Abstract
We evaluated neuropathological findings in two studies of AAV2-GDNF efficacy and safety in naive aged (>20 years) or MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-lesioned rhesus macaques. In the first study, a total of 17 animals received one of two doses of AAV2-GDNF into either putamen or substantia nigra (SN). To control for surgical variables, all animals received identical putaminal and nigral infusions in which phosphate-buffered saline was substituted for vector as appropriate. All 17 aged monkeys were studied for 6 months before necropsy. In a separate study, 11 MPTP-lesioned rhesus macaques with extensive lesions in the right SN and mild lesions in the left SN received bilateral infusions of AAV2-GDNF (9.9 × 1011 vector genomes) or PBS into the putamen and were then studied for up to 14 months. In the current analysis, we addressed safety issues regarding AAV2-GDNF administration. An extensive series of assessments of in-life behavioral and clinical parameters was conducted. No overt histopathology or immune responses were detected in any experimental monkey. However, the delivery of AAV2-GDNF to the SN of aged monkeys caused a marked and significant loss of body weight (−19.4%). No weight loss was observed in the MPTP-lesioned monkeys despite bilateral axonal transport of glial cell line-derived neurotrophic factor (GDNF) to the SN from the putamen. These findings indicate that putaminal administration of AAV2-GDNF by convection-enhanced delivery shows therapeutic promise without any apparent side effects. Importantly, nigral administration of AAV2-GDNF caused significant weight loss that raises substantial concern for clinical application of this approach.
Introduction
Glial cell line-derived neurotrophic factor (GDNF) plays an important role in the postnatal survival of mesencephalic dopamine neurons (Granholm et al., 2000; Pascual et al., 2008). A variety of experiments in rodent and nonhuman primate (NHP) models have shown that direct infusion of recombinant GDNF protein or virus-mediated GDNF gene elevation in the substantia nigra or striatum results in the protection of dopaminergic neurons in neurotoxicant models (Kearns and Gash, 1995; Tomac et al., 1995; Kordower et al., 2000; Maswood et al., 2002; Grondin et al., 2003). Despite these promising data, however, clinical studies of putaminal infusion of recombinant GDNF for the treatment of Parkinson's disease (PD) have consistently failed to demonstrate clinical benefit (Nutt et al., 2003; Lang et al., 2006). One possible explanation for discordant results within patient cohorts and across clinical trials may have been poor or inappropriate distribution of the infused growth factor within the target region. Nevertheless, enthusiasm persists for the development of GDNF-based therapies for PD because of its obvious therapeutic potential in animal models and limited numbers of patients. However, studies with recombinant GDNF in NHP found cerebellar lesions characterized by Purkinje cell loss, molecular layer atrophy, and granule cell loss (Hovland et al., 2007). In addition, several patients with PD in a randomized controlled trial of putaminal GDNF infusion developed neutralizing anti-GDNF antibodies (Lang et al., 2006). Thus, a thorough experimental evaluation of safety associated with the GDNF delivery treatment protocol, anatomical targeting, and therapeutic dose is warranted.
Our laboratory has studied infusion of AAV2-GDNF into the nigrostriatal pathway via convection-enhanced delivery (CED) in aged and parkinsonian rhesus macaques (Eberling et al., 2009; Johnston et al., 2009). In the present study, we addressed safety issues arising from AAV2-GDNF administration in these prior studies. An extensive series of assessments was conducted of in-life behavioral and clinical parameters, serum and cerebrospinal fluid (CSF) levels of GDNF protein, GDNF antibody and AAV2 neutralizing antibody, organ histopathology, and peripheral and CNS immune/inflammatory reaction to AAV2 and/or GDNF protein. No evidence of any adverse neuropathology was found, with the notable exception of a marked (grade 2) loss in body weight in aged animals that received nigral, but not putaminal, AAV2-GDNF. In the same subjects, GDNF levels were elevated in frontal cortical regions (Johnston et al., 2009), suggesting that increased dopamine turnover in the mesolimbic system occurred. These data provide insight into the safety and tolerability of AAV2-GDNF delivery, and dose and transgene expression; in addition, they call attention to potential safety-related aspects of AAV2-GDNF treatment related to the anatomical locus of delivery.
Materials and Methods
Animals and surgery
Seventeen aged rhesus monkeys (20–25 years of age) were infused only with phosphate-buffered saline (PBS, formulation buffer) (PBS control, n = 3), or with a high dose of AAV2-GDNF to the right putamen (1.65 × 1012 vector genomes [VG], HD Put, n = 5) or the right substantia nigra (5.5 × 1011 VG, HD SN, n = 3), or with a lower dose of AAV2-GDNF (1.65 × 1011 VG) to the right putamen (LD Put, n = 3). All aged monkeys received a total of six infusions: two infusion sites per putamen (75 μl/site) and one infusion per substantia nigra (50 μl) with equivalent volumes of PBS infused into sites that did not receive AAV2-GDNF (Table 1). All animals were killed after 6 months and were subjected to a battery of in vivo and postmortem analyses summarized in Table 2.
Table 1.
Experimental Design
| |
Putamen |
Nigra |
||
|---|---|---|---|---|
| Group | L | R | L | R |
| Aged NHP | ||||
| HD Put | PBS | GDNF | PBS | PBS |
| LD Put | PBS | GDNF | PBS | PBS |
| HD SN | PBS | PBS | PBS | GDNF |
| PBS | PBS | PBS | PBS | PBS |
| Parkinsonian NHP | ||||
| GDNF, 1 month | GDNF | GDNF | — | — |
| PBS, 1 month | PBS | PBS | — | — |
| GDNF, 6 months | GDNF | GDNF | — | — |
| PBS, 6 months | PBS | PBS | — | — |
| GDNF, 14 months | GDNF | GDNF | — | — |
| PBS, 14 months | PBS | PBS | — | — |
Abbreviations: GDNF, glial cell line-derived neurotrophic factor; HD, high dose; L, left; LD, low dose; NHP, nonhuman primates; PBS, phosphate-buffered saline; R, right.
Table 2.
Summary of Analyses
| Tissue | Assay(s) |
|---|---|
| Body | Weight measurement |
| Serum | AAV2 neutralization antibody |
| Blood | White blood cell count |
| GDNF ELISA | |
| GDNF antibody ELISA | |
| CSF | GDNF ELISA |
| GDNF antibody ELISA | |
| Brain | |
| Striatum | IHC: TH, GDNF, CD68, Iba1, and GFAP; H&E |
| Substantia nigra | IHC: TH, GDNF, CD68, Iba1, and GFAP |
| Cerebellum | H&E |
Abbreviations: AAV2, adeno-associated virus type 2; CSF, cerebrospinal fluid; GDNF, glial cell line-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; H&E, hematoxylin and eosin staining; IHC, immunohistochemistry; TH, tyrosine hydroxyase.
Eleven young adult rhesus monkeys (7–10 years of age) were lesioned with one or two right intracarotid artery infusions of 2–4 mg of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-HCl, followed by additional intravenous administrations of 0.2- to 0.5-mg/kg doses of MPTP-HCl. Intravenous dosing with MPTP continued until the animal showed bilateral parkinsonian signs and a clinical rating scale (CRS) score between 21 and 26 as previously described (Eberling et al., 1998, 2009). After the MPTP-lesioned animals achieved a stable behavioral disability rating in more than 10 assessments over a 4-month period, they received bilateral administration (Table 1) of either AAV2-GDNF (9.9 × 1011 VG) or PBS into the putamen (75 μl/site, two sites per hemisphere). All animals were subjected to a battery of in vivo and postmortem analyses summarized in Table 2.
Food consumption, body weight, and behavioral observations
Behavioral observations and monitoring of food consumption (number of biscuits consumed) were conducted daily. Body weight was recorded every 2–4 weeks. CRS assessments were conducted throughout the in-life study by an observer blind to treatment groups as previously described (Eberling et al., 2009).
Necropsy
After sedation with ketamine, an intravenous overdose of sodium pentobarbital solution greater than 86 mg/kg was administered. Cardiac perfusion was performed with approximately 2 liters of PBS. The brain was harvested, sectioned into 3-mm slabs with a customized brain matrix; alternating slices were fixed by immersion in neutral 10% buffered formalin or freshly frozen in dry ice-cooled isopentane.
Histochemistry
Midbrain blocks containing the putamen or substantia nigra were postfixed in Zamboni's fixative and sectioned coronally (40 μm). Sets of serial sections 400 μm apart were stained with antibodies against GDNF, tyrosine hydroxylase (TH), glial fibrillary acidic protein (GFAP), CD68, and ionized calcium-binding adaptor molecule-1 (Iba1). All slides were evaluated by our laboratory as well as by two independent board-certified neuropathologists (E.J.H. and H.S.L.) blind to the treatment groups.
Hematoxylin and eosin staining
Free-floating sections were rehydrated and stained with hematoxylin (Surgipath Medical Industries, Richmond, IL) for 15 sec. Sections were washed with tap water and then differentiated in 0.5% glacial acetic acid–70% alcohol followed by staining in bluing solution. After incubation in eosin (Surgipath Medical Industries), sections were dehydrated in alcohol and xylene.
GDNF, TH, GFAP, CD68, and Iba1 staining
Sections were washed three times in PBS for 5 min each followed by treatment with 1% H2O2 (v/v) in PBS for 20 min at room temperature (∼22°C). Sections were incubated in Sniper blocking solution (Biocare Medical, Concord, CA) for 30 min at room temperature followed by incubation with primary antibodies (GDNF, diluted 1:500 [R&D Systems, Minneapolis, MN]; TH, diluted 1:600 [Chemicon, Billerica, MA]; CD68, diluted 1:1000 [Dako, Glostrup, Denmark]; Iba1, diluted 1:1000 [Biocare Medical]; GFAP, diluted 1:15,000 [Chemicon]) in Da Vinci diluent (Biocare Medical) overnight at room temperature. After three rinses in PBS for 5 min each at room temperature, sections were incubated in Mach 2 horseradish peroxidase (HRP) polymer (Biocare Medical) for 1 hr at room temperature, followed by several washes and colorimetric development (3,3′-diaminobenzidine [DAB]; Vector Laboratories, Burlingame, CA). Immunostained sections were mounted on slides and sealed with Cytoseal (Richard-Allan Scientific, Kalamazoo, MI).
Quantification of TH-positive profiles
For each monkey, TH-positive profiles in the striatum were counted in two sections, one containing the nucleus accumbens and the other containing the anterior commissure. The striatum was precisely outlined at low magnification ( × 2.5 objective) on a Zeiss Axioskop microscope (Carl Zeiss, Thornwood, NY) by the optical fractionator technique (Stereo Investigator 7 software; MBF, Williston, VT). The counting frame of the optical fractionator was defined in 100-μm squares and systematic sampling was performed with a sampling grid of 200-μm squares. The sample sites were automatically generated by the computer and examined with a × 20 objective. The counting frame displays inclusion and exclusion lines. Only whole TH-positive profiles falling within the inclusion area of the counting frame, and not in contact with the exclusion lines, were counted. In addition, a profile was enumerated in the counting frame only if it came into focus within the predetermined optical dissector (8 μm), with a 2-μm guard zone set to the top of the section. Section thickness was monitored with a Zeiss Axioskop microcator.
Microarray
Tissue punches (2 × 3 mm; 10–20 mg wet weight) were collected from putamen. Total RNA was isolated by a modified TRIzol extraction method, using an AutoGenprep 245T (Autogen, Holliston, MA) according to the manufacturer's instructions. RNA sample preparation, labeling, and array hybridizations were performed according to standard protocols from the University of California, San Francisco (UCSF) Shared Microarray Core Facilities (http://www.arrays.ucsf.edu) and Agilent Technologies (Santa Clara, CA; http://www.agilent.com). Total RNA quality was assessed with a Pico chip on a 2100 bioanalyzer (Agilent Technologies). RNA was amplified and labeled with Cy3–CTP by means of the Agilent low RNA input fluorescence linear amplification kit according to the protocol of the manufacturer (Agilent Technologies). Labeled cRNA was quantitated with a Nanodrop ND-100 (Nanodrop Technologies, Wilmington DE), and equal amounts of Cy3-labeled target were hybridized to whole-mouse genome 4 × 44K inkjet arrays (Agilent Technologies). Hybridizations were incubated for 14 hr, according to the manufacturer's protocol. Arrays were scanned with an Agilent microarray scanner and raw signal intensities were extracted with Feature Extraction version 9.1 software.
Neutralizing antibody assay
HEK-293A cells (Invitrogen, Carlsbad, CA) were plated at a density of 2 × 104 cells per well in 24-well multiwells and incubated overnight at 37°C in 5% CO2–95% air. The next day, serum samples, collected from nonhuman primates (either pre- or postsurgery), were incubated at 56°C for 45 min to inactivate complement. Samples were serially diluted in Dulbecco's modified Eagle's medium (DMEM)–10% fetal bovine serum (FBS). AAV2-eGFP standard was mixed with serum dilutions at a 1:1 (v/v) ratio. Mixtures contained 1 × 108 transducing units (TU) of AAV2-eGFP with each serum dilution. Virus and serum mixtures were prepared in triplicate, incubated at 37°C for 1 hr, and subsequently used to infect 293A cells. Cells were then incubated at 37°C for 48 hr. After incubation, growth medium was removed and cells were treated with 200 μl of trypsin–EDTA per well at 37°C for 5 min. Trypsin was neutralized by the addition of 300 μl of DMEM–10% FBS and the contents of each well were filtered through a cell strainer before flow cytometric analysis. Individual cells were analyzed by flow cytometry on a FACSCalibur instrument (BD Biosciences, San Jose, CA) and the fraction of enhanced green fluorescent protein (eGFP)-expressing cells compared with the total number of cells scanned was determined. To express the percentage of neutralized virus, virus/serum sample readings were subtracted from positive control sample (no serum) readings and expressed as the percentage of rAAV2-eGFP that had been neutralized.
Results
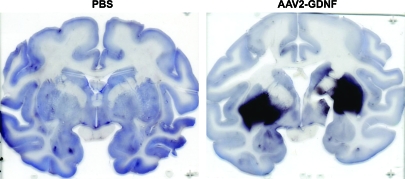
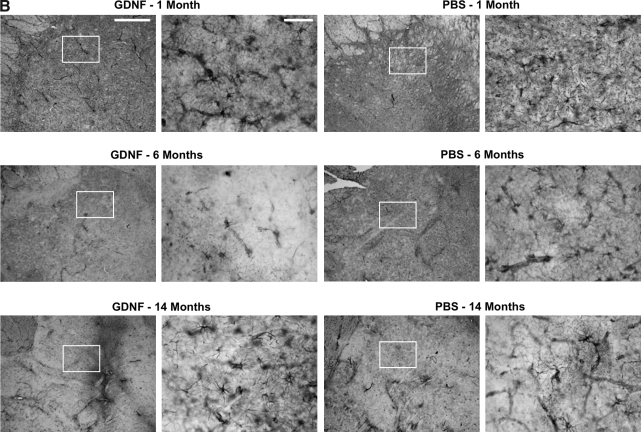
Seventeen aged naive rhesus macaques (20–25 years of age) were divided into 4 treatment groups as previously described (Johnston et al., 2009). The right putamen of these NHP received PBS, low-dose AAV2-GDNF (LD Put), or high-dose AAV2-GDNF (HD Put). Monkeys with PBS infusion in the putamen additionally received either PBS or high-dose AAV2-GDNF in the right substantia nigra (HD SN) (Table 1). These NHP were observed for 6 months before necropsy. In addition, 11 young adult MPTP-lesioned NHP (parkinsonian NHP, 7–10 years of age), with extensive nigral lesions in the right hemisphere and mild lesions in the left hemisphere (Eberling et al., 2009), received bilateral infusions of AAV2-GDNF or PBS into the putamen and then were observed for 1, 6, or 14 months (Table 1). CED facilitated a broad distribution of vector throughout the targeted region as indicated by GDNF immunohistochemistry in a typical NHP 1 month after putaminal AAV2-GDNF infusion (Fig. 1). Related issues regarding putaminal and nigral expression of GDNF are dealt with further in Fig. 8.
FIG. 1.
Distribution of glial cell line-derived neurotrophic factor (GDNF) after AAV2-GNDF administration. Coronal sections through the striatum illustrate positive labeling for GDNF within the putamen of a typical parkinsonian nonhuman primate (NHP) (right) and control NHP (left) 1 month after vector infusion. Similarly intense GDNF expression was also seen in aged NHP 6 months after vector infusion (see Fig. 8). Color images available online at www.liebertonline.com/hum.
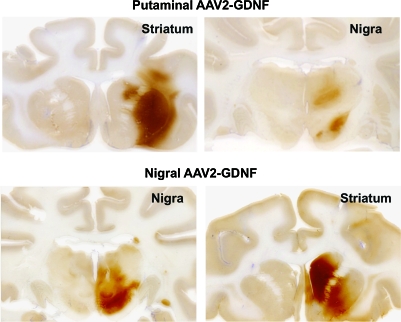
FIG. 8.
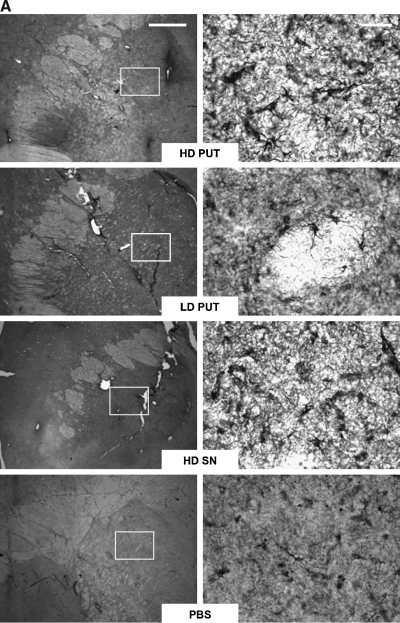
GDNF expression in the putamen and nigra of aged primates after either putaminal or nigral infusion of high-dose AAV2-GDNF. Top: GDNF staining of putamen (left) and the corresponding GDNF expression in the substantia nigra (right) in an animal that received putaminal AAV2-GDNF. Bottom: Similar staining of nigra and putamen in a representative animal in which AAV2-GDNF was infused into the substantia nigra. Note the prominent caudal staining after nigral delivery in contrast to the slight staining seen in the caudate after putaminal delivery. Color images available online at www.liebertonline.com/hum.
Peripheral immunity to AAV2
Preexisting humoral immunity to AAV2 vector or a humoral response resulting from AAV2-GDNF administration may reduce transgene expression (Sanftner et al., 2004) or cause inflammation (Peden et al., 2004, 2009). To quantify possible neutralizing antibodies directed against AAV2 capsid, serum was collected from all animals before and after surgery and antibody titers were measured. Ten monkeys had no detectable antibody titer, and no other animals had neutralizing titers greater than 1:1280, indicating good containment of vector within the CNS, consistent with results from previous studies (Cunningham et al., 2008; Herzog et al., 2009).
A high white blood cell (WBC) count can be an indicator of infection or inflammation. Blood was collected 2 months after AAV2-GDNF administration. The hematological results showed that the WBC count of all animals was within normal limits (5.4–8.9 × 103/μl). Furthermore, the WBC differential (percentage of neutrophils, lymphocytes, monocytes, eosinophils, and basophils) of the treatment groups was comparable to the control group, suggesting no apparent peripheral immune reaction attributable to AAV2 administration.
Humoral response to GDNF in CSF and blood
To evaluate possible elicitation of a humoral response to the transgene product, GDNF, protein, and antibodies against GDNF were quantified in samples of cerebrospinal fluid (CSF) and blood by ELISA. No GDNF or anti-GDNF antibody was detected in any of the treatment groups in either study at the time of necropsy (data not shown), indicating no evidence of a humoral response to GDNF.
Brain histopathology
Cellular inflammatory responses to AAV2-GDNF
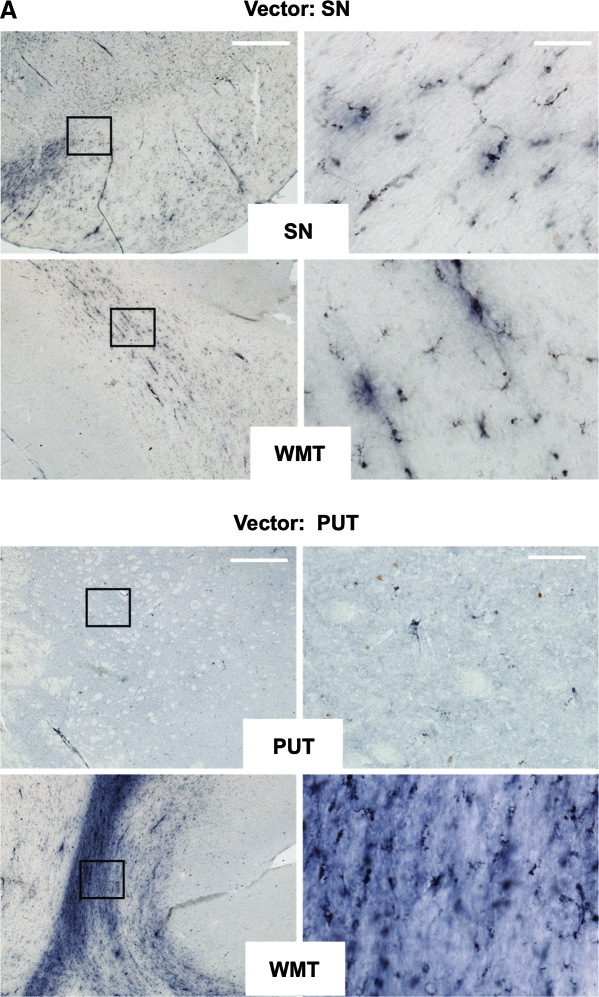
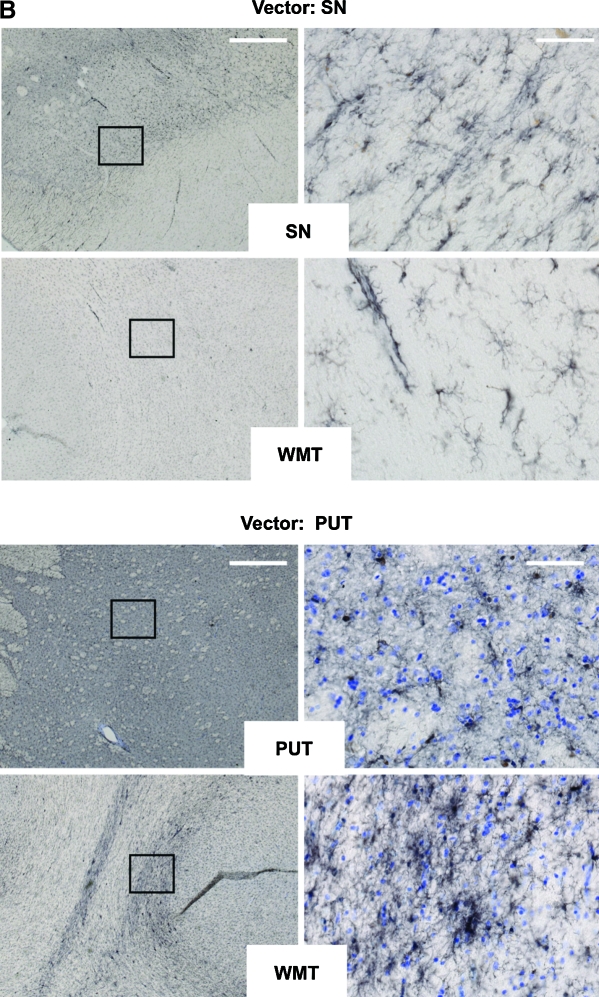
We evaluated NHP brain sections, particularly the substantia nigra (SN) and putamen (PUT), for signs of inflammation. Because microglia/macrophages play an important role in cellular inflammatory responses in the brain, the microglia/macrophage markers CD68 (microglia/macrophage phagocytic marker) and Iba1 (microglia/macrophage marker) were evaluated in nigral and striatal tissues. Figure 2A (top) shows that, in the monkeys with nigral infusion, few CD68+ cells were localized to the SN. Along the cannula track in the subcortical white matter (WMT), moderate CD68 immunoreactivity was observed in some monkeys in the treatment group (Fig. 2A) as well as in the PBS group (data not shown). Few CD68+ cells were present in the putamen of monkeys that received a striatal infusion (Fig. 2A, bottom), whereas moderate to severe increases in CD68+ cells were observed along the cannula track in the WMT. Quantification of CD68+ microglia/macrophage cells by optical densitometry in the nigral and striatal sections revealed no detectable difference in CD68 immunoreactivity between the AAV2-GDNF treatment groups and the control group (data not shown). Figure 2B illustrates representative Iba1 staining images of monkeys with either nigral (Fig. 2B, top) or striatal (Fig. 2B, bottom) delivery of AAV2-GDNF. Typical inactive Iba1+ cells (small cell bodies and thin processes) were observed in the delivery sites (SN or PUT). In the WMT, a number of activated Iba1+ cells (larger cell bodies and thick processes) were present along the cannula track in some AAV2-GDNF-treated or PBS control monkeys. Together, these results indicate that infiltration or activation of microglia/macrophages in the subcortical white matter is unlikely to be due to viral proteins, but rather to the surgical procedure.
FIG. 2.
(A) Microglial CD68 staining in substantia nigra and subcortical white matter in aged NHP that received AAV2-GDNF infusions into the substantia nigra or putamen. Immunohistochemistry with anti-CD68 was performed on coronal cortical and nigral sections. Left: Representative low-magnification staining under each condition. Right: High-magnification images of the delineated boxes from the images on the left. Vector was infused either into substantia nigra (Vector: SN) or putamen (Vector: PUT). White matter tracts (WMT), putamen (PUT), or substantia nigra (SN) was stained with antibody as indicated in the open box at the base of each pair of images. No substantial increase in the presence of activated microglia/macrophages was observed in any experimental subject. Scale bars: left, 1 mm; right, 50 μm. (B) Microglial Iba1 staining in substantia nigra and subcortical white matter in aged NHP that received AAV2-GDNF infusions into the substantia nigra or putamen. Immunohistochemistry with anti-Iba1 was performed on coronal cortical and nigral sections. Left: Representative low-magnification staining under each condition. Right: High-magnification images of the delineated boxes from the left-hand images. Vector was infused either into substantia nigra (Vector: SN) or into putamen (Vector: PUT). White matter tracts (WMT), putamen (PUT), or substantia nigra (SN) was stained with antibody as indicated in the white box at the base of each pair of images. No substantial increase the presence of activated microglia/macrophages was observed in any experimental subject. Scale bars: left, 1 mm; right, 50 μm. Color images available online at www.liebertonline.com/hum.
Astrocytes have important physiological properties with respect to CNS homeostasis. It has been appreciated that astrocytes can be activated and function as immunocompetent cells in the CNS, a process involving chemokine/cytokine production and antigen presentation (Dong and Benveniste, 2001). Reactive astrocytes have both hypertrophic processes, as well as soma that overexpress intermediate filaments, glial fibrillary acidic protein (GFAP), vimentin, and nestin (Pekny et al., 2007). GFAP immunostaining revealed that GFAP+ cells appeared to be relatively active (thicker main processes and larger soma) in both the left and right putamen (away from the cannula track) of aged primates in the AAV2-GDNF treatment groups compared with the PBS control group (Fig. 3A). In contrast, no overt difference in GFAP immunoreactivity between AAV2-GDNF treatment groups and the PBS control group was detected in the parkinsonian primates, and GFAP+ cells in the putamen had resolved over time (Fig. 3B). Neuropathological assessment of sections revealed no significant difference in the number of GFAP+ cells between left and right putamen of aged and parkinsonian primates (Table 3). However, in aged primates there were more GFAP+ cells in the AAV2-GDNF treatment groups than in the PBS controls; for parkinsonian primates, the number of GFAP+ cells decreased over time.
FIG. 3.
(A) Expression of glial fibrillary acidic protein (GFAP)-positive astrocytes in the putamen of aged NHP. Coronal striatal sections were analyzed by immunohistochemistry with anti-GFAP antibody. For each group, representative images of the right putamen are shown. Left: Representative low-magnification staining under each condition. Right: High-magnification images of the delineated boxes from the left-hand images. Right and left hemispheres were indistinguishable to a blinded observer. Scale bars: top, left-hand column, for lower magnification figures, 1 mm; top, right-hand column, for higher magnification figures, 50 μm. (B) Expression of GFAP-positive astrocytes in the putamen of parkinsonian NHP. Coronal striatal sections were analyzed by immunohistochemistry with anti-GFAP antibody. For each group of either PBS-infused or AAV2-GDNF-treated NHP at the indicated times after treatment, representative images of the right, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-lesioned putamen are shown. Scale bars: top, first column, for lower magnification figures (first and third columns), 1 mm; top, second column, for higher magnification figures (second and fourth columns), 50 μm.
Table 3.
Tabulated Assessment of Glial Fibrillary Acidic Protein Staining
| Brain region | PUT La | PUT Ra |
|---|---|---|
| Aged NHP | ||
| HD Put | +++ | +++ |
| LD Put | ++ | ++ |
| HD SN | ++ | ++ |
| PBS | + | + |
| Parkinsonian NHP | ||
| GDNF, 1 month | +++ | +++ |
| PBS, 1 month | ++++ | ++++ |
| GDNF, 6 months | +++ | +++ |
| PBS, 6 months | ++ | +++ |
| GDNF, 14 months | + | + |
| PBS, 14 months | ++ | ++ |
Abbreviations: GDNF, glial cell line-derived neurotrophic factor; HD, high dose; L, left; LD, low dose; NHP, nonhuman primates; PBS, phosphate-buffered saline; PUT, putamen; R, right; SN, substantia nigra.
+ = <10, ++ = 11–20, +++ = 21–30, ++++ = >30 GFAP-positive cells per × 40 field of view.
The functional consequences of astrocyte reactivity depend very much on the molecular pathway involved that in turn may result in the elaboration of inflammatory cytokines/chemokines or neurotrophic functions (Dong and Benveniste, 2001; Escartin and Bonvento, 2008). To determine whether expression of inflammatory cytokines/chemokines was altered in the AAV2-GDNF treatment groups, tissue punches (n = 4 or 5 per treatment group and n = 6 for the control group) obtained from the putamen were subjected to RNA microarray analysis. No significant increase in mRNA expression of inflammatory cytokines/chemokines, such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and monocyte chemotactic protein (MCP)-1, for the treatment groups and the controls was detected (data not shown).
Hematoxylin and eosin stain histology
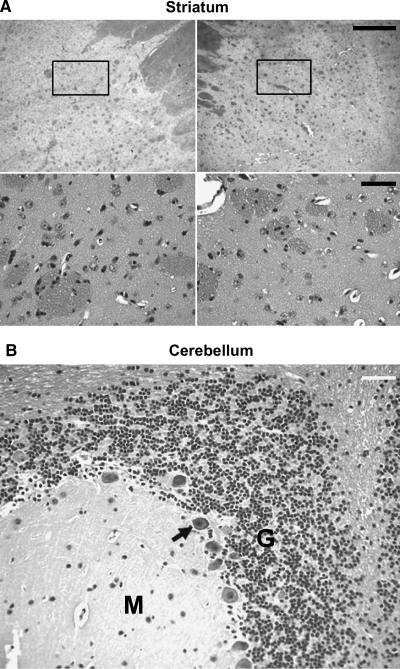
Fixed brain tissue, stained with hematoxylin and eosin (H&E), revealed no evidence of overt pathology, such as encephalitis, infarcts, and/or cellular infiltrates, at the delivery sites (Fig. 4A) of all experimental subjects. Furthermore, in contrast to previous studies indicating that multifocal cerebellar Purkinje cells were lost after putaminal infusion of GDNF protein in NHP (Hovland et al., 2007), cerebella of all subjects remained intact, as revealed by the presence of normal Purkinje cells (Fig. 4B, arrow), along with a densely populated granule cell layer (G) and uniform molecular (M) layer (Fig. 4B).
FIG. 4.
Hematoxylin and eosin (H&E) staining in the striatum and cerebellum of parkinsonian NHP. H&E staining revealed no obvious adverse histology in the striatum and cerebellum in any of the experimental subjects. (A) Representative images of H&E staining in the left and right striatum at lower magnification (scale bar: top row, 1 mm) and higher magnification (scale bar: bottom row, 50 μm). (B) Normal Purkinje cells (arrow), densely populated granule cell layer (G), and uniform molecular layer (M) in the cerebellum. H&E staining in aged NHP gave results identical to those shown here.
Presence of TH-positive profiles
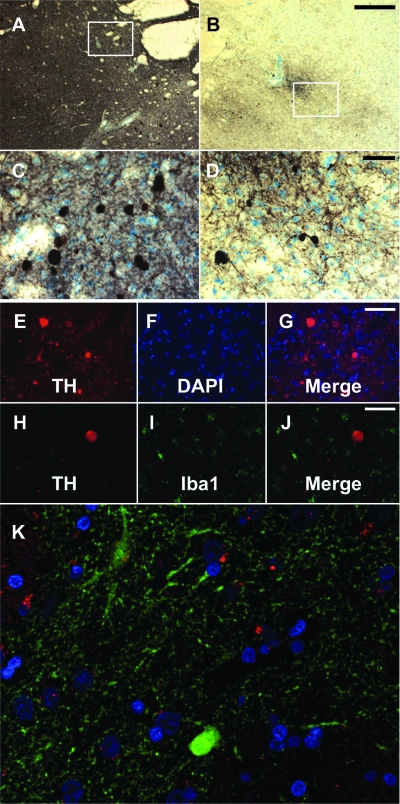
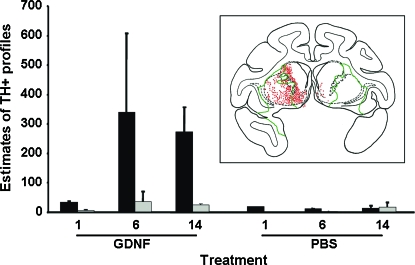
In MPTP-lesioned parkinsonian primates, especially those that received AAV2-GDNF, enhanced expression of TH-positive profiles (Kaiya and Namba, 1981; Marti et al., 2002; Miner et al., 2003) in the striatum was observed (Fig. 5A and B). Figure 5A and C shows representative TH staining in the putamen of the mildly lesioned hemisphere at low (Fig. 5A) and high (Fig. 5C) magnification, and Fig. 5B and D depicts representative low-magnification (Fig. 5B) and high-magnification (Fig. 5D) TH staining in the severely lesioned putamen. Compared with normal TH-positive fibers, these profiles were larger in size, with diameters greater than 20 μm (Fig. 5C and D). Costaining for TH and cell nuclei (with 4′,6-diamidino-2-phenylindole [DAPI]) showed that TH did not colocalize with nuclei (Fig. 5E–G), excluding the possibility that these profiles were cell bodies, and they were not associated with activated microglia (Fig. 5H–J). Costaining with anti-TH and anti-ubiquitin antibodies (red, Fig. 5K) indicated that they were unlikely to be dystrophic neurites (Olanow and McNaught, 2006). Abundant profiles were present in the striata of 6- and 14-month GDNF groups compared with the 1-month GDNF group and all PBS groups, and a greater number of these profiles were localized to the left striatum, which had received only a mild MPTP lesion (solid columns, Fig. 6), but they were uncommon in the more severely lesioned hemisphere (gray columns, Fig. 6) and rare in PBS control animals. Profiles accumulated over the first 6 months but appeared to remain stable thereafter. We will have more data regarding this issue when NHP at the 2-year time point are analyzed. The inset schematic in Fig. 6 indicates the distribution of profiles in a representative coronal section of the putamen from a parkinsonian NHP, demonstrating the preponderance of profiles (red dots) in the less severely lesioned putamen.
FIG. 5.
Presence of tyrosine hydroxylase (TH)-positive profiles in the striatum of parkinsonian primates TH immunostaining revealed the presence of TH-positive profiles in the putamen of parkinsonian NHP of all GDNF-treated animals. (A–D) Representative images of TH-positive profiles in the left, mildly lesioned (A and C) and right, severely lesioned (B and D) putamen. (C and D) Higher magnification images of the boxed areas indicated in (A) and (B), respectively. Scale bars: for lower magnification (A and B), 1 mm; for higher magnification (C and D), 50 μm. Costaining for TH and cell nuclei (4′,6-diamidino-2-phenylindole; DAPI) indicated that TH-positive profiles (E) did not colocalize with nuclei (F) as revealed by the merged image (G). Costaining for TH (H) and Iba1 (I) indicated no activated Iba1-positive microglia surrounding the profiles (J). Immunofluorescence staining of TH-rich sections from NHP 14 months after receiving AAV2-GDNF showed that TH-positive profiles (green) did not colocalize with ubiquitin deposits (red) or nuclei (blue) (K).
FIG. 6.
Quantitative distribution of TH-positive profiles in the left and right striatum of parkinsonian primates. TH-positive profiles were quantitated as described in Materials and Methods. A greater number of these profiles were localized to the left striatum, which had received only a mild MPTP lesion (solid columns), but they were uncommon in the more severely lesioned hemisphere (gray columns). Coronal reconstructions of the number and location of TH-positive profiles (red dots) in the striatum of 6-month GDNF parkinsonian NHP is illustrated schematically in the inset. The border of GDNF immunoreactivity in this primate is outlined by the green line. Color images available online at www.liebertonline.com/hum.
The idea that the lesioning process itself enhances the appearance of TH-positive profiles is supported by the observation that GDNF treatment resulted in few such structures in normal, aged NHP (data not shown). Our current hypothesis is that these profiles represent enhanced TH-containing fibers induced by the combined effects of MPTP lesion and GDNF treatment on partially degenerated dopaminergic fibers. In this scenario, dopaminergic synaptic degeneration in the MPTP-treated striatum yields fibers that do not resemble mature, integrated neurons but are nevertheless able to respond to GDNF. It is important to note, however, that the abundance of these profiles does not correlate with any long-term behavioral (our unpublished observations) or histological abnormalities, and thus do not appear to present safety concerns.
Body weight and food consumption assessments
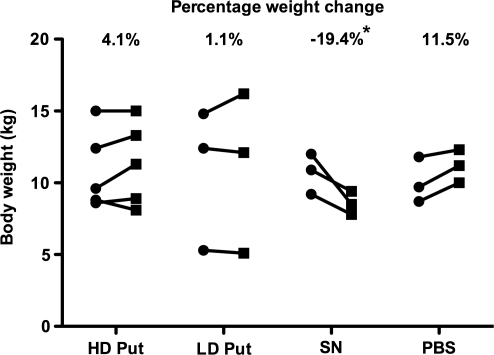
Body weight of and food consumption by aged and parkinsonian NHP were closely monitored throughout the study. All subjects except the three aged animals in the HD SN group maintained their body weight over a period of 6 months (Fig. 7). In contrast, the HD SN group displayed a gradual and continuous decrease in weight after the surgery, and had lost 19.4% of their body weight (∼2 kg) at the time of necropsy. In addition, these HD SN group animals displayed transient but significantly reduced food intake (∼30%) for 4 weeks after AAV2-GDNF administration compared with the PBS control group. This finding parallels the observation of Manfredsson and colleagues that nigral rAAV-GDNF administration induced similar weight loss in rats (Manfredsson et al., 2009). A perplexing feature of these studies was the fact that expression of GDNF was seen in both putamen and nigra, regardless of whether vector was infused into nigra or putamen (Fig. 8). Clearly, the most striking consequence of nigral vector infusion was strong expression throughout the caudate nucleus that was not seen in animals receiving putaminal vector. Staining was also seen in the thalamus, frontal cortex, and ventral tegmental area (Johnston et al., 2009). In view of the well-established role of these regions in affective aspects of dopaminergic function, one might speculate that GDNF-mediated alterations in dopaminergic transmission in the mesolimbic system may play a central role in effects of GDNF on body weight.
FIG. 7.
Body weight change 6 months after AAV2-GDNF delivery in aged primates. *Significant weight loss compared with PBS control (p < 0.05, n = 3). No weight loss was observed in parkinsonian primates 6 months after treatment.
Discussion
We have previously reported expression of GDNF by means of AAV2-GDNF infusion by CED in the dopaminergic nigrostriatal pathway of aged NHP (Johnston et al., 2009), and demonstrated the potential clinical utility in MPTP-lesioned NHP (Eberling et al., 2009). In the present study, we evaluated safety issues regarding AAV2-GDNF administration to NHP brain. We demonstrate, in both models, an absence of tissue pathology with the exception of minor surgical trauma and a mild increase in anti-AAV2 capsid antibodies commonly seen in convective delivery procedures in NHP (Cunningham et al., 2008) and rodents (Sanftner et al., 2004). As shown in these earlier studies and in the present work, this type of infusion of AAV2 into striatum induces no cellular immune response, and this appears to be driven in part by the neuronal specificity of AAV2 in the brain (Bankiewicz et al., 2000). Little microglial/macrophage-mediated neuroinflammation in either putamen or substantia nigra was observed in the present study, except for that occurring in proximity to cannula tracks. Similarly, GFAP immunostaining did not show any strong correlation of GDNF upregulation with increased expression of GFAP-positive astrocyte in the putamen of all experimental subjects. In the aged primates, it seemed that the magnitude of GFAP immunoreactivity was greater in the GDNF treatment groups compared with the PBS controls. However, in the MPTP-lesioned primates, no detectable difference was observed between the GDNF treatment groups and PBS controls. It should be noted that MPTP lesioning before GDNF or PBS injection in these monkeys may confound the effect of GDNF on astrocytic activation (Chen et al., 2002). In the 14-month MPTP-lesioned primates, including both GDNF treatment and PBS control subjects, astrocytic activation appeared to resolve over time. Because there was no significant increase in proinflammatory cytokines/chemokines detected at the RNA level in the putamen of all treatment groups compared with the control group, increased GFAP+ astrocytes appear not to have conveyed any adverse effects.
The most important adverse finding was that nigral administration of AAV2-GDNF caused significant weight loss and increased GDNF levels in frontal cortical regions. This observation is in accordance with previous reports of weight loss in rats with nigral or hypothalamic delivery of AAV2-GDNF (Tumer et al., 2006; Manfredsson et al., 2009) and with reports describing similar, although transient, weight loss after intracerebroventricular (ICV) infusion of recombinant GDNF protein (Martin et al., 1996; Zhang et al., 1997). In our study, all the aged NHP received the same pattern of both nigral and putaminal injections of either AAV2-GDNF or PBS, indicating that surgery itself is unlikely to have caused weight loss in the SN group. The presence of viral vector itself (AAV2) in the nigra is also unlikely to be the cause of the weight loss; Manfredsson and colleagues showed that AAV2-GFP injected into the nigra as control had no effect. The SN group displayed reduced food intake for ∼4 weeks after surgery, whereas most of the remaining NHP did not show decreased appetite, although a few animals showed brief (<1 week) postoperative loss of appetite, perhaps consistent with a prolonged postsurgical recovery period in aged NHP. Manfredsson and colleagues found a specific loss of adipose tissue, but not muscle, in GDNF-treated rats. They suggested a potential mechanism via stimulation by GDNF of a small population of corticotrophin-releasing factor (corticotrophin-releasing hormone [CRH]) neurons located in the medial parvocellular division (MPD) of the paraventricular nucleus of the hypothalamus. We have not, however, explicitly addressed this putative mechanism in our studies. Nevertheless, the fact that such significant weight loss can be induced by nigral GDNF expression is disturbing. Our findings, together with the work of others cited previously, suggest the need for considerable caution in proposing clinical studies in which GDNF or its homolog, neurturin, would be specifically directed to the substantia nigra. Such clinical protocols should be well supported by direct experiments with the vector system in question specifically designed to rule out weight loss as an adverse event.
Interestingly, putaminal GDNF does not cause weight loss either in intact or lesioned NHP, even though we have demonstrated considerable transport of GDNF from putamen to substantia nigra. We suspect that anterograde transport of AAV2-GDNF (and probably GDNF protein as well) to other areas of the midbrain are responsible. In lesioned animals, where the dopaminergic projections to the striatum have been substantially eliminated, transport of vector/gene product to the nigra is comparable to that seen in unlesioned animals. This suggests that anterograde, rather than retrograde, transport is the predominant means by which GDNF is disseminated to distal locations, although clearly retrograde transport of AAV2 also occurs under some conditions (Kaspar et al., 2003; Kells et al., 2009).
Our finding of TH-positive profiles in the MPTP-lesioned primates is interesting. This is the first report, to our knowledge, of the presence of TH-positive profiles in MPTP primates after GDNF administration. These enhanced TH-positive profiles, more evident in mildly lesioned hemispheres, were increased in those monkeys that received GDNF. It is possible that such profiles represent cross-sections of enlarged TH-positive fibers as previously reported by Suwelack and colleagues (2004) in 6-OHDA-lesioned rats treated with a recombinant adenovirus containing GDNF. The fact that profiles are more abundant in the mildly lesioned hemisphere and in the ventral striatum and nucleus accumbens (partially affected by MPTP on the ipsilateral side) suggests that TH-positive profiles are in fact enlarged dopaminergic fibers. Whether these TH-positive profiles or “hypertrophic fibers” convey any beneficial or detrimental effect remains to be investigated. However, they did not appear to elicit any inflammatory responses, because no activated microglia surrounding these fibers were detected.
Taken together, the present findings demonstrated that putaminal administration of AAV2-GDNF at the indicated doses in aged and MPTP-lesioned NHP induced no significant immunological responses and no observed adverse side effects. Our findings support putaminal, but not nigral, AAV2-GDNF gene therapy as a safe therapeutic strategy for Parkinson's disease. This work raises substantial safety concerns for any proposed clinical studies in which nigral, in contrast to putaminal, delivery of GDNF or similar transforming growth factor-β family growth factors are contemplated.
Acknowledgments
This study was supported by an NIH-NINDS Cooperative Research Agreement (U54) (NS045309). The authors thank Sheryl Osborne for helpful input on the design of this study and Dr. William J. Bowers and Wade Narrow for technical assistance on neutralizing antibody assay.
Author Disclosure Statement
No competing financial interests exist.
References
- Bankiewicz K.S. Eberling J.L. Kohutnicka M. Jagust W. Pivirotto P. Bringas J. Cunningham J. Budinger T.F. Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys: In vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp. Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Chen L.W. Wei L.C. Qiu Y. Liu H.L. Rao Z.R. Ju G. Chan Y.S. Significant up-regulation of nestin protein in the neostriatum of MPTP-treated mice: Are the striatal astrocytes regionally activated after systemic MPTP administration? Brain Res. 2002;925:9–17. doi: 10.1016/s0006-8993(01)03253-x. [DOI] [PubMed] [Google Scholar]
- Cunningham J. Pivirotto P. Bringas J. Suzuki B. Vijay S. Sanftner L. Kitamura M. Chan C. Bankiewicz K.S. Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain. Mol. Ther. 2008;16:1267–1275. doi: 10.1038/mt.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. Benveniste E.N. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Eberling J. Kells A.P. Pivirotto P. Beyer J. Bringas J. Federoff H.J. Forsayeth J. Bankiewicz K. Functional effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in parkinsonian rhesus monkeys. Hum. Gene Ther. 2009;20:511–518. doi: 10.1089/hum.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling J.L. Jagust W.J. Taylor S. Bringas J. Pivirotto P. Vanbrocklin H.F. Bankiewicz K.S. A novel MPTP primate model of Parkinson's disease: Neurochemical and clinical changes. Brain Res. 1998;805:259–262. doi: 10.1016/s0006-8993(98)00710-0. [DOI] [PubMed] [Google Scholar]
- Escartin C. Bonvento G. Targeted activation of astrocytes: A potential neuroprotective strategy. Mol. Neurobiol. 2008;38:231–241. doi: 10.1007/s12035-008-8043-y. [DOI] [PubMed] [Google Scholar]
- Granholm A.C. Reyland M. Albeck D. Sanders L. Gerhardt G. Hoernig G. Shen L. Westphal H. Hoffer B. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J. Neurosci. 2000;20:3182–3190. doi: 10.1523/JNEUROSCI.20-09-03182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin R. Cass W.A. Zhang Z. Stanford J.A. Gash D.M. Gerhardt G.A. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog C.D. Brown L. Gammon D. Kruegel B. Lin R. Wilson A. Bolton A. Printz M. Gasmi M. Bishop K.M. Kordower J.H. Bartus R.T. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson's disease. Neurosurgery. 2009;64:602–612. doi: 10.1227/01.NEU.0000340682.06068.01. discussion 612–603. [DOI] [PubMed] [Google Scholar]
- Hovland D.N., Jr. Boyd R.B. Butt M.T. Engelhardt J.A. Moxness M.S. Ma M.H. Emery M.G. Ernst N.B. Reed R.P. Zeller J.R. Gash D.M. Masterman D.M. Potter B.M. Cosenza M.E. Lightfoot R.M. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF) in rhesus monkeys. Toxicol. Pathol. 2007;35:1013–1029. doi: 10.1177/01926230701481899. [DOI] [PubMed] [Google Scholar]
- Johnston L.C. Eberling J. Pivirotto P. Hadaczek P. Federoff H.J. Forsayeth J. Clinically relevant effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys. Hum. Gene Ther. 2009;20:497–510. doi: 10.1089/hum.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiya H. Namba M. Two types of dopaminergic nerve terminals in the rat neostriatum: An ultrastructural study. Neurosci. Lett. 1981;25:251–256. doi: 10.1016/0304-3940(81)90400-6. [DOI] [PubMed] [Google Scholar]
- Kaspar B.K. Llado J. Sherkat N. Rothstein J.D. Gage F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Kearns C.M. Gash D.M. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- Kells A.P. Hadaczek P. Yin D. Bringas J. Varenika V. Forsayeth J. Bankiewicz K.S. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower J.H. Emborg M.E. Bloch J. Ma S.Y. Chu Y. Leventhal L. McBride J. Chen E.Y. Palfi S. Roitberg B.Z. Brown W.D. Holden J.E. Pyzalski R. Taylor M.D. Carvey P. Ling Z. Trono D. Hantraye P. Deglon N. Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Lang A.E. Gill S. Patel N.K. Lozano A. Nutt J.G. Penn R. Brooks D.J. Hotton G. Moro E. Heywood P. Brodsky M.A. Burchiel K. Kelly P. Dalvi A. Scott B. Stacy M. Turner D. Wooten V.G. Elias W.J. Laws E.R. Dhawan V. Stoessl A.J. Matcham J. Coffey R.J. Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Manfredsson F.P. Tumer N. Erdos B. Landa T. Broxson C.S. Sullivan L.F. Rising A.C. Foust K.D. Zhang Y. Muzyczka N. Gorbatyuk O.S. Scarpace P.J. Mandel R.J. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol. Ther. 2009;17:980–991. doi: 10.1038/mt.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M.J. Saura J. Burke R.E. Jackson-Lewis V. Jimenez A. Bonastre M. Tolosa E. Striatal 6-hydroxydopamine induces apoptosis of nigral neurons in the adult rat. Brain Res. 2002;958:185–191. doi: 10.1016/s0006-8993(02)03694-6. [DOI] [PubMed] [Google Scholar]
- Martin D. Miller G. Fischer N. Diz D. Cullen T. Russell D. Glial cell line-derived neurotrophic factor: The lateral cerebral ventricle as a site of administration for stimulation of the substantia nigra dopamine system in rats. Eur. J. Neurosci. 1996;8:1249–1255. doi: 10.1111/j.1460-9568.1996.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Maswood N. Grondin R. Zhang Z. Stanford J.A. Surgener S.P. Gash D.M. Gerhardt G.A. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged rhesus monkeys. Neurobiol. Aging. 2002;23:881–889. doi: 10.1016/s0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Miner L.H. Schroeter S. Blakely R.D. Sesack S.R. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J. Comp. Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Nutt J.G. Burchiel K.J. Comella C.L. Jankovic J. Lang A.E. Laws E.R., Jr. Lozano A.M. Penn R.D. Simpson R.K., Jr. Stacy M. Wooten G.F. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Olanow C.W. McNaught K.S. Ubiquitin–proteasome system and Parkinson's disease. Mov. Disord. 2006;21:1806–1823. doi: 10.1002/mds.21013. [DOI] [PubMed] [Google Scholar]
- Pascual A. Hidalgo-Figueroa M. Piruat J.I. Pintado C.O. Gomez-Diaz R. Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Peden C.S. Burger C. Muzyczka N. Mandel R.J. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden C.S. Manfredsson F.P. Reimsnider S.K. Poirier A.E. Burger C. Muzyczka N. Mandel R.J. Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented. Mol. Ther. 2009;17:524–537. doi: 10.1038/mt.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M. Wilhelmsson U. Bogestal Y.R. Pekna M. The role of astrocytes and complement system in neural plasticity. Int. Rev. Neurobiol. 2007;82:95–111. doi: 10.1016/S0074-7742(07)82005-8. [DOI] [PubMed] [Google Scholar]
- Sanftner L.M. Suzuki B.M. Doroudchi M.M. Feng L. McClelland A. Forsayeth J.R. Cunningham J. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol. Ther. 2004;9:403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Suwelack D. Hurtado-Lorenzo A. Millan E. Gonzalez-Nicolini V. Wawrowsky K. Lowenstein P.R. Castro M.G. Neuronal expression of the transcription factor Gli1 using the Tα1 α-tubulin promoter is neuroprotective in an experimental model of Parkinson's disease. Gene Ther. 2004;11:1742–1752. doi: 10.1038/sj.gt.3302377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A. Lindqvist E. Lin L.F. Ogren S.O. Young D. Hoffer B.J. Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tumer N. Scarpace P.J. Dogan M.D. Broxson C.S. Matheny M. Yurek D.M. Peden C.S. Burger C. Muzyczka N. Mandel R.J. Hypothalamic rAAV-mediated GDNF gene delivery ameliorates age-related obesity. Neurobiol. Aging. 2006;27:459–470. doi: 10.1016/j.neurobiolaging.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Miyoshi Y. Lapchak P.A. Collins F. Hilt D. Lebel C. Kryscio R. Gash D.M. Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J. Pharmacol. Exp. Ther. 1997;282:1396–1401. [PubMed] [Google Scholar]