Abstract
The ability of recombinant adeno-associated viral (rAAV) vectors to exhibit minimal immunogenicity and little to no toxicity or inflammation while eliciting robust, multiyear gene expression in vivo are only a few of the salient features that make them ideally suited for many gene therapy applications. A major hurdle for the use of rAAV in sizeable research and clinical applications is the lack of efficient and versatile large-scale production systems. Continued progression toward flexible, scalable production techniques is a prerequisite to support human clinical evaluation of these novel biotherapeutics. This review examines the current state of large-scale production methods that employ the herpes simplex virus type 1 (HSV) platform to produce rAAV vectors for gene delivery. Improvements have substantially advanced the HSV/AAV hybrid method for large-scale rAAV manufacture, facilitating the generation of highly potent, clinical-grade purity rAAV vector stocks. At least one human clinical trial employing rAAV generated via rHSV helper-assisted replication is poised to commence, highlighting the advances and relevance of this production method.
Introduction
Despite its small size and relatively simple genome, adeno-associated virus (AAV) has become one of the most scrutinized viral vectors for gene transfer, primarily because of its lack of human pathogenicity. Gene transfer vectors based on recombinant AAV (rAAV) have consistently demonstrated transduction of dividing and quiescent cells, long-term gene expression in vivo, and little or no toxicity and inflammation, making them the vectors of choice for many gene therapy applications. However, many promising preclinical studies along with successful clinical trials have highlighted a series of challenges that will need to be addressed to support continued rAAV use for human gene therapy (Mueller and Flotte, 2008b). A major hurdle to rAAV gene therapy stems from the inherent replication deficiency of the parental virus, and therefore the production systems for its viral vectors. Blankinship and colleagues estimate that as much as 4 × 1014 rAAV vector genomes (VG) per dose for systemic diseases such as Duchenne muscular dystrophy would be necessary to achieve therapeutic protein expression via gene transfer for even a 10-kg child (Blankinship et al., 2006). Generating such amounts of rAAV is daunting, even with advancements in baculovirus-based manufacturing of rAAV (Cecchini et al., 2008). Therefore, continued human clinical evaluation of rAAV will be intricately linked to the advancement of manufacturing systems capable of delivering vector quantities sufficient to meet the expanding clinical demand.
Large-scale viral vector technology is not trivial, and meeting current good manufacturing practice (cGMP) regulations is a long, cumbersome, and costly process. It is imperative that the development of scalable systems for rAAV production be performed in accordance with GMP requirements to avoid unnecessary delays and unexpected setbacks. This review aims at presenting the current state-of-the-art methods that employ the herpes simplex virus type 1 (HSV) platform to produce rAAV vectors for gene therapy. Recent advancements have demonstrated that at least one recombinant HSV (rHSV)-based system is capable of supporting large-scale production of rAAV batches, generating more than 2 × 1015 DNA-containing particles from a single 10-liter culture run. Furthermore, the HSV/AAV hybrid manufacturing system is about to be executed under GMP, and should be evaluated in humans by the year 2010. Taken together, these advancements suggest that application to systemic diseases such as α1-antitrypsin deficiency and Duchenne muscular dystrophy may currently be within the reach of the most advanced HSV/AAV hybrid manufacturing system.
Viral Helper Functions Required for rAAV Production
Recombinant AAV vectors typically bear the gene of interest and expression regulators in lieu of the wild-type virus rep and cap open reading frames (ORFs) (Hermonat and Muzyczka, 1984; McLaughlin et al., 1988; Samulski et al., 1989). The rep ORF encodes four nonstructural Rep proteins involved in every step of the viral life cycle, whereas the cap ORF encodes the three structural proteins, VP1, VP2, and VP3, that form the icosahedral capsid. The only viral sequences that are retained in the recombinant vector genome are the inverted terminal repeats (ITRs), which constitute the minimal cis-acting sequences required for AAV DNA replication and packaging. AAV is a naturally replication-defective human parvovirus, requiring the presence of a helper virus to trigger its replication and the generation of progeny virions. In the absence of helper assistance, AAV integrates its genome site-specifically within the host cell chromosome, where it persists indefinitely unless rescued via cellular infection with a helper virus (Schultz and Chamberlain, 2008). As a consequence, production of recombinant AAV relies entirely on the presence of two types of functions: the AAV endogenous functions of Rep and Cap, and the helper virus functions. Viruses of the Adenoviridae and Herpesviridae families have been shown to provide these essential trans functions for AAV replication. Historically, rAAV production methods are based on adenoviral (Ad) helper functions, and the Ad genes that are required for promoting AAV replication have been identified (E1A and E1B, E2A, VA, and E4orf6) and subsequently cloned into helper plasmids, except for the E1 ORF, which is often provided by the producer cell line. AAV and Ad elements, provided from either two separate helper plasmids or combined into one (Grimm and Kleinschmidt, 1999; Zolotukhin et al., 2002), are supplied in trans by standard transfection methods in 293 cells, along with the vector plasmid, as reviewed (Aucoin et al., 2008). Despite their versatility and relative affordability (e.g., they require only plasmid DNA manufacture), transfection-based methods have limited efficiency in generating high particle-per-cell yields, and more importantly, are inherently difficult to scale up.
Although Ad has been the prototypical helper virus for AAV-2, HSV was shown three decades ago to be a fully competent helper virus for rAAV-2 replication and packaging (Handa and Carter, 1979; Buller et al., 1981). Interestingly, AAV uses both helpers differently to achieve productive replication in an opportunistic manner. Ad promotes AAV replication both directly, through trans-activators that stimulate AAV rep gene expression, and indirectly by forcing the cell to enter S phase, which in turn provides AAV with active cellular replication machinery. In contrast to adenovirus, the gene products of which are not directly involved in AAV DNA synthesis, HSV replication proteins can be directly used by AAV for efficient genome replication and packaging. The minimal set of HSV genes required includes UL5, UL8, and UL52, which encode components of the HSV helicase–primase complex, and UL29, which encodes a single-stranded DNA-binding protein (Mishra and Rose, 1990; Weindler and Heilbronn, 1991). These four proteins are components of the HSV core replication machinery, along with the HSV polymerase UL30, the polymerase accessory factor UL42, and the origin-binding protein UL9. The mechanistic differences between the helper assistance provided by adenovirus and HSV may result from the major differences in how these viruses interact with host cells. Infection with HSV usually downregulates cellular functions (Taddeo et al., 2004), forcing AAV to use the HSV replication complex to propagate instead of the cellular machinery alone (Ward et al., 2001; Slanina et al., 2006). Interestingly, AAV-2 replication and packaging can occur in the absence of HSV DNA replication, so long as HSV early gene expression occurs (Weindler and Heilbronn, 1991; Ward et al., 2001). AAV itself can also negatively interfere with HSV-induced cellular DNA amplification and HSV DNA replication through Rep78 and Rep68 activities (Heilbronn et al., 1990; Glauser et al., 2007). These observations led to the development of a cell-free system for AAV DNA replication by Ward and colleagues, in which the set of HSV proteins UL5, UL8, UL29, and UL52, along with the HSV polymerase complex and accessory proteins (UL30 and UL42 genes), and AAV Rep68 are provided (Ward et al., 2001).
Hybrid HSV/AAV Vector Technology
Vectors based on HSV
HSV vectors carrying foreign DNA have long been explored for gene therapy applications due to a series of unique features that make them particularly attractive for the central nervous system. Describing the myriad of HSV vector systems employed for gene therapy is not our focus; however, the features that made the development of HSV-based complementation systems for rAAV production possible are addressed here. In contrast to AAV, the HSV genome is substantially larger and much more complex. Its 152-kb-long, double-stranded, linear DNA carries more than 80 genes organized into unique long (UL) and unique short (US) segments, each flanked by inverted repeats (Blits and Bunge, 2006). HSV gene expression is precisely and temporally regulated as HSV infection progresses. However, more than 50% of the genome encodes nonessential gene products and can be deleted without jeopardizing viral amplification. Deletion of nonessential viral DNA sequences allows for the insertion of large foreign DNA sequences, typically a gene of interest and regulatory elements (Epstein et al., 2005; Hibbitt and Wade-Martins, 2006). In general, two types of HSV vector have been developed: recombinant HSV (rHSV) vectors that retain most of the wild-type genome and carry the expression cassette of interest, and plasmid-based amplicon systems that do not retain any other HSV DNA than the two cis-acting elements for replication and packaging, the origin of replication (oriS), and the packaging signal (sequence a or pac) (Epstein et al., 2005).
Recombinant HSV vectors
Because HSV is, in opposition to AAV, a serious human pathogen (Schleiss, 2009), and cytotoxic to cells, rHSV vectors usually lack essential viral replication genes, rendering them replication incompetent. As a result, replication-defective recombinants also display significantly reduced cytotoxicity for the producer cells. Immediate-early (IE) genes encoding infected cell proteins (ICP) 0, 4, 22, 27, and 47 are the most commonly deleted, and are often deleted in combination to confer additional safety. In particular, the role of ICP27 in cellular toxicity has been well documented (Sandri-Goldin, 2008). ICP27 negatively interferes with cellular messenger RNA splicing, polyadenylation, and stability, and inhibits the transcription of many genes. In addition, ICP27 is also involved in the downregulation of HSV early gene expression, some of which are essential to AAV replication. As a consequence, the production of replication-incompetent rHSV vectors requires adequate complementing cell lines for providing in trans the missing replication and packaging functions of rHSV. However, rHSV vectors modified with multiple replication deficiencies to enhance their safety profiles and reduce their cellular toxicity exhibit substantially reduced titer relative to that of the wild-type virus, sometimes up to 3 logs (Warnock et al., 2006; Osten et al., 2007). Because rHSV has promising features for gene therapy, many efforts have been invested in the development of large-scale methods for the production of high-titer stocks (Ozuer et al., 2002b; Wechuck et al., 2002; Knop and Harrell, 2007), and transfer to GMP settings (Burton et al., 2005; Mandel et al., 2008).
HSV amplicon systems
The first generation of rAAV complementation systems employing rHSV helper functions used HSV amplicons. A major advantage of HSV amplicons, relative to rHSV viruses, is that they carry no viral genes and therefore result in a less immunogenic safety profile with reduced toxicity. Also, because the only HSV elements retained are two short sequences (origin of replication and packaging signal), amplicons can accommodate large foreign DNA sequences (up to 150 kb) (Oehmig et al., 2007). Amplicons are typically produced by transfecting the plasmid containing the amplicon DNA into a cell along with superinfection with a replication-deficient HSV mutant to provide the trans elements required for replicating and packaging the amplicon genomes. The major disadvantage of amplicons is that they usually result in decreased yields of helpers relative to rHSV vectors (Epstein et al., 2005).
Hybrid HSV/AAV as gene transfer vectors
Using HSV as a support for rAAV manufacture, as later described, is only one of many possible applications resulting from a combination of HSV and rAAV vector technology. HSV/AAV amplicon vectors have been created by inserting a recombinant AAV genome within the HSV amplicon plasmid backbone and used as a delivery tool for testing gene expression in vitro and in vivo. These hybrid vectors advantageously combine the large delivery capacity of HSV with the unique ability of AAV to mediate site-specific integration within the human genome (Kotin et al., 1992). To that end, the AAV-2 large Rep ORF, the trans function required for site-specific integration, was added to the HSV amplicon and placed outside of the AAV ITRs (the only cis element required for site-specific integration). HSV/AAV-Rep/rAAV amplicons have been used to transduce diverse cell types and can support the integration of a >100-kb foreign DNA sequence within the target cell chromosome (Johnston et al., 1997; Costantini et al., 1999; Lam et al., 2002; Wang et al., 2002; Liu et al., 2006; Oehmig et al., 2007).
HSV-Based AAV Production Systems
HSV amplicon-based system
The first HSV-based method developed to produce rAAV relied on an amplicon system. The AAV-2 rep and cap genes and their native promoters (p5, p19, and p40) were inserted into a plasmid carrying the HSV origin of replication and packaging signal (Conway et al., 1997). To generate HSV particles carrying the rep and cap genes, the resulting pHSV-RC plasmid was transfected into Vero cells with either wild-type HSV DNA (KOS strain) or infected with wild-type HSV. In this system, wild-type HSV was used as a helper to provide the missing trans factors required for HSV amplicon DNA replication and packaging into HSV particles. HSV particles generated during this process, both from amplicon and wild-type HSV sources, were further amplified through serial infection passages. Finally, HSV-RC stocks were used to infect either proviral cell lines that contained an integrated rAAV-2 genome or cells transfected with an rAAV-2 plasmid or infected with rAAV-2. The amplicon system demonstrated HSV-supported rescue and replication of rAAV; however, it also suffered from many disadvantages. First, it relied on the input of three separate components: the HSV-RC, wild-type HSV, and rAAV. Second, the presence and propagation of wild-type HSV were undesirable from both safety and yield limitation perspectives; indeed, wild-type HSV rapidly induced a significant cytopathic effect in infected cells, resulting in early cell death and inefficient rAAV packaging. Finally, amplification through passaging favored wild-type HSV, resulting in successive loss of HSV-RC. In the same study, cellular toxicity could be significantly reduced, and rAAV-2 yields increased, by using a mutant HSV strain deleted for ICP27 (d27-1) instead of wild-type HSV (Conway et al., 1997).
Taken together, these preliminary experiments demonstrated that an HSV-based system that combines both AAV and HSV trans functions was capable of rescuing and replicating all forms of rAAV genomes, including integrated proviral genomes. It also highlighted that (1) replication of the HSV stocks was not substantially inhibited by the AAV-2 Rep proteins and that (2) rAAV production was further improved by using the replication-defective rHSV-RC-d27.1, significantly reducing cell toxicity; and last but not least, (3) no detectable wild-type AAV-2 or rcAAV-2 was generated during the process.
As a variation of this strategy, other investigators used a disabled, single-cycle (DISC) herpesvirus for rAAV replication from an amplicon-based system (Zhang et al., 1999; Feudner et al., 2001). DISC-HSV lacked expression of HSV glycoprotein H (gH) and infectious particles could be generated only from a complementing gH-expressing cell line, conferring an increment of safety. The rAAV-2 genome and AAV-2 rep and cap genes were both incorporated into HSV amplicons, either separately (HSV-rc and HSV-rAAV) or combined (HSV-rc-rAAV). Similar to the system of Conway and colleagues, rAAV production was initiated by amplicon transfection of baby hamster kidney (BHK) cells, followed by superinfection with DISC-HSV. Despite their limited productivity (<7 × 102 transducing units [TU]/cell), presumably due to continued reliance on low-efficiency transfection, Feudner and colleagues demonstrated the potency of HSV amplicon-produced rAAV in vivo for the first time by efficiently transducing photoreceptors in the murine retina (Feudner et al., 2001).
Recombinant HSV-based systems
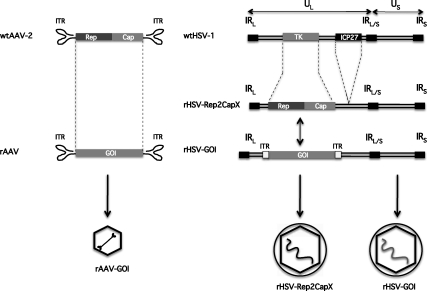
Efficient production of rAAV requires both AAV rep and cap gene products as well as the helper virus gene products; therefore an HSV-based AAV production platform would ideally combine the requisite AAV elements (ITRs, rep and cap genes) and HSV helper functions. To this end, the second-generation system of Conway and colleagues simplified HSV-based rAAV production by replacing HSV amplicon usage with recombinant HSV vector infection (Conway et al., 1999). The AAV-2 rep and cap genes were inserted into the thymidine kinase (TK) locus of a replication-defective HSV vector, d27-1, by homologous recombination (see Fig. 1). The resulting d27.1-rc vector was propagated in V27 cells, a complementing Vero-derived cell line that expresses HSV ICP27 (Rice and Knipe, 1990). rAAV was generated, from either a proviral cell line or producer cells either transfected with a proviral AAV plasmid or infected with rAAV, by infection with d27.1-rc. In all cases, the rAAV genome was successfully rescued, replicated, and packaged into fully infectious particles. There were several advantages to this method: (1) both AAV and HSV helper trans functions were provided by a single infectious vector (d27.1-rc), which was more amenable to scale-up; (2) there was no competition or ratio variation with wild-type HSV particles during production of the d27.1-rc vector; and (3) the lack of ICP27 probably contributed to higher rAAV yields by reducing cell toxicity and diminishing AAV Rep and Cap mRNA splicing.
FIG. 1.
Schematic representations of adeno-associated virus (AAV) and herpes simplex virus (HSV) and vectors. Genomic organization of wild-type AAV-2 (left, top) and wild-type HSV-1 (right, top) viruses is shown with major genetic elements (not to scale); genomic organization of recombinant vectors thereof (left/right, middle); resulting viral vectors (left/right, bottom). ITR, inverted terminal repeat; Rep, AAV nonstructural protein open reading frame; Cap, AAV structural protein open reading frame; X, AAV serotype 1–10; GOI, gene of interest in the rAAV backbone; rHSV-GOI, rHSV carrying rAAV-GOI; IR, inverted repeat; L/S, long and short; U, unit; TK, thymidine kinase open reading frame; ICP, infection cell protein.
An important parameter that was addressed in this study was the timing and dosing of Rep expression, which were directly correlated to the multiplicity of infection (MOI) used for the rHSV d27.1-rc vector. In addition, 293 and Vero cell lines were identified to be more efficient AAV vector producers than the complementing V27. Using these optimized conditions, rAAV yields were about 4 × 102 TU/cell for 293 cells transfected with pTRUF5, a plasmid carrying an rAAV genome expressing green fluorescent protein (GFP), and up to 5 × 102 TU/cell when a proviral cell line was infected with the rHSV d27.1-rc vector (Table 1). These results were comparable to yields obtained from standard transfection methods based on the Ad helper systems used at the time. Importantly, no replication-competent AAV particles were detected, although detection was limited to Hirt-extracted DNA. Specific productivity was also maintained on scaling more than 600-fold from a 10-cm culture dish to a Nunclon Δ cell factory (Nunc/Thermo Fisher Scientific, Rochester, NY). Despite these promising results, the system scalability was ultimately limited by reliance on adherent cell culture and either a transfection step to provide the rAAV or the creation of a proviral cell line for each rAAV construct.
Table 1.
Summary of Various HSV-Based Methods to Produce rAAV
| |
|
|
|
|
Vector genome (harvest)a |
Infectious units (harvest)b |
|
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| Method | rAAV | Construct | Helper construct | Cells (scale) | VG/cell | Total VG | IU/cell | Total IU | VG:IU ratio | Reference |
| Transfection + infection | rAAV2-GFP | N/A | HSV-Rep2Cap2 | GFP-92c (∼5.7 × 107) | 3.2 × 102 | 1.8 × 1010 | N/D | N/D | N/D | Conway et al., 1997 |
| Transfection + infection | rAAV2-GFP | pTRUF5d | rHSV-Rep2Cap2 | 293 (3 × 107) | N/D | N/D | 3.8 × 102 | 1.14 × 1010 | N/D | Conway et al., 1999 |
| Infection | rAAV2-GFP | N/A | rHSV-Rep2Cap2 | GFP-92 (1 × 109) | N/D | N/D | 3.8 × 102 | 3.8 × 1011 | N/D | |
| Transfection | rAAV2-GFP | pHAV5 (rAAV-GFP) | pHAV7.3(rep2cap2) + HSV-1 (PS1) | BHK (2 × 105) | N/D | N/D | 6.6 × 102 | 2 × 108 | N/D | Feudner et al., 2001 |
| Transfection + infection | rAAV5-LacZ | pTR5-LacZ or rAAV5-LacZ | rHSV-Rep2Cap5rHSV-Rep2Cap5 | 293 (∼1 × 106) | 8 × 103 | 8 × 109 | N/D | N/D | N/D | Wustner et al., 2002 |
| Coinfection | rAAV2-GFP | rHSV-GFP | rHSV-Rep2Cap2 | BHK (1 × 107) | N/De | N/D | 40 | 4 × 108 | N/De | Booth et al., 2004 |
| rAAV2-GFP | rHSV-GFP | rHSV-Rep2Cap2 | 293 (1 × 107) | 1.3 × 105 | 1.3 × 1012 | 9.4 × 103 | 9.4 × 1010 | 14 | ||
| Coinfection | rAAV1-AAT | rHSV-AAT | rHSV-Rep2Cap1 | 293 (5 × 109) | 8.5 × 104 | 4.3 × 1014 | N/D | N/D | 1.2 × 102 | Kang et al., 2009 |
| rAAV9-AAT | rHSV-AAT | rHSV-Rep2Cap9 | 293 (2 × 109) | N/D | N/D | N/D | N/D | N/D | ||
| Coinfection | rAAV2-sFLT01 | rHSV-sFLT01 | rHSV-Rep2Cap2 | sBHK (3.5 × 107) | 7.3 × 104 | 1.0 × 1011 | 1.2 × 104 | 4.3 × 1011 | 6.0 | Thomas et al., 2009 |
| rAAV1-AAT | rHSV-AAT | rHSV-Rep2Cap1 | sBHK (2.2 × 1010) | 1.1 × 105 | 2.4 × 1015 | 5.4 × 102 | 1.2 × 1013 | 2.1 × 102 | ||
| rAAV5-sFLT01 | rHSV-sFLT01 | rHSV-Rep2Cap5 | sBHK (3.7 × 107) | 5.5 × 104 | 8.3 × 1010 | 6.8 × 102 | 2.5 × 1010 | 82 | ||
| rAAV8-AAT | rHSV-AAT | rHSV-Rep2Cap8 | sBHK (5.8 × 107) | 1.2 × 105 | 2.7 × 1011 | N/D | N/D | N/D | ||
Abbreviations: AAT, α1-antitrypsin gene; BHK, baby hamster kidney cells; GFP, green fluorescent protein; HSV, herpes simplex virus; IU, infectious units; N/A, not applicable; N/D, not determined; sBHK, suspension baby hamster kidney cells; sFLT01, Flt-1 domain 2 linked by nine glycines to the human IgG1 heavy-chain Fc region; VG, vector genomes.
Vector genome titers as reported by the various groups. Methods used to determine vector genome and infectious unit titers may differ.
For the sake of clarity, infectious units also refers to transduction and expression units.
GFP-92, 293 cells stably transformed with rAAV2-GFP DNA.
pTRUF5, plasmid carrying the rAAV-CMV-GFP genome.
Physical capsid titer as determined by ELISA: 1.5 × 105 capsids/cell, 1.5 × 1012 total capsids, and capsid:IU ratio of 3.6 × 103.
The advent of rAAV manufacturing systems based solely on HSV infection was the next step in rHSV-based rAAV production, and generated a transfection-free system that circumvented proviral cell line generation. This was achieved by developing rHSV vectors carrying all the cis- and trans-acting elements necessary for rAAV replication and packaging (Hwang et al., 2003; Booth et al., 2004), and by producing rAAV via a single infection step. Building on the work of Conway and coworkers, Hwang and colleagues used the replication-deficient HSV d27.1 to generate an ITR-GOI (gene of interest) construct, denoted rHSV/AAV-GFP, which bore the humanized GFP gene flanked by the AAV-2 ITRs in the TK gene of the HSV backbone, and an rHSV-rep2/cap2 construct carrying the AAV-2 rep and cap genes. The two HSV vectors were subsequently used to coinfect 293 cells in culture flasks. A significant increase in rAAV yield was observed relative to transfection or single rHSV infection, with more than 6 × 103 TU/cell produced, and 1.5 × 105 VG/cell generated at a scale of 1 × 109 cells. This corresponded to about a 30-fold yield increment from a typical transfection production (Hwang et al., 2003). Similarly, Booth and colleagues reported an HSV coinfection system in which one vector contained the AAV-2 rep and cap genes and a second HSV vector bore the rAAV proviral cassette (Booth et al., 2004). Despite a highly stable genome during serial expansion of the rHSV helper vectors, specific rAAV yields (40 TU/cell) were not increased relative to transient transfection (Table 1). Booth and colleagues also reported the first in vivo evaluation of HSV-made rAAV, in which transfection-made rAAV was administered as a potency control (Booth et al., 2004). rAAV stocks produced by both methods were shown to generate robust gene expression when administered to the rat brain.
More recently, HSV-based rAAV manufacture has benefited from several advances, encompassing production of larger batches of concentrated rHSV, production of multiple rAAV serotypes, the use of suspension cells, and streamlined, scalable purification and associated characterization methods for quality control testing and in vivo evaluation. The production of high-titer, infectious rHSV stocks can be challenging because of several features inherent to HSV biology: (1) the production efficiency and the product safety profile are usually inversely correlated, in that rendering HSV vectors replication incompetent by genetic deletions also typically reduces rHSV yield; and (2) HSV particles are highly sensitive to production and processing conditions (e.g., temperature, shear, solvents, and detergents) and can easily be inactivated during manipulation.
To facilitate scale-up, herpes simplex viral vectors for both gene therapy (serotype 1) and vaccine (serotype 2) applications have been produced in bioreactors (Zecchini and Smith, 1999; Ozuer et al., 2002a; Knop and Harrell, 2007). In vitro culture of the adherence-dependent Vero and Vero-derived cell lines relies on a solid support, even in bioreactors. This can be achieved with microcarriers (such as Cytodex 1; GE Healthcare Life Sciences, Piscataway, NJ), macrocarriers (such as FibraCel; New Brunswick Scientific, Edison, NJ), or multilayered culture vessels (such as a CellCube; Corning Life Sciences, Lowell, MA) that permit medium perfusion (Zecchini and Smith, 1999; Ozuer et al., 2002a; Knop and Harrell, 2007). With rAAV manufacture in mind, Knop and Harrell scaled HSV helper vector production from T-225 flasks (∼3 × 107 V27 cells) to cell factories (∼1 × 109 cells) and finally to 3.5-liter working volume bioreactors (∼3 × 1010 cells) across an 800-fold surface area scale-up. In the latter, a CelliGen Plus packed-bed bioreactor (New Brunswick Scientific) was run using fed-batch vector production for 3 days postinfection, and the resulting rHSV-rep2/cap2 vector was recovered from the supernatant, the cells, or both. Culture supernatant typically yielded in excess of 1 × 1012 plaque-forming units (PFU) of rHSV, and postprocessing (filtration and concentration) vector stocks attained titers of about 1–2 × 109 PFU/ml (Knop and Harrell, 2007). Importantly, this streamlined, bench-top system was suitable to GMP settings.
Using rHSV produced by the FibraCel bioreactor method, Kang and colleagues produced concentrated stocks of rAAV in an adherent cell platform by rHSV coinfection (Kang et al., 2009). This method relied on coinfecting 293 cells with rHSV-rep2/capX (where X was serotype 1, 2, or 9) and rHSV-GOI at MOIs of 12 and 2, respectively, and harvesting at 52 hr postinfection. The rAAV vector genome (VG) and/or infectious particle (IP) titers were determined from cell lysates by real-time quantitative polymerase chain reaction (qPCR) and a green cell assay, respectively. More than 1 × 105 VG/cell were obtained for rAAV2-GFP production, with a VG:IP ratio of 14 (Kang et al., 2009). In this study, an rAAV construct carrying a therapeutic gene (α1-antitrypsin, or AAT) was also produced with a similar specific yield (vector genomes per cell) as rAAV2-GFP. A critical parameter of rAAV production systems is the yield and ratio of the four AAV Reps. The rHSV-rep2/cap2-infected 293 cells displayed Rep protein expression patterns similar to those observed for wild-type AAV-2 (Mendelson et al., 1986; Trempe et al., 1987). The system was scaled up to cell factories for the production of rAAV-AAT in serotypes 1 and 9 (see below). AAV-1 was further purified from crude cell lysates by ion-exchange chromatography using a three-column process to ensure high final product purity, which is requisite for clinical-grade vector batches. AAV-9 was purified by the standard iodixanol density gradient centrifugation (Zolotukhin et al., 1999). Concentration and formulation were achieved by tangential flow filtration (TFF) and diafiltration. This method was capable of producing more than 8.5 × 1013 AAV-1 VG (∼8.5 × 104 VG/cell) from one cell factory (Table 1). Importantly, the HSV-derived vector potency was conserved, and even improved, as indicated by in vitro assays (VG:IP ratio) and in vivo protein expression when compared with transfection-made rAAV-1 as a control. Despite the yield and purity improvements, any production system that relies on adherent cell culture in flasks is inherently limited in scale; making suspension cell culture requisite for scale-up. In addition, the high total MOI (14) for rHSV helper vector input reported required potentially prohibitive quantities of HSV.
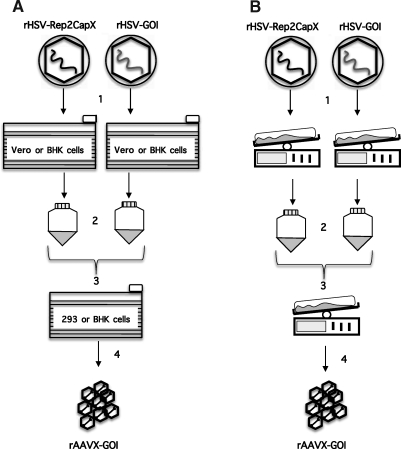
To date, only a few studies have reported rAAV production from mammalian cells adapted to grow in suspension (Park et al., 2006; Durocher et al., 2007; Feng et al., 2007; Hildinger et al., 2007). Across these studies, specific yield of vector (vector genomes per cell) continues to be an impediment. Thomas and colleagues have published a scalable version of the HSV coinfection process in which an adherent 293 cell culture is supplanted with a suspension culture of the BHK cell line (Thomas et al., 2009). The system proved to be highly advantageous for two reasons: rAAV yield was sustained over a wide range of cell densities at the time of infection, which contrasts with adherent cell-based methods; and rAAV yield was insensitive to the input MOI of the rHSV-rep2/capX helper (i.e., MOIs between 12 and 4), permitting a 3-fold reduction of this helper construct relative to the previous adherent cell production method (Kang et al., 2009; Thomas et al., 2009). Taken together, these improvements increased the concentration of producer cells by 2-fold (∼2 × 109 cells/liter), while reducing rHSV-rep2/capX vector input 3-fold, resulting in an overall increase in rAAV yield without increasing the medium volume or the specific virus load. The rHSV coinfection of suspension BHK (sBHK) cells was scaled up from 25-ml spinner cultures to 10-liter working volume disposable bioreactors with consistent specific yield ranging from about 7 × 104 to 1.1 × 105 VG/cell (Table 1). This resulted in greater than 2 × 1014 VG/liter at harvest, or more than 2 × 1015 VG for a single 10-liter disposable bioreactor run. A multistep purification process consisting of clarification and concentration steps (depth filtration, dead-end filtration, tangential flow filtration, and diafiltration) followed by two-column chromatography purification, and a final concentration/buffer exchange, provided vector in sufficient purity for administration to animals. Furthermore, the most up-to-date methods to ascertain the titer, purity, and potency of rAAV vectors were employed to characterize the final rAAV stocks generated (Kang et al., 2009; Thomas et al., 2009). Characterization of purified rAAV stocks consisted of silver staining of SDS–PAGE separation of proteins (purity), ratio of rAAV full capsids (qPCR) to infectious particles (TCID50), residual HSV protein (ELISA), and residual HSV DNA (qPCR). Quality control concluded that vector stocks were of high purity and potency, and were substantially free of HSV protein and DNA. The potency of formulated rAAV1-hAAT vector preparations was evaluated in mice and resulted in serum hAAT protein concentrations similar to that observed for rAAV1 produced in adherent 293 cells (Kang et al., 2009; Thomas et al., 2009). These improvements render the rHSV complementation system amenable to large-scale rAAV production for use in human clinical trials, and catapult this promising production technology to the forefront of rAAV manufacture. Figure 2 illustrates two large-scale production schemes using both adherent and suspension methods.
FIG. 2.
rAAV production methods by coinfection with rHSVs. (A) Adherent cell production of rAAV via rHSV coinfection. Cells are seeded and infected in 10-tray cell factories. (B) Suspension cell production of rAAV via rHSV coinfection. Cells are seeded and expanded in disposable bioreactors. (1) Generation of rHSV stocks; (2) rHSV vector recovery and concentration to high titer; (3) coinfection with rHSV vectors; (4) harvest and recovery of rAAV from cellular lysates. BHK, baby hamster kidney cells.
Production of Alternative rAAV Serotypes by the Recombinant HSV System
It has become clear that the use of AAV serotype 2 will be limited to specific gene therapy trials depending on the targeted cell type(s). Alternative AAV serotypes have now garnered increased attention because of their superior efficacy, kinetics, and diverse tissue tropism in numerous tissue/disease-specific applications when compared with AAV-2. For instance, AAV-1 (α1-antitrypsin deficiency, cardiac failure, epilepsy, limb girdle muscular dystrophy), AAV-5 (Duchenne muscular dystrophy), and AAV-6 (cardiac failure) have been employed in human clinical studies to deliver therapeutic genes to target tissues for correction of a wide variety of genetic deficiencies (Mueller and Flotte, 2008a). Furthermore, our laboratory has evaluated multiple AAV serotypes for cardiac and systemic disease correction in preclinical animal studies (Pacak et al., 2006; Mah et al., 2007). In particular, these studies have revealed that AAV-9 has an excellent expression profile in cardiac muscle (Pacak et al., 2006), and our laboratory is exploring the development of AAV-9-based vectors for future trials targeting heart diseases. As with many trials, these will require a large amount of AAV vector to be therapeutically successful, necessitating a manufacturing system capable of producing multiple AAV serotypes with minimal genetic manipulations.
Three reports have addressed the production of AAV serotypes other than AAV-2 by an HSV-based approach. In 2002, Wustner and colleagues published a method for producing rAAV5 in which an ICP27-deleted rHSV vector bore the AAV-5 rep and cap genes. rAAV-5 was produced by transfecting an rAAV-5 proviral plasmid into 293 cells followed by infection with rHSV-rep5cap5 (Wustner et al., 2002). The resulting rAAV yields were modest, potentially due to HSV-induced cytopathic effect, reliance on transfection, suboptimal AAV rep and cap expression, or a combination thereof. Higher specific yields (vector genomes per cell) of multiple AAV serotypes have been reported for rHSV vector coinfection of mammalian cells in both adherent and suspension systems for serotypes 1, 5, 8, and 9 (Kang et al., 2009; Thomas et al., 2009). Different AAV capsid sequences were inserted in lieu of the AAV-2 cap gene to generate rHSV-rep2/cap(1, 5, 8, or 9). Specific productivity (vector genomes per cell) was maintained across the serotypes, with serotype 5 productivity being the lowest and serotype 8 productivity the highest in the sBHK system, and serotypes 1 and 9 were shown to be biologically active in vivo (Kang et al., 2009; Thomas et al., 2009).
Quality Control and Quality Assurance
A series of acceptance criteria must be defined in order to assess the safety, identity, and potency of rAAV vector batches generated by any HSV-based method. Tests will be performed according to SOPs and analyzed on the basis of validated methods to assess (1) the rAAV vector genome titer, (2) the rAAV infectious titer, and (3) the presence of residual process contaminants such as rcAAV, rcHSV, HSV and cellular proteins, Benzonase, and HSV and cellular DNA.
Residual HSV DNA and HSV protein have been consistently detected in purified stocks prepared by the HSV coinfection of mammalian cells (Kang et al., 2009; Thomas et al., 2009). If replication-competent HSV is present in rHSV stocks generated from the replication-defective mutant d27.1, this could diminish the safety profile of rAAV derived from these helper vectors in a clinical setting. Fortunately, the role of rHSV vectors in the process as intermediate reagents during rAAV production should permit rAAV purification process clearance of any replication-competent HSV revertants generated during the rHSV amplification steps and introduced into the rAAV production process. In that context, clinical manufacture should tolerate the use of the ICP27-deleted mutants, which have the advantage of yielding higher titer rAAV than other, “safer” replication-defective rHSV vectors. Despite this, acceptance criteria for rcHSV and rHSV in the final product will need to be precisely defined in preclinical toxicology studies to avoid human patient consequences. An independent quality assurance unit will need to authorize release of the manufactured product on reviewing assay results against predefined acceptance criteria.
Use of HSV-Produced rAAV in Clinical Settings
The use of material made by any rHSV-based production method in clinical trials will require intensive testing before release for human application. However, this transition will be facilitated by the knowledge acquired in clinical trials employing transfection-generated rAAV. Moreover, it will be critical to establish a side-by-side comparison of safety and efficacy for rAAV generated by each of the two methods. Animal studies should assess the safety of HSV-generated rAAV in nonhuman primates through extensive biodistribution and toxicology analyses. As mentioned, preclinical investigations with different serotypes have already provided valuable observations concerning the use of HSV-generated rAAV (Kang et al., 2009; Thomas et al., 2009). The in vivo efficacy of two therapeutic rAAVs, rAAV1-AAT and rAAV9-AAT, was assessed in mice and directly compared with transfection-generated rAAV1-AAT, with rAAV-9-derived hAAT expression attaining clinically relevant levels (Kang et al., 2009).
Clinical trials will also rely on the production of rAAV under strict manufacturing procedures based on U.S. Food and Drug Administration (FDA) guidelines, ensuring safety and consistency of the product injected into patients. A significant amount of time and funds must be invested to establish fully characterized master and working cell and viral banks. In the case of rHSV, substantial challenges can arise from the generation of master viral banks (MVBs) and working viral banks (WVBs) for each rHSV. In addition, master cell banks (MCBs) and working cell banks (WCBs) must be produced for rHSV-complementing cell line(s) before rHSV MVB and WVB generation. By contrast, transfection-based methods require only plasmid preparations for upstream DNA input, which are relatively easy and inexpensive to make under GMP compliance, test, and release in a GLP (good laboratory practice) setting.
One potential concern when generating rHSV MVBs or WVBs is rHSV instability during serial expansion to large volumes, requiring full batch testing at each amplification step. However, the HSV systems reviewed here are at least as stable as other, clinical-stage manufacturing systems, such as Ad- or baculovirus-based production methods (Booth et al., 2004; Kohlbrenner et al., 2005; Kang et al., 2009). Multiple authors have demonstrated the genetic stability of serially passaged rHSV vectors for rAAV production (Booth et al., 2004; Kang et al., 2009). In addition, the minimal genetic stability of eight passages reported by Kang and colleagues for rHSV helper constructs would permit infection of more than 2.7 × 1022 sBHK cells (or 1.4 × 1013 liters) and produce more than 2 × 1027 rAAV VG, assuming only a modest rHSV serial expansion titer of 4 × 107 PFU/ml. On the other hand, once a large batch of rHSV is produced and tested, the same batch can be used in multiple production runs provided long-term storage and stability conditions are identified and implemented. The end result would be a significant reduction in repetitive production and testing costs.
The appropriateness of cell line selection for MCBs for both rHSV and rAAV are also of critical importance. Vero and Vero-based cell lines, for instance, are already approved by the World Health Organization (WHO, Geneva, Switzerland) and used in GMP production of vaccines. The use of BHK cells has been approved in a phase 1 trial for Huntington disease as well as for the commercial production of recombinant factor VIII for the treatment of hemophilia A (Boedeker, 2001; Bloch et al., 2004).
Our GMP facility at the Powell Gene Therapy Center (University of Florida, Gainesville, FL) has produced multiple lots of clinical-grade rAAV by the transfection method. On average, construct-dependent yields of 1–3 × 1014 total VG of purified rAAV were produced, requiring from 20 to 90 cell factories (∼2 × 1010 to 9 × 1010 cells), or about 25 to 100 liters of cell culture. By contrast, the herpes-based production of rAAV-1/AAT can generate the same amount of purified rAAV (3 × 1014 VG) from about 3.4 liters of sBHK culture or about 9 liters of adherent 293 cells, requiring an input of as little as 8 ml (rHSV-rep2/cap1) and 4 ml (rHSV-AAT) of the rHSV helpers.
Conclusion
Multiple scalable systems have now been developed for the production of rAAV, including adenovirus-based, baculovirus-based, and rHSV-based methods (Aucoin et al., 2008). The most significant innovation in rAAV manufacture in recent years is the advent of baculovirus infection of insect cells. Yields reported using baculoviral helpers are comparable to HSV complementation, with successful baculovirus production of rAAV scale-up to 10-, 40-, and 100-liter bioreactors accomplished (Negrete et al., 2007a,b; Cecchini et al., 2009). The method has been improved by Zolotukhin and colleagues by developing packaging cell lines that contain AAV rep and cap integrated into the host insect cell chromosome, permitting multiple serotypes to be produced by a single baculovirus infection of serotype-specific cell lines (Aslanidi et al., 2009). The final hurdles for baculovirus rAAV manufacture needing to be addressed are the apparent instability of the baculoviral constructs during serial passage amplification and the significantly higher particle-to-infectivity ratios reported (>1000) as compared with HSV-derived rAAV.
In comparison, sBHK mammalian cell culture production of rAAV is the most advanced rHSV complementation system reported to date, and is poised for use in the GMP manufacture of human clinical trial vector (Thomas et al., 2009). The salient features of rHSV manufacture of rAAV include the generation of high-titer rAAV stocks possessing excellent infectivity ratios, accommodation of multiple AAV capsid serotypes without loss of infectivity, stability during serial expansion of helper constructs, and the synergistic effect of increasing cell density and specific yields, which can elicit excellent volumetric productivities of more than 2 × 1014 VG/liter. Challenges remaining for rHSV-based rAAV manufacture include removal of animal-derived components, continued refinement and scale-up of rHSV vector manufacturing technology, and demonstrating scale-up to larger (>10-liter) batches in a GMP setting.
Acknowledgments
The authors thank Mr. Brian Cleaver (Human Applications Laboratory, UFL) for providing yields for clinical-grade rAAV preparations. This work was funded by NIH grant HL59412 and DK58327 (to B.J.B.).
Author Disclosure Statement
B.J. Byrne and D. Knop hold share options in AGTC and have a conflict of interest to the extent that this work potentially increases their personal financial interests. N. Clément does not have a conflict of interest. B.J.B is an inventor of AAV technology owned by UFL and licensed to AGTC Inc. As a founder of AGTC there is a potential conflict of interest.
References
- Aslanidi G. Lamb K. Zolotukhin S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5059–5064. doi: 10.1073/pnas.0810614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucoin M.G. Perrier M. Kamen A.A. Critical assessment of current adeno-associated viral vector production and quantification methods. Biotechnol. Adv. 2008;26:73–88. doi: 10.1016/j.biotechadv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Blankinship M.J. Gregorevic P. Chamberlain J.S. Gene therapy strategies for Duchenne muscular dystrophy utilizing recombinant adeno-associated virus vectors. Mol. Ther. 2006;13:241–249. doi: 10.1016/j.ymthe.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Blits B. Bunge M.B. Direct gene therapy for repair of the spinal cord. J. Neurotrauma. 2006;23:508–520. doi: 10.1089/neu.2006.23.508. [DOI] [PubMed] [Google Scholar]
- Bloch J. Bachoud-Levi A.C. Deglon N. Lefaucheur J.P. Winkel L. Palfi S. Nguyen J.P. Bourdet C. Gaura V. Remy P. Brugieres P. Boisse M.F. Baudic S. Cesaro P. Hantraye P. Aebischer P. Peschanski M. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: Results of a phase I study. Hum. Gene Ther. 2004;15:968–975. doi: 10.1089/hum.2004.15.968. [DOI] [PubMed] [Google Scholar]
- Boedeker B.G. Production processes of licensed recombinant factor VIII preparations. Semin. Thromb. Hemost. 2001;27:385–394. doi: 10.1055/s-2001-16891. [DOI] [PubMed] [Google Scholar]
- Booth M.J. Mistry A. Li X. Thrasher A. Coffin R.S. Transfection-free and scalable recombinant AAV vector production using HSV/AAV hybrids. Gene Ther. 2004;11:829–837. doi: 10.1038/sj.gt.3302226. [DOI] [PubMed] [Google Scholar]
- Buller R.M. Janik J.E. Sebring E.D. Rose J.A. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J. Virol. 1981;40:241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton E.A. Fink D.J. Glorioso J.C. Replication-defective genomic HSV gene therapy vectors: Design, production and CNS applications. Curr. Opin. Mol. Ther. 2005;7:326–336. [PubMed] [Google Scholar]
- Cecchini S. Negrete A. Kotin R.M. Toward exascale production of recombinant adeno-associated virus for gene transfer applications. Gene Ther. 2008;15:823–830. doi: 10.1038/gt.2008.61. [DOI] [PubMed] [Google Scholar]
- Cecchini S. Virag T. Negrete A. Kotin R.M. Production and processing of rAAVU7smOPT in 100 L bioreactors for canine models of Duchenne muscular dystrophy. Mol. Ther. 2009;17:S17. [Google Scholar]
- Conway J.E. Zolotukhin S. Muzyczka N. Hayward G.S. Byrne B.J. Recombinant adeno-associated virus type 2 replication and packaging is entirely supported by a herpes simplex virus type 1 amplicon expressing Rep and Cap. J. Virol. 1997;71:8780–8789. doi: 10.1128/jvi.71.11.8780-8789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J.E. Rhys C.M. Zolotukhin I. Zolotukhin S. Muzyczka N. Hayward G.S. Byrne B.J. High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap. Gene Ther. 1999;6:986–993. doi: 10.1038/sj.gt.3300937. [DOI] [PubMed] [Google Scholar]
- Costantini L.C. Jacoby D.R. Wang S. Fraefel C. Breakefield X.O. Isacson O. Gene transfer to the nigrostriatal system by hybrid herpes simplex virus/adeno-associated virus amplicon vectors. Hum. Gene Ther. 1999;10:2481–2494. doi: 10.1089/10430349950016825. [DOI] [PubMed] [Google Scholar]
- Durocher Y. Pham P.L. St-Laurent G. Jacob D. Cass B. Chahal P. Lau C.J. Nalbantoglu J. Kamen A. Scalable serum-free production of recombinant adeno-associated virus type 2 by transfection of 293 suspension cells. J. Virol. Methods. 2007;144:32–40. doi: 10.1016/j.jviromet.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Epstein A.L. Marconi P. Argnani R. Manservigi R. HSV-1-derived recombinant and amplicon vectors for gene transfer and gene therapy. Curr. Gene Ther. 2005;5:445–458. doi: 10.2174/156652305774329285. [DOI] [PubMed] [Google Scholar]
- Feng L. Guo M. Zhang S. Chu J. Zhuang Y. Optimization of transfection mediated by calcium phosphate for plasmid rAAV-LacZ (recombinant adeno-associated virus-β-galactosidase reporter gene) production in suspension-cultured HEK-293 (human embryonic kidney 293) cells. Biotechnol. Appl. Biochem. 2007;46:127–135. doi: 10.1042/BA20060143. [DOI] [PubMed] [Google Scholar]
- Feudner E. De Alwis M. Thrasher A.J. Ali R.R. Fauser S. Optimization of recombinant adeno-associated virus production using an herpes simplex virus amplicon system. J. Virol. Methods. 2001;96:97–105. doi: 10.1016/s0166-0934(01)00298-1. [DOI] [PubMed] [Google Scholar]
- Glauser D.L. Strasser R. Laimbacher A.S. Saydam O. Clement N. Linden R.M. Ackermann M. Fraefel C. Live covisualization of competing adeno-associated virus and herpes simplex virus type 1 DNA replication: Molecular mechanisms of interaction. J. Virol. 2007;81:4732–4743. doi: 10.1128/JVI.02476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D. Kleinschmidt J.A. Progress in adeno-associated virus type 2 vector production: Promises and prospects for clinical use. Hum. Gene Ther. 1999;10:2445–2450. doi: 10.1089/10430349950016799. [DOI] [PubMed] [Google Scholar]
- Handa H. Carter B.J. Adeno-associated virus DNA replication complexes in herpes simplex virus or adenovirus-infected cells. J. Biol. Chem. 1979;254:6603–6610. [PubMed] [Google Scholar]
- Heilbronn R. Burkle A. Stephan S. Zur Hausen H. The adeno-associated virus rep gene suppresses herpes simplex virus-induced DNA amplification. J. Virol. 1990;64:3012–3018. doi: 10.1128/jvi.64.6.3012-3018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P.L. Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: Transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbitt O.C. Wade-Martins R. Delivery of large genomic DNA inserts >100 kb using HSV-1 amplicons. Curr. Gene Ther. 2006;6:325–336. doi: 10.2174/156652306777592054. [DOI] [PubMed] [Google Scholar]
- Hildinger M. Baldi L. Stettler M. Wurm F.M. High-titer, serum-free production of adeno-associated virus vectors by polyethyleneimine-mediated plasmid transfection in mammalian suspension cells. Biotechnol. Lett. 2007;29:1713–1721. doi: 10.1007/s10529-007-9441-3. [DOI] [PubMed] [Google Scholar]
- Hwang K.K. Mandell T. Kintner H. Zolotukhin S. Snyder R. Byrne B.J. High titer recombinant adeno-associated virus production using replication deficient herpes simplex viruses type 1. Mol. Ther. 2003;7:S14–S15. [Google Scholar]
- Johnston K.M. Jacoby D. Pechan P.A. Fraefel C. Borghesani P. Schuback D. Dunn R.J. Smith F.I. Breakefield X.O. HSV/AAV hybrid amplicon vectors extend transgene expression in human glioma cells. Hum. Gene Ther. 1997;8:359–370. doi: 10.1089/hum.1997.8.3-359. [DOI] [PubMed] [Google Scholar]
- Kang W. Wang L. Harrell H. Liu J. Thomas D.L. Mayfield T.L. Scotti M.M. Ye G.J. Veres G. Knop D.R. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009;16:229–239. doi: 10.1038/gt.2008.158. [DOI] [PubMed] [Google Scholar]
- Knop D.R. Harrell H. Bioreactor production of recombinant herpes simplex virus vectors. Biotechnol. Prog. 2007;23:715–721. doi: 10.1021/bp060373p. [DOI] [PubMed] [Google Scholar]
- Kohlbrenner E. Aslanidi G. Nash K. Shklyaev S. Campbell-Thompson M. Byrne B.J. Snyder R.O. Muzyczka N. Warrington K.H., Jr. Zolotukhin S. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol. Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin R.M. Linden R.M. Berns K.I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P. Hui K.M. Wang Y. Allen P.D. Louis D.N. Yuan C.J. Breakefield X.O. Dynamics of transgene expression in human glioblastoma cells mediated by herpes simplex virus/adeno-associated virus amplicon vectors. Hum. Gene Ther. 2002;13:2147–2159. doi: 10.1089/104303402320987842. [DOI] [PubMed] [Google Scholar]
- Liu Q. Perez C.F. Wang Y. Efficient site-specific integration of large transgenes by an enhanced herpes simplex virus/adeno-associated virus hybrid amplicon vector. J. Virol. 2006;80:1672–1679. doi: 10.1128/JVI.80.4.1672-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah C. Pacak C.A. Cresawn K.O. Deruisseau L.R. Germain S. Lewis M.A. Cloutier D.A. Fuller D.D. Byrne B.J. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol. Ther. 2007;15:501–507. doi: 10.1038/sj.mt.6300100. [DOI] [PubMed] [Google Scholar]
- Mandel R.J. Burger C. Snyder R.O. Viral vectors for in vivo gene transfer in Parkinson's disease: Properties and clinical grade production. Exp. Neurol. 2008;209:58–71. doi: 10.1016/j.expneurol.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S.K. Collis P. Hermonat P.L. Muzyczka N. Adeno-associated virus general transduction vectors: Analysis of proviral structures. J. Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson E. Trempe J.P. Carter B.J. Identification of the trans-acting Rep proteins of adeno-associated virus by antibodies to a synthetic oligopeptide. J. Virol. 1986;60:823–832. doi: 10.1128/jvi.60.3.823-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L. Rose J.A. Adeno-associated virus DNA replication is induced by genes that are essential for HSV-1 DNA synthesis. Virology. 1990;179:632–639. doi: 10.1016/0042-6822(90)90130-j. [DOI] [PubMed] [Google Scholar]
- Mueller C. Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008a;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Mueller C. Flotte T.R. Gene therapy for cystic fibrosis. Clin. Rev. Allergy Immunol. 2008b;35:164–178. doi: 10.1007/s12016-008-8080-3. [DOI] [PubMed] [Google Scholar]
- Negrete A. Esteban G. Kotin R.M. Process optimization of large-scale production of recombinant adeno-associated vectors using dielectric spectroscopy. Appl. Microbiol. Biotechnol. 2007a;76:761–772. doi: 10.1007/s00253-007-1030-9. [DOI] [PubMed] [Google Scholar]
- Negrete A. Yang L.C. Mendez A.F. Levy J.R. Kotin R.M. Economized large-scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. J. Gene Med. 2007b;9:938–948. doi: 10.1002/jgm.1092. [DOI] [PubMed] [Google Scholar]
- Oehmig A. Cortes M.L. Perry K.F. Sena-Esteves M. Fraefel C. Breakefield X.O. Integration of active human β-galactosidase gene (100 kb) into genome using HSV/AAV amplicon vector. Gene Ther. 2007;14:1078–1091. doi: 10.1038/sj.gt.3302960. [DOI] [PubMed] [Google Scholar]
- Osten P. Grinevich V. Cetin A. Viral vectors: A wide range of choices and high levels of service. Handb. Exp. Pharmacol. 2007;178:177–202. doi: 10.1007/978-3-540-35109-2_8. [DOI] [PubMed] [Google Scholar]
- Ozuer A. Wechuck J.B. Goins W.F. Wolfe D. Glorioso J.C. Ataai M.M. Effect of genetic background and culture conditions on the production of herpesvirus-based gene therapy vectors. Biotechnol. Bioeng. 2002a;77:685–692. doi: 10.1002/bit.10162. [DOI] [PubMed] [Google Scholar]
- Ozuer A. Wechuck J.B. Russell B. Wolfe D. Goins W.F. Glorioso J.C. Ataai M.M. Evaluation of infection parameters in the production of replication-defective HSV-1 viral vectors. Biotechnol. Prog. 2002b;18:476–482. doi: 10.1021/bp010176k. [DOI] [PubMed] [Google Scholar]
- Pacak C.A. Mah C.S. Thattaliyath B.D. Conlon T.J. Lewis M.A. Cloutier D.E. Zolotukhin I. Tarantal A.F. Byrne B.J. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Park J.Y. Lim B.P. Lee K. Kim Y.G. Jo E.C. Scalable production of adeno-associated virus type 2 vectors via suspension transfection. Biotechnol. Bioeng. 2006;94:416–430. doi: 10.1002/bit.20776. [DOI] [PubMed] [Google Scholar]
- Rice S.A. Knipe D.M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus α protein ICP27. J. Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R.J. Chang L.S. Shenk T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R.M. The many roles of the regulatory protein ICP27 during herpes simplex virus infection. Front. Biosci. 2008;13:5241–5256. doi: 10.2741/3078. [DOI] [PubMed] [Google Scholar]
- Schleiss M.R. Persistent and recurring viral infections: the human herpesviruses. Curr. Probl. Pediatr. Adolesc. Health Care. 2009;39:7–23. doi: 10.1016/j.cppeds.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Schultz B.R. Chamberlain J.S. Recombinant adeno-associated virus transduction and integration. Mol. Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina H. Weger S. Stow N.D. Kuhrs A. Heilbronn R. Role of the herpes simplex virus helicase–primase complex during adeno-associated virus DNA replication. J. Virol. 2006;80:5241–5250. doi: 10.1128/JVI.02718-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeo B. Esclatine A. Roizman B. Post-transcriptional processing of cellular RNAs in herpes simplex virus-infected cells. Biochem. Soc. Trans. 2004;32:697–701. doi: 10.1042/BST0320697. [DOI] [PubMed] [Google Scholar]
- Thomas D.L. Wang L. Niamke J. Liu J. Kang W. Scotti M.M. Ye G.J. Veres G. Knop D.R. Scalable recombinant adeno-associated virus production using recombinant herpes simplex virus type 1 coinfection of suspension-adapted mammalian cells. Hum. Gene Ther. 2009 doi: 10.1089/hum.2009.004. [DOI] [PubMed] [Google Scholar]
- Trempe J.P. Mendelson E. Carter B.J. Characterization of adeno-associated virus Rep proteins in human cells by antibodies raised against Rep expressed in Escherichia coli. Virology. 1987;161:18–28. doi: 10.1016/0042-6822(87)90166-8. [DOI] [PubMed] [Google Scholar]
- Wang Y. Camp S.M. Niwano M. Shen X. Bakowska J.C. Breakefield X.O. Allen P.D. Herpes simplex virus type 1/adeno-associated virus rep+ hybrid amplicon vector improves the stability of transgene expression in human cells by site-specific integration. J. Virol. 2002;76:7150–7162. doi: 10.1128/JVI.76.14.7150-7162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. Falkenberg M. Elias P. Weitzman M. Linden R.M. Rep-dependent initiation of adeno-associated virus type 2 DNA replication by a herpes simplex virus type 1 replication complex in a reconstituted system. J. Virol. 2001;75:10250–10258. doi: 10.1128/JVI.75.21.10250-10258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock J.N. Merten O.W. Al-Rubeai M. Cell culture processes for the production of viral vectors for gene therapy purposes. Cytotechnology. 2006;50:141–162. doi: 10.1007/s10616-005-5507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechuck J.B. Ozuer A. Goins W.F. Wolfe D. Oligino T. Glorioso J.C. Ataai M.M. Effect of temperature, medium composition, and cell passage on production of herpes-based viral vectors. Biotechnol. Bioeng. 2002;79:112–119. doi: 10.1002/bit.10310. [DOI] [PubMed] [Google Scholar]
- Weindler F.W. Heilbronn R. A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J. Virol. 1991;65:2476–2483. doi: 10.1128/jvi.65.5.2476-2483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wustner J.T. Arnold S. Lock M. Richardson J.C. Himes V.B. Kurtzman G. Peluso R.W. Production of recombinant adeno-associated type 5 (rAAV5) vectors using recombinant herpes simplex viruses containing rep and cap. Mol. Ther. 2002;6:510–518. doi: 10.1006/mthe.2002.0695. [DOI] [PubMed] [Google Scholar]
- Zecchini T.A. Smith R.J. Production of high titre disabled infectious single cycle (DISC) HSV from a microcarrier culture. Cytotechnology. 1999;30:203–210. doi: 10.1023/A:1008005200711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. De Alwis M. Hart S.L. Fitzke F.W. Inglis S.C. Boursnell M.E. Levinsky R.J. Kinnon C. Ali R.R. Thrasher A.J. High-titer recombinant adeno-associated virus production from replicating amplicons and herpes vectors deleted for glycoprotein H. Hum. Gene Ther. 1999;10:2527–2537. doi: 10.1089/10430349950016861. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S. Byrne B.J. Mason E. Zolotukhin I. Potter M. Chesnut K. Summerford C. Samulski R.J. Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S. Potter M. Zolotukhin I. Sakai Y. Loiler S. Fraites T.J., JR. Chiodo V.A. Phillipsberg T. Muzyczka N. Hauswirth W.W. Flotte T.R. Byrne B.J. Snyder R.O. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]