, there is increasing consensus that pediatric bipolar disorder is a valid and prevalent disorder associated with serious functional impairment (Carlson and Kelly 1998; Wozniak et al. 2003; Geller et al. 2004; Wozniak et al. 2005; Birmaher et al. 2006; McClellan et al. 2007; Moreno et al. 2007) and continuity into early adulthood (Geller et al. 2008). A retrospective study of 480 adult bipolar patients found that those reporting childhood-onset bipolar illness had more lifetime episodes of mania and depression, more co-morbid mental illness, and more rapid cycling than patients with adult onset bipolar disorder (Leverich et al. 2007). Similar findings were reported in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study of 1000 adult bipolar patients, finding that early-onset bipolar patients reported greater co-morbid anxiety and substance abuse, more recurrences of mood episodes, shorter periods of euthymia, and greater incidence of suicide attempts and violence (Perlis et al. 2004).
Many studies indicate that pediatric bipolar disorder causes significant emotional, academic, and social impairment in preschool, prepubertal, and adolescent youth (Wozniak et al. 1995; Geller et al. 2000a; Geller et al. 2000b; Wilens et al. 2003a; Craney and Geller 2003; Pavuluri et al. 2005; Axelson et al. 2006; Kowatch and DelBello 2006; Danielyan et al. 2007; Goldstein et al. 2009). Studies across many sites document the utility of pharmacotherapy treatment for pediatric bipolar disorder (Kowatch et al. 2000; Delbello et al. 2002b; Findling et al. 2003; Kowatch et al. 2003; Biederman et al. 2005b. Biederman et al. 2005c; Findling et al. 2005; Biederman et al. 2007a; Biederman et al. 2007b; DelBello et al. 2006; Findling et al. 2006).
Although to date, only lithium, risperidone, and aripiprazole have Food and Drug Administration (FDA) approval for the treatment of pediatric bipolar disorder, in 2007 the American Academy of Child and Adolescent Psychiatry released a practice parameter recommending treatment for pediatric bipolar disorder with a traditional mood stabilizer such as lithium or valproic acid and/or an atypical antipsychotic medication (McClellan et al. 2007). In clinical practice, children and adults with bipolar disorder are commonly treated with more than one medicine. For example, we reported that children with pediatric bipolar disorder receive on average three medicines (Wozniak et al. 2003). There is an emerging research base supporting the efficacy of multiple medications in pediatric bipolar disorder, with combined approaches targeting symptoms of mania and psychiatric co-morbidity (Kafantaris et al. 2001a; Kafantaris et al. 2001b; DelBello et al. 2002a; Delbello et al. 2002b; Findling et al. 2003; Kowatch et al. 2003; Pavuluri et al. 2004b; Scheffer et al. 2005; Findling et al. 2006; Findling et al. 2007). Indeed, a study applying an evidence-based pharmacotherapy algorithm to pediatric bipolar disorder patients found that few patients were able to remain on monotherapy with lithium, divalproex, or a second-generation antipsychotic over the course of 18 months, and the majority required combination therapy to achieve significant stabilization (Pavuluri et al. 2004c). The use of multiple medicines in the management of adult bipolar disorder has also been documented in the STEP-BD study (Ghaemi et al. 2006). Yet uncertainties remain as to the extent of this practice and the reasons underlying combined pharmacotherapy. In addition, safety concerns persist as to the long-term use of antipsychotics and mood stabilizers in children (Correll 2007; Correll 2008).
Few studies address the safety and efficacy of combined pharmacotherapy for the attendant co-morbid conditions commonly seen in pediatric bipolar disorder (Scheffer et al. 2005; Chang et al. 2006). Pediatric bipolar disorder is a highly co-morbid condition (Kowatch et al. 2005a) with high rates of conduct disorder (Biederman et al. 1999; Wozniak et al. 2001), anxiety disorders (Birmaher et al. 2002; Wozniak et al. 2002; Harpold et al. 2005; Masi et al. 2007), and attention-deficit/hyperactivity disorder (ADHD) (Borchardt and Bernstein 1995; Geller et al. 1995; Lewinsohn et al. 1995; Biederman et al. 1996; West et al. 1995a; West et al. 1995b; Wozniak et al. 1995; Wozniak and Biederman 1996; Post et al. 2004; Reich et al. 2005), all of which might be responsive to additional pharmacological intervention.
The main aim of this study was to quantify the pharmacological approaches in the management of pediatric bipolar disorder by child and adolescent psychiatrists practicing in a pediatric psychopharmacology specialty clinic. To this end, we conducted a naturalistic study consisting of a retrospective audit of the clinical records of pediatric patients with bipolar and bipolar spectrum disorders. Our aim was to assess the number of psychotropics used in individual patients, determine the reason for their use, estimate the degree of improvement, and calculate the adverse effect burden of the medications. Based on recent trends in the pharmacotherapy of pediatric bipolar disorder, we hypothesized that the most frequent medication prescribed to pediatric bipolar disorder patients would be second-generation antipsychotic medications. Furthermore, we hypothesized that the presence of co-morbid disorders accounted for the majority of additional medications used in this population.
Methods
We conducted a retrospective, unblinded chart review to gather information regarding the psychopharmacologic treatment of youth with bipolar and bipolar spectrum disorder and performed a cross-sectional examination of the outcomes. Permission to conduct this research with deidentified data was obtained from the institutional review board.
The subjects were children and adolescents currently under the care of board-certified child and adolescent psychiatrists (J.B., T.W., and J.W.) in the outpatient pediatric psychopharmacology clinic at a major university center, Massachusetts General Hospital. We reviewed electronic medical records with the treating psychiatrists to identify active patients (seen within the last year) ranging in age from 4 to 19 years old who were diagnosed with bipolar disorder or bipolar spectrum disorder. All diagnoses were made by the treating psychiatrists using Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria (American Psychiatric Association 1994; American Psychiatric Association 2000). We generated an alphabetical patient list for each clinician and collected data for the first 10–20 patients on the list that carried the diagnosis of bipolar disorder and bipolar spectrum disorder. Each clinician contributed data on no more than 20 patients to avoid skewing the data toward any one clinician's prescribing practices. We restricted the study to those seen at least once over the last year to ensure that clinicians would be able to easily recall the cases and assign accurate severity and improvement scores, which would be compromised if the patient had been seen over a year previously. In addition, we wanted to minimize biases that might occur in reporting on the last 10–20 patients seen, because these could represent frequently seen high-acuity patients. There were no exclusion criteria.
The data obtained for each subject included age, gender, psychotropic medications, doses, and adverse events (obtained by spontaneous patient report as well as by clinician query during treatment) associated with each medication. For each medication, we asked clinicians to identify a target disorder for each medication to the best of their ability (bipolar mania/mixed state, depression, ADHD, or anxiety). For example, if the target symptom for a specific medication was irritability, clinicians were asked to identify to the best of their ability whether they assessed the irritability to be from mania, mixed state, or depression. The severity of each disorder at the time the subject entered the clinic was recorded using the National Institute of Mental Health Clinical Global Impressions Scale–Severity (CGI-S) [1 = not ill; 7 = extremely ill]. The improvement on the current treatment was measured using the Clinical Global Impressions Scale–Improvement (CGI-I) [1 = very much improved; 7 = very much worse] (Conners and Barkley 1985). Ratings were made retrospectively at the time of the study by the treating clinicians based on chart notes and knowledge of the patients.
Data were gathered to determine the number and types of medications used to treat psychopathology in the subjects, the reason for their use, the rates of improvement (meaningful improvement was defined as CGI-I ≤2) of each disorder with medication treatment and the severity of adverse events related to the medications (rated by using a separate CGI-S for Adverse Events). All data are presented as percentages, absolute numbers, or mean ± standard deviation (SD) unless otherwise described.
Results
Sociodemographic and clinical characteristics of sample
Data on therapeutics from 53 consecutive charts of youths with bipolar disorder or bipolar spectrum disorder were evaluated. Table 1 summarizes demographical and clinical information of the sample. The mean age of the sample was 13 ± 3.6 years (range 4–19 years); 18 (34%) were children <12 years of age and 39 (74%) were male. Seventeen patients (32%) were receiving pharmacological treatment for mania compared with 35 patients (67%) for mixed state; 1 patient was receiving only cognitive behavioral therapy (CBT) to address bipolar symptoms (plus psychotropic medication to address psychiatric co-morbidity). Thirty six (68%) youths were receiving an additional medication for treatment of a co-morbid diagnosis—18 (34%) for depression, 32 (60%) for ADHD, and 14 (26%) for an anxiety disorder. The syndrome-specific pretreatment mean CGI-S scores ranged from 4.8 to 5.6, indicating moderate to marked severity of illness (see Table 1 for details).
Table 1.
Demographics and Clinical Characteristics
| Number of Patients | Percent of Patients | |
|---|---|---|
| Total | 53 | |
| <12 years old | 18 | 34% |
| Males | 39 | 74% |
| Treated for co-morbid diagnosis | 32 | 68% |
| Treated for Depression | 18 | 34% |
| Treated for ADHD | 32 | 60% |
| Treated for Anxiety | 14 | 26% |
| Mean | Standard Deviation | |
| Mean Age (years) | 13 | ±3.6 |
| Mean number of psychotropic medications | 3 | ±1.6 |
| CGI-S Mania/Mixed state | 5.6 | ±0.69 |
| CGI-S Depression | 5.3 | ±0.64 |
| CGI-S ADHD | 4.8 | ±0.82 |
| CGI-S Anxiety | 5 | ±0.69 |
Abbreviations: ADHD = Attention-deficit/hyperactivity disorder; CGI-S = Clinical Global Impressions—Severity.
The mean number of current psychotropic medications per patient was 3.0 ± 1.6. Medications included (not mutually exclusive) second-generation antipsychotics (n = 46 [87%]), central nervous system stimulants (n = 19 [36%]), mood stabilizers (lithium carbonate, divalproex sodium, and carbamazepine) (n = 11 [21%]), nonstimulant anti-ADHD medications (n = 9 [17%]), α-2 agonists (n = 9 [17%]), benzodiazepines (n = 7 [13%]), selective serotonin reuptake inhibitors (SSRI) (n = 5 [9.4%]), non-SSRI antidepressants (n = 5 [9.4%]), and other antiepileptic medications (n = 3 [6%]).
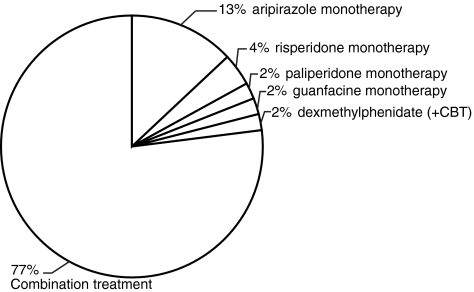
As depicted in Fig. 1, 12 patients (23%) were treated with monotherapy. Of patients receiving monotherapy, 10 patients (19% of all patients) were treated with second-generation antipsychotic monotherapy: 7 Patients (13% of all patients) with aripiprazole, 2 patients (4%) with risperidone, and 1 patient (2%) with paliperidone. Of the remaining 2 patients on monotherapy, 1 patient was treated with guanfacine and the other patient with dexmethylphenidate (bipolar symptoms were managed with cognitive behavioral therapy alone). None of the patients was on monotherapy with a mood stabilizer.
FIG. 1.
Distribution of patients on monotherapy compared to combination pharmacotherapy. The majority of patients required combination treatment. Aripiprazole was the most commonly used medication for monotherapy. CBT, Cognitive behavioral therapy.
Medications specifically targeting mania/mixed state
The mean number of medications used specifically to target mania/mixed state per patient was 1.4 ± 0.9. These medications included second-generation antipsychotics (n = 46 [87%]), mood stabilizers (n = 11 [21%]), other antiepileptic medications (oxcarbazepine) (n = 3 [6%]), first-generation antipsychotics (n = 3 [6%]), guanfacine (n = 1 [2%]), and a β-blocker (n = 1 [2%]).
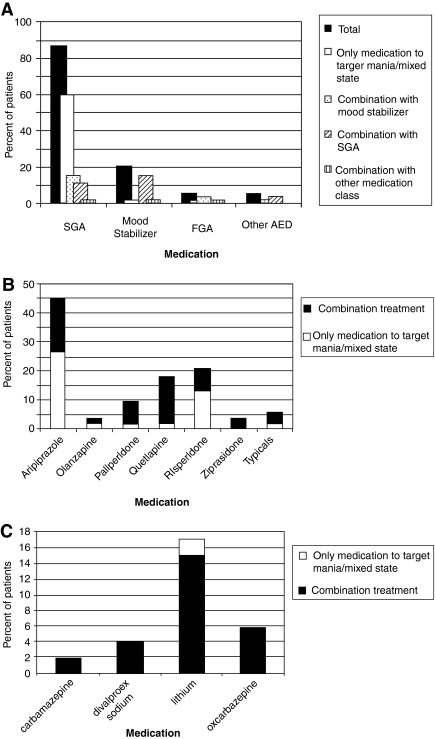
As shown in Fig. 2A, 60% (n = 32) of all patients were being treated with a second-generation antipsychotic as the only medication to address mania/mixed state specifically versus 2% (n = 1) of patients who were being treated with a mood stabilizer (lithium) as the only treatment to target mania/mixed state. Eighty seven percent (n = 46) of patients were on at least one second-generation antipsychotic medication compared with 21% (n = 11) of patients who were on at least a mood stabilizer to target mania/mixed state. Eleven percent (n = 6) of patients were on a combination of second-generation antipsychotic medications and 15% (n = 8) were on a combination of a second-generation antipsychotic plus mood stabilizer.
FIG. 2.
Medications used specifically to target mania/mixed state. (A) All medication classes used to target mania/mixed state. The majority of patients were on at least one second-generation antipsychotic medication. Similarly, of patients receiving only one medication to target mania/mood symptoms, a majority received a second-generation antipsychotic medication. (B) The use of specific antipsychotic agents in the treatment of mania/mixed state. Aripiprazole was the most commonly used antipsychotic medication in both monotherapy and in combined regimens. (C) Use of mood stabilizers and antiepileptic medications. Mood stabilizers and AEDs were almost universally used in combined regimens. Lithium was the most commonly used mood stabilizer. SGA, second generation antipsychotic; FGA, first generation antipsychotic; AEDs, anti-epileptic drugs (oxcarbazepine).
Figure 2, B and C, demonstrates the use of specific antipsychotic medications and mood stabilizers, respectively. As shown in Fig. 2B, 45% (n = 24) of all patients were being treated with aripiprazole; 26% (n = 14) of all patients were on aripiprazole as the only psychopharmacologic treatment to address mania/mixed state.
Table 2 shows the average doses of second-generation antipsychotic medications and mood stabilizers used to target mania/mixed state.
Table 2.
Doses of Medications to Treat Mania/Mixed state
| |
|
Mean daily dose (mg/day) |
||
|---|---|---|---|---|
| Medication | Dose range (mg/day) | Total | <12 years old | ≥12 years old |
| Second-generation antipsychotic medications | ||||
| Aripiprazole (n = 24) | 1.5–40 | 20 ± 13 | 7.9 ± 5.0 | 29 ± 10 |
| Risperidone (n = 11) | 0.5–6.0 | 2.8 ± 1.8 | 2.7 ± 1.9 | 2.8 ± 1.9 |
| Quetiapine (n = 9) | 100–800 | 328 ± 191 | 200 ± 141 | 364 ± 195 |
| Paliperidone (n = 4) | 3.0–12 | 6.0 ± 4.2 | N/A | 6.0 ± 4.2 |
| Olanzapine (n = 2) | 2.5–15 | 8.8 ± 8.8 | N/A | 8.8 ± 8.8 |
| Ziprasidone (n = 2) | 40–120 | 80 ± 57 | N/A | 80 ± 57 |
| Mood stabilizers and AEDs | ||||
| Lithium (n = 8) | 300–1800 | 656 ± 500 | N/A | 656 ± 500 |
| Oxcarbazepine (n = 3) | 600–900 | 800 ± 173 | N/A | 800 ± 173 |
| Divalproex sodium (n = 2) | 625–1000 | 813 ± 265 | 1000 | 635 |
| Carbamazepine (n = 1) | 600 | 600 | N/A | 600 |
Abbreviations: N/A = not applicable; AEDs = antiepileptic drugs.
Medications for co-morbid conditions
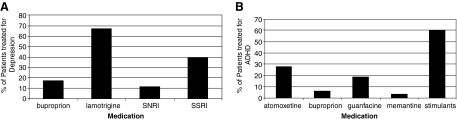
Medications used to target depression and ADHD are shown in Fig. 3, a and b, respectively. The mean number of medications used specifically to treat depression was 1.2 ± 0.43 and ADHD was 1.2 ± 0.42. Sixty seven percent of patients (n = 12) with depression were treated with lamotrigine (Fig. 3A) and 60% of patients (n = 19) with ADHD were treated with stimulants (Fig. 3B).
FIG. 3.
Medications used to target depression (A, n = 18) and ADHD (B, n = 32). Lamotrigine was the most commonly used medication to target depression, and stimulants were the most common medication class for ADHD. ADHD, Attention-deficit/hyperactivity disorder; SNRI, Serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Response to pharmacotherapy
A CGI-I score of ≤2 (much or very much improved) defined meaningful improvement. Using this definition of response, as shown in Table 3, there was meaningful improvement in overall dimensions of illness in 66% of patients (with mean pretreatment CGI-S of 5.2 ± 0.8 and mean CGI-I of 2.2 ± 0.7). There was meaningful improvement of mania in 80% of patients (with mean pretreatment CGI-S of 5.4 ± 0.6 and mean CGI-I of 2.0 ± 0.8), of mixed state in 57% of patients (with mean pretreatment CGI-S of 5.7 ± 0.7 and mean CGI-I of 2.3 ± 0.7) of ADHD in 56% of patients (with mean pretreatment CGI-S of 4.8 ± 0.8 and mean CGI-I of 2.4 ± 0.7), and of depression in 91% of patients (with mean pretreatment CGI-S of 5.3 ± 0.6 and mean CGI-I of 1.8 ± 0.6). Second-generation antipsychotic medications led to meaningful improvement in 73% of patients compared to 64% of patients on mood stabilizers in treating mania/mixed state, although, as noted above, second-generation antipsychotic medications were often used as the only treatment to address bipolar disorder, whereas mood stabilizers typically were used in combination with other antimanic agents including second-generation antipsychotic medications.
Table 3.
CGI Scores for Specific Disorders
| Diagnosis | CGI-S | CGI-I | CGI-I ≤ 2 |
|---|---|---|---|
| ADHD | 4.8 ± 0.8 | 2.4 ± 0.7 | 56% |
| Depression | 5.3 ± 0.6 | 1.8 ± 0.6 | 91% |
| Mania | 5.4 ± 0.6 | 2.0 ± 0.8 | 80% |
| Mixed state | 5.7 ± 0.7 | 2.3 ± 0.7 | 57% |
| Overall | 5.2 ± 0.8 | 2.2 ± 0.7 | 66% |
Listed are mean CGI-S scores pretreatment (at time of entry into the clinic) and CGI-I scores (at the time of the chart review) for individual disorders as well as overall illness. The table also includes the percent of patients with meaningful improvement on the current treatment regimen (defined by CGI-I ≤2).
Abbreviations: CGI-S = Clinical Global Impressions–Severity; CGI-I, Clinical Global Impressions-Improvement; ADHD = attention-deficit/hyperactivity disorder.
Adverse events
A similar percentage of subjects experienced adverse effect burden on second-generation antipsychotic medications and mood stabilizers (54% vs. 60%, respectively). Table 4 lists the medication adverse events that were identified as well as the severity score (on a scale of 1 to 7, 1 = not severe, 7 = extremely severe) and the adverse event burden for each medication. Among the second-generation antipsychotic medications, adverse events included weight gain, sedation, and extrapyramidal symptoms (EPS). Adverse events for lithium included tremor, weight gain, acne, polyuria/polydipsia, and hair thinning. Divalproex sodium was associated with sedation.
Table 4.
Adverse Events
| Medication | Adverse Event (AE) | Severity of AE | n (with AE) | Total | AE burden (%) |
|---|---|---|---|---|---|
| Aripiprazole | any adverse event | 3.7 | 13 | 24 | 54% |
| weight gain | 3.4 | 9 | 24 | 38% | |
| sedation | 3.5 | 4 | 24 | 17% | |
| EPS | 4 | 2 | 24 | 8% | |
| nausea | 6 | 1 | 24 | 4% | |
| Olanzapine | any adverse event | 2.8 | 2 | 2 | 100% |
| sedation | 2.5 | 2 | 2 | 100% | |
| weight gain | 3 | 1 | 2 | 50% | |
| Paliperidone | weight gain | 4 | 2 | 4 | 50% |
| Quetiapine | any adverse event | 3.3 | 5 | 9 | 56% |
| sedation | 3 | 4 | 9 | 44% | |
| weight gain | 4 | 2 | 9 | 22% | |
| Risperidone | weight gain | 3.5 | 6 | 11 | 55% |
| Ziprasidone | activation | 4 | 1 | 2 | 50% |
| Lithium | any adverse event | 4.1 | 6 | 8 | 75% |
| tremor | 4 | 2 | 7 | 29% | |
| acne | 4.5 | 2 | 7 | 29% | |
| polyuria, polydypsia | 3 | 1 | 7 | 14% | |
| weight gain | 5 | 1 | 7 | 14% | |
| hair thinning | 4 | 1 | 7 | 14% | |
| Divalproex sodium | sedation | 2 | 1 | 2 | 50% |
| Clonidine | sedation | 5 | 1 | 3 | 33% |
| Concerta | sleep problems | 3 | 1 | 8 | 13% |
| Daytrana | self-excoriation | 3 | 1 | 1 | 100% |
| Dexedrine | any adverse event | 4 | 1 | 1 | 100% |
| decreased appetite | 4 | 1 | 1 | 100% | |
| irritability | 4 | 1 | 1 | 100% | |
| Guanfacine | sedation | 3 | 1 | 6 | 17% |
| Clonazepam | disinhibition | 4 | 1 | 4 | 25% |
| Lorazepam | sedation | 3 | 1 | 4 | 25% |
| Neurontin | sedation | 2.7 | 3 | 3 | 100% |
| Paroxetine | irritability | 4 | 1 | 1 | 100% |
Listed are adverse events related to treatment with specific medications (if a medication is not listed, no associated adverse events were reported in this study). CGI-S refers to the severity of the adverse event (1 = not severe; 7 = extremely severe). AE burden refers to the percent of patients with that particular adverse event on that medication.
Abbreviations: EPS Extrapyramidal symptoms; CGI-S = Clinical Global Impressions–Severity.
Combined treatment
The most common reason for combined therapy was to treat co-morbid ADHD (n = 32, 60%). In addition to treatment of co-morbid psychiatric diagnoses (ADHD, depression, and anxiety), 13% (n = 7) of patients were treated for weight gain with medications including topiramate, naltrexone, and metformin, 3.8% (n = 2) of patients were treated for insomnia with clonidine, and 3.8% (n = 2) of patients were treated for EPS with benztropine.
Discussion
This was a retrospective audit of the pharmacologic treatment of pediatric bipolar disorder in a naturalistic setting by child and adolescent psychiatrists practicing in a pediatric psychopharmacology specialty clinic. In our sample, the majority of patients were moderately or markedly ill as indicated by their CGI severity at time of referral and 68% carried a co-morbid diagnosis. Patients were most frequently prescribed second-generation antipsychotics followed by central nervous system stimulants and mood stabilizers. The majority of patients demonstrated improvement on the most recent assessment of their clinical status, although this assessment was retrospective rather than prospective in nature. The clinical characteristics of this sample are consistent with prior studies documenting high co-morbidity of child and adolescent mania with depression (Conners and Barkley 1985; Geller et al. 1994; Geller et al. 2001), disruptive behavior disorders (Spencer et al. 2001), substance use disorders (Biederman et al. 2000a; Wilens et al. 2008), and anxiety disorders (Geller et al. 1995). A recent review of national trends found that 52.7% of outpatients with pediatric bipolar disorder carried a co-morbid diagnosis (Moreno et al. 2007).
The finding that patients in this chart review were prescribed an average of three medications is consistent with our previous study documenting a similarly high rate of combined pharmacotherapy in the management of pediatric bipolar disorder (Wozniak et al. 2003). Combination pharmacotherapy is common among adult patients with bipolar disorder (Frye et al. 2000). Combination pharmacotherapy has also been studied in the treatment of pediatric bipolar disorder. This includes studies of antipsychotic and mood stabilizer combinations (Delbello et al. 2002b; Pavuluri et al. 2004b), combinations of mood stabilizers (Findling et al. 2006), and the combination of a mood stabilizer with a mood stabilizer, stimulant, antidepressant, or antipsychotic (Kowatch et al. 2003). The most common reasons for combined pharmacotherapy in our series were for the treatment of depression, co-morbid ADHD, and co-morbid anxiety. The rationale for combined pharmacotherapy in child psychiatry includes partial response to a single agent, synergistic effects of combined agents, and diagnoses associated with the need for a specific combination (Wilens et al. 1995).
The overall magnitude of meaningful improvement of 66% observed in this sample is consistent with findings in other studies. A study of 263 youth with bipolar I, bipolar II, or bipolar not otherwise specified by Birmaher et al. found that 68% recovered from their index episode (Birmaher et al. 2006). Another longitudinal study of 89 bipolar children treated naturalistically in the community found that 65% recovered from mania (Geller et al. 2004). Of note, mania was more treatment responsive than mixed state in our sample. This is consistent with emerging adult bipolar literature, which suggests that patients with even subsyndromal manic symptoms during depressive episodes were at greater risk of earlier onset of symptoms, rapid cycling, and lifetime suicide attempts than bipolar individuals without mixed features (Goldberg et al. 2009). Analysis of specific medication classes used to treat pediatric bipolar disorder revealed that second-generation antipsychotic medications outnumbered mood stabilizers by a ratio of 4:1. Moreover, nearly 1 in 5 patients were maintained on a regimen of second-generation antipsychotic monotherapy, whereas not a single patient was on mood stabilizer monotherapy. The preference for second-generation antipsychotic medications over mood stabilizers was due to several reasons. First, there were clinical concerns regarding the difficulty of obtaining blood levels and the narrow therapeutic index of mood stabilizers. Indeed, our previous work as well as that of Kowatch et al. has suggested limited efficacy of mood stabilizers in pediatric bipolar disorder (Biederman et al. 1998; Kowatch et al. 2000). Second, the use of second-generation antipsychotic monotherapy is consistent with an emerging literature consisting of chart reviews, open-label trials, and increasing numbers of randomized trials supporting the efficacy of second-generation antipsychotic monotherapy in pediatric bipolar disorder (Frazier et al. 2001; Masi et al. 2002; Biederman et al. 2005c; Barzman et al. 2006; Biederman et al. 2007a; Biederman et al. 2007b; Tohen et al. 2007) and adult bipolar disorder (Perlis et al. 2006). Furthermore, the use of second-generation antipsychotics supports recent pediatric bipolar disorder practice guidelines that indicate that first-line treatment is monotherapy with conventional mood stabilizers or second-generation antipsychotics.
Among the second-generation antipsychotics, aripiprazole was the most frequently prescribed medication, followed by risperidone. Because these medications are FDA approved for the treatment of pediatric bipolar disorder, this prescription pattern is consistent with the recommendations of the American Academy of Child and Adolescent Psychiatry (AACAP) practice parameters as well as a recent treatment guideline for pediatric bipolar disorder (Kowatch et al. 2005b; McClellan et al. 2007). Regarding aripiprazole, the adult bipolar literature suggests that aripiprazole monotherapy is efficacious in the treatment of mania in both the acute and maintenance phase (Vieta et al. 2005; Keck et al. 2007). In addition, a recent analysis of 516 adult bipolar I patients found that aripiprazole improved outcomes, even in groups that are traditionally treatment resistant (Suppes et al. 2008). Regarding the pediatric literature, two chart reviews (Barzman et al. 2004; Biederman et al. 2005a) and one small open-label trial (Biederman et al. 2007b) support the efficacy and safety of aripiprazole in the treatment of pediatric bipolar disorder.
In this sample, 18 patients were treated for bipolar depression and 90% achieved significant improvement on varied treatment regimens. The two most commonly prescribed medications for bipolar depression were lamotrigine and SSRIs. There is minimal evidence guiding the treatment of pediatric bipolar depression. An open-label trial of lamotrigine in adolescents with bipolar depression suggested that it may be effective in acute treatment (Chang et al. 2006). SSRIs remain controversial, with one chart review in children and adolescents suggesting that they improve bipolar depression but also increase risk of relapse into mania (Biederman et al. 2000b; Wilens et al. 2003b).
In contrast, only 56% of the subjects treated for ADHD improved. The most commonly prescribed medications for ADHD were stimulants and atomoxetine. One randomized controlled trial found that mixed amphetamine salts were beneficial and safe for ADHD when manic symptoms were stabilized with divalproex (Scheffer et al. 2005). A second randomized controlled trial found that methylphenidate in combination with a thymoleptic agent in stabilized youth was also safe and effective in children and adolescents with ADHD and bipolar disorder (Findling et al. 2007). Taken together, these results support the AACAP practice parameter that advocates for the cautious use of stimulants to target ADHD after adequate mood stabilization (McClellan et al. 2007).
These results need to be interpreted in light of methodological limitations. This was a relatively small number of patients and co-morbidity was high. Because assessments of baseline severity and improvement were done retrospectively using the CGI by treating clinicians rather than prospectively using rating scales, they were subject to assessment bias. In a similar vein, CGI severity after treatment was not assessed, so we could not directly compare CGI severity at baseline and after treatment. Instead, we focused on CGI improvement as a measure of treatment response. Adverse events were obtained in the course of standard clinical care by clinicians via spontaneous patient report and clinician query. However, systematic collection of adverse events was not part of this study, and this naturalistic approach could have led to a lower number of reported adverse events. In addition, the authors are colleagues and psychopharmacology research collaborators, so their prescribing practices may not be representative of prescribing practices in other pediatric specialty clinics. Because only currently active patients were included in the analysis, results may have appeared more favorable because of the exclusion of patients that had not been seen in more than a year. This may have excluded patients that dropped out of treatment due to adverse effects or lack of improvement or patients whose symptoms improved to such a degree that they did not require active treatment. In addition, duration of treatment was not gathered, so the length of time on the different medications could have differed. However, this study design was chosen to focus on a cross-sectional view of the patient's clinical status rather than a longitudinal view. The study also did not gather information on adjuvant psychosocial treatment of pediatric bipolar disorder and its co-morbidities, which may facilitate the long term management of symptoms (Pavuluri et al. 2004a; West et al. 2007). Finally, we presume that clinicians discontinued medications that were not effective in treating target conditions or that resulted in significant side effects that would affect both the CGI improvement scores as well as the adverse events ratings.
Despite these limitations, our analysis of treatment patterns in a specialized pediatric psychopharmacology clinic suggests the importance of combined pharmacotherapy in the management of pediatric bipolar disorder and its co-morbidities. At present, clinical treatment is guided by Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) nosology rather than a sophisticated understanding of the underlying etiology of the disorder. In the future, treatments may be guided by a greater understanding of the genetic and neural circuitry dysfunction responsible for the symptoms of pediatric bipolar disorder. More systematic research is needed to expand the clinical evidence base of the safety and efficacy of treatments for pediatric bipolar disorder and associated disorders.
Footnotes
There was no support from any grant, funding source, or commercial interest regarding this paper.
Disclosures
Mona P. Potter, M.D., Howard Y. Liu, M.D., and Carly Henderson, B.A. have no conflicts of interest or financial ties to disclose. Michael Monuteaux, Sc.D, was a speaker for a symposium sponsored by Shire. Janet Wozniak, M.D., is a Consultant to Pfizer, Shire Pharmaceuticals, and Eli Lilly; receives research funding from Eli Lilly and the National Institute of Mental Health; and is on the speakers bureaus of Eli Lilly and Janssen.Timothy E. Wilens, M.D., is a consultant for Abbott, McNeil, Lilly, the National Institutes of Health National/Institute on Drug Abuse, Novartis, Merck, and Shire; receives research funding from Abbott, McNeil, Lilly, National Institutes of Health National/Institute on Drug Abuse, Merck, and Shire; and is on the speakers bureau for Lilly, McNeil, Novartis, and Shire. Joseph Biederman, M.D., is a consultant/advisory board member for Janssen, McNeil, Novartis, and Shire; receives research funding from Alza, AstraZeneca, Bristol Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals Inc., McNeil, Merck, Organon, Otsuka, Shire, National Institute of Mental Health, and National Institute of Child Health and Human Development; and is on the speakers bureau for Janssen, McNeil, Novartis, Shire, and UCB Pharma, Inc. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker's fees for/from the following additional sources: Abbott, AstraZeneca, Celltech, Cephalon, Eli Lilly and Co., Esai, Forest, Glaxo, Gliatech, NARSAD, National Institute on Drug Abuse, New River, Novartis, Noven, Neurosearch, Pfizer, Pharmacia, The Prechter Foundation, The Stanley Foundation, and Wyeth.
References
- American Psychiatric Association. Diagnostic, Statistical Manual of Mental Disorders, 4th edition (DSM-IV) Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic, Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- Axelson D. Birmaher B. Strober M. Gill MK. Valeri S. Chiappetta L. Ryan N. Leonard H. Hunt J. Iyengar S. Bridge J. Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Barzman DH. DelBello MP. Kowatch RA. Gernert B. Fleck DE. Pathak S. Rappaport K. Delgado SV. Campbell P. Strakowski SM. The effectiveness and tolerability of aripiprazole for pediatric bipolar disorders: A retrospective chart review. J Child Adolesc Psychopharmacol. 2004;14:593–1600. doi: 10.1089/cap.2004.14.593. [DOI] [PubMed] [Google Scholar]
- Barzman DH. DelBello MP. Adler CM. Stanford KE. Strakowski SM. The efficacy and tolerability of quetiapine versus divalproex for the treatment of impulsivity and reactive aggression in adolescents with co-occurring bipolar disorder and disruptive behavior disorder(s) J Child Adolesc Psychopharmacol. 2006;16:665–670. doi: 10.1089/cap.2006.16.665. [DOI] [PubMed] [Google Scholar]
- Biederman J. Faraone S. Mick E. Wozniak J. Chen L. Ouellette C. Marrs A. Moore P. Garcia J. Mennin D. Lelon E. Attention-deficit hyperactivity disorder and juvenile mania: An overlooked comorbidity? J Am Acad Child Adolesc Psychiatry. 1996;35:997–1008. doi: 10.1097/00004583-199608000-00010. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Bostic JQ. Prince J. Daly J. Wilens TE. Spencer T. Garcia-Jetton J. Russell R. Wozniak J. Faraone SV. The naturalistic course of pharmacologic treatment of children with maniclike symptoms: A systematic chart review. J Clin Psychiatry. 1998;59:628–637. doi: 10.4088/jcp.v59n1111. quiz 638. [DOI] [PubMed] [Google Scholar]
- Biederman J. Faraone SV. Chu MP. Wozniak J. Further evidence of a bidirectional overlap between juvenile mania and conduct disorder in children. J Am Acad Child Adolesc Psychiatry. 1999;38:468–476. doi: 10.1097/00004583-199904000-00021. [DOI] [PubMed] [Google Scholar]
- Biederman J. Faraone SV. Wozniak J. Monuteaux MC. Parsing the association between bipolar, conduct, and substance use disorders: A familial risk analysis. Biol Psychiatry. 2000a;48:1037–1044. doi: 10.1016/s0006-3223(00)00906-9. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Spencer TJ. Wilens TE. Faraone SV. Therapeutic dilemmas in the pharmacotherapy of bipolar depression in the young. J Child Adolesc Psychopharmacol. 2000b;10:185–192. doi: 10.1089/10445460050167296. [DOI] [PubMed] [Google Scholar]
- Biederman J. McDonnell MA. Wozniak J. Spencer T. Aleardi M. Falzone R. Mick E. Aripiprazole in the treatment of pediatric bipolar disorder: A systematic chart review. CNS Spectr. 2005a;10:141–148. doi: 10.1017/s1092852900019489. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Hammerness P. Harpold T. Aleardi M. Dougherty M. Wozniak J. Open-label, 8-week trial of olanzapine and risperidone for the treatment of bipolar disorder in preschool-age children. Biol Psychiatry. 2005b;58:589–594. doi: 10.1016/j.biopsych.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Wozniak J. Aleardi M. Spencer T. Faraone SV. An open-label trial of risperidone in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2005c;15:311–317. doi: 10.1089/cap.2005.15.311. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Spencer T. Dougherty M. Aleardi M. Wozniak J. A prospective open-label treatment trial of ziprasidone monotherapy in children and adolescents with bipolar disorder. Bipolar Disord. 2007a;9:888–894. doi: 10.1111/j.1399-5618.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Spencer T. Doyle R. Joshi G. Hammerness P. Kotarski M. Aleardi M. Wozniak J. An open-label trial of aripiprazole monotherapy in children and adolescents with bipolar disorder. CNS Spectr. 2007b;12:683–689. doi: 10.1017/s1092852900021519. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Kennah A. Brent D. Ehmann M. Bridge J. Axelson D. Is bipolar disorder specifically associated with panic disorder in youths? J Clin Psychiatry. 2002;63:41–419. doi: 10.4088/jcp.v63n0507. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Axelson D. Strober M. Gill MK. Valeri S. Chiappetta L. Ryan N. Leonard H. Hunt J. Iyegar S. Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt CM. Bernstein GA. Comorbid disorders in hospitalized bipolar adolescents compared with unipolar depressed adolescents. Child Psychiatry Hum Dev. 1995;26:11–18. doi: 10.1007/BF02353226. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Kelly KL. Manic symptoms in psychiatrically hospitalized children—What do they mean? J Affect Disord. 1998;51:123–135. doi: 10.1016/s0165-0327(98)00211-0. [DOI] [PubMed] [Google Scholar]
- Chang K. Saxena K. Howe M. An open-label study of lamotrigine adjunct or monotherapy for the treatment of adolescents with bipolar depression. J Am Acad Child Adolesc Psychiatry. 2006;45:298–304. doi: 10.1097/01.chi.0000194566.86160.a3. [DOI] [PubMed] [Google Scholar]
- Conners CK. Barkley RA. Rating scales and checklists for child psychopharmacology. Psychopharmacol Bull. 1985;21:809–843. [PubMed] [Google Scholar]
- Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: A systematic review and pooled analysis of short-term trials. J Am Acad Child Adolesc Psychiatry. 2007;46:687–700. doi: 10.1097/chi.0b013e318040b25f. [DOI] [PubMed] [Google Scholar]
- Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69(Suppl 4):26–36. [PubMed] [Google Scholar]
- Craney JL. Geller B. A prepubertal and early adolescent bipolar disorder-I phenotype: Review of phenomenology and longitudinal course. Bipolar Disord. 2003;5:243–256. doi: 10.1034/j.1399-5618.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- Danielyan A. Pathak S. Kowatch RA. Arszman SP. Johns ES. Clinical characteristics of bipolar disorder in very young children. J Affect Disord. 2007;97:51–59. doi: 10.1016/j.jad.2006.05.028. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Kowatch RA. Warner J. Schwiers ML. Rappaport KB. Daniels JP. Foster KD. Strakowski SM. Adjunctive topiramate treatment for pediatric bipolar disorder: A retrospective chart review. J Child Adolesc Psychopharmacol. 2002a;12:323–330. doi: 10.1089/104454602762599862. [DOI] [PubMed] [Google Scholar]
- Delbello MP. Schwiers ML. Rosenberg HL. Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002b;41:1216–1223. doi: 10.1097/00004583-200210000-00011. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Kowatch RA. Adler CM. Stanford KE. Welge JA. Barzman DH. Nelson E. Strakowski SM. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45:305–313. doi: 10.1097/01.chi.0000194567.63289.97. [DOI] [PubMed] [Google Scholar]
- Findling RL. McNamara NK. Gracious BL. Youngstrom EA. Stansbrey RJ. Reed MD. Demeter CA. Branicky LA. Fisher KE. Calabrese JR. Combination lithium and divalproex sodium in pediatric bipolarity. J Am Acad Child Adolesc Psychiatry. 2003;42:895–901. doi: 10.1097/01.CHI.0000046893.27264.53. [DOI] [PubMed] [Google Scholar]
- Findling RL. McNamara NK. Youngstrom EA. Stansbrey R. Gracious BL. Reed MD. Calabrese JR. Double-blind 18-month trial of lithium versus divalproex maintenance treatment in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:409–417. doi: 10.1097/01.chi.0000155981.83865.ea. [DOI] [PubMed] [Google Scholar]
- Findling RL. McNamara NK. Stansbrey R. Gracious BL. Whipkey RE. Demeter CA. Reed MD. Youngstrom EA. Calabrese JR. Combination lithium and divalproex sodium in pediatric bipolar symptom re-stabilization. J Am Acad Child Adolesc Psychiatry. 2006;45:142–148. doi: 10.1097/01.chi.0000189135.05060.8a. [DOI] [PubMed] [Google Scholar]
- Findling RL. Short EJ. McNamara NK. Demeter CA. Stansbrey RJ. Gracious BL. Whipkey R. Manos MJ. Calabrese JR. Methylphenidate in the treatment of children and adolescents with bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1445–1453. doi: 10.1097/chi.0b013e31814b8d3b. [DOI] [PubMed] [Google Scholar]
- Frazier JA. Biederman J. Tohen M. Feldman PD. Jacobs TG. Toma V. Rater MA. Tarazi RA. Kim GS. Garfield SB. Sohma M. Gonalez-Heydrich J. Risser RC. Nowlin ZM. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2001;11:239–250. doi: 10.1089/10445460152595568. [DOI] [PubMed] [Google Scholar]
- Frye MA. Ketter TA. Leverich GS. Huggins T. Lantz C. Denicoff KD. Post RM. The increasing use of polypharmacotherapy for refractory mood disorders: 22 years of study. J Clin Psychiatry. 2000;61:9–15. doi: 10.4088/jcp.v61n0104. [DOI] [PubMed] [Google Scholar]
- Geller B. Fox LW. Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year-old depressed children. J Am Acad Child Adolesc Psychiatry. 1994;33:461–468. doi: 10.1097/00004583-199405000-00003. [DOI] [PubMed] [Google Scholar]
- Geller B. Sun K. Zimerman B. Luby J. Frazier J. Williams M. Complex and rapid-cycling in bipolar children and adolescents: A preliminary study. J Affect Disord. 1995;34:259–268. doi: 10.1016/0165-0327(95)00023-g. [DOI] [PubMed] [Google Scholar]
- Geller B. Bolhofner K. Craney JL. Williams M. DelBello MP. Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry. 2000a;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- Geller B. Zimerman B. Williams M. Bolhofner K. Craney JL. Delbello MP. Soutullo CA. Diagnostic characteristics of 93 cases of a prepubertal and early adolescent bipolar disorder phenotype by gender, puberty and comorbid attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2000b;10:157–164. doi: 10.1089/10445460050167269. [DOI] [PubMed] [Google Scholar]
- Geller B. Zimerman B. Williams M. Bolhofner K. Craney JL. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158:125–127. doi: 10.1176/appi.ajp.158.1.125. [DOI] [PubMed] [Google Scholar]
- Geller B. Tillman R. Craney JL. Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61:459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- Geller B. Tillman R. Bolhofner K. Zimerman B. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemi SN. Hsu DJ. Thase ME. Wisniewski SR. Nierenberg AA. Miyahara S. Sachs G. Pharmacological treatment patterns at study entry for the first 500 STEP-BD participants. Psychiatr Serv. 2006;57:660–665. doi: 10.1176/ps.2006.57.5.660. [DOI] [PubMed] [Google Scholar]
- Goldberg JF. Perlis RH. Bowden CL. Thase ME. Miklowitz DJ. Marangell LB. Calabrese JR. Nierenberg AA. Sachs GS. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: Findings From the STEP-BD. Am J Psychiatry. 2009;166:173–181. doi: 10.1176/appi.ajp.2008.08050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein TR. Birmaher B. Axelson D. Goldstein BI. Gill MK. Esposito-Smythers C. Ryan ND. Strober MA. Hunt J. Keller M. Psychosocial functioning among bipolar youth. J Affect Disord. 2009;114:174–183. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold TL. Wozniak J. Kwon A. Gilbert J. Wood J. Smith L. Biederman J. Examining the association between pediatric bipolar disorder and anxiety disorders in psychiatrically referred children and adolescents. J Affect Disord. 2005;88:19–26. doi: 10.1016/j.jad.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Kafantaris V. Coletti DJ. Dicker R. Padula G. Kane JM. Adjunctive antipsychotic treatment of adolescents with bipolar psychosis. J Am Acad Child Adolesc Psychiatry. 2001a;40:1448–1456. doi: 10.1097/00004583-200112000-00016. [DOI] [PubMed] [Google Scholar]
- Kafantaris V. Dicker R. Coletti DJ. Kane JM. Adjunctive antipsychotic treatment is necessary for adolescents with psychotic mania. J Child Adolesc Psychopharmacol. 2001b;11:409–413. doi: 10.1089/104454601317261582. [DOI] [PubMed] [Google Scholar]
- Keck PE., Jr. Calabrese JR. McIntyre RS. McQuade RD. Carson WH. Eudicone JM. Carlson BX. Marcus RN. Sanchez R. Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: A 100-week, double-blind study versus placebo. J Clin Psychiatry. 2007;68:1480–1491. doi: 10.4088/jcp.v68n1003. [DOI] [PubMed] [Google Scholar]
- Kowatch RA. Suppes T. Carmody TJ. Bucci JP. Hume JH. Kromelis M. Emslie GJ. Weinberg WA. Rush AJ. Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:713–720. doi: 10.1097/00004583-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Kowatch RA. Sethuraman G. Hume JH. Kromelis M. Weinberg WA. Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry. 2003;53:978–984. doi: 10.1016/s0006-3223(03)00067-2. [DOI] [PubMed] [Google Scholar]
- Kowatch RA. Fristad M. Birmaher B. Wagner KD. Findling RL. Hellander M. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005a;44:213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Kowatch RA. Youngstrom EA. Danielyan A. Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 2005b;7:483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Kowatch RA. DelBello MP. Pediatric bipolar disorder: emerging diagnostic and treatment approaches. Child Adolesc Psychiatr Clin N Am. 2006;15:73–108. doi: 10.1016/j.chc.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Leverich GS. Post RM. Keck PE., Jr. Altshuler LL. Frye MA. Kupka RW. Nolen WA. Suppes T. McElroy SL. Grunze H. Denicoff K. Moravec MK. Luckenbaugh D. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM. Klein DN. Seeley JR. Bipolar disorders in a community sample of older adolescents: Prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- Masi G. Mucci M. Millepiedi S. Clozapine in adolescent inpatients with acute mania. J Child Adolesc Psychopharmacol. 2002;12:93–99. doi: 10.1089/104454602760219135. [DOI] [PubMed] [Google Scholar]
- Masi G. Perugi G. Millepiedi S. Toni C. Mucci M. Pfanner C. Berloffa S. Pari C. Akiskal HS. Bipolar co-morbidity in pediatric obsessive-compulsive disorder: Clinical and treatment implications. J Child Adolesc Psychopharmacol. 2007;17:475–486. doi: 10.1089/cap.2006.0107. [DOI] [PubMed] [Google Scholar]
- McClellan J. Kowatch R. Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107–125. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- Moreno C. Laje G. Blanco C. Jiang H. Schmidt AB. Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Graczyk PA. Henry DB. Carbray JA. Heidenreich J. Milklowitz DJ. Child- and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: Development and preliminary results. J Am Acad Child Adolesc Psychiatry. 2004a;43:528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Henry DB. Carbray JA. Sampson G. Naylor MW. Janicak PG. Open-label prospective trial of risperidone in combination with lithium or divalproex sodium in pediatric mania. J Affect Disord. 2004b;82(Suppl 1):S103–S111. doi: 10.1016/j.jad.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Henry DB. Devineni B. Carbray JA. Naylor MW. Janicak PG. A pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2004c;43:859–867. doi: 10.1097/01.chi.0000128790.87945.2f. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Birmaher B. Naylor MW. Pediatric bipolar disorder: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Perlis RH. Miyahara S. Marangell LB. Wisniewski SR. Ostacher M. DelBello MP. Bowden CL. Sachs GS. Nierenberg AA. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Perlis RH. Welge JA. Vornik LA. Hirschfeld RM. Keck PE., Jr. Atypical antipsychotics in the treatment of mania: A meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67:509–516. doi: 10.4088/jcp.v67n0401. [DOI] [PubMed] [Google Scholar]
- Post RM. Chang KD. Findling RL. Geller B. Kowatch RA. Kutcher SP. Leverich GS. Prepubertal bipolar I disorder and bipolar disorder NOS are separable from ADHD. J Clin Psychiatry. 2004;65:898–902. doi: 10.4088/jcp.v65n0703. [DOI] [PubMed] [Google Scholar]
- Reich W. Neuman RJ. Volk HE. Joyner CA. Todd RD. Comorbidity between ADHD and symptoms of bipolar disorder in a community sample of children and adolescents. Twin Res Hum Genet. 2005;8:459–466. doi: 10.1375/183242705774310105. [DOI] [PubMed] [Google Scholar]
- Scheffer RE. Kowatch RA. Carmody T. Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry. 2005;162:58–64. doi: 10.1176/appi.ajp.162.1.58. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. Biederman J. Wozniak J. Faraone SV. Wilens TE. Mick E. Parsing pediatric bipolar disorder from its associated comorbidity with the disruptive behavior disorders. Biol Psychiatry. 2001;49:1062–1070. doi: 10.1016/s0006-3223(01)01155-6. [DOI] [PubMed] [Google Scholar]
- Suppes T. Eudicone J. McQuade R. Pikalov A., 3rd Carlson B. Efficacy and safety of aripiprazole in subpopulations with acute manic or mixed episodes of bipolar I disorder. J Affect Disord. 2008;107:145–154. doi: 10.1016/j.jad.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Tohen M. Kryzhanovskaya L. Carlson G. Delbello M. Wozniak J. Kowatch R. Wagner K. Findling R. Lin D. Robertson-Plouch C. Xu W. Dittmann RW. Biederman J. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164:1547–1556. doi: 10.1176/appi.ajp.2007.06111932. [DOI] [PubMed] [Google Scholar]
- Vieta E. Bourin M. Sanchez R. Marcus R. Stock E. McQuade R. Carson W. Abou-Gharbia N. Swanink R. Iwamoto T. Effectiveness of aripiprazole v. haloperidol in acute bipolar mania: Double-blind, randomised, comparative 12-week trial. Br J Psychiatry. 2005;187:235–242. doi: 10.1192/bjp.187.3.235. [DOI] [PubMed] [Google Scholar]
- West AE. Henry DB. Pavuluri MN. Maintenance model of integrated psychosocial treatment in pediatric bipolar disorder: A pilot feasibility study. J Am Acad Child Adolesc Psychiatry. 2007;46:205–212. doi: 10.1097/01.chi.0000246068.85577.d7. [DOI] [PubMed] [Google Scholar]
- West SA. McElroy SL. Strakowski SM. Keck PE., Jr. McConville BJ. Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry. 1995a;152:271–273. doi: 10.1176/ajp.152.2.271. [DOI] [PubMed] [Google Scholar]
- West SA. Strakowski SM. Sax KW. Minnery KL. McElroy SL. Keck PE., Jr. The comorbidity of attention-deficit hyperactivity disorder in adolescent mania: Potential diagnostic and treatment implications. Psychopharmacol Bull. 1995b;31:347–351. [PubMed] [Google Scholar]
- Wilens TE. Spencer T. Biederman J. Wozniak J. Connor D. Combined pharmacotherapy: An emerging trend in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 1995;34:110–112. doi: 10.1097/00004583-199501000-00021. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Biederman J. Forkner P. Ditterline J. Morris M. Moore H. Galdo M. Spencer TJ. Wozniak J. Patterns of comorbidity and dysfunction in clinically referred preschool and school-age children with bipolar disorder. J Child Adolesc Psychopharmacol. 2003a;13:495–505. doi: 10.1089/104454603322724887. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Biederman J. Kwon A. Chase R. Greenberg L. Mick E. Spencer TJ. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003b;13:143–152. doi: 10.1089/104454603322163862. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Biederman J. Adamson JJ. Henin A. Sgambati S. Gignac M. Sawtelle R. Santry A. Monuteaux MC. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: A controlled study. Drug Alcohol Depend. 2008;95:188–198. doi: 10.1016/j.drugalcdep.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J. Biederman J. A pharmacological approach to the quagmire of comorbidity in juvenile mania. J Am Acad Child Adolesc Psychiatry. 1996;35:826–828. doi: 10.1097/00004583-199606000-00023. [DOI] [PubMed] [Google Scholar]
- Wozniak J. Biederman J. Kiely K. Ablon JS. Faraone SV. Mundy E. Mennin D. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry. 1995;34:867–876. doi: 10.1097/00004583-199507000-00010. [DOI] [PubMed] [Google Scholar]
- Wozniak J. Biederman J. Faraone SV. Blier H. Monuteaux MC. Heterogeneity of childhood conduct disorder: further evidence of a subtype of conduct disorder linked to bipolar disorder. J Affect Disord. 2001;64:121–131. doi: 10.1016/s0165-0327(00)00217-2. [DOI] [PubMed] [Google Scholar]
- Wozniak J. Biederman J. Monuteaux MC. Richards J. Faraone SV. Parsing the comorbidity between bipolar disorder and anxiety disorders: A familial risk analysis. J Child Adolesc Psychopharmacol. 2002;12:101–111. doi: 10.1089/104454602760219144. [DOI] [PubMed] [Google Scholar]
- Wozniak J. Monuteaux M. Richards J. K EL. Faraone SV. Biederman J. Convergence between structured diagnostic interviews and clinical assessment on the diagnosis of pediatric-onset mania. Biol Psychiatry. 2003;53:938–944. doi: 10.1016/s0006-3223(03)00344-5. [DOI] [PubMed] [Google Scholar]
- Wozniak J. Biederman J. Kwon A. Mick E. Faraone S. Orlovsky K. Schnare L. Cargol C. van Grondelle A. How cardinal are cardinal symptoms in pediatric bipolar disorder? An examination of clinical correlates. Biol Psychiatry. 2005;58:583–588. doi: 10.1016/j.biopsych.2005.08.014. [DOI] [PubMed] [Google Scholar]