Abstract
Objective
The aim of this study was to examine the association between the CYP2D6 and CYP2C19 genotype-predicted combined phenotypes and short-term measures of psychotropic efficacy and toxicity.
Methods
A rater-blinded, retrospective genotype association design examined a cohort of hospitalized pediatric psychiatric patients genotyped for CYP2D6 and CYP2C19 as part of clinical care. These combined genotypes were used to predict a combined phenotype. The primary efficacy outcome measure was the behavior intervention score (BIS), a function of the number of recorded timeouts/seclusions, therapeutic holds, and physical restraints. Drug tolerability was defined as the total number of recorded adverse drug reactions.
Results
Primary analysis was performed on 279 pediatric patients taking CYP2D6- or CYP2C19- dependent psychotropics. Combined phenotype was associated with BIS (p = 0.01) and number of adverse drug reactions (p = 0.03). Combined poor metabolizers treated with psychotropics had the lowest BIS (highest efficacy) and the highest number of adverse drug reactions. Combined ultrarapid metabolizers had the highest BIS (lowest efficacy) and the lowest number of adverse drug reactions.
Conclusion
Common variants in CYP2D6 and CYP2C19 are associated with the short-term efficacy and tolerability of psychotropic medications in hospitalized pediatric patients.
Introduction
Mental health problems among American children have been referred to as a national public health crisis by the President's New Freedom Commission on Mental Health, subcommittee on Children and Family (New Freedom Commission on Mental Health 2003). Mental illness, significant enough to impair normal development and functioning, affects approximately 10% of children ages 9–17 years (see Goldman et al. 1999). Many children with mental illness require more than outpatient treatment; as a result, psychiatric disorders are the leading cause for hospitalizations for children between 5 and 19 years old (Geller and Biebel 2006). Aggression, a common symptom of many psychiatric diagnoses and often the reason for hospitalization, poses a significant risk and cost to patient and caretaker (Jensen et al. 2007).
Patient response to commonly used psychotropic medications demonstrates significant variability; only 30–75% of patients experience efficacy, whereas 65–75% encounter adverse events (Kirchheiner et al. 2004; Emslie et al. 2006; Kratochvil et al. 2006; Hetrick et al. 2007). Many psychotropic medications are metabolized by cytochrome P450 (CYP) enzymes coded for by the polymorphic genes CYP2D6 (+124030) and CYP2C19 (*124020). The relationships between the distinct CYP2D6 or CYP2C19 genotypes and the pharmacokinetics of psychotropic medications are the basis for genotype-based dosing recommendations for some medications (Kirchheiner et al. 2001).
Despite the prevalence of the problem, the contribution of drug-metabolizing enzyme genotypes to the variability in aggressive behavior treatment response in hospitalized pediatric psychiatric patients is unknown. The purpose of this study was to examine the association between CYP2D6 and CYP2C19 genotype-predicted metabolizing phenotypes and short-term measures of drug efficacy (incidence of behavioral interventions for aggressive behavior) and toxicity (adverse drug reactions) in an inpatient pediatric psychiatric population. We hypothesized common variants in these two genes would be important factors in psychotropic medication clinical response variability in these patients.
Method
The study used a rater-blinded, retrospective genotype association design that involved a cohort of hospitalized psychiatric patients genotyped for CYP2D6 and CYP2C19 as part of clinical care. The study was approved by the first author's Institutional Review Board.
Inclusion/exclusion criteria
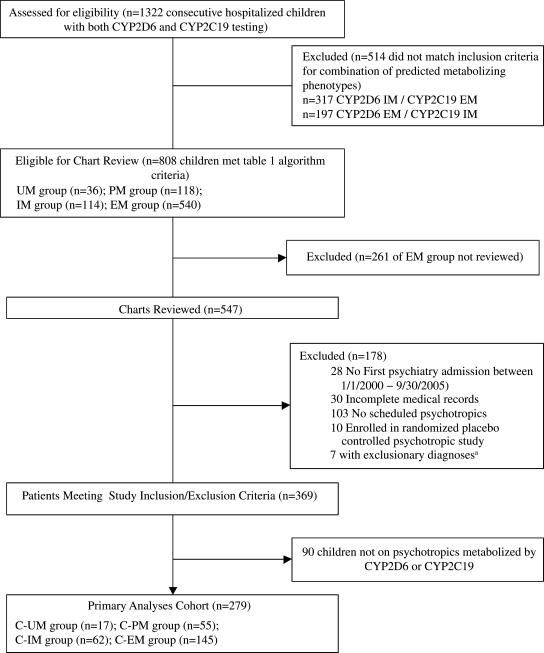
Figure 1 details the sampling procedure. Patients were eligible for inclusion in the study if they had genotyping for both CYP2D6 and CYP2C19 between January 1, 2005, and September 30, 2005; had their first inpatient admission to the psychiatric service at the authors' institution between January 1, 2000, and September 30, 2005; and had a combination of CYP2D6/CYP2C19-predicted metabolizing phenotypes that matched one of four (out of a possible six) predetermined combinations (Table 1, stage 1). Patients were excluded if: (1) Complete records from their first inpatient psychiatric hospitalization were not available; (2) the patient was enrolled in a blinded placebo-controlled clinical drug trial; or (3) the patient had a primary medical condition that could contribute to psychiatric symptoms (e.g., substance abuse, brain injury, congenital brain abnormality).
FIG. 1.
Identification of study cohort. aExclusionary diagnoses: Cerebellar hypoplasia, closed head injury, microcephaly, resected brain tumor, severe mental retardation, primary diagnosis of substance abuse. C-PM = Combined poor metabolizing phenotype; C-IM = combined intermediate metabolizing phenotype; C-EM = combined extensive metabolizing phenotype; C-UM = combined ultrarapid metabolizing phenotype.
Table 1.
Algorithm to Determine Patients' Combined Phenotype Groups
| Stage (step) | Process | Rationale |
|---|---|---|
| 1 | Single gene predicted metabolizing phenotype | Laboratory used the CYP2D6- and CYP2C19- predicted metabolizing phenotypes to identify patients fitting one of the following combinations: |
| CYP2D6 | CYP2D6 EM and CYP2C19 EM | |
| • EM: Two functional alleles (*1/*1) | CYP2D6 IM and CYP2C19 IM | |
| • PM: Two nonfunctional alleles (*3, *4, *5) | CYP2D6 UM and CYP2C19 EM or IM | |
| • IM: Combination of *1 and *3 or *4 or *5 | CYP2D6 PM and CYP2C19 PM | |
| • UM: more than two copies of *1 allele | CYP2D6 PM and CYP2C19 EM or IM | |
| CYP2C19 | CYP2D6 EM or IM and CYP2C19 PM | |
| • EM: Two functional alleles (*1/*1) | Medical record numbers of patients meeting these criteria were provided by the laboratory to the first author for the study. | |
| • PM: Two nonfunctional alleles (*2/*2) | ||
| • IM: Combination of *1 and *2 | ||
| 2 | Classified patients into one of four combined genotype predicted metabolizing phenotype groups matched to psychotropics (C-UM, C-PM, C-IM, C-EM) | Maximize the number of patients allocated to predicted phenotype groups considered at the highest risk for poor efficacy from or poor tolerability of routinely prescribed psychotropics |
| 2 (1) | Determined C-UM group | More psychotropics are metabolized by CYP2D6 than CYP2C19. Patients with C-UM phenotype are expected to need higher psychotropic doses to achieve therapeutic response (Kirchheiner et al. 2001). C-UM phenotype is the less common of the two extreme phenotypes (Bernard et al. 2006). |
| • Patients with CYP2D6 *1 duplication and taking a CYP2D6 dependent psychotropic were classified as a C-UM regardless of CYP2C19 genotype. | ||
| 2 (2) | C-PM classification decision from remaining cohort:• CYP2D6 PM and taking CYP2D6 dependent psychotropic; or• CYP2C19 PM and taking CYP2C19 dependent psychotropic; or• Both of the above | Patients with C-PM phenotype are expected to need lower doses due to increased risk for drug toxicity with standard doses (Kirchheiner et al. 2001). |
| 2 (3) | C-IM classification decision from remaining cohort: CYP2D6 IM- and CYP2C19 IM-predicted phenotypes and taking at least one psychotropic dependent on either enzyme | Limited prior studies on impact of IM phenotype on efficacy and tolerability. IM/IM strategy examined effect regardless of whether patient took a CYP2D6- or CYP2C19-dependent psychotropic. |
| 2 (4) | C-EM classification decision from remaining cohort of patients: CYP2D6 EM- and CYP2C19 EM-predicted phenotypes and taking a least one psychotropic dependent on either enzyme | EM/EM comparison group for the variant phenotypes without regard to whether they were taking a CYP2D6- or CYP2C19-metabolized medication |
Abbreviations: C-PM = combined poor metabolizing phenotype; C-IM = combined intermediate metabolizing phenotype; C-EM = combined extensive metabolizing phenotype; C-UM = combined ultrarapid metabolizing phenotype.
CYP2D6 and CYP2C19 genotyping and phenotype prediction were completed prior to this study as part of clinical care. Genotyping was performed in a Clinical Laboratory Improvement Amendments (CLIA)-approved, College of American Pathologists (CAP)-certified laboratory using DNA extracted from peripheral blood or buccal swab samples. The TaqMan® allelic discrimination system (Applied Biosystems, Forest City, CA) and long polymerase chain reaction (PCR) (Lovlie et al. 1996) were used to analyze DNA for the alleles *1, *3,*4, *5 (deletion) and duplication in CYP2D6 and the *1, *2 alleles in CYP2C19. Genotype results were used to classify a patient's predicted metabolizing phenotype (Table 1, stage 1).
Data collection
Data were collected from the medical records of the patient's first inpatient psychiatric hospitalization. All chart reviewers were trained by the principal investigator (PI) and were blinded to the patient's CYP2D6 and CYP2C19 genotype and corresponding predicted metabolizing phenotype. The PI and study psychiatric pharmacist were blinded when they re-reviewed >50% of the charts to assure integrity and consistency of extracted data.
Clinical diagnoses
Each patient's discharge diagnoses, using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) multiaxial classification system were taken from the medical record. After all patient data were entered but before the blind was broken, the study's child and adolescent psychiatrist categorized all abstracted axis I diagnoses into one of nine major diagnostic categories (Table 2).
Table 2.
Demographic and Clinical Characteristics of the Study Cohort
| Characteristic | Study cohort (n = 279) |
|---|---|
| Age, mean years (SD) | 12.7 (3.2) |
| Sex, female n (%) | 137 (49.1%) |
| Race | |
| Black | 63 (22.6%) |
| White | 202 (72.4%) |
| Other | 14 (5.0%) |
| Diagnostic Categorya | |
| Mood disorders | 224 (80.3%) |
| Disruptive behavior disorders | 132 (47.3%) |
| Anxiety disorders | 77 (27.6%) |
| Impulse control disorders | 24 (8.6%) |
| Psychotic disorders | 19 (6.8%) |
| Pervasive developmental disorders | 17 (6.1%) |
| Eating disorders | 7 (2.5%) |
| Adjustment disorders | 5 (1.8%) |
| Other | 76 (27.2%) |
| Admit GAF, mean (SD)† | 21.5 (6.4) |
| Number of psychotropics used | |
| 1 | 99 (35.5%) |
| 2 | 80 (28.7%) |
| 3 | 45 (16.1%) |
| 4 | 29 (10.4%) |
| ≥5 | 26 (9.3%) |
Several diagnostic categories may have been made for 1 patient.
Two patients missing admit GAF score.
Abbreviations: SD = Standard deviation; GAF = Global Assessment of Functioning score.
Medication classification
A psychotropic medication was classified as a CYP2D6 and/or CYP2C19 substrate if two or more of the following references listed it as a substrate: Drug-Interaction Table (Flockhart 2007), Lexi-Comp Drug Information Handbook (Lacy et al. 2005), and Micromedex (Micromedex Healthcare Series). The number of psychotropics was obtained by totaling all scheduled psychotropics a patient was taking during the admission.
Algorithm for determining combined genotype-predicted metabolizing phenotype groups
An algorithm to classify patients by their genotype-predicted metabolizing phenotype groups (called “combined phenotype” groups) was developed to identify patients considered at highest risk for poor efficacy or poor tolerability to routinely prescribed psychotropics (Table 1, stage 2, steps 1–4).
Treatment outcome measures
Efficacy
The study's primary efficacy outcome was a measure of aggression severity. Because the clinicians had not administered any objective aggression rating scale prospectively, a transformation was used based on the sum of the recorded behavioral interventions (BI) for disruptive/aggressive behavior and included the number of timeouts or seclusions (BI1), therapeutic holds (BI2), and physical restraints (BI3). A weight-free approach combined the individual recorded behavioral interventions and was called the Behavioral Intervention Score (BIS),
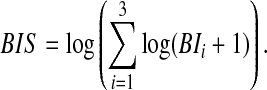 |
This score is considered weight-free because it is unaffected by different weights applied to the individual BI items (Elston 1963).
Three secondary efficacy outcome measures included the patient's total number of as-needed (PRN) psychotropic medication doses, length of stay (LOS), and change in global assessment of functioning (GAF) score (American Psychiatric Association 2000) from admission to discharge.
Tolerability
The study's primary tolerability outcome measure was the number of recorded adverse drug reactions (ADRs). Symptoms were counted as ADRs if documented during the time frame when scheduled psychotropics were administered and there was: (1) Documentation by a physician/nurse that a symptom was an ADR; or (2) documentation that parent or patient reported the symptom was drug related and there was no physician documentation of an alternative etiology for the symptom; or (3) no documented history of a symptom prior to hospital admission and no documented explanation for the symptom; or (4) a history of a symptom, but during the medication course the symptom increased in frequency and/or severity. Each ADR was classified as mild or severe by the study's child and adolescent psychiatrist. The operational definition for a severe ADR was the need for physician evaluation and intervention.
Statistical analysis
Descriptive statistics were calculated for patients' characteristics. The following values were used to reduce the influence of outliers: Number of PRN doses >30 = 30, number of time outs/seclusions >20 = 20, number of holds >5 = 5, number of restraints >3 = 3, number of ADRs >10 = 10, and LOS >31 days = 31.
Statistical models were developed for all outcomes and modeled as a function of age, sex (Dean et al. 2007), measure of severity (multiple diagnoses), number of psychotropic medications (Jensen et al. 2007), admit GAF (American Psychiatric Association 2000), and the primary predictor of interest, combined phenotype. Battery reduction was performed to reduce the dimensionality of the nine diagnosis categories to produce valid statistical models (D'Agostino et al.1995). In all regression models, predictors were transformed using either logs (admit GAF score) or natural splines (age, number of psychotropic medications, and predicted phenotypes) to relax the linearity assumption. Nonlinear terms were removed if the p value was greater than 0.20.
For the primary statistical analysis using combined phenotype as well as the subset analyses using individual CYP2D6- and CYP2C19-predicted phenotypes, multivariable regression models were fit to examine the effects of the predictors (described above) on the primary efficacy (BIS) and primary tolerability (number of ADRs) outcome variables. With the advice of the study psychiatrist, race was not included as a model predictor because race was not considered a contributing factor to clinical course variability. Multivariable models were also used to examine the same model predictors on the secondary outcomes (total number of PRN psychotropic doses during admission, change in GAF score, and LOS).
A standard formula using a one-way analysis of variance (ANOVA) with a planned contrast was used to determine sample size and suggested 376 subjects would give investigators 81% power to detect a linear trend among the combined phenotype groups with moderate effect sizes between the combined poor metabolizer (C-PM) and combined extensive metabolizer (C-EM) groups. To maximize the potential of detecting a linear trend among the groups, all patients in the C-PM, combined intermediate metabolizer (C-IM), and combined ultrarapid metabolizer (C-UM) groups (n = 268) and 279 patients from the C-EM group (a number roughly equal to the total of the other three groups) were included for eligibility review (total n = 547; Fig. 1). The subset of C-EM group patients' charts was selected by the study statisticians using a list generated by a simple randomization and presented along with the other charts to the raters with the genotype information concealed.
The primary analysis was performed on patients meeting inclusion/exclusion criteria who received psychotropics metabolized by either CYP2D6 or CYP2C19. All statistical analyses were conducted using S-Plus version 8.0 (TIBCO Software INC, Palo Alto, CA). The Hmisc and Design Libraries (Harrell 2009) were used for statistical modeling.
Results
Patient population
As part of routine clinical care, 1322 (78%) of the 1701 unique patients admitted to the inpatient psychiatric service between January 1, 2005, and September 30, 2005, were genotyped for both CYP2D6 and CYP2C19. A total of 369 children met study inclusion/exclusion criteria; the primary analyses were performed on the subset of 279 patients (ages 3–18 years, median age 13 years) who received psychotropic medications metabolized by CYP2D6 or CYP2C19 or both (Fig. 1). The clinical characteristics are shown in Table 2. The majority of patients were on either one (36%) or two (29%) psychotropics. The ten most commonly used psychotropic medications metabolized by CYP2D6 and/or CYP2C19 are listed in Table 3. Battery reduction, used to reduce dimensionality, indicated that four diagnostic categories (psychotic disorders, anxiety disorders, impulse control disorders, pervasive developmental disorder) accounted for 77% of the original variance. These were selected as predictors in the subsequent analyses.
Table 3.
Top 10 Scheduled Psychotropics Metabolized by CYP2D6 and/or CYP2C19 (n = 279)
| Drug | Number of patients | CYP2D6 substrate | CYP2C19 substrate |
|---|---|---|---|
| Risperidone | 87 | X | |
| Fluoxetine | 70 | X | X |
| Aripiprazole | 51 | X | |
| Escitalopram | 33 | X | |
| Atomoxetine | 32 | X | |
| Amphetamine plus dextroamphetamine salts | 30 | X | |
| Olanzapine | 18 | X | |
| Paroxetine | 18 | X | |
| Citalopram | 13 | X | |
| Haloperidol | 6 | X |
At least one ADR was experienced by 50% of patients. The most common were sleep disturbances (28%), gastrointestinal symptoms (15%), headache (13%), and difficulty concentrating (8%); all were rated as mild. Severe ADRs were less common and included mood change (8%), dizziness (4%), extrapyramidal symptoms (4%), aggressive behavior (2%), rash (1%), and shortness of breath (1%). There were single episodes of neuroleptic malignant syndrome and convulsions.
One or more PRN psychotropics were used by 60%. The most common PRN medication was diphenhydramine (92%) followed by (in descending frequency ranging between 11% and 3%) olanzapine, risperidone, quetiapine, ziprasidone, melatonin, and haloperidol.
Combined phenotype groups
Individuals from the subset of 279 patients were placed into one of four possible phenotype groups using the algorithm described in Table 1, stage 2. Slightly over half (52%) of the patients had a predicted C-EM phenotype, 22% had a C-IM phenotype, 20% had a C-PM phenotype, and 6% had a C-UM phenotype . There were no differences among combined phenotype groups in sex, diagnostic categories, admit GAF score, or the distribution of the total number of scheduled psychotropic drugs used. There was greater use of scheduled psychotropic medications classified as CYP2D6 substrates in the C-UM and C-PM phenotype groups (both 100%) than the C-IM phenotype group (87%) and the C-EM phenotype group (89%) (p = 0.03). There was no difference among the four combined phenotype groups in the use of scheduled psychotropic medications classified as CYP2C19 substrates.
For each patient, the maximal dose of each scheduled psychotropic medication was identified. Among the four combined phenotype groups, there was no difference in the maximal dose for the four most commonly used CYP2D6 substrate psychotropics (risperidone, fluoxetine, aripiprazole, atomoxetine) and the two most commonly used CYP2C19 substrate psychotropics (fluoxetine, escitalopram).
Efficacy outcomes
After adjustment for model predictors, there was a significant relationship between the model and the study's primary efficacy outcome variable, BIS (p < 0.0001, r2 = 0.30). Five of the factors were independently significant—age, sex, number of psychotropics, impulse control disorder diagnosis, and the combined phenotype.
There was a statistically significant relationship between combined phenotype and BIS (p = 0.01, Table 4) when adjusted for other predictors in the model. Patients in the C-PM group had lower BIS (higher efficacy), whereas patients in the C-UM group had the highest BIS (lowest efficacy). Although none of the secondary outcome variables reached significance, the total number of PRN medication doses (p = 0.14) showed a steady increase across groups (from C-PM to C-UM) that paralleled the effect detected by the BIS. There was no difference among groups in change in GAF scores (p = 0.90).
Table 4.
Adjusted Means and the 95% Confidence Intervals of Efficacy and Tolerability Outcomes by Combined Phenotype Groups
| |
Means (95% CI)aCombined metabolizing subgroup (n = 279) |
||||
|---|---|---|---|---|---|
| C-PM (n = 55) | C-IM (n = 62) | C-EM (n = 145) | C-UM (n = 17) | p Value for trend | |
| Efficacy outcomes | |||||
| Behavioral Intervention Score | 0.20 (0.04–0.39) | 0.31 (0.18–0.46) | 0.44 (0.30–0.59) | 0.57 (0.37–0.80) | 0.01 |
| Number of PRN doses | 1.07 (0.61–1.67) | 1.27 (0.89–1.72) | 1.48 (1.08–1.97) | 1.72 (1.14–2.47) | 0.14 |
| Length of stay | 6.36 (5.43–7.45) | 6.58 (5.87–7.38) | 6.81 (6.09–7.61) | 7.04 (6.05–8.19) | 0.38 |
| Change of GAF | 22.7 (21.0–24.4) | 22.7 (21.5–23.9) | 22.6 (21.5–23.8) | 22.6 (21.0–24.2) | 0.90 |
| Tolerability outcome | |||||
| Number of ADRs | 1.44 (0.96–2.00) | 1.19 (0.88–1.54) | 0.96 (0.69–1.26) | 0.75 (0.43–1.13) | 0.03 |
Data presented as adjusted means and 95% confidence interval (CI) with adjusters set at the mean or reference category (sex = female, Age = 13, admit GAF = 20, number of psychiatric drugs = 2, psychotic disorder = no, anxiety disorder = no, impulse control disorder = no, pervasive developmental disorder = no).
Abbreviations: C-PM = combined poor metabolizing phenotype; C-IM = combined intermediate metabolizing phenotype; C-EM =combined extensive metabolizing phenotype; C-UM = combined ultrarapid metabolizing phenotype; PRN = as needed; GAF = Global Assessment of Functioning score; ADRs = adverse drug reactions.
As a secondary analysis, the effect of each gene's predicted metabolizing phenotype was examined (Table 5). A relationship between CYP2D6-predicted metabolizing phenotype and BIS was noted (p = 0.01). In contrast, no relationship was detected between CYP2C19-predicted metabolizing phenotype and BIS (p = 0.57).
Table 5.
Adjusted Means, 95% Confidence Intervals of Efficacy and Tolerability Outcomes by CYP2D6 and CYP2C19 Subgroups
| |
Adjusted means (95% CI)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
CYP2D6-predicted metabolizing phenotype (n = 255) |
CYP2C19-predicted metabolizing phenotype (n = 127) |
|||||||
| PM (n = 45) | IM (n = 56) | EM (n = 137) | UM (n = 17) | p value | PM (n = 11) | IM (n = 34) | EM (n = 82) | p value | |
| Efficacy Outcomes | |||||||||
| Behavioral Intervention Score | 0.20 (0.03–0.41) | 0.32 (0.18–0.48) | 0.45 (0.30–0.62) | 0.60 (0.37–0.86) | 0.01 | 0.42 (0.06–0.91) | 0.54 (0.29–0.85) | 0.40 (0.23–0.59) | 0.57 |
| Number of PRN doses | 1.01 (0.53–1.66) | 1.22 (0.82–1.72) | 1.45 (1.02–1.98) | 1.71 (1.09–2.52) | 0.14 | 1.33 (0.28–3.26) | 1.15 (0.41–2.28) | 1 (0.41–1.84) | 0.81 |
| Length of stay | 6.14 (5.18–7.28) | 6.41 (7.24–8.04) | 6.68 (7.53–8.48) | 6.97 (8.19–9.93) | 0.30 | 7.04 (4.74–10.5) | 7.11 (5.58–9.05) | 6.70 (5.63–7.96) | 0.88 |
| Change of GAF | 22.7 (20.8–24.5) | 22.7 (21.4–24.0) | 22.7 (21.4–23.9) | 22.7 (21.0–24.4) | 0.98 | 23.4 (19.4–27.3) | 25.9 (23.2–28.7) | 25.2 (22.9–27.6) | 0.41 |
| Tolerability outcome | |||||||||
| Number of ADRs | 1.27 (0.81–1.85) | 1.14 (0.81–1.52) | 1.01 (0.72–1.36) | 0.89 (0.53–1.35) | 0.27 | 2.04 (0.95–3.73) | 1.07 (0.58–1.71) | 0.67 (0.37–1.02) | 0.01 |
Data presented as adjusted means and 95% confidence interval (CI) with adjusters set at the mean or reference category (sex = female, age = 13, admit GAF = 20, number of psychiatric drugs = 2, psychotic disorder = no, anxiety disorder = no, impulse control disorder = no, pervasive developmental disorder = no).
Abbreviations: PM = Individual gene predicted poor metabolizing phenotype; IM = individual gene predicted intermediate metabolizing phenotype; EM = individual gene predicted extensive metabolizing phenotype; UM = individual gene predicted ultra-rapid metabolizing phenotype; PRN = as needed; GAF = Global Assessment of Functioning score; ADRs = adverse drug reactions.
A total of 90 out of 369 patients met all inclusion/exclusion criteria but did not receive psychotropics dependent on CYP2D6 or CYP2C19 (Fig. 1). These 90 patients were analyzed using statistical methods identical to the study cohort to determine if a similar linear relationship between BIS and genotype existed in patients taking psychotropics not metabolized by either CYP2D6 or CYP2C19. No difference among the four combined phenotype groups was detected (p = 0.42).
Tolerability outcome
There was a significant relationship between combined predicted phenotype and the number of ADRs (primary tolerability variable, p = 0.03; Table 4) when adjusted for other predictors in the model. As a secondary analysis, a relationship between CYP2C19-predicted metabolizing phenotype and number of ADRs was noted (p = 0.01; Table 5). An association between CYP2C19-predicted metabolizing phenotype and the type of ADRs (severe vs. mild vs. none) was also seen using the nonparametric Kruskal–Wallis test (p = 0.04).
Discussion
This is the first study to detect a pharmacogenetic association between drug-metabolizing enzyme genotypes and short-term psychotropic medication efficacy and tolerability in hospitalized children. As metabolizing capability increased across the four combined metabolizing phenotype groups, the BIS increased steadily, indicating decreased drug efficacy (p = 0.01), and the number of adverse drug reactions (ADRs) decreased, indicating less drug toxicity (p = 0.03). When only the CYP2C19-predicted phenotype was considered, there was a significant increase in the total number (p = 0.01) and severe ADRs (p = 0.04) as metabolizing ability decreased, indicating increased drug toxicity.
Aggressive behavior is a common nonspecific symptom in many psychiatric disorders (Masters et al. 2002). In one study, 33% of youths in an inpatient psychiatric facility were involved in an assault on hospital staff (Ryan et al. 2004). Because patients' aggressive behaviors put them, their families, and their inpatient caretakers at great risk, limiting and decreasing aggressive behavior is a key objective of patients' hospitalization3 (Foster et al. 2007; Jensen et al. 2007) This study used BIS as the measure of short-term drug efficacy because as medication efficacy improves, the BIS should decrease.
Clinicians at the study site did not rate aggression in the medical records using a standard scale, which created the need to use a proxy, BIS, as the measure of short-term drug efficacy. The BIS is based on the objective, reliable, and verifiable interventions of time outs, seclusions, therapeutic holds, and physical restraints. By hospital policy, these four interventions are accepted clinical interventions for aggression against others or self, and each use of any one of these must be documented in the patient's hospital chart. As such, retrospective identification of any of these four interventions is a reliable, identifiable proxy for patient aggression. In general, a child requiring a time out was considered to have less severe aggression than a child requiring a therapeutic hold or physical restraint making it unreasonable to simply sum the total number of behavioral interventions for each child. A search of the literature and consultation with the study's child adolescent psychiatrist did not provide evidence for assigning differential weights to BI1, BI2, and BI3. Therefore, a composite BIS was calculated using a weight-free index approach,
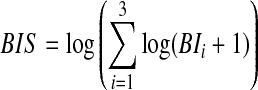 |
that does not change under different weights applied to the individual BI items (Elston 1963). Internal consistency of the individual BI items demonstrated a Cronbach alpha coefficient of 0.72, which is considered acceptable (Nunnaly 1978) when combining items. As scheduled psychotropics effectively treat the underlying diagnoses and associated aggression, the need for behavioral interventions should decrease as clinical condition improves. Therefore, BIS is a logical measure of short-term drug efficacy: As medication efficacy improves, BIS should decrease.
The study found that patients with C-UM phenotypes had the highest BIS and fewest ADRs; as metabolizing ability decreased across combined phenotype groups, the BIS decreased (improved efficacy) and ADRs increased (greater toxicity). Two potential explanations include: (1) An underlying relationship between CYP2D6/CYP2C19 genotype and psychiatric disease severity or (2) a genotype–pharmacokinetic association where differential drug exposure occurs across combined phenotype groups. Two findings from this study indicate a genotype–pharmacokinetic relationship is more likely than a genotype–disease severity phenotype: (1) The 90 patients who did not receive CYP2D6- or CYP2C19-dependent psychotropic medications showed no significant differences in BIS across the four combined phenotype groups and (2) the maximal dose for six of the most commonly used CYP2D6/CYP2C19-dependent psychotropics was the same across combined phenotype groups.
Specifically, inadequate drug efficacy, as measured by the BIS, coupled with low ADR rates particularly in the C-UM patients strongly suggests these patients are being underdosed. The reverse is true for C-PM patients. These results are similar to analyses of pooled data from children and adolescents who were treated with atomoxetine in any of four acute, double-blind, and placebo-controlled studies (Michelson et al. 2007). The investigators found that patients who had genotypes consistent with CYP2D6-predicted poor metabolism had greater symptom reduction when compared to CYP2D6 EM patients at the cost of a modestly greater increase in some adverse events. The investigators noted that more EM patients discontinued because of lack of efficacy than PMs who discontinued due to adverse events.
This is the first study to identify a significant relationship between the total number of ADRs (p = 0.01) and severe ADRs (p = 0.04) across CYP2C19 genotypes for the subgroups of patients taking psychotropics metabolized through the CYP2C19 pathway. While not statistically significant, a trend of increasing ADRs from CYP2D6 UMs to CYP2D6 PMs was demonstrated. Similar to this study, negative CYP2D6 results have been noted by others (Binder and Holsboer 2006). Yet, some studies (using smaller sample sizes, different methodologies, and patient populations) detected a relationship between the CYP2D6 genotype and the incidence of ADRs to medications metabolized through the CYP2D6 pathway (Chen et al. 1996; de Leon et al. 1998; Chou et al. 2000; Kirchheiner et al. 2001; Mrazek 2006).
In this study, CYP2D6 substrates were mostly atypical antipsychotics and selective serotonin reuptake inhibitor (SSRI) antidepressants rather than typical antipsychotics or tricyclic antidepressants. The most commonly occurring ADRs with these psychotropics are weight gain and metabolic effects (Correll and Carlson 2006) and were likely underreported in this study due to insufficient LOS. Common, yet highly subjective, ADRs such as akathisia were not detected frequently, possibly due to underreporting or inadequate documentation in the medical record.
This study is limited by its retrospective design, the number of different medications patients received, the lack of long-term follow up, the lack of standardized aggression and ADR scale use, the exclusion of certain “mixed” genotype combinations (Fig.1), and the limited number of CYP2D6 and CYP2C19 alleles tested. Retrospective studies have the potential for more bias than prospective studies. We attempted to minimize bias by using the rater-blinded methodology. The lack of differences across combined phenotype groups in both the medications used and their maximal drug doses implies that medication selection and short-term usage patterns were similar across the groups. This reduces, but does not eliminate, the potential bias introduced by lack of a prospective protocol-driven medication selection and titration schedule approach. Clearly, prospective, longitudinal studies in pediatric patients would contribute to further understanding of the combined phenotype–clinical response relationships. Although ideal, standardized ADR and aggression scales are not commonly used in the routine inpatient psychiatric clinical care setting and were not available for this study.
The CYP2D6 and CYP2C19 genotype data used in this study presented the results of a genetic analysis performed for clinical care not research purposes. As such, the panel of alleles tested in 2005 was not comprehensive but represented a balance between established clinical relevance of allelic variation (Bradford 2002), need for results within a 2-day window, and cost. Future prospective studies should include a larger panel of CYP2D6 alleles (e.g., the functional allele CYP2D6*2 (Gaedigk et al. 2003) and the reduced-function alleles that occur more commonly in non-Caucasian populations, for example CYP2D6*10 (Luo et al. 2005; Sistonen et al. 2007), CYP2D6*17, CYP2D6*41 (Cai et al. 2006; Gaedigk et al. 2007), along with CYP2C19*17, a recently discovered polymorphism associated with CYP2C19 ultrarapid metabolism (Sim et al. 2006; Rudberg et al. 2008)).
Conclusion
Identifying factors underlying the variability in drug efficacy or tolerability is a key component for optimizing the patient's response to therapy. This is the first study to demonstrate that, for children hospitalized for psychiatric conditions, CYP2D6 and CYP2C19 genetic variation contributes to psychotropic medication's short-term efficacy against aggression and the occurrence of ADRs. Consideration of a patient's genotype at the onset of a psychiatric hospitalization could play a significant role in personalizing and improving subsequent therapy. Prospective longitudinal studies are necessary to better inform how to optimally incorporate this genetic information into the medical management of patients with aggression. In summary, this study indicates that CYP2D6 and CYP2C19 genotypes are important in the clinical care of children with psychiatric diagnoses requiring medications that are metabolized through these two enzyme pathways.
Disclosures
The manuscript's authors disclose the following corporate/commercial relations that might pose a conflict of interest: Cynthia A. Prows is a consultant for PTC Therapeutics; Sanjeev Pathak currently works at AstraZeneca Pharmaceuticals, Wilmington, DE, and during study planning, implementation, and results interpretation was a psychiatrist at Cincinnati Children's Hospital Medical Center; Alexander A. Vinks is a consultant for NPS pharmaceuticals, has grant support from Roche Laboratories, and has a laboratory contract with Isotechnika, Inc.; Tracy A. Glauser is a consultant for UCB Pharma, Ortho-McNeil Neurologics, Eisai Pharmaceuticals, and Jazz Pharmaceutical; Todd G. Nick, Shannon N. Saldana, Kejian Zhang, Chunyan Liu, and Zachary S. Daniels have no conflicts of interest or financial ties to disclose.
Footnotes
Supported in part by NIH grant 5U10H0037249=10(SNS, AAV, TA6).
Acknowledgments
The authors would like to thank: Richard Wenstrup, M.D., for his advice during the development and implementation of the study; Marie Malgaz, Kasia Bryc, B.S., and Steve Fordyce for their assistance with identifying eligible patients; Debbie Baker, Luke Botting, B.A., and Carol Hetteberg, M.S.N., for their tireless and careful chart reviews; and Thomas Boat, M.D., Michael Sorter, M.D., Robert Kowatch, M.D., Ph.D., and Gregory Grabowski, M.D., for their critical and insightful reviews.
References
- American Psychiatric Association: Diagnostic and Statistical Manual, 4th edition, Text Revision (DSM-IV-TR) Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- Bernard S. Neville KA. Nguyen AT. Flockhart DA. Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: Clinical implications. Oncologist. 2006;11:126–135. doi: 10.1634/theoncologist.11-2-126. [DOI] [PubMed] [Google Scholar]
- Binder EB. Holsboer F. Pharmacogenomics and antidepressant drugs. Ann Med. 2006;38:82–94. doi: 10.1080/07853890600551045. [DOI] [PubMed] [Google Scholar]
- Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3:229–243. doi: 10.1517/14622416.3.2.229. [DOI] [PubMed] [Google Scholar]
- Cai WM. Nikoloff DM. Pan RM. de Leon J. Fanti P. Fairchild M. Koch WH. Wedlund PJ. CYP2D6 genetic variation in healthy adults and psychiatric African-American subjects: Implications for clinical practice and genetic testing. Pharmacogenomics J. 2006;6:343–350. doi: 10.1038/sj.tpj.6500378. [DOI] [PubMed] [Google Scholar]
- Chen S. Chou WH. Blouin RA. Mao Z. Humphries LL. Meek QC. Neill JR. Martin WL. Hays LR. Wedlund PJ. The cytochrome P450 2D6 (CYP2D6) enzyme polymorphism: Screening costs and influence on clinical outcomes in psychiatry. Clin Pharmacol Ther. 1996;60:522–534. doi: 10.1016/S0009-9236(96)90148-4. [DOI] [PubMed] [Google Scholar]
- Chou WH. Yan FX. de Leon J. Barnhill J. Rogers T. Cronin M. Pho M. Xiao V. Ryder TB. Liu WW. Teiling C. Wedlund PJ. Extension of a pilot study: Impact from the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clin Psychopharmacol. 2000;20:246–251. doi: 10.1097/00004714-200004000-00019. [DOI] [PubMed] [Google Scholar]
- Correll CU. Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- D'Agostino R. Belanger A. Markson E. Kelly-Hayes M. Wolf P. Development of health risk appraisal functions in the presence of multiple indicators: The Framingham Study nursing home institutionalization model. Stat Med. 1995;14:1757–1770. doi: 10.1002/sim.4780141605. [DOI] [PubMed] [Google Scholar]
- de Leon J. Barnhill J. Rogers T. Boyle J. Chou WH. Wedlund PJ. Pilot study of the cytochrome P450-2D6 genotype in a psychiatric state hospital. Am J Psychiatry. 1998;155:1278–1280. doi: 10.1176/ajp.155.9.1278. [DOI] [PubMed] [Google Scholar]
- Dean AJ. Duke SG. George M. Scott J. Behavioral management leads to reduction in aggression in a child and adolescent psychiatric inpatient unit. J Am Acad Child Adolesc Psychiatry. 2007;46:711–720. doi: 10.1097/chi.0b013e3180465a1a. [DOI] [PubMed] [Google Scholar]
- Elston RC. A weight-free index for the purpose of ranking or selection with respect to several traits at a time. Biometrics. 1963;19:85–97. [Google Scholar]
- Emslie G. Kratochvil C. Vitiello B. Silva S. Mayes T. McNulty S. Weller E. Waslick B. Casat C. Walkup J. Pathak S. Rohde P. Posner K. March J. Treatment for Adolescents with Depression Study (TADS): Safety results. J Am Acad Child Adolesc Psychiatry. 2006;45:1440–1455. doi: 10.1097/01.chi.0000240840.63737.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockhart DA. Drug interaction table (cytochrome P450 system) [Nov 15;2007 ]. http://medicine.iupui.edu/flockhart/ http://medicine.iupui.edu/flockhart/
- Foster C. Bowers L. Nijman H. Aggressive behaviour on acute psychiatric wards: Prevalence, severity and management. J Adv Nurs. 2007;58:140–149. doi: 10.1111/j.1365-2648.2007.04169.x. [DOI] [PubMed] [Google Scholar]
- Gaedigk A. Ryder DL. Bradford LD. Leeder JS. CYP2D6 poor metabolizer status can be ruled out by a single genotyping assay for the−1584G promoter polymorphism. Clin Chem. 2003;49(6 Pt 1):1008–1011. doi: 10.1373/49.6.1008. [DOI] [PubMed] [Google Scholar]
- Gaedigk A. Ndjountche L. Divakaran K. Dianne Bradford L. Zineh I. Oberlander TF. Brousseau DC. McCarver DG. Johnson JA. Alander SW. Wayne Riggs K. Steven Leeder J. Cytochrome P4502D6 (CYP2D6) gene locus heterogeneity: Characterization of gene duplication events. Clin Pharmacol Ther. 2007;81:242–251. doi: 10.1038/sj.clpt.6100033. [DOI] [PubMed] [Google Scholar]
- Geller JL. Biebel K. The premature demise of public child and adolescent inpatient psychiatric beds: Part I: Overview and current conditions. Psychiatr Q. 2006;77:251–271. doi: 10.1007/s11126-006-9012-0. [DOI] [PubMed] [Google Scholar]
- Goldman HH. Rye P. Sirovatka P. Chapter 3: Children and mental health. http://www.surgeongeneral.gov/library/mentalhealth/chapter3/sec1.html/ In Mental. 1999;Health:A. [Google Scholar]
- Harrell FE. The Hmisc and Design Libraries. [Jan 24;2009 ]. http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/RS/ http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/RS/
- Hetrick S. Merry S. McKenzie J. Sindahl PMP. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database of Systematic Reviews (3):Art. No.: CD004851. 2007;DOI:10. doi: 10.1002/14651858.CD004851.pub2. [DOI] [PubMed] [Google Scholar]
- Jensen PS. Youngstrom EA. Steiner H. Findling RL. Meyer RE. Malone RP. Carlson GA. Coccaro EF. Aman MG. Blair J. Dougherty D. Ferris C. Flynn L. Green E. Hoagwood K. Hutchinson J. Laughren T. Leve LD. Novins DK. Vitiello B. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: Implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46:309–322. doi: 10.1097/chi.0b013e31802f1454. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J. Brosen K. Dahl ML. Gram LF. Kasper S. Roots I. Sjoqvist F. Spina E. Brockmoller J. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: A first step towards subpopulation-specific dosages. Acta Psychiatr Scand. 2001;104:173–192. doi: 10.1034/j.1600-0447.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J. Nickchen K. Bauer M. Wong ML. Licinio J. Roots I. Brockmoller J. Pharmacogenetics of antidepressants and antipsychotics: The contribution of allelic variations to the phenotype of drug response. Mol Psychiatry. 2004;9:442–473. doi: 10.1038/sj.mp.4001494. [DOI] [PubMed] [Google Scholar]
- Kratochvil C. Emslie G. Silva S. McNulty S. Walkup J. Curry J. Reinecke M. Vitiello B. Rohde P. Feeny N. Casat C. Pathak S. Weller E. May D. Mayes T. Robins M. March J. Acute time to response in the Treatment for Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45:1412–1418. doi: 10.1097/01.chi.0000237710.73755.14. [DOI] [PubMed] [Google Scholar]
- Lacy CF. Armstrong LL. Goldman MP. Lance LL. Drug information handbook. Hudson (Ohio): Lexi-Comp; 2005. [Google Scholar]
- Lovlie R. Daly AK. Molven A. Idle JR. Steen VM. Ultrarapid metabolizers of debrisoquine: characterization and PCR-based detection of alleles with duplication of the CYP2D6 gene. FEBS Lett. 1996;392:30–34. doi: 10.1016/0014-5793(96)00779-x. [DOI] [PubMed] [Google Scholar]
- Luo HR. Gaedigk A. Aloumanis V. Wan YJ. Identification of CYP2D6 impaired functional alleles in Mexican Americans. Eur J Clin Pharmacol. 2005;61:797–802. doi: 10.1007/s00228-005-0044-4. [DOI] [PubMed] [Google Scholar]
- Masters KJ. Bellonci C. Bernet W. Arnold V. Beitchman J. Benson RS. Bukstein O. Kinlan J. McClellan J. Rue D. Shaw JA. Stock S. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 Suppl):4S–25S. doi: 10.1097/00004583-200202001-00002. [DOI] [PubMed] [Google Scholar]
- Michelson E. Read HA. Ruff DD. Witcher J. Zhang S. McCracken J. CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46:242–251. doi: 10.1097/01.chi.0000246056.83791.b6. [DOI] [PubMed] [Google Scholar]
- Micromedex Healthcare Series. [Nov 17;2006 ]. http://mcmicromed1/home/dispatch/ http://mcmicromed1/home/dispatch/
- Mrazek DA. Incorporating pharmacogenetics into clinical practice: Reality of a new tool in psychiatry. The context of genetic testing in clinical psychiatric practice. CNS Spectr. 2006;11(3 Suppl 3):3–4. doi: 10.1017/s1092852900025578. [DOI] [PubMed] [Google Scholar]
- New Freedom Commission on Mental Health: Achieving the Promise: Transforming Mental Health Care in America. Final Report. Rockville (Maryland): 2006. [Google Scholar]
- Nunnally JC. Psychometric Theory. New. 1978;York:McGraw. [Google Scholar]
- Rudberg I. Mohebi B. Hermann M. Refsum H. Molden E. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther. 2008;83:322–327. doi: 10.1038/sj.clpt.6100291. [DOI] [PubMed] [Google Scholar]
- Ryan EP. Hart VS. Messick DL. Aaron J. Burnette M. A prospective study of assault against staff by youths in a state psychiatric hospital. Psychiatr Serv. 2004;55:665–670. doi: 10.1176/appi.ps.55.6.665. [DOI] [PubMed] [Google Scholar]
- Sim SC. Risinger C. Dahl ML. Aklillu E. Christensen M. Bertilsson L. Ingelman-Sundberg M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103–113. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sistonen J. Sajantila A. Lao O. Corander J. Barbujani G. Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics. 2007;17:93–101. doi: 10.1097/01.fpc.0000239974.69464.f2. [DOI] [PubMed] [Google Scholar]