Abstract
Objective
The aim of this study was to conduct a prospective safety and tolerability study of aripiprazole for the treatment of tics in children and adolescents with Tourette's disorder (TD).
Method
Eleven subjects (10 males) with TD (age 9–19 years, mean 13.36, standard deviation [SD] 3.33) who did not respond or were unable to tolerate previous tic medication were treated with aripiprazole in an open-label, flexible-dosing study over 10 weeks. Tic severity was rated using the Yale Global Tic Severity Scale (YGTSS) and the Clinical Global Impressions Scale for tics (CGI-Tics) at baseline and at follow-up.
Results
The mean (±SD) daily dose for aripiprazole was 4.5 ± 3.0 mg. Mean (±SD) YGTSS Global Severity scores reduced from 61.82 ± 13.49 at baseline to 33.73 ± 15.18 at end point; mean YGTSS total tic scores reduced from 28.18 ± 7.74 at baseline to 16.73 ± 7.54 at end point. Mean (±SD) CGI-Tic severity scores reduced from 4.45 ± 0.52 (moderate-marked) at baseline to 3.18 ± 0.60 (mild) at end point. On the CGI-Tic improvement scale, 10 (91%) subjects achieved 1 (“very much improved”) or 2 (“much improved”) at end point. Most common adverse effects included appetite increase and weight gain in 5 subjects, mild extrapyramidal effects in 7 subjects, and headaches and tiredness/fatigue in 7 subjects; 1 subject experienced akathisia and muscle cramps.
Conclusion
Aripiprazole appears to be a safe and tolerable treatment in children and adolescents with TD that appears to reduce tics; it should be further investigated as a treatment option in controlled trials.
Introduction
Tourette's disorder (TD) is a childhood-onset neuropsychiatric disorder characterized by multiple motor and vocal tics (American Psychiatric Association 2000). The majority of clinically referred individuals with TD also meet criteria for one or more comorbid psychiatric disorders, including obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), mood disorders, and non-OCD anxiety disorders (Coffey et al. 2000). The disorder is often chronic and associated with significant impairment.
Currently, the only medications formally approved for use in TD are haloperidol and pimozide. However, significant side effects, including extrapyramidal symptoms (EPS) such as acute dystonic reactions, Parkinsonism, akathisia, and tardive dyskinesia (Shapiro et al. 1973), have led to use of the newer atypical neuroleptics. In recent years, the atypical neuroleptics have been used frequently to treat tics in youth with TD; however, reports have emerged demonstrating serious adverse effects, including substantial weight gain, development of abnormal glucose and lipid metabolism, elevated serum prolactin levels, and/or cardiac effects such as prolongation of the QTc interval (Green 2001). α-Adrenergic agonists, such as clonidine and guanfacine, are also used to treat tics in TD, but are not formally approved for treatment. Nevertheless, many youths with TD are unresponsive or do not tolerate the α-adrenergic agents due to their adverse effects of sedation, dysphoria, and hypotension.
The aim of the study was to explore the use and tolerability of aripiprazole as a treatment for tics in youth with TD. Aripiprazole is an atypical antipsychotic that differs from other atypical antipsychotics because it is a dopamine partial agonist and it is indicated for treatment of schizophrenia and bipolar disorder in adults and adolescents. Aripiprazole has been reported to be less likely to cause weight gain in adults compared to other atypical neuroleptics (Kolotkin et al. 2008). Recently, several case series and one open-label study have reported on the use of aripiprazole in children and adolescents with TD or chronic tic disorder, but to date, no controlled trials have been reported (Hounie et al. 2004; Dehning et al. 2005; Kastrup et al. 2005; Murphy et al. 2005; Bubl et al. 2006; Constant et al. 2006; Davies et al. 2006; Duane 2006; Fountoulakis et al. 2006; Yoo et al. 2006; Ben Djebara et al. 2008; Budman et al. 2008; Kawohl et al. 2008; Seo et al. 2008; Stenstrom and Sindo 2008; Winter et al. 2008). These case reports and open-label series have suggested that aripiprazole at doses of 2.5–15 mg daily appears to reduce tic severity as judged by clinician rating scales. In addition, these studies on aripiprazole and TD primarily report efficacy measures and sometimes report weight and body mass index (BMI). There is very little information regarding parameters such as lipid profiles, prolactin levels, and other blood abnormalities. We report here a new prospective, open-label safety and tolerability study of aripiprazole that incorporates these measures, including dosing, tolerability, and treatment response in 11 children and adolescents with TD.
Methods
Subjects
Design of the study was an investigator initiated, prospective, 10 week, open-label, safety and tolerability study of patients who met Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) (American Psychiatric Association 2000) criteria for TD. Subjects were recruited through the Institute for Tourette's and Tic Disorders, referrals from local professionals, and the Tourette Syndrome Association. All subjects were evaluated with a comprehensive medical and psychiatric assessment by the senior author with expertise in the diagnosis and treatment of Tourette's disorder. All subjects had been treated in the past with conventional tic medications, including α-adrenergic agonists and typical or atypical neuroleptics.
Recruitment of subjects occurred between September, 2005 and August, 2008. Twenty-five subjects were screened for the study, and 14 were screen failures as a result of failure to meet inclusion and/or exclusion criteria. Specific reasons for screen failures included medical contraindication, complications with medical history, success on other medication, clinical concerns with ADHD (not TD), aripiprazole prescribed in the past with intolerable side effects, patient was not interested, or the patient did not have an adequate trial of clonidine.
Subjects were eligible for inclusion if they met the following inclusion criteria: (1) Age 7–18 years of age (inclusive) when informed consent was obtained; (2) met full DSM-IV-TR diagnostic criteria for TD or chronic motor tic disorder by clinical interview on examination by a physician investigator, and confirmed by Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997); (3) had failed to respond or been unable to tolerate an adequate trial, as determined by the investigator, of clonidine, guanfacine, or neuroleptic medication in the past; (4) tics were causing significant distress or impairment, as determined by parent/subject and principal investigator, on current treatment regimen; (5) no significant abnormalities in laboratory results, including serum chemistries, hematology, and urinalysis; (6) able to swallow pills; (7) had normal intelligence in the judgment of the investigator.
Subjects were excluded from the study if they met any of the following exclusion criteria: (1) Organic brain disease such as traumatic brain injury residua; (2) met criteria for mental retardation as defined by the DSM-IV-TR; (3) history of seizure disorder (other than febrile seizure); (4) history of Sydenham's chorea; (5) autism, schizophrenia, other psychotic disorder, or bipolar disorder; (6) primary diagnosis of a major mood disorder that required ongoing psychiatric treatment; (7) neurological disorder other than a tic disorder; (8) at least one explosive outburst per week, or four explosive outbursts during a 1-month period of time; (9) a major medical illness; (10) females of child bearing age unwilling to use birth control or who were pregnant, as determined by serum pregnancy test at baseline assessment, or lactating; (11) past or current history of substance dependence and/or a current history of substance abuse or who fail baseline urine toxicology screen; (12) any clinically significant abnormal laboratory results at baseline screening, including electrocardiogram (EKG) or blood tests; and (13) history of ongoing or previously undisclosed child abuse.
Concomitant psychotropic medications for comorbid disorders were allowed if the agent(s) and dose(s) had been stable for at least 1 month prior to treatment and were held constant during the entire period of study observation. Subjects were allowed to enroll and cross taper previous tic medication if it had provided at least some therapeutic benefit in the past, but their tics were still causing significant distress or impairment at the time of assessment.
Eleven subjects (10 males) were enrolled in the study. Subjects ranged in age from 9 to 19 years; parents provided written informed consent for use of aripiprazole for subjects less than 18 years, and subjects age 18 or older provided their own written consent. All subjects less than age 18 provided assent. The study protocol was approved by the New York University Institutional Review Board (IRB).
Procedures
All subjects underwent comprehensive medical and psychiatric assessment at baseline, which included physical examination, serum hematology and chemistry evaluation, and electrocardiograms. Lifetime diagnoses were established using the K-SADS-PL (Kaufman et al. 1997), a semistructured diagnostic interview administered by the principal investigator and senior author. All laboratory data were reviewed by the senior author and had to be within normal limits before initiation of treatment.
Outcome measures
Symptom severity was assessed by the principal investigator. Tic severity was assessed at pretreatment baseline, at weekly or biweekly intervals during the study, and at 10 weeks posttreatment using the Yale Global Tic Severity Scale (YGTSS) (Leckman et al. 1989) and the Clinical Global Impressions Scale for tics scale (CGI-Tics) (Berk et al. 2008), the primary outcome measures. Operational definition of CGI-Tics severity was as follows: (1) Normal or no tics at all; (2) borderline, tics may or may not be present; (3) mild, observable motor and/or vocal tics that may or may not be noticed, would not call attention to the individual, and are associated with no distress or impairment; (4) moderate, observable motor and/or vocal tics that would always be noticed, would call attention to the individual, and may be associated with some distress or impairment; (5) marked, exaggerated motor and/or vocal tics that are disruptive, would always call attention to the individual, and are always associated with significant distress or impairment; (6) severe, extremely exaggerated motor and/or vocal tics that are disruptive, would always call attention to the individual, and are associated with injury or inability to carry out daily functions.
Secondary outcome measures were administered at pretreatment, weekly, or biweekly intervals during the study, and at 10 weeks posttreatment and included the Children's Global Assessment Scale (C-GAS) administered by the principal investigator, Attention Deficit Hyperactivity Disorder Rating Scale (ADHD-RS) parent rating version, Children's Depression Rating Scale Revised (CDRS) administered by the principal investigator, Clinical Global Impressions Scale for Obsessive Compulsive Disorder (CGI-OCD), Clinical Global Impressions Scale for Attention-Deficit Hyperactivity Disorder (CGI-ADHD), Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) administered by the principal investigator, and the Multidimensional Anxiety Scale for Children (MASC), a self-report scale.
Dosing and visit schedule
All medication and treatment decisions were made by the study principal investigator. Previous tic medication was tapered and discontinued during the screening and washout period between visit 1 (Screening) and visit 2 (Medication Initiation). Most subjects were off clonidine or guanfacine for at least 2 weeks, and typical or atypical neuroleptics for at least 4 weeks prior to starting study medication. However, if it was clinically unfeasible, in the judgment of the investigator, for the subject to remain off previous tic medication for the duration of the washout phase, aripiprazole could be cross-tapered after visit 2 during the first 2–4 weeks of treatment. Clinically unfeasible was defined as, in the judgment of investigator and parents, the subject's tics would be highly likely to cause significant distress or impairment during a washout phase.
Subjects on medication for a comorbid condition (such as a stimulant for ADHD or a selective serotonin reuptake inhibitor [SSRI] for OCD) could remain on the medication during the study, but agreed to remain on the same dosage throughout the duration of the study. One subject was receiving citalopram and one was on escitalopram for the duration of the study.
Aripiprazole was initiated at doses of 1.25 mg for preadolescents or 2.5 mg for adolescents daily. Subjects between 25 and 50 kg were started on 1.25 mg/day and between 50 and 70 kg were started on 2.5 mg/day. Subjects were flexibly titrated by 1.25–2.5 mg every 5–7 days as tolerated and clinically indicated. Subjects were assessed at weekly intervals during the first 6 weeks and then biweekly for the second 4 weeks.
Dosage reductions were allowed at any time throughout the trial for potential adverse events of at least moderate severity. Anticholinergic medication was allowed for subjects who experienced extrapyramidal side effects.
Safety measures
Potential adverse effects were discussed in detail prior to initiation of treatment with aripiprazole, and were closely monitored at each office visit by review of systems using the Safety Monitoring Uniform Report Form (SMURF) (Greenhill et al. 2004). In addition to the SMURF, safety assessments included vital signs, weight, BMI, waist circumference, Abnormal Involuntary Movement Scale (AIMS), clinical hematological and chemistry laboratory measures, and electrocardiograms. A physical exam was performed on all subjects at baseline and the last visit. Clinical laboratory measures included qualitative urine human chorionic gonadotropin (hCG) (for females), conjugated bilirubin and prolactin panel, comprehensive metabolic panel and lipid profile, and complete blood count (CBC) with differential. Adverse effects were monitored and documented at all postbaseline visits.
Analytic methods
Study data were examined with respect to distribution, outliers, and missing values. The demographic and clinical characteristics of samples are described in terms of means, standard deviations, range, and proportions as needed. Appropriate transformations are applied where the data are not normally distributed.
This study was an open-label design, with each subject contributing baseline and posttreatment measures. Therefore, Wilcoxon signed rank tests for paired data were used to compare pretreatment (baseline) and posttreatment (end point) scores on all primary outcome measures. Exploratory analyses were conducted to compare baseline and end-point scores on measures of global functioning, and psychiatric comorbidity. Significance is judged at level p = 0.05, two-sided; p values are reported unadjusted for multiple comparisons. Analyses were performed using SPSS version 16.
Results
Description of sample
Sociodemographic characteristics of the study sample are described in Tables 1 and 2. Subjects included 10 males (91%) and 1 female (9%); age range was 9–19 years old with mean (±SD) 13.36 ± 3.33 years. High rates of psychiatric comorbidity were observed in these subjects: 9 (82%) met lifetime criteria for ADHD, 3 (27%) for OCD, and 2 (18%) for non-OCD anxiety disorders. One subject (9%) met lifetime criteria for major depressive disorder. Two (18%) subjects took other psychotropic medication (citalopram and escitalopram, respectively) for their comorbid psychiatric disorders during the study, but the dosages of these medications remained stable during the study.
Table 1.
Demographics and Comorbid Diagnoses (N = 11)
| N (%) | |
|---|---|
| Males | 10 (91%) |
| Females | 1 (9%) |
| Comorbid diagnosis | |
| ADHD | 9 (82%) |
| OCD | 3 (27%) |
| MDD | 1 (9%) |
| Non-OCD Anxiety | 2 (18%) |
| Mean age (range) | 13.36 (9–19) |
Abbreviations: ADHD = Attention-deficit/hyperactivity disorder; OCD = obsessive-compulsive disorder; MDD = major depressive disorder.
Table 2.
Concomitant Medications: Subjects Crossed Over from Previous Tic Medications to Aripiprazole (N = 5)
| Drug | Number of participants on drug |
|---|---|
| Haloperidol | 2 |
| Clonidine | 2 |
| Risperidone (only for first week) | 1 |
Dosing, range, and compliance
The dose range for aripiprazole was 1.25–13.75 mg daily; mean (±SD) daily dose was 4.5 ± 3.0 mg. Treatment duration was approximately 10 weeks (mean 78 days, SD ± 21.82) with dosing titrated to therapeutic range within 4–6 weeks. The entire sample had a mean medication compliance rate of 93% (SD ± 9.90) that ranged from 75% to 109%.
Five subjects (45%) were cross-tapered from previous tic medication to aripiprazole during the first 2–4 weeks of the study; that is, they began treatment with aripiprazole while taking their previous tic medication which was tapered and discontinued. Two subjects (18%) were cross-tapered from clonidine, 2 subjects (18%) were cross-tapered from haloperidol, and 1 subject (9%) was cross-tapered from risperidone. Six subjects (55%) were on no medication and received aripiprazole only during the trial. Four subjects (36%) received benztropine and 1 (9%) lorazepam for extrapyramidal adverse effects during the study.
Primary outcome measures: Tic effects
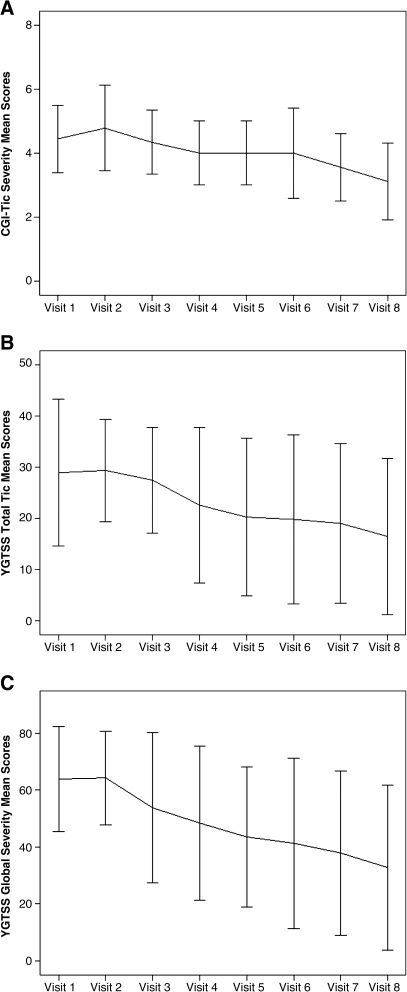
Mean (±SD) pretreatment (baseline) YGTSS Global Severity score (61.82 ± 13.49) (marked) declined significantly to end point (33.73 ± 15.18; p = 0.003) (mild) (Table 3) while YGTSS Total Tic scores also declined significantly from baseline (28.18 ± 7.74) (moderate) to end point (16.73 ± 7.54; p = 0.003) (minimal). Mean (±SD) CGI-Tic severity scores reduced significantly from 4.45 ± 0.52 (moderate-marked) at baseline to 3.18 ± 0.60 (mild) at end point (p = 0.004). Ten (91%) subjects achieved CGI-Tic Improvement scores of 1 (“very much improved”) or 2 (“much improved”) at end point (Fig. 1).
Table 3.
Tic Effects in TD Subjects (N = 11)
| Rating | Baseline (mean ± SD) | End point (mean ± SD) | Difference (mean ± SD) | Effect size (r) | p value |
|---|---|---|---|---|---|
| CGI-Tic | |||||
| Severity | 4.45 (0.52) | 3.18 (0.60) | −1.27 (0.65) | 0.616 | 0.004 |
| YGTSS | |||||
| Motor tic | 15.82 (4.40) | 9.73 (2.76) | −6.09 (4.41) | 0.598 | 0.005 |
| Vocal tic | 12.36 (7.10) | 7.00 (5.76) | −5.36 (4.57) | 0.569 | 0.008 |
| Total tic | 28.18 (7.74) | 16.73 (7.54) | −11.45 (6.23) | 0.626 | 0.003 |
| Global Severity | 61.82 (13.49) | 33.73 (15.18) | −28.09 (11.83) | 0.626 | 0.003 |
Abbreviations: TD = Tourette's disorder; SD = standard deviation; CGI-Tic = Clinical Global Impressions Scale for tics; YGTSS = Yale Global Tic Severity Scale.
FIG. 1.
(A) The mean CGI-Tic Severity scores from baseline to end point (N = 11). (B) The mean YGTSS Total Tic scores from baseline to end point (N = 11). (C) The mean YGTSS Global Severity scores from baseline to end point (N = 11). Error bars represent the standard deviation (±2.0), in these measurements. CGI-Tic Severity = Clinical Global Impressions–Tic Severity; YGTSS = Yale Global Tic Severity Scale.
Outcome measures were explored for patients with severe TD and those with nonsevere TD. Patients with severe TD were defined as those that had scores of 25 or higher on the YGTSS total tic subscale, whereas those who were defined as having nonsevere TD had scores of 24.99 or lower. Patients with severe TD had mean (±SD) pretreatment (baseline) YGTSS Global Severity scores (69.14 ± 5.90) (marked) that declined significantly to end point (40.71 ± 13.01; p = 0.018) (moderate) (Table 4), whereas YGTSS Total Tic scores also declined significantly from baseline (33.43 ± 2.30) (moderate) to end point (20.43 ± 6.35; p = 0.017) (minimal). Mean (±SD) CGI-Tic severity scores reduced significantly from 4.57 ± 0.54 (moderate-marked) at baseline to 3.43 ± 0.54 (mild-moderate) at end point (p = 0.023).
Table 4.
Tic Effects in TD Subjects with Severe YGTSS Total Tic Scores at Visit 1 (N = 7)
| Rating | Baseline (mean ± SD) | End point (mean ± SD) | Difference (mean ± SD) | Effect size (r) | p value |
|---|---|---|---|---|---|
| CGI-Tic | |||||
| Severity | 4.57 (0.54) | 3.43 (0.54) | −1.14 (0.69) | 0.607 | 0.023 |
| YGTSS | |||||
| Motor tic | 17.00 (3.22) | 11.29 (1.89) | −5.71 (4.07) | 0.587 | 0.028 |
| Vocal tic | 16.43 (2.44) | 9.14 (5.61) | −7.29 (4.35) | 0.634 | 0.018 |
| Total tic | 33.43 (2.30) | 20.43 (6.35) | −13.00 (7.19) | 0.637 | 0.017 |
| Global Severity | 69.14 (5.90) | 40.71 (13.01) | −28.43 (12.43) | 0.632 | 0.018 |
Abbreviations: TD = Tourette's disorder; YGTSS = Yale Global Tic Severity Scale; SD = standard deviation; CGI-Tic = Clinical Global Impressions Scale for tics.
Patients with nonsevere TD had mean (±SD) pretreatment (baseline) YGTSS Global Severity scores (49.00 ± 13.88) (moderate) that declined to end point (21.50 ± 10.79; p = 0.068) (mild) (Table 5), while YGTSS Total Tic scores also declined from baseline (19.00 ± 10.25) (minimal) to end point (10.25 ± 4.57; p = 0.068) (minimal). Mean (±SD) CGI-Tic severity scores reduced significantly from 4.25 ± 0.50 (moderate-marked) at baseline to 2.75 ± 0.50 (borderline-mild) at end point (p = 0.063). Differences between baseline and end-point YGTSS Global Severity scores, YGTSS Total Tic scores, and CGI-Tic severity scores only trended toward significance in these patients with nonsevere TD. Effect sizes for all primary outcome measures ranged from 0.47 to 0.66 (Tables 3–5).
Table 5.
Tic Effects in TD Subjects with Nonsevere YGTSS Total Tic Scores at Visit 1 (N = 4)
| Rating | Baseline (mean ± SD) | End point (mean ± SD) | Difference (mean ± SD) | Effect size (r) | p value |
|---|---|---|---|---|---|
| CGI-Tic | |||||
| Severity | 4.25 (0.50) | 2.75 (0.50) | −1.50 (0.58) | 0.657 | 0.063 |
| YGTSS | |||||
| Motor tic | 13.75 (5.91) | 7.00 (1.63) | −6.09 (4.41) | 0.646 | 0.068 |
| Vocal tic | 5.25 (7.09) | 3.25 (4.27) | −5.36 (4.57) | 0.474 | 0.180 |
| Total tic | 19.00 (3.56) | 10.25 (4.57) | −11.45 (6.23) | 0.646 | 0.068 |
| Global Severity | 49.00 (13.88) | 21.50 (10.79) | −28.09 (11.83) | 0.646 | 0.068 |
Abbreviations: TD = Tourette's disorder; YGTSS = Yale Global Tic Severity Scale; SD = standard deviation; CGI-Tic = Clinical Global Impressions Scale for tics.
Secondary outcome measures: Effects on global assessment and comorbid disorder severity
Exploratory analyses were conducted to assess effects of aripiprazole on secondary outcomes, including OCD (CYBOCS), ADHD (ADHD-RS), C-GAS, depression (CDRS), and anxiety (MASC), and are shown in Table 6.
Table 6.
Assessment of Global and Comorbid Disorders (N = 11)
| Baseline mean (SD) | Baseline range | End-point mean (SD) | End-point range | Difference mean (SD) | Effect size (r) | p value | |
|---|---|---|---|---|---|---|---|
| C-GAS | 53.36 (4.03) | 48–60 | 60.64 (4.63) | 52–65 | 7.27 (4.92) | 0.608 | 0.004 |
| ADHD-RS | |||||||
| Inattention | 14.80 (6.30) | 0–24 | 11.20 (6.89) | 0–22 | −3.60 (3.92) | 0.471 | 0.035 |
| Hyperactivity-Impulsivity | 13.60 (7.65) | 1–22 | 9.30 (6.70) | 0–18 | −4.30 (2.67) | 0.599 | 0.007 |
| Total | 28.40 (12.80) | 1–41 | 20.5 (11.58) | 1–36 | −7.90 (5.36) | 0.599 | 0.007 |
| CDRS-R | 39.09 (8.88) | 30–61.5 | 35.82 (5.46) | 30–49.5 | −3.27 (5.94) | 0.329 | 0.123 |
| CGI-OCD | 2.55 (0.93) | 1–4 | 2.00 (0.94) | 1–3 | −0.60 (0.70) | 0.474 | 0.034 |
| CGI-ADHD | 3.36 (0.92) | 1–4 | 2.70 (1.25) | 1–4 | −0.60 (0.84) | 0.415 | 0.063 |
| CY-BOCS | |||||||
| Obsession | 3.91 (3.30) | 0–11 | 1.09 (1.81) | 0–6 | −2.82 (3.82) | 0.451 | 0.035 |
| Compulsion | 4.27 (3.04) | 0–10 | 2.36 (2.50) | 0–8 | −1.91 (3.05) | 0.367 | 0.085 |
| Total | 8.18 (6.15) | 0–21 | 3.45 (3.05) | 0–9 | −4.73 (6.15) | 0.448 | 0.035 |
| MASC total score | 45.78 (17.02) | 19–75 | 47.86 (12.06) | 30–57 | −1.00 (16.19) | 0.030 | 0.917 |
Abbreviations: C-GAS = Children's Global Assessment Scale; CDRS-R = Children's Depression Rating Scale, Revised; CGI-OCD = Clinical Global Impressions Scale for Obsessive Compulsive Disorder; CGI-ADHD = Clinical Global Impressions Scale for Attention-Deficit Hyperactivity Disorder; CY-BOCS = Children's Yale-Brown Obsessive Compulsive Scale; MASC = Multidimensional Anxiety Scale for Children; ADHD-RS = Attention Deficit Hyperactivity Disorder Rating Scale.
Overall functioning as measured on the C-GAS showed significant improvement with an increase from mean (±SD) C-GAS score at pretreatment (baseline) of 53.36 ± 4.03 (some noticeable problems) to end point to 60.64 ± 4.63; p = 0.004 (some problems).
There was a significant decrease from baseline to end point in mean (±SD) ADHD-RS inattention scores (14.80 ± 6.30 vs. 11.20 ± 6.89; p = 0.035), ADHD-RS hyperactivity-impulsivity scores (13.60 ± 7.65 vs. 9.30 ± 6.70; p = 0.007), and ADHD-RS total scores (28.40 ± 12.80 vs. 20.50 ± 11.58; p = 0.007). CGI-ADHD scores reduced from baseline to end point (mild to borderline ill) (3.36 ± 0.92 vs. 2.70 ± 1.25; p = 0.063), but this was not statistically significant.
There was a significant decrease from baseline to end point in mean (SD) CGI-OCD scores (2.55 ± 0.93 vs. 2.00 ± 0.94; p = 0.034) (borderline ill). There was a significant reduction in mean (±SD) CY-BOCS Total score (8.18 ± 6.15 vs. 3.45 ± 3.05; p = 0.035) and mean (±SD) CY-BOCS Obsession scores (3.91 ± 3.30 vs. 1.09 ± 1.81; p = 0.035) but no significant reduction in CY-BOCS Compulsion scores (4.27 ± 3.04 vs. 2.36 ± 2.50; p = 0.085) from baseline to end point.
There were no significant changes in measures of anxiety (MASC) and depression (CDRS-R) from baseline to end point. Mean (±SD) baseline MASC Total score was 45.78 ± 17.02 versus 47.86 ± 12.06 end point (p = 0.917); mean (±SD) baseline CDRS-R score was 39.09 ± 8.88 versus 35.82 ± 5.46 at end point (p = 0.123). Effect sizes for all secondary outcome measures ranged from 0.030 to 0.608 (Table 6).
Adverse effects
The majority of subjects tolerated aripiprazole well. In most cases, adverse effects emerged when the dose was increased in an attempt to target symptoms that did not respond to lower dosage. Titrations were made in 1.25- to 2.5-mg increments only.
Most common adverse effects were reported to be mild and included appetite increase and weight gain in 7 subjects and extrapyramidal side effects (EPS) (muscle, bone, or joint pain conditions on the SMURF) in 10 subjects (Table 7). Other common adverse effects were headaches, experienced by all 11 patients, and tiredness/fatigue in 8 subjects (Table 7). One subject (patient 8; see tables in Appendix) dropped out before 10 weeks due to akathisia and muscle cramps unresponsive to dosage reduction and anticholinergic medication.
Table 7.
Adverse Effects (SMURF) (N = 11)
| Type of adverse effects reported | Subjects reported N (%) | Frequency of adverse effect N | Frequency possibly relateda to study drug N (%) |
|---|---|---|---|
| Headache | 11 (100%) | 34 | 1 (3%) |
| Muscle, bone, or joint pain/condition | 10 (91%) | 31 | 18 (56%) |
| Appetite increase/weight gain | 7 (64%) | 27 | 25 (93%) |
| Stomach discomfort | 9 (82%) | 26 | 7 (27%) |
| Tiredness/fatigue | 8 (73%) | 23 | 19 (83%) |
| Dizziness | 9 (82%) | 18 | 11 (61%) |
| Appetite decrease/weight loss | 6 (55%) | 12 | 10 (85%) |
| Drowsiness/sedation | 7 (64%) | 13 | 10 (77%) |
| Dry mouth | 4 (36%) | 10 | 10 (100%) |
Possibility as determined by principal investigator.
Weight
Overall, there was not a significant difference between the means (±SD) of weight in pounds at baseline (131.54 ± 52.65 vs. 133.69 ± 55.88; p = 0.286) and at end point. Subjects gained an average of 2.16 pounds (SD ± 8.63) over the course of the study; the 6 subjects who reported weight gain as an adverse event gained 4.3 pounds (SD ± 11.24) (Table 8). The data were stratified by pubertal status as shown in Table 8. Stratification of subjects by developmental stage (preadolescents age 7–11; and adolescents age 12–19) had no effect on overall findings in this small number of subjects. It is important to note that the 5 subjects (45%) who were cross-tapered from previous tic medications, including clonidine, haloperidol, and risperidone, to aripiprazole during the first half of the study began with a mean weight of 130.80 ± 57.23 and ended with a mean weight of 128.4 ± 60.83 for an average weight loss of 2.4 pounds ± 7.76 (p = 0.893) during the study. Those 3 subjects (27%), who underwent cross-taper from previous neuroleptic medications, including haloperidol and risperidone, to aripiprazole during the first half of the study, began with a mean weight of 146.0 ± 75.29 and ended with a mean weight of 141.17 ± 82.27 for an average weight loss of 4.83 pounds ± 9.88 (p = 0.593) during the study.
Table 8.
Effects on Height and Weight
| Baseline mean (SD) | Baseline range | End-point mean (SD) | End-point range | Difference mean (SD) | Effect size (r) | p value | |
|---|---|---|---|---|---|---|---|
| Whole sample, n = 11 | |||||||
| Weight (pounds) | 131.54 (52.65) | 73.00–232.00 | 133.69 (55.88) | 71.50–236.0 | 2.16 (8.63) | 0.227 | 0.286 |
| BMI | 22.69 (5.89) | 15.26–35.27 | 22.91 (6.55) | 14.59–36.41 | 0.22 (1.48) | 0.085 | 0.689 |
| Height (inches) | 62.97 (6.97) | 53.10–78.70 | 63.20 (7.35) | 53.50–81.00 | 0.23 (.93) | 0.186 | 0.383 |
| Waist (inches) | 32.77 (7.94) | 25.00–46.00 | 32.50 (7.43) | 25.00–46.00 | −0.27 (2.13) | 0.181 | 0.498 |
| Preadolescents (age 6–12), n = 6 | |||||||
| Weight (pounds) | 96.82 (16.04) | 73.0–114.00 | 96.08 (14.57) | 71.50–114.00 | −0.73 (9.21) | 0.091 | 0.753 |
| BMI | 20.35 (4.34) | 15.26–27.92 | 20.16 (4.47) | 14.59–28.00 | −0.18 (1.47) | 0.091 | 0.753 |
| Height (inches) | 58.06 (2.65) | 53.10–61.00 | 58.18 (2.32) | 53.50–59.70 | 0.12 (.81) | 0.182 | 0.527 |
| Waist (inches) | 27.23 (3.15) | 25.00–31.90 | 27.75 (2.75) | 25.00–31.00 | 0.53 (2.36) | 0.651 | 1.00 |
| Adolescents (13–18), n = 5 | |||||||
| Weight (pounds) | 173.20 (51.29) | 104.00–232.00 | 178.82 (53.61) | 109.50–236.00 | 5.62 (7.26) | 0.554 | 0.080 |
| BMI | 25.50 (6.72) | 18.07–35.27 | 26.21 (7.57) | 18.79–36.41 | 0.71 (1.50) | 0.343 | 0.279 |
| Height (inches) | 68.86 (5.76) | 63.60–78.70 | 69.22 (6.72) | 64.00–81.00 | 0.36 (1.14) | 0.058 | 0.854 |
| Waist (inches) | 40.17 (5.53) | 35.00–46.00 | 38.83 (7.01) | 32.00–46.00 | −1.33 (1.53) | 0.655 | 0.109 |
Abbreviations: SD = Standard deviation; BMI = body mass index.
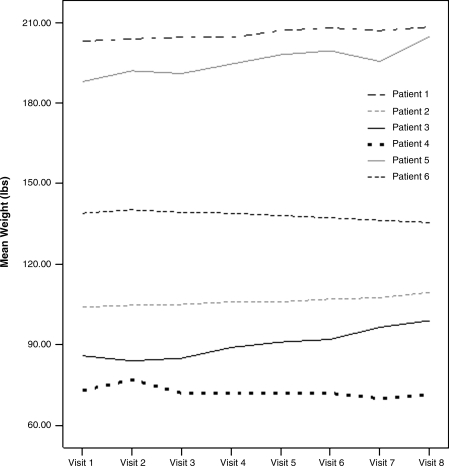
Weights of the 6 patients treated only with aripiprazole during the study are shown in Fig. 2. It is notable that they began with a baseline mean weight of 132.15 ± 54.07 and ended with a mean weight of 138.10 ± 56.86 for an average weight gain of 5.95 pounds ± 7.93 (p = 0.116).
FIG. 2.
Weights (pounds) of patients only on aripiprazole (n = 6). The weights are shown plotted weekly for each of the 6 patients that were not on any other antipsychotic medications and were only treated with aripiprazole during the study. There was no significant overall weight gain noted.
Vital signs and laboratory measures
There were no significant differences between baseline and end point in vital signs or any laboratory measurements including heart rate, blood pressure, hematology, lipid profiles, glucose, or blood chemistries, other than a very slight but significant increase in chloride (102.8 ± 0.98 vs. 104.0 ± 2.16; p = 0.031). There was a significant decrease in prolactin (15.1 ± 11.85 vs. 5.8 ± 5.89; p = 0.037) from baseline to end point (see tables in Appendix). This decrease was observed in 80% of the subjects regardless of whether they were cross-tapered from a typical neuroleptic, such as haloperidol, or atypical neuroleptic, such as risperidone, to aripiprazole. There were no significant changes in EKGs between baseline and endpoint (Table 9).
Table 9.
Safety Laboratory Test Results (N = 11)
| Lab test | Baseline value | End-point value | Mean difference (±SD) | p value |
|---|---|---|---|---|
| Heart rate | 76.8 | 75.3 | −1.29 | 0.933 |
| QTc intervals | 412.8 | 411.9 | −0.38 | 0.726 |
| Systolic blood pressure | 113.6 | 118.4 | −4.73 | 0.168 |
| Diastolic blood pressure | 68.1 | 67.6 | 0.55 | 0.858 |
| Chloride | 102.8 | 104.0 | −1.30 | 0.031a |
| Prolactin | 15.1 | 5.8 | −11.78 | 0.037a |
Less than p = 0.05.
Abbreviation: SD = Standard deviation.
Discussion
Results of this prospective, open-label, safety and tolerability study suggest that aripiprazole was beneficial in reduction of tics in a small sample of children and adolescents with TD who had failed to respond or been unable to tolerate previous tic treatment. Exploratory analyses revealed beneficial effects on global functioning, ADHD symptoms and OCD symptoms. Mean dose was in the low range when compared to the typical dose range of 15–30 mg used to treat schizophrenia and bipolar disorder. Most subjects tolerated the medication well.
Adverse effects were generally manageable. In this sample, 1 subject discontinued treatment due to the emergence of intolerable akathisia unresponsive to dosage reduction or to anticholinergic medication. Interestingly, this subject had been treated for several years with moderate doses of haloperidol (3–4 mg); this experience may have rendered him less responsive to a D2 partial agonist after relatively long exposure to a potent D2 antagonist. Treatment with haloperidol may have also sensitized his receptors to extrapyramidal effects. There were no other notable features in this individual that could explain the intolerable akathisia.
The frequency of extrapyramidal side effects in this sample of children with TD appears to be somewhat higher than expected, compared with the frequency of such symptoms reported in studies of adults with schizophrenia (Marder et al. 2003). However, given aripiprazole's partial dopamine agonist–antagonist effects, and the putative therapeutic mechanism of action involving D2 receptor blockade, it is possible that youths with TD, with cortico-striato-thalamic-cortical tract disinhibition, may be particularly vulnerable to extrapyramidal side effects of this medication.
In our series, 7 (64%) of 11 subjects experienced appetite increase and weight gain during treatment, although the weight change did not reach statistical significance. This trend was a somewhat unexpected finding, because previous studies using aripiprazole monotherapy for treatment of schizophrenia in adults resulted in a modest weight loss over an 8-week study duration (Casey et al. 2003). However, more recent studies involving children have suggested that children are more likely to experience metabolic effects of aripiprazole than adults (Correll 2008).
Limitations
Some limitations of this study design must be taken into account. Regarding our sample, all subjects had failed to respond or been unable to tolerate previous tic medications, so they could be considered to have unique or treatment-refractory symptoms. Furthermore, this was a small sample of subjects treated within a specialty program, and ascertainment bias is possible that could reduce the generalizability of our findings. With regard to methodology, this was an open-label study, so we did not have a comparison group nor were we blinded to treatment. Open-label trials are susceptible to non-medication-related effects, including placebo response and response to more frequent visits with the doctor as part of the trial. It is important to note that the treatment and assessments (using systematic, standardized ratings) were performed by several investigators, but they always included the principal investigator (B.J.C.).
Because two of our subjects were taking concomitant medication for psychiatric comorbid disorders, we cannot rule out drug interactions as contributing to adverse effects observed, nor can we exclude the possibility that particular concomitant medication combinations might be contributing to synergism or additive therapeutic effects.
Despite these limitations, findings in our exploratory study indicate that aripriprazole may be a potentially beneficial and tolerable treatment for tics in children and adolescents with TD. One additional open-label aripiprazole study published recently has also reported a reduction in tics with aripiprazole in children and adolescents with TD or chronic tic disorder (Seo et al. 2008). The mean dose in our study (4.5 ± 3.0 mg) is less than that reported in this and other prior studies. Taken together, these studies suggest that relatively low-dose aripiprazole is beneficial in reduction of tic symptoms in children and adolescents with TD and chronic tic disorders.
Because both studies had an open-label design, future controlled studies are indicated in TD. To date, there are several controlled trials that have demonstrated the efficacy of typical neuroleptics (haloperidol and pimozide) and atypical neuroleptics (risperidone) for the treatment of TD (Sallee et al. 1997; Bruggeman et al. 2001; Pringsheim and Marras 2009), although these medications have also been shown to have adverse effects such as weight gain and extrapramidal symptoms. Given the relatively neutral side-effect profile observed in this and other open-label studies of aripiprazole, aripiprazole could be considered as an additional treatment option. However, definitive evidence concerning efficacy, effectiveness, and adverse effects profile of aripiprazole awaits controlled comparative studies.
Disclosures
Dr. Coffey receives research support from Boehringer Ingelheim, Bristol Myers Squibb, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, Tourette Syndrome Association, and American Academy of Child and Adolescent Psychiatry; is on the Advisory Boards of Jazz Pharmaceuticals and Novartis; and is on the Research/Advisory Board of Eli Lilly. Dr. Lyon receives research support from Tourette Syndrome Association and the American Academy of Child and Adolescent Psychiatry. Dr. Jummani, Dr. Hirsch, Mr. Spirgel, Ms. Goldman, and Ms. Samar have no financial ties or conflicts of interest to disclose.
Appendix: Dosage Titrations
Patient 1
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 203.00 | 5.00 | |
| 2 | 203.94 | Aripiprazole 2.5 mg | |
| 3 | 204.50 | Aripiprazole 5 mg | |
| 4 | 204.40 | Aripiprazole 6.25 mg | |
| 5 | 207.00 | Aripiprazole 8.75 mg | |
| 6 | 208.00 | Aripiprazole 11.25 mg | |
| 7 | 207.00 | Aripiprazole 11.25 mg | |
| 8 | 208.30 | Aripiprazole 13.75 mg | 3.00 |
Patient 2
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 104.00 | 7.10 | |
| 2 | 104.70 | Aripiprazole 2.5 mg | |
| 3 | 105.00 | Aripiprazole 2.5 mg | |
| 4 | 106.00 | Aripiprazole 2.5 mg | |
| 5 | 106.00 | Aripiprazole 2.5 mg | |
| 6 | 107.00 | Aripiprazole 2.5 mg | |
| 7 | 107.50 | Aripiprazole 2.5 mg | |
| 8 | 109.50 | Aripiprazole 2.5 mg | 15.30 |
Patient 3
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 85.90 | 10.10 | |
| 2 | 84.00 | Aripiprazole 1.25 mg | |
| 3 | 85.00 | Aripiprazole 2.5 mg | |
| 4 | 89.00 | Aripiprazole 2.5 mg | |
| 5 | 91.00 | Aripiprazole 2.5 mg | |
| 6 | 92.00 | Aripiprazole 3.75 mg | |
| 7 | 96.50 | Aripiprazole 3.75 mg | |
| 8 | 99.00 | Aripiprazole 5 mg | 0.60 |
Patient 4
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 188.00 | 8.60 | |
| 2 | 192.00 | Aripiprazole 1.25 mg | |
| 3 | 191.00 | Aripiprazole 2.5 mg | |
| 4 | 194.50 | Aripiprazole 5 mg | |
| 5 | 198.00 | Aripiprazole 5 mg | |
| 6 | 199.50 | Aripiprazole 6.25 mg | |
| 7 | 195.50 | Aripiprazole 6.25 mg | |
| 8 | 204.80 | Aripiprazole 7.5 mg | 7.90 |
Patient 5
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 139.00 | 12.20 | |
| 2 | 140.25 | Aripiprazole 2.5 mg | |
| 3 | 139.25 | Aripiprazole 1.25 mg | |
| 4 | 139.00 | Aripiprazole 1.25 mg | |
| 5 | 135.50 | Aripiprazole 2.5 mg | 10.20 |
Patient 6
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 73.00 | Pimozide 3 mg | 46.80 |
| 2 | 77.00 | Aripiprazole 1.25 mg | |
| 3 | 72.00 | Aripiprazole 2.5 mg | |
| 4 | 72.00 | Aripiprazole 6.25 mg | |
| 5 | 72.00 | Aripiprazole 7.5 mg | |
| 6 | 72.00 | Aripiprazole 8.75 mg | |
| 7 | 70.00 | Aripiprazole 8.75 mg | |
| 8 | 71.50 | Aripiprazole 8.75 mg | 3.30 |
Patient 7
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 92.00 | Haloperidol 2 mg | 13.80 |
| 2 | 93.50 | Aripiprazole 1.25 mg, Haloperidol 2 mg | |
| 3 | 95.50 | Aripiprazole 2.5 mg, Haloperidol 0.5 mg | |
| 4 | 92.50 | Aripiprazole 3.75 mg | |
| 5 | 91.00 | Aripiprazole 5 mg | |
| 6 | 90.50 | Aripiprazole 6.25 mg | |
| 7 | 92.00 | Aripiprazole 6.25 mg | |
| 8 | 89.00 | Aripiprazole 10 mg | 1.60 |
Patient 8
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 104.00 | Clonidine 0.2 mg | 6.90 |
| 2 | 104.50 | Aripiprazole 1.25 mg, Clonidine 0.125 mg | |
| 3 | 105.50 | Aripiprazole 1.25 mg, Clonidine 0.125 mg | |
| 4 | 104.50 | Aripiprazole 1.25 mg, Clonidine 0.125 mg | |
| 5 | 104.50 |
Patient 9
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 232.00 | Haloperidol 4 mg, Clonidine 0.2 mg | 14.10 |
| 2 | 231.00 | Aripiprazole 1.25 mg, Haloperidol 4 mg | |
| 3 | 235.00 | Aripiprazole 2.5 mg, Haloperidol 3 mg | |
| 4 | 234.00 | Aripiprazole 2.5 mg, Haloperidol 2 mg | |
| 5 | 241.00 | Aripiprazole 3.75 mg, Haloperidol 1 mg | |
| 6 | 239.00 | Aripiprazole 5.0 mg | |
| 7 | 236.00 | Aripiprazole 6.25 mg | |
| 8 | Aripiprazole 7.5 mg | 14.90 |
Patient 10
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 112.00 | Clonidine 0.4 mg | 17.30 |
| 2 | 111.00 | Aripiprazole 1.25 mg, Clonidine 0.05 mg | |
| 3 | 109.50 | Aripiprazole 2.5 mg | |
| 4 | 111.50 | Aripiprazole 2.5 mg | |
| 5 | 113.50 | Aripiprazole 2.5 mg | |
| 6 | 114.00 | Aripiprazole 2.5 mg | |
| 7 | Aripiprazole 2.5 mg | ||
| 8 | Aripiprazole 2.5 mg | 0.80 |
Patient 11
| Visit Number | Weight (lbs) | Drug Dosage | Prolactin |
|---|---|---|---|
| 1 | 114.00 | Risperidone 1 mg | 24.20 |
| 2 | 108.00 | Aripiprazole 1.25 mg, Risperidone 0.5 mg | |
| 3 | 107.00 | Aripiprazole 2.5 mg | |
| 4 | 104.00 | Aripiprazole 2.5 mg | |
| 5 | 102.00 | Aripiprazole 2.5 mg | |
| 6 | 101.50 | Aripiprazole 3.75 mg | |
| 7 | 99.00 | Aripiprazole 3.75 mg | |
| 8 | 98.50 | Aripiprazole 3.75 mg | 0.20 |
Footnotes
This was an investigator-initiated study supported by a grant from Bristol Myers Squibb to the corresponding author (B.J.C.). The funding source played no role in the study design, data analysis, or writing of the manuscript. All laboratory work associated with this study was funded by National Center for Research Resources (NCRR) M01 RR00096.
Acknowledgments
We wish to thank Eva Petkova, Ph.D., for her assistance with the biostatistical analysis.
References
- American Psychiatric Association. Diagnostic, Statistical Manual of Mental Disorders 4th edition, Text Revision (DMS-IV-TR) Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- Ben Djebara M. Worbe Y. Schupbach M. Hartmann A. Aripiprazole: A treatment for severe coprolalia in “refractory” Gilles de la Tourette syndrome. Mov Disord. 2008;23:438–440. doi: 10.1002/mds.21859. [DOI] [PubMed] [Google Scholar]
- Berk M. Ng F. Dodd S. Callaly T. Campbell S. Bernardo M. Trauer T. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract. 2008;14:979–983. doi: 10.1111/j.1365-2753.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- Bruggeman R. van der Linden C. Buitelaar JK. Gericke GS. Hawkridge SM. Temlett JA. Risperidone versus pimozide in Tourette's disorder: A comparative double-blind parallel-group study. J Clin Psychiatry. 2001;62:50–56. doi: 10.4088/jcp.v62n0111. [DOI] [PubMed] [Google Scholar]
- Bubl E. Perlov E. Tebartz Van Elst L. Aripiprazole in patients with Tourette syndrome. World J Biol Psychiatry. 2006;7:123–125. doi: 10.1080/15622970500474770. [DOI] [PubMed] [Google Scholar]
- Budman C. Coffey BJ. Shechter R. Schrock M. Wieland N. Spirgel A. Simon E. Aripiprazole in children and adolescents with Tourette disorder with and without explosive outbursts. J Child Adolesc Psychopharmacol. 2008;18:509–515. doi: 10.1089/cap.2007.061. [DOI] [PubMed] [Google Scholar]
- Casey DE. Carson WH. Saha AR. Liebeskind A. Ali MW. Jody D. Ingenito GG. Switching patients to aripiprazole from other antipsychotic agents: A multicenter randomized study. Psychopharmacology (Berl) 2003;166:391–399. doi: 10.1007/s00213-002-1344-3. [DOI] [PubMed] [Google Scholar]
- Coffey BJ. Biederman J. Spencer T. Geller DA. Faraone SV. Bellordre CA. Informativeness of structured diagnostic interviews in the identification of Tourette's disorder in referred youth. J Nerv Ment Dis. 2000;188:583–588. doi: 10.1097/00005053-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Constant EL. Borras L. Seghers A. Aripiprazole is effective in the treatment of Tourette's Disorder. Int J Neuropsychopharmacol. 2006;21:773–774. doi: 10.1017/S1461145706006833. [DOI] [PubMed] [Google Scholar]
- Correll C. Antipsychotic use in children and adolescents: Minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47:9–20. doi: 10.1097/chi.0b013e31815b5cb1. [DOI] [PubMed] [Google Scholar]
- Davies L. Stern JS. Agrawal N. Robertson MM. A case series of patients with Tourette's syndrome in the United Kingdom treated with aripiprazole. Hum Psychopharmacol. 2006;21:447–453. doi: 10.1002/hup.798. [DOI] [PubMed] [Google Scholar]
- Dehning S. Riedel M. Muller N. Aripiprazole in a patient vulnerable to side effects. Am J Psychiatry. 2005;162:625. doi: 10.1176/appi.ajp.162.3.625. [DOI] [PubMed] [Google Scholar]
- Duane DD. Aripiprazole in childhood, adolescence for Tourette syndrome. J Child Neurol. 2006;21:358. doi: 10.1177/08830738060210041303. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN. Siamouli M. Kantartzis S. Panagiotidis P. Iacovides A. Kaprinis GS. Acute dystonia with low-dosage aripiprazole in Tourette's disorder. Ann Pharmacother. 2006;40:775–777. doi: 10.1345/aph.1G331. [DOI] [PubMed] [Google Scholar]
- Green WH. Child, Adolescent Clinical Psychopharmacology. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Greenhill LL. Vitiello B. Fisher P. Levine J. Davies M. Abikoff H. Chrisman AK. Chuang S. Findling RL. March J. Scahill L. Walkup J. Riddle MA. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2004;43:1488–1496. doi: 10.1097/01.chi.0000142668.29191.13. [DOI] [PubMed] [Google Scholar]
- Hounie A. De Mathis A. Sampaio AS. Mercadante MT. Aripiprazole, Tourette syndrome. Rev Bras Psiquiatr. 2004;26:213. doi: 10.1590/s1516-44462004000300015. [DOI] [PubMed] [Google Scholar]
- Kastrup A. Schlotter W. Plewnia C. Bartels M. Treatment of tics in Tourette syndrome with aripiprazole. J Clin Psychopharmacol. 2005;25:94–96. doi: 10.1097/01.jcp.0000150229.69124.66. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kawohl W. Schneider F. Vernaleken I. Neuner I. Aripiprazole in the pharmacotherapy of Gilles de la Tourette syndrome in adult patients. World J Biol Psychiatry. 2008:1–5. doi: 10.1080/15622970701762544. [DOI] [PubMed] [Google Scholar]
- Kolotkin RL. Corey-Lisle PK. Crosby RD. Kan HJ. McQuade RD. Changes in weight and weight-related quality of life in a multicentre, randomized trial of aripiprazole versus standard of care. Eur Psychiatry. 2008;23:561–566. doi: 10.1016/j.eurpsy.2008.01.1421. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Riddle MA. Hardin MT. Ort SI. Swartz KL. Stevenson J. Cohen DJ. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Marder SR. McQuade RD. Stock E. Kaplita S. Marcus R. Safferman AZ. Saha A. Ali M. Iwamoto T. Aripiprazole in the treatment of schizophrenia: Safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003;61:123–136. doi: 10.1016/s0920-9964(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Murphy TK. Bengtson MA. Soto O. Edge PJ. Sajid MW. Shapira N. Yang M. Case series on the use of aripiprazole for Tourette syndrome. Neuropsychopharmacol. 2005;8:489–490. doi: 10.1017/S1461145705005365. [DOI] [PubMed] [Google Scholar]
- Pringsheim T. Marras C. Pimozide for tics in Tourette's syndrome. Cochrane Database Syst Rev. 2009:CD006996. doi: 10.1002/14651858.CD006996.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR. Nesbitt L. Jackson C. Sine L. Sethuraman G. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette's disorder. Am J Psychiatry. 1997;154:1057–1062. doi: 10.1176/ajp.154.8.1057. [DOI] [PubMed] [Google Scholar]
- Seo WS. Sung HM. Sea HS. Bai DS. Aripiprazole treatment of children, adolescents with Tourette disorder or chronic tic disorder. J Child Adolesc Psychopharmacol. 2008;8:197–205. doi: 10.1089/cap.2007.0064. [DOI] [PubMed] [Google Scholar]
- Shapiro AK. Shapiro E. Wayne HL. Treatment of Gilles de la Tourette's syndrome with haloperidol: Review of 34 cases. Arch Gen Psychiatry. 1973;28:702–723. doi: 10.1001/archpsyc.1973.01750310070010. [DOI] [PubMed] [Google Scholar]
- Stenstrom AD. Sindo I. Aripiprazole for the treatment of Tourette's syndrome. Ugeskr Laeger. 2008;170:58. [PubMed] [Google Scholar]
- Winter C. Heinz A. Kupsch A. Strohle A. Aripiprazole in a case presenting with Tourette syndrome. obsessive-compulsive disorder. J Clin Psychopharmacol. 2008;28:452–454. doi: 10.1097/JCP.0b013e31817d86cc. [DOI] [PubMed] [Google Scholar]
- Yoo HK. Kim JY. Kim CY. A pilot study of aripiprazole in children and adolescents with Tourette's disorder. J Child Adolesc Psychopharmacol. 2006;16:505–506. doi: 10.1089/cap.2006.16.505. [DOI] [PubMed] [Google Scholar]