Abstract
Objective
The aim of this study was to evaluate the tolerability of adding OROS methylphenidate (MPH) to children who are partial responders to atomoxetine (ATMX) in the treatment of attention-deficit/hyperactivity disorder (ADHD).
Methods
This was a two-phase, 7-week, open study in children aged 6–17 years. Phase 1 initiated ATMX for a minimum of 4 weeks. Phase 2 entered partial responders to ATMX and added OROS MPH to their regimen. Safety was assessed using blood pressure and heart rate measurements, electrocardiogram readings, AEs, laboratories, and ATMX levels.
Results
Fifty subjects who were partial responders to ATMX received the combination therapy, with 41 subjects completing the entire protocol. As reported elsewhere (Wilens et al., 2009), OROS MPH added to partial responders of ATMX was accompanied by a 40% reduction in the ADHD rating scale score and improvements in executive functioning. However, the combination of ATMX plus OROS MPH was associated with greater rates of insomnia, irritability, and loss of appetite compared to ATMX alone. A small significant increase in diastolic blood pressure was observed during adjunctive OROS MPH, with no clinically meaningful changes in electrocardiogram (ECG) parameters during the study. ATMX levels and liver function tests did not significantly change during the combination treatment.
Conclusion
Adjunct OROS MPH in ATMX partial responders yielded an additive adverse effect burden in this short-term study. Further controlled research with larger samples of children is warranted.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurobehavioral disorder in the United States. Psychopharmacological intervention is among the most efficacious treatments for pediatric ADHD (MTA Cooperative Group 1999; Pliszka 2007). However, due to the presence of residual ADHD symptoms, adverse effects (AEs), or psychiatric co-morbidities, clinicians commonly employ a combination of medications for youths with ADHD (Safer et al. 2003). Given the proven efficacy, and variable mechanisms of action of stimulant and nonstimulant (atomoxetine [ATMX]) agents (Wilens 2006), combination treatment with these two classes of agents makes conceptual sense. However, to date, there is a lack of safety and tolerability data for this regimen in children; the literature is limited to case series or clinical reviews of the use of combination ATMX and stimulant therapy in children and adults (Brown 2004; Carlson et al. 2007).
In one prior report of combination ATMX and the extended-release stimulant OROS methylphenidate (MPH) in a small sample of children with ADHD (Carlson et al. 2007), numerically fewer treatment emergent AEs were observed in the combined ATMX plus MPH group compared with ATMX monotherapy (ATMX/placebo). However, patients in the combined ATMX/MPH group did have a mean decrease in weight of 0.89 kg, as compared to a small increase in weight with ATMX monotherapy. The most common treatment-emergent AEs for children in this study were insomnia, vomiting, headache, and nausea. No significant impact on blood pressure or heart rate was observed in those receiving combination therapy. While useful, the small sample size and a priori inclusion of children with prior inadequate stimulant response limit the generalization of the data.
As part of a comprehensive examination of adjunct OROS MPH and ATMX (Wilens et al., 2009), we studied the safety profile of this combination, as determined by adverse event monitoring, vital sign assessments (blood pressure, heart rate, weight), electrocardiography (ECG), laboratories (liver test, blood count), and pharmacokinetics (serum ATMX level).
We hypothesized that the combination of OROS MPH plus ATMX would be associated with greater rates of AEs common to these medications, as well as a greater increase in blood pressure and heart rate than observed with ATMX alone. We further hypothesized that ECG parameters or blood chemistry laboratories would not be significantly altered during combination treatment, nor would adjunct OROS MPH significantly impact ATMX levels.
Methods
Subjects
As part of a larger comprehensive examination of adjunct OROS MPH in ATMX partial responders (Wilens et al., 2009), we specifically examined tolerability and pharmacokinetics of this combined treatment. Eligible subjects had a diagnosis of ADHD by clinical interview supported by structured psychiatric interview. Excluded from the study were potential subjects who were on other psychotropic medications, had clinically significant chronic medical condition(s) such as history of structural cardiac defects or cardiovascular symptoms (palpitations or syncope) (Gutgesell et al. 1999), clinically significant abnormal baseline laboratory values, pregnant or nursing females, mental retardation, and organic brain disorders. Likewise, youths with a lifetime diagnosis of a psychotic disorder, bipolar disorder, or a current diagnosis(es) of major depressive disorder, panic disorder or generalized anxiety disorder, Tourette's disorder, or substance use disorder were not enrolled. Subjects with a history of no response or intolerable AEs to either ATMX or MPH were excluded for ethical reasons. The study was approved by the Partner's Human Research Committee. Parents of subjects completed an informed consent and all subjects 7 years old and older completed an assent prior to study entry.
Clinical trial
The study was a single site, open, 4-week treatment of ATMX, with partial ADHD responders completing a subsequent 3-week study of adjunct OROS MPH. After screening for ADHD, youths underwent a comprehensive clinical interview, supplemented by a structured psychiatric assessment and neuropsychological battery. Eligible ADHD youths who had been washed out from their previous treatment (if applicable) were then started on ATMX openly for a minimum of 4 weeks; partial responders received OROS for 3 additional weeks, as described in Wilens et al. (2009).
In brief, ATMX was initiated using a weight-based nomogram at 0.5 mg/kg per day, to be administered daily for 2 weeks, and then increased to 1.2 mg/kg per day (or 100 mg maximum) for 2 weeks. ATMX could be administered as daily (a.m) or twice-daily dosing (a.m. and p.m.) based on tolerability. Subjects already on a stable regimen of ATMX (maximum dosage of 1.4 mg/kg) who manifested a partial response could enter directly into the trial and be placed on adjunct OROS MPH. OROS MPH was titrated openly in 18-mg increments weekly to a target dose of 54 mg.
Safety assessments
Prior to beginning study medication, a 12-lead ECG, blood laboratories (complete blood count, liver function tests), and vital signs (systolic blood pressure [SBP], diastolic blood pressure [DBP], and heart rate [HR]) were collected for all subjects. Vital signs were obtained by oscillometry, from subjects at rest, in the sitting position, using an Omron HEM-907 blood pressure monitor (Omron Healthcare, Bannockburn, IL). Heart rate was derived from vital sign assessment.
During the study, AEs and concomitant medications were recorded at each visit. AEs were assessed according to a general query by the treating physician, monitoring emergent, and/or ongoing adverse effects. AEs were systematically rated according to severity (mild, moderate, severe, serious), relationship to study medication (unrelated, unlikely, possible, probable, definite), frequency, treatment (e.g., pharmacologic, nonpharmacologic), and effect on study medication (e.g., none, discontinued). Use of concomitant medications was recorded at every visit.
Vital signs were assessed at each study visit, as a single, first reading, typically 7–10 hours after morning administration of medication, during after school office visits. Electrocardiogram, laboratories, and ATMX levels were collected at the end of each study period (4 weeks of ATMX monotherapy; 3 weeks of combined ATMX and OROS MPH, or at last study visit for premature discontinuation). Serum for ATMX level was collected on site and sent via Massachusetts General Hospital laboratory to MEDTOX Diagnostics, Inc., for analysis by liquid chromatography/tandem mass spectrometry, with a reporting limit of 10 ng/mL. Efforts were made to be consistent across all subjects in the collection of ATMX levels, approximately 7–10 hours after morning administration (during after school study appointments). For those children who were taking ATMX at twice-daily dosing at study end point, serum levels were obtained prior to the second dose of ATMX (typically administered in the evening time).
Statistical analysis
As described (Wilens et al., 2009), consistent with our main aim to examine adjunct OROS MPH in ATMX partial responders, we included in our analyses only those subjects who were entered into phase 2 (n = 50). In these 50 subjects, all analyses were intent to treat (ITT) (at least one dose of OROS MPH) with last observation carried forward (LOCF). Differences between continuous variables at the start and end of each phase were analyzed using paired t-tests. The McNemar test was used to assess differences among binary variables at the start and end of each phase. An α level of 0.05 was used to assert statistical significance; all statistical tests were two-tailed. Data are presented as mean ± standard deviation (SD), unless otherwise stated. We calculated all statistics using STATA 10.0.
Results
In all, 82 subjects were exposed to ATMX and 50 were exposed to the combination of ATMX and OROS-MPH. Of the 50 subjects enrolled into phase 2, 40 started at study baseline, whereas 10 were already on ATMX and entered directly into the beginning of phase 2. Demographic features of the sample are described by Wilens et al. (this issue). Briefly, subjects were predominately male subjects with the combined subtype of ADHD. Nearly half of subjects (44%) had previous medication exposure. Oppositional defiant disorder was the most common lifetime co-morbidity, reported in 37% of the sample.
Of the 50 subjects who entered into phase 2, 41 subjects completed the protocol (8 subjects discontinued due to AEs and 1 subject dropped out because of a physical illness unrelated to medication). There were no differences between included and dropped subjects in phase 2 on any baseline demographic variables, including co-morbidity. The mean weight-corrected dose of ATMX remained 1.1 mg/kg per day throughout phase 2 of the study. The mean weight-corrected dose of OROS MPH at study end point was 1.0 mg/kg per day. Wilens et al. (2009) described a 40% reduction in ADHD rating scale score during the period of combination treatment and improvements in executive functioning.
Adverse effects
There were no serious AEs (e.g., death, life-threatening event, event requiring inpatient hospitalization) during this study. There were no subjective reports of suicidality or suicide attempts during the study. Eight subjects (8/50) discontinued early from the combined trial (phase 2) due to AEs. The majority of subjects (6 of 8) who discontinued did so following 1 week of combined ATMX + OROS MPH (18 mg) due to a combination of AEs including insomnia, gastrointestinal (GI) upset or appetite loss, and/or changes in mood.
AEs for the sample that began phase 2 (n = 50) are presented in Table 1; ATMX monotherapy was most commonly associated with mild-to-moderate GI AEs, fatigue, and headache. Compared to ATMX alone, adjunct OROS MPH was associated with significantly greater rates of mild to moderate insomnia, irritability, loss of appetite, and lower rates of fatigue (Table 1). Although no difference in weight occurred during ATMX monotherapy, weight significantly declined (mean 1.8 pounds) during combined treatment (p < 0.005). We failed to find a significant relationship between AEs that increased significantly during adjunct OROS MPH (loss of appetite, insomnia, or irritability) and a subject's baseline age or weight, or ATMX level during the study.
Table 1.
Adverse Effects for Atomoxetine Monotherapy (Phase 1 and with Adjunct OROS-Methylphenidate (Phase 2)
| Adverse effect | Start of phase 2 n (%) | End of phase 2 n (%) | OR | 95% CI | χ2 | p value |
|---|---|---|---|---|---|---|
| Fatigue | 17 (34) | 5 (10) | 0 | 0–0.36 | 12.00 | p < 0.0005 |
| Gastrointestinal | 18 (36) | 20 (40) | 1.25 | 0.44–3.64 | 0.22 | p = 0.64 |
| Headache | 12 (24) | 11 (22) | 0.86 | 0.24–2.98 | 0.08 | p = 0.78 |
| Insomnia | 7 (14) | 26 (52) | 7.33 | 2.20–38.27 | 14.44 | p < 0.0001 |
| Irritable | 8 (16) | 16 (32) | 5.0 | 1.10–46.93 | 5.33 | p = 0.02 |
| Loss of appetite | 7 (14) | 22 (44) | 6.0 | 1.75–31.80 | 10.71 | p < 0.001 |
| Rhinitis | 10 (20) | 11 (22) | 1.17 | 0.34–4.20 | 0.08 | p = 0.78 |
| Othera | 7 (14) | 15 (30) | 3.67 | 0.97–20.47 | 4.57 | p = 0.03 |
Includes enuresis (3), talking fast/on edge (2), nosebleed (1), itchy eyes/dilated pupils (2), anxious (1), mouth pain (1), irregular mood/decreased personality (2), dry mouth (2), pale (1), arm pain (1), and urinary (1).
Abbreviations: OR = odds ratio; CI = confidence interval.
Within the “Other” group of AEs (Table 1), noteworthy reports during combined ATMX + OROS MPH treatment included “talking fast”/“on edge” in 2 children, and “irregular mood”/“decreased personality” in 2 children. Of this group, 1 child with coincident irritability discontinued after the first week of combined treatment.
On the basis of the qualitative inspection of the data, the pattern and frequency of AEs were similar among the 40 subjects who started at study baseline and the 10 who were already on a stable dose of ATMX and entered directly into the beginning of phase 2. For these 10 subjects, most common AEs during phase 2 were insomnia (40%), loss of appetite (40%), GI AEs (40%), irritability (20%), and headache (20%).
Cardiovascular indices
Cardiovascular indices for the sample that began phase 2 (n = 50) are presented in Table 2. There were no serious cardiovascular AEs (e.g., sudden cardiac death, myocardial infarction, stroke) reported during ATMX monotherapy or during combination therapy, and no subject discontinued due to cardiovascular AEs.
Table 2.
Cardiovascular Effects of Atomoxetine Monotherapy (Phase 1) and with Adjunct OROS-Methylphenidate (Phase 2)
| Vital signs | Baseline | Start of phase 2 | t (Baseline vs. start phase 2) | p value | End point | t (Start phase 2 vs. end point) | p value | t (Baseline vs. end point) | P value |
|---|---|---|---|---|---|---|---|---|---|
| SBP | 103.1 ± 9.1 | 104.5 ± 9.4 | −0.33 | 0.75 | 104.8 ± 10.6 | −0.13 | 0.90 | −0.71 | 0.48 |
| DBP | 62.3 ± 8.9 | 64.5 ± 9.2 | −2.18 | 0.04 | 67.3 ± 7.8 | −2.02 | 0.05 | −3.92 | 0.0003 |
| Heart rate | 84.7 ± 10.9 | 93.3 ± 12.7 | −4.50 | 0.0001 | 95.0 ± 14.2 | −0.70 | 0.49 | −4.31 | 0.0001 |
| ECG Indices | |||||||||
| PR | 133.9 ± 25.5 | 132.7 ± 19.7 | 0.43 | 0.67 | 129.3 ± 18 | 2.31 | 0.03 | 2.11 | 0.04 |
| QRS | 82.3 ± 9.8 | 83.8 ± 10.0 | −1.65 | 0.11 | 83.8 ± 9.1 | −0.13 | 0.90 | −2.56 | 0.01 |
| QT | 358.6 ± 23.0 | 348.8 ± 22.9 | 2.28 | 0.03 | 344.7 ± 26 | 1.57 | 0.12 | 4.34 | 0.0001 |
| QTc | 412.6 ± 16.4 | 416.6 ± 17.1 | −1.85 | 0.07 | 418.6 ± 14.8 | −0.87 | 0.39 | −2.64 | 0.01 |
Abbreviations: SBP = systolic blood pressure; DBP = diastolic blood pressure; ECG = electro cardiogram.
There was no significant change in SBP observed over the course of the study. Specifically, SBP did not significantly increase during adjunctive OROS MPH. Conversely, ATMX monotherapy was associated with a significant increase in DBP (+2.2 mmHg; p =0.04), with an additional significant increase (+2.8 mmHg) during adjunctive OROS MPH (p = 0.05). In total, DBP significantly increased by 5 mmHg over the course of the study. ATMX monotherapy was also associated with significant increases in heart rate (+8.6 bpm; p = 0.0001). Heart rate did not increase significantly with the addition of OROS MPH.
Changes in ECG parameters during the study are reported in Table 2. At study end point, small reductions in the PR interval (4.8 ± 2.3 msec) and increases in QRS (1.7 ± 0.7 msec) and QTc (5.8 ± 2.2 msec) from baseline reached statistical significance (p < 0.04).
Outlier analysis
There were no outliers with SBP >140 mmHg or DBP >90 mmHg during the study. One 6-year-old subject had a heart rate >120 beats per minute (bpm) during combined treatment (121 bpm). No subjects had QRS >120 msec or PR >200 msec during combined treatment. One child had QTc intervals >460 msec in two separate ECGs during combined treatment (460 msec, 463 msec); these ECGs were found not to be clinically significant per review of an independent cardiologist. Baseline QTc for this child was 457 msec.
Laboratories/pharmacokinetics
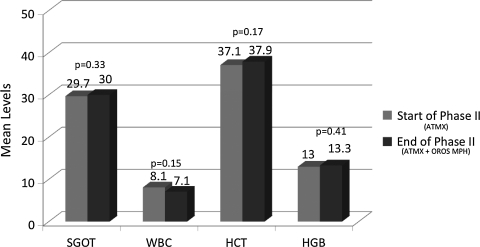
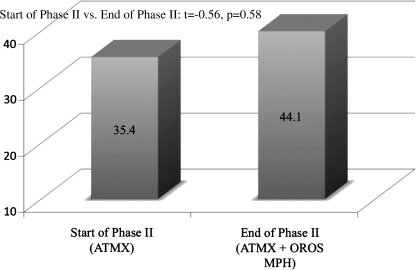
As can be seen in Fig. 1, measures of liver functioning (serum glutamic oxaloacetic transaminase [SGOT]) and hematologies (white blood cells [WBC], hematocrit [HCT], hemoglobin [HGB]) were not significantly changed during combination treatment. We then examined the impact of adjunctive OROS MPH on ATMX serum levels. ATMX levels did not significantly change during the combination treatment compared to ATMX alone (Fig. 2). For those children who took ATMX at twice-daily dosing (n = 20), serum levels were obtained prior to the second dose of ATMX (typically administered in the evening time).
FIG. 1.
Mean levels of laboratories for ATMX monotherapy and with adjunct OROS MPH. ATMX = Atomoxetine; MPH = methylphenidate.
FIG. 2.
ATMX Serum Levels for ATMX monotherapy and with adjunct OROS MPH. ATMX = Atomoxetine; MPH = methylphenidate.
Discussion
This analysis of adjunct OROS MPH in ATMX partial responders finds improvements in ADHD symptomatology and executive functioning (Wilens et al., 2009), and an additive adverse effect burden during short-term treatment. Although there were no serious general or cardiovascular AEs in this study, adjunct OROS MPH was associated with statistically and clinically significant increased rates of appetite suppression, insomnia, and irritability as compared to ATMX monotherapy. A small statistically significant increase in DBP was observed during adjunct OROS MPH without clinically meaningful change in ECG parameters. As hypothesized, there was no impact of adjunct OROS MPH on ATMX serum levels or laboratories.
As hypothesized, the combination of OROS MPH plus ATMX was associated with greater rates of AEs than ATMX monotherapy; 16% of subjects discontinued early from the combined trial due to AEs. The majority of subjects who discontinued early did so following the first week of combined ATMX + OROS MPH. Clinical recommendations, based on this data, are to be watchful for AEs in the first week of combined treatment, because we did not find other variables such as a subject's baseline age or weight, or ATMX level to be correlated with increased AEs with adjunctive OROS MPH.
The frequency of appetite suppression (44%) and insomnia (52%) during combination treatment in this study is approximately twice the rates of these common AEs typically reported in short-term studies of OROS MPH monotherapy (Wolraich et al. 2001; Stein et al. 2003; Wilens et al. 2006b; Newcorn et al. 2008). Despite the fact that ATMX treatment in children has been associated with sedation as opposed to insomnia in previous placebo-controlled studies, initial treatment with ATMX did not appear to protect against the possibility of stimulant-induced insomnia.
In this study, irritability also occurred at a significantly higher rate during combination treatment (32%), as compared to ATMX alone (16%). The rate of irritability exceeds that typically observed with either ATMX or OROS MPH alone (Swanson et al. 2004; Wilens et al. 2006; Newcorn et al. 2008). The one prior study of combined ATMX and OROS MPH found no occurrences of irritability; however, only 9 children were exposed to combination treatment (Carlson et al. 2007).
This elevated rate of irritability may reflect in part the young mean age of our sample. A meta-analysis of short-term controlled trials of ATMX found significantly greater rates of irritability in children treated with ATMX as compared to placebo (7.7% vs. 4.1%; p = 0.042), whereas rates of irritability did not statistically separate from placebo in adolescents (4.7% vs. 4.3%; p = 0.476) (Wilens et al. 2006a). Moreover, the short-term nature of the study limits conclusions, because mood AEs may have lessened over time for those who remain in treatment (Wehmeier et al. 2008). In our study, 8 out of 50 subjects discontinued early from the trial due to AEs; irritability was reported in half of these subjects.
Fatigue was the sole adverse effect to decline significantly during adjunct OROS MPH, as compared to ATMX monotherapy. However, the rate of fatigue during combination treatment was consistent with, not lower than, prior reports of fatigue in short-term ATMX monotherapy (Wilens et al. 2006a).
Cardiovascular changes observed during combination therapy were consistent with increases in blood pressure documented in children with ADHD treated with stimulants (Findling et al. 2005; Wilens et al. 2005; Donner et al. 2007) and ATMX (Michelson et al. 2001; Wernicke et al. 2003). In addition, there were no serious cardiovascular AEs during combination therapy, and no subject discontinued due to cardiovascular AEs. However, given the small numbers in this study, we will briefly discuss the effects observed on blood pressure and heart rate, because it would be premature to make clinical recommendations based on these data.
In our study, 2–3 mmHg increases in DBP were additive during ATMX monotherapy and subsequent combined treatment, thus yielding an increase in DBP of 5 mmHg over the course of the study. Conversely, there was no significant change in SBP. In a prior small and controlled study, Carlson et al. (2007) reported a 2.1 mmHg mean increase in SBP and a 3 mmHg mean increase in DBP associated with OROS MPH in combination with ATMX.
Of interest, differential effects on SBP and DBP have been observed during OROS MPH treatment in youths. In a very recent examination of cardiovascular effects of relatively high-dose OROS MPH monotherapy in a large sample of adolescents with ADHD followed to 6 months, DBP was significantly increased in the short term; however, it returned toward baseline between the 6-week and 6-month end points (Hammerness et al. 2008). Conversely, SBP did not significantly increase in the short-term end point, but was significantly increased at the 6-month longer-term end point. Analysis of cardiovascular effects of ATMX suggests that short-term blood pressure increases may stabilize over time (Wernicke et al. 2003).
The significant change in HR (+10.3 bpm) during the study was largely accounted for by the 8.6-bpm increase in HR during ATMX monotherapy, consistent with prior reports of HR increase during treatment with ATMX (Wernicke et al. 2003). In their one small prior study of combined ATMX and OROS MPH, Carlson et al. (2007) reported a 5-bpm increase in HR.
There were no discontinuations due to cardiovascular AEs in our study compared to the 1 patient who discontinued in the combined ATMX/MPH group with supraventricular extrasystoles in the Carlson et al. trial (Carlson et al. 2007). Current advisories from the American Academy of Pediatrics (AAP), American Academy of Child and Adolescent Psychiatry (AACAP), and American Heart Association (AHA) suggest the need for routine monitoring of cardiovascular-based vital signs (SBP, DBP, HR), but not ECG, during ADHD monotherapy. However, it is not clear from these preliminary findings whether combined ATMX + stimulant will yield greater impact on cardiovascular parameters than either medication alone.
Consistent with safety data on ATMX, there was no evidence of hepatoxicity in this study. A recent summary of clinical trial data from nearly 8000 pediatric and adult patients treated with ATMX found that increases in liver function tests are rare events (Bangs et al. 2008). Albeit limited by a small sample size of short duration, adjunct MPH did not alter liver function testing. These data support the current recommendations to rely on clinical monitoring of symptoms indicative of liver dysfunction and not liver function testing in youth receiving ATMX.
We also failed to find pharmacokinetically relevant drug interaction with MPH in this study; although the mean absolute ATMX level was increased, this was not statistically significant. Changes in ATMX level during the study may reflect the lack of control of timing of ascertainment in a small sample. ATMX levels were not significantly altered with adjunct OROS MPH. The lack of a pharmacokinetic drug interaction between ATMX and MPH is not surprising. MPH has a substantial first-pass effect with a major pathway of metabolism through plasma based de-esterification to the inactive ritalinic acid (Patrick et al. 1987; Markowitz and Patrick 2001; Markowitz et al. 2003). De-esterification is rapid and is unaffected by hepatic induction or inhibition (DeVane et al. 2000). Thus, MPH would not be expected to have pharmacokinetic drug interactions with hepatically metabolized medications such as ATMX (Markowitz et al. 1999; DeVane et al. 2000; Markowitz and Patrick 2001), consistent with our current findings.
Study Limitations
There are a number of methodological limitations to our study. The open nature of this study did not allow for a placebo comparator. This design did not allow for a maintenance phase on stable combined therapy. Thus, AEs from the initiation of ATMX may have carried over into the combined phase, and, moreover, the adverse effect profile may have been influenced by OROS MPH titration. Treatment-related AEs with ATMX have been shown to taper rapidly following 3 months of treatment (Wilens et al. 2006c), and relatively lower rates of common stimulant AEs (insomnia and anorexia) have also been observed in youth during maintenance with OROS MPH (McGough et al. 2006). It would be particularly useful to follow combination treatment into a maintenance phase to determine if a similar pattern of reduced AEs over time occurs during combination therapy. Our inclusion of subjects directly into phase 2 who were already on ATMX for clinical reasons may have confounded these results. However, visual inspection of the data did not show meaningful differences in the AE profile between these subjects.
In addition, we excluded youths with any significant medical and/or cardiovascular history and youths with current psychiatric co-morbidity, thus restricting the generalization of the findings to more co-morbid, clinically relevant populations. We used a general query for AEs of study medication, rather than an AE rating scale, and we did not use an independent rater for AEs and efficacy. The timing of blood pressure measurement in this study might not have been at the peak of medication levels and may have varied throughout the study, although the majority of visits occurred 7–10 hours after morning administration of medication. Blood pressure and HR were measured by a single determination obtained through an automated oscillometric device. Although automated blood pressure devices reduce observer bias, they tend to report blood pressure values 4–12 mmHg higher than blood pressure obtained by the auscultatory method (Park et al. 2001; Park et al. 2005).
Conclusions
Despite these limitations, combining the effectiveness (Wilens et al., 2009) and the current systematic examination of tolerability, it appears that the combination of ATMX and OROS MPH is a reasonable clinical approach meriting further controlled study. As described elsewhere (Wilens et al., 2009), a 40% reduction in ADHD rating scale score occurred during the period of combination treatment, with improvements in executive functioning. On the basis of the systematic review of tolerability data in this small open study, clinicians should monitor for possible additive AEs and continue to monitor cardiovascular vital signs as per AHA guidelines. Further controlled research is warranted to determine the efficacy and AE profile of combined ATMX and stimulant class medications in both short- and longer-term trials.
Footnotes
Funding for this study was made through an investigator-initiated grant (T. Wilens) from Ortho-McNeil Pediatrics.
Disclosures
Dr. Hammerness has been a speaker for, received research funds, or participated in continuing medical education (CME) activities/professional talks supported by the following pharmaceutical companies: Abbott, Eli Lilly & Company, Forest Research Institute, Ortho-McNeil-Janssen, and Shire. Dr. Hammerness has also received research funds from Elminda Ltd. and has also received honoraria from Reed Medical Education (a company working as a logistics collaborator for the MGH Psychiatry Academy). The education programs conducted by the MGH Psychiatry Academy are supported through Independent Medical Education (IME) grants from pharmaceutical companies co-supporting programs along with participant tuition. Commercial entities supporting the MGH Psychiatry Academy are listed on the Academy's website, www.mghcme.org/ten. Dr. Hammerness has also participated, as an investigator, in research studies in the past 2 years funded by the following pharmaceutical companies: Bristol Myers Squibb, Cephalon, Eli Lilly, GlaxoSmithKline, Johnson&Johnson, McNeil, New River, Novartis, Organon, Otsuka, Pfizer, Shire, and Takeda. Dr. Georgiopoulos received in 2005 an unrestricted educational grant for a book project from GlaxoSmithKline. She also anticipates payment for a CME presentation for Pri-Med 10/18/08 supported by an educational grant from McNeil Pediatrics Division-PPC, Inc., administered by Ortho-McNeil Janssen Scientific Affairs, LLC. Dr. Doyle is on the Speakers Bureau for Shire, McNeil, Janssen, and Novartis and does CME with PriMed, the Neuroeducational Institute, and the Danemiller Foundation. He is on the Advisory Boards of Shire and Novartis. Ms. Utzinger, Ms.Schillinger, Ms. Martelon, and Ms. Brodziak have no financial ties or conflicts of interst nothing to disclose. Dr. Joseph Biederman is currently receiving research support from the following sources: Alza, AstraZeneca, Bristol Myers Squibb, Eli Lilly and Co., Janssen Pharmaceuticals Inc., McNeil, Merck, Organon, Otsuka, Shire, National Institute of Mental Health (NIMH), and National Institute of Child Health and Human Development (NICHD). Dr. Biederman is currently a consultant/advisory board member for the following pharmaceutical companies: Janssen, McNeil, Novartis, and Shire. He is currently a speaker for the following speaker's bureaus: Janssen, McNeil, Novartis, Shire, and UCB Pharma, Inc. In previous years, Dr. Biederman received research support, consultation fees, or speaker's fees for/from the following additional sources: Abbott, AstraZeneca, Celltech, Cephalon, Eli Lilly and Co., Esai, Forest, Glaxo, Gliatech, NARSAD, NIDA, New River, Novartis, Noven, Neurosearch, Pfizer, Pharmacia, The Prechter Foundation, The Stanley Foundation, and Wyeth. Dr. Timothy Wilens receives grant support from the following sources: Abbott, McNeil, Lilly, National Institutes of Health (NIH) (NIDA), Merck, and Shire. He is a speaker for the following speaker's bureaus: Lilly, McNeil, Novartis, and Shire. Dr. Wilens is a consultant for: Abbott, McNeil, Lilly, NIH (NIDA), Novartis, Merck, and Shire.
References
- Bangs ME. Jin L. Zhang S. Desaiah D. Allen AJ. Read HA. Regev A. Wernicke JF. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31:345–354. doi: 10.2165/00002018-200831040-00008. [DOI] [PubMed] [Google Scholar]
- Brown TE. Atomoxetine and stimulants in combination for treatment of attention deficit hyperactivity disorder: Four case reports. J Child Adolesc Psychopharmacol. 2004;14:129–136. doi: 10.1089/104454604773840571. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Dunn D. Kelsey D. Ruff D. Ball S. Ahrbecker L. Allen AJ. A pilot study for augmenting atomoxetine with methylphenidate: safety of concomitant therapy in children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2007;1:10. doi: 10.1186/1753-2000-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVane CL. Markowitz JS. Carson SW. Boulton DW. Gill HS. Nahas Z. Risch SC. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20:347–349. doi: 10.1097/00004714-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Donner R. Michaels MA. Ambrosini PJ. Cardiovascular effects of mixed amphetamine salts extended release in the treatment of school-aged children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:706–712. doi: 10.1016/j.biopsych.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Findling RL. Biederman J. Wilens T. Spencer T. McGough J. Lopez FA. Tulloch S. Short and Long-term cardiovascular effects of mixed amphetamine salts extended release (Adderall XR) in Children. J Pediatr. 2005;47:348–354. doi: 10.1016/j.jpeds.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Gutgesell H. Atkins D. Barst R. Buck M. Franklin W. Humes R. Ringel R. Shaddy R. Taubert KA. AHA scientific statement: Cardiovascular monitoring of children and adolescents receiving psychotropic drugs. J Am Acad Child Adolesc Psychiatry. 1999;38:979–982. doi: 10.1097/00004583-199908000-00022. [DOI] [PubMed] [Google Scholar]
- Hammerness P. Wilens TE. Mick E. Spencer T. Doyle R. McCreary M. Levine M. Becker J. Biederman J. Cardiovascular effects of high doses of OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr. 2009;155:84–89. doi: 10.1016/j.jpeds.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Markowitz JS. Patrick KS. Pharmacokinetic and pharmacodynamic drug ineractions in the treatment of ADHD. Clin Pharmacokinet. 2001;40:753–772. doi: 10.2165/00003088-200140100-00004. [DOI] [PubMed] [Google Scholar]
- Markowitz JS. Morrison SD. DeVane CL. Drug interactions with psychostimulants. Int Clin Psychopharmacol. 1999;14:1–18. doi: 10.1097/00004850-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Markowitz JS. Straughn AB. Patrick KS. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: Focus on methylphenidate formulations. Pharmacotherapy. 2003;23:1281–1299. doi: 10.1592/phco.23.12.1281.32697. [DOI] [PubMed] [Google Scholar]
- McGough JJ. McBurnett K. Bukstein O. Wilens TE. Greenhill L. Lerner M. Stein M. Once-daily OROS® methylphenidate is safe and well tolerated in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2006;16:351–356. doi: 10.1089/cap.2006.16.351. [DOI] [PubMed] [Google Scholar]
- Michelson D. Faries D. Wernicke J. Kelsey D. Kendrick K. Sallee FR. Spencer T. Atomoxetine in the treatment of children, adolescents with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled, dose-response study. Pediatrics. 2001;108:E83. doi: 10.1542/peds.108.5.e83. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group: A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD [see comments] Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Newcorn JH. Kratochvil CJ. Allen AJ. Casat CD. Ruff DD. Moore RJ. Michelson D. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: Acute comparison and differential response. Am J Psychiatry. 2008;165:721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- Park MK. Menard SW. Yuan C. Comparison of auscultatory and oscillometric blood pressures. Arch Pediatr Adolesc Med. 2001;155:50–53. doi: 10.1001/archpedi.155.1.50. [DOI] [PubMed] [Google Scholar]
- Park MK. Menard SW. Schoolfield J. Oscillometric blood pressure standards for children. Pediatr Cardiol. 2005;26:601–607. doi: 10.1007/s00246-004-0828-9. [DOI] [PubMed] [Google Scholar]
- Patrick KS. Caldwell RW. Ferris RM. Breese GR. Pharmacology of the enantiomers of threo-methylphenidate. J Pharmocol Exp Therapeut. 1987;241:152–158. [PubMed] [Google Scholar]
- Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Safer DJ. Zito JM. DosReis S. Concomitant psychotropic medication for youths. Am J Psychiatry. 2003;160:438–449. doi: 10.1176/appi.ajp.160.3.438. [DOI] [PubMed] [Google Scholar]
- Stein MA. Sarampote CS. Waldman ID. Robb AS. Conlon C. Pearl PL. Black DO. Seymour KE. Newcorn JH. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics. 2003;112:e404. doi: 10.1542/peds.112.5.e404. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Wigal S. Wigal T. Sonuga-Barke E. Greenhill L. Biederman J. Kollins S. Nguyen AS. DeCory HH. Dirksen SJ. Hatch SJ. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (The Comacs Study) Pediatrics. 2004;113:E206–E216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- Wehmeier PM. Schacht A. Lehmann M. Dittmann RW. Silva SG. March JS. Emotional well-being in children, adolescents treated with atomoxetine for attention-deficit/hyperactivity disorder: Findings from a patient, parent, physician perspective using items from the pediatric adverse event rating scale (PAERS) Child Adolesc Psychiatry Ment Health. 2008;2:11. doi: 10.1186/1753-2000-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke JF. Faries D. Girod D. Brown J. Gao H. Kelsey D. Quintana H. Lipetz R. Michelson D. Heiligenstein J. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003;26:729–740. doi: 10.2165/00002018-200326100-00006. [DOI] [PubMed] [Google Scholar]
- Wilens T. Mechanism of action of agents used in ADHD. J Clin Psychiatry. 2006;67:32–37. [PubMed] [Google Scholar]
- Wilens TE. Spencer TJ. Biederman J. Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in adolescents with ADHD. CNS Spectrums. 2005;10(Suppl 15):22–30. doi: 10.1017/s109285290000242x. [DOI] [PubMed] [Google Scholar]
- Wilens T. Kratochvil C. Newcorn J. Gao H. Do children and adolescents with ADHD respond differently to atomoxetine? J Am Acad Child Adolesc Psychiatry. 2006a;45:149–157. doi: 10.1097/01.chi.0000190352.90946.0b. [DOI] [PubMed] [Google Scholar]
- Wilens T. McBurnett K. Bukstein O. McGough J. Greenhill L. Lerner M. Stein MA. Conners CK. Duby J. Newcorn J. Bailey CE. Kratochvil CJ. Coury D. Casat C. Denisco MJ. Halstead P. Bloom L. Zimmerman BA. Gu J. Cooper K. Lynch JM. Multisite, controlled trial of OROS® methylphenidate (CONCERTA®) in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2006b;160:82–90. doi: 10.1001/archpedi.160.1.82. [DOI] [PubMed] [Google Scholar]
- Wilens T. Newcorn J. Kratochvil CJ. Gao H. Thomason CK. Rogers AK. Feldman PD. Levine LR. Longer-term atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Pediatr. 2006c;149:112–119. doi: 10.1016/j.jpeds.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Hammerness P. Utzinger L. Schillinger M. Georgiopoulous A. Doyle R. Martelon M. Brodziak K. An Open Study of Adjunct OROS-Methylphenidate in Children, Adolescents who are Atomoxetine Partial Responders: I. Effectiveness. J Child Adolesc Psychopharmacol. 2009;19:485–492. doi: 10.1089/cap.2008.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich ML. Greenhill LL. Pelham W. Swanson J. Wilens T. Palumbo D. Atkins M. McBurnett K. Bukstein O. August G. Randomized, controlled trial of oros methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:883–892. doi: 10.1542/peds.108.4.883. [DOI] [PubMed] [Google Scholar]