Abstract
Cell transplantation for the treatment of joint disease is an important clinical tool. Genetic modification of cells before transplantation has shown enhanced healing. Ex vivo genetic modification of joint tissue cells with various adeno-associated virus (AAV) serotypes has not been investigated. The transduction efficiencies of self-complementary AAV serotypes (1–6 and 8) were determined in joint tissue containing chondrocytes and synoviocytes isolated from equine models. When comparing scAAV serotypes for efficient transduction ex vivo, in chondrocytes versus synoviocytes, serotypes 6 and 2, and serotypes 3 and 2, respectively, appeared superior for gene expression. Unlike adenoviral vectors, no upregulation of inflammatory markers, such as matrix metalloproteinases and aggrecanase, was seen on treatment of joint tissue with AAV vectors ex vivo. Our findings also corroborate that ex vivo transduction of joint tissue can result in high transgene protein levels over time, and transplantation modalities might be feasible using AAV vectors in the treatment of joint-related diseases.
The field of gene therapy has shown much promise in the area of musculoskeletal diseases, specifically arthritis (Evans and Robbins, 1994; Ghivizzani et al., 1998; Goater et al., 2000; Kafienah et al., 2003; Evans, 2004; Evans et al., 2004; Goodrich et al., 2006, 2007). There are myriad proteins that have been identified as decreasing catabolism and increasing anabolism in joints through targeting the main cell types, chondrocytes and synoviocytes (Ulrich-Vinther, 2007). Gene therapy using both in vivo and ex vivo approaches offers the potential of longer term protein expression than recombinant proteins and benefits such as steady state protein expression, delivery of proteins to cells surrounded by matrix such as chondrocytes in the deep layers of cartilage, and higher overall levels of therapeutic proteins. In addition, further development of stem cell therapy strengthens the need to maximize ex vivo gene delivery.
Various viral and nonviral vectors have been studied in the field of gene therapy for arthritis (Evans, 2004; Evans et al., 2004; Gouze et al., 2002, 2003, 2004). Recombinant adeno-associated viral (AAV) vectors are highly attractive vectors for the treatment of joint diseases by virtue of their ability to promote long-term expression and to infect nondividing cells, and because of the broad tissue tropism exhibited by AAV serotypes (Ulrich-Vinther, 2007). To date, most studies of joint tissues have used recombinant AAV (rAAV) serotype 2 (Madry et al., 2003, 2005; Cucchiarini and Madry, 2005; Ulrich-Vinther et al., 2005; Dai and Rabie, 2007a). However, the prevalence of antibodies against this serotype is high in the human population (Grimm and Kay, 2003) and one study by Adriaansen and colleagues reported a significantly greater immune response to rAAV2 compared with rAAV5 when injected into the ankle joints of rats (Adriaansen et al., 2005). At present, at least 12 naturally occurring serotypes of AAV have been isolated, cloned, and sequenced (AAV1 to AAV12) (Choi et al., 2005; Gao et al., 2005; Schmidt et al., 2008). Studies conducted in various skeletal tissues have revealed a wide array of tissue transduction efficiencies depending on serotype (Riviere et al., 2006; Dai and Rabie, 2007b). In 2001, McCarty and colleagues reported increased efficacy of rAAV vectors by synthesizing a self-complementary recombinant AAV vector (scAAV) that spontaneously reanneals, alleviating the requirement rAAV has for host cell DNA synthesis (McCarty et al., 2001). Bypassing rate-limiting second-strand DNA synthesis within the host cell resulted in 5- to 140-fold more efficient transduction than conventional rAAV (McCarty et al., 2001). This step becomes more important when considering cell types that have little to no DNA replication taking place (i.e., neurons, cardiomyocytes, and chondrocytes). This has direct implications for the successful use of scAAV vectors to treat joint diseases because the cells of joints have a low rate of cell division.
Previous studies have shown that ex vivo genetic modification of cells before implantation can enhance cartilage healing (Hidaka et al., 2003; Goodrich et al., 2005, 2007; Madry et al., 2005). No investigations have been performed that evaluate the efficacy of various scAAV serotypes ex vivo, before transplantation. Typically, these cells are implanted into a cartilage defect 48–72 hr after transduction (Hidaka et al., 2003; Goodrich et al., 2007). Establishment of the appropriate scAAV serotype to maximize transduction of these cells would be of great interest to clinicians and researchers using this important clinical tool.
In an effort to determine the maximal ex vivo transduction efficiency of scAAV vectors in joint tissues, we tested scAAV serotypes 1, 2, 3, 4, 5, 6, and 8 (scAAV1–6 and scAAV8) in chondrocytes and synoviocytes. Initial screening tests were first performed to evaluate transduction. Further testing was performed with optimal serotypes to evaluate transduction efficiency, appropriate viral dose, potential cell toxicity, length of protein expression, and cytokine response to serotypes. To the best of our knowledge, this is the first study to evaluate scAAV serotypes in chondrocytes and synoviocytes. The data described herein will be relevant for the design of future ex vivo transduction studies involving scAAV vectors in therapeutic gene transfer to joint tissue.
All scAAV vectors used in this study were provided by the Gene Therapy Center Vector Core Facility (Chapel Hill, CA). The scAAVGFP construct (pHpa-trs-SK) used in this study has been described previously (McCarty et al., 2001) and cross-packaging in various serotype capsids was performed as described (Rabinowitz et al., 2002). Statistical analysis involved one-way analysis of variance (ANOVA) of all continuous data with Tukey post-hoc comparison when statistical significance was detected. Ordinal data (viability and cells scores) were analyzed by Kruskal–Wallis test. Statistical significance was determined when p ≤ 0.05 and a trend was noted when 0.05 < p < 0.1.
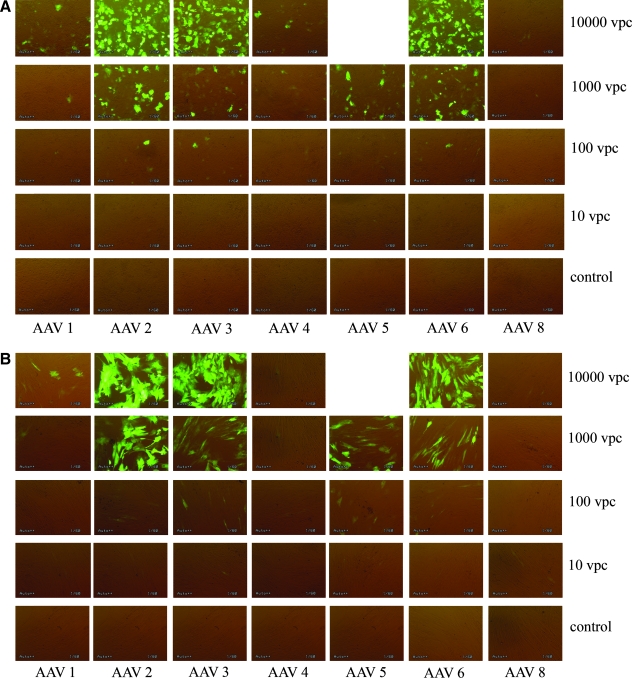
For preliminary screening of AAV serotypes, chondrocytes and synoviocytes were harvested from femoropatellar joints of neonatal foals (1 to 2 days old) at postmortem, and stored in liquid nitrogen until ready for use (Nixon et al., 1992). Cells were thawed for plating as previously described (Brower-Toland et al., 2001; Saxer et al., 2001) and monolayer cultures in HEPES-buffered F-12 medium with 10% fetal bovine serum (FBS) (GIBCO, Grand Island, NY) at 50% confluence were established in 24-well plates. One day after seeding, cells were transduced with scAAV1–6 and scAAV8 packaging the green fluorescent protein (GFP)-encoding transgene driven by the cytomegalovirus (CMV) promoter at various levels of viral particles per cell (VPC). Fluorescence micrographs were obtained every 3 days for the first 30 days and then weekly for up to 90 days to monitor GFP expression. As shown in Fig. 1, preliminary screening of scAAVGFP serotypes revealed that scAAV2, 3, 5, and 6 transduced chondrocyte/synoviocyte cultures at high efficiency by day 7. scAAV1, 4, and 8 had minimal GFP expression at all of the viral doses tested in both chondrocytes and synoviocytes. Transduction efficiencies of serotypes at 10,000 VPC followed the order scAAV2 > scAAV6 > scAAV3 in chondrocytes (Fig. 1A) and scAAV2 > scAAV3 > scAAV6 in synoviocytes (Fig. 1B). Although scAAV5GFP was not tested at this VPC dose (due to low titer), these vectors appeared to transduce joint tissue with efficiency similar to scAAV3 and scAAV6 vectors at 1000 VPC.
FIG. 1.
Initial screening of scAAVGFP1, 2, 3, 4, 5, 6 and 8 in (A) chondrocytes and (B) synoviocytes in monolayer cell culture on day 7. scAAV2, 3, 5, and 6 had the greatest amount of GFP expression. The viral dose of 10,000 viral particles per cell (VPC) had the highest percentage transduction. Bottom labels indicate serotypes and right column labels indicate the VPC. Original magnification, × 40.
This observed trend is in general agreement with other studies that demonstrate serotype selectivity in skeletal muscle (Riviere et al., 2006) or chondrocytes and bone marrow-derived mesenchymal stem cells (Dai and Rabie, 2007a). In a later study by Dai and colleagues, however, rAAV serotype 1 appeared to transduce rat chondrocytes efficiently (Dai and Rabie, 2007b), which is in contrast to our findings in the equine model. This can likely be attributed to species-specific differences in tissue tropism of AAV serotypes (Duan et al., 2000; Sanlioglu et al., 2001), and stresses the need to test various AAV serotypes in the species of interest before testing therapeutic transgenes.
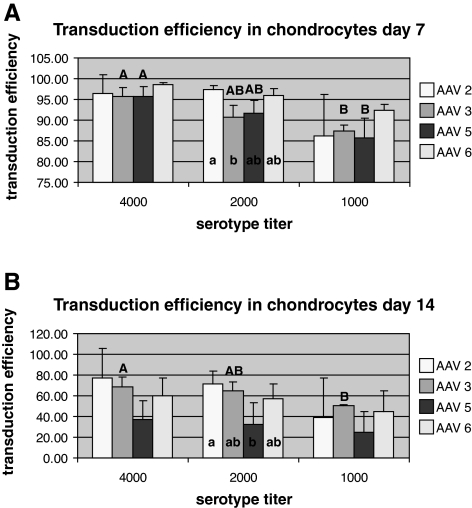
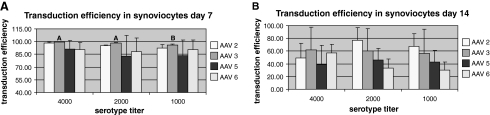
On the basis of this preliminary screening, we quantitated the transduction efficiency of scAAV2, 3, 5, and 6 in chondrocytes and synoviocytes by flow cytometry-assisted cell sorting on days 7 and 14 posttransduction. Briefly, sorting was conducted with a MoFlo cell sorter/analyzer (Beckman Coulter, Fullerton, CA) with a 100-μm flow cell tip used at a sheath pressure of 30 psi and a flow rate of 12,000 events per second, with a laser line of 488 nm and a laser power of 110. Cells were sorted on the basis of GFP fluorescence with a 530/540 bandpass filter, preceded by a neutral-density 2.0 filter and high voltage of 400–450 with a log signal. Summit software (version 4.0; Beckman Coulter) was used to collect histograms and set sort parameters. As shown in Fig. 2A, on day 7, 95–99% GFP-positive cells were detected for scAAV2, 3, 5, and 6 at 4000 VPC in chondrocytes. No significant differences were seen in transduction efficiencies between the serotypes (~85–90% GFP-positive cells) for scAAV2, 3, 5, and 6 at a dose of 4000 VPC. Of the dosing groups, AAV3 and AAV5 had significantly better transduction efficiencies at 4000 VPC than at 1000 VPC and within the dosing groups, AAV2 at 2000 VPC had significantly greater transducing efficiency than AAV3. However, as seen in Fig. 2B, analysis of samples on day 14 revealed a statistically significant lower transduction efficiency for scAAV5 (~36% GFP-positive cells) in comparison with scAAV2 at 2000 VPC. Dose did not affect serotype transduction efficiency on day 14 except for AAV3, for which 4000 VPC was better than 1000 VPC. In synoviocytes, a transduction efficiency of 85–100% (GFP-positive cells) was seen for each serotype tested on day 7 posttransduction (Fig. 3A). AAV3 had a higher transduction efficiency at 4000 and 2000 VPC than at 1000 VPC. At 14 days there were no significant differences between AAV serotypes 2, 3, 5, and 6 at any dose. Further, there were no significant differences within any serotype at doses of 4000, 2000, and 1000 VPC (Fig. 3B). For scAAV2, 3, 5, and 6, fluorescence appeared to begin approximately 2 days after transduction and reached maximal fluorescence at approximately 5 days after transduction.
FIG. 2.
Quantitative analysis (flow cytometry-assisted cell sorting) of the transduction efficiency of scAAVGFP2, 3, 5, and 6. Transduction was performed on chondrocytes in monolayer at (A) 7 days and (B) 14 days. Flow cytometry was performed on days 7 and 14 after transduction with 4000, 2000, and 1000 VPC. Capital letters denote significant differences in titer within serotypes, and uncapitalized letters denote significant differences between serotypes within each titer tested.
FIG. 3.
Quantitative analysis (flow cytometry-assisted cell sorting) of the transduction efficiency of scAAVGFP2, 3, 5, and 6. Transduction was performed on synoviocytes in monolayer at (A) 7 days and (B) 14 days. Flow cytometry was performed on days 7 and 14 after transduction with 4000, 2000, and 1000 VPC. Capital letters denote significant differences in titers within serotypes.
At 14 days both chondrocytes and synoviocytes were approximately doubled in number from day 7, which accounts for the 50% decrease in the number of cells emitting GFP. This decrease in GFP-positive cell number over time can be attributed to dilution of AAV vector genomes and is expected because of the persistence of AAV vector genomes in episomal rather than integrated form (Bartlett et al., 2000). At 51 days, both chondrocytes and synoviocytes had at least 30% GFP-positive cells when transduced with AAV2 or AAV6 (data not shown). This long-term expression is encouraging for therapeutic gene transfer applications in cartilage healing, osteoarthritis, and rheumatoid arthritis, for which the healing time required is prolonged compared with soft tissue (months vs. days) (Sams and Nixon, 1993; Hendrickson et al., 1994).
For cell viability studies, trypan blue staining and morphological studies were carried out on days 0, 3, 7, 10, and 14 posttransduction. Cells were given a score of 0 through 5 on the basis of morphological characteristics. Abnormal morphological characteristics were considered to be rounding, crenation, or lifting off the plate. A score of 1 = 1–20% of the cells had abnormal cell morphology, 2 = 21–40, 3 = 41–60, 4 = 61–80, and 5 = 81–100%. Five views at × 200 magnification were assessed and an average score was determined. As outlined in Table 1, scAAV2 vectors appear to have some negative impact on both chondrocytes and synoviocytes, based on the observation that several cells became rounded, crenated, or detached from the plate. A trend (0.05 < p < 0.1) was detected on days 7, 10, and 14, wherein scAAV2GFP had the highest score (greatest morbidity) at each time point. The reason behind this effect is not clear. No significant differences were detected for viability between any serotype or titer.
Table 1.
Average Cell Viability and Cell Scoresa for Chondrocytes and Synoviocytes over 14 Days
| |
scAAV2 |
scAAV3 |
scAAV5 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Control | 4000 VPC | 2000 VPC | 1000 VPC | Control | 4000 VPC | 2000 VPC | 1000 VPC | Control | 4000 VPC | 2000 VPC | 1000 VPC |
| Chondrocyte Morphology Scores | ||||||||||||
| 3 | 0 (0) | 1.7 (2.1) | 0.7 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.7 (1.2) | 0 (0) | 0 (0) |
| 7 | 0 (0) | 2.7 (1.2)b | 2.3 (0.6)b | 2.0 (1) | 0 (0) | 0.3 (0.6) | 0.3 (0.6) | 0.7 (0.6) | 0 (0) | 0.3 (0.6) | 0 (0) | 0.3 (0.6) |
| 10 | 0 (0) | 3 (1)b | 2.7 (1.2)b | 2.3 (1.5) | 0 (0) | 1.3 (0.6) | 1.0 (0) | 1.0 (0) | 0 (0) | 1.3 (1.2) | 1 (1.0) | 1 (1.0) |
| 14 | 0 (0) | 3 (1)b | 2.7 (1.2)b | 2.3 (1.5) | 0 (0) | 1.3 (0.6) | 0.7 (0.6) | 0.7 (0.6) | 0 (0) | 1.0 (1.0) | 0.7 (0.6) | 0.7 (0.6) |
| Chondrocyte Viability | ||||||||||||
| 7 | 94 (2) | 81 (21) | 87 (5) | 94 (2) | 90 (7) | 94 (3) | 94 (4) | 91 (5) | 96 (4) | 85 (9) | 86 (14) | 73 (17) |
| 14 | 91 (9) | 79 (20) | 85 (5) | 94 (5) | 86 (10) | 91 (5) | 83 (14) | 88 (3) | 85 (8) | 93 (6) | 92 (7) | 79 (16) |
| Synoviocyte Morphology Scores | ||||||||||||
| 3 | 0 (0) | 2.7 (0.6)c | 1.7 (0.6)b | 1.0 (1.0) | 0 (0) | 1.0 (1.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 7 | 0 (0) | 2.7 (0.6)c | 2.0 (0)b | 1.3 (0.6) | 0 (0) | 0.3 (0.6) | 0 (0) | 0.3 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 10 | 0 (0) | 2.3 (1.2)c | 1.7 (0.6)b | 1.3 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.3 (0.6) | 0.3 (0.6) | 0 (0) |
| 14 | 0 (0) | 2.0 (1.0)c | 1.7 (0.6)b | 1.3 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.3 (0.6) | 0.3 (0.6) | 0 (0) |
| Synoviocyte Viability | ||||||||||||
| 7 | 92 (2) | 66 (18) | 81 (9) | 80 (22) | 94 (2) | 83 (12) | 87 (6) | 85 (3) | 90 (6) | 81 (13) | 87 (5) | 95 (4) |
| 14 | 85 (4) | 63 (28) | 83 (14) | 75 (17) | 85 (11) | 83 (15) | 79 (11) | 73 (18) | 77 (10) | 67 (14) | 68 (30) | 36 (12) |
Abbreviations: scAAV2, 3, and 5; self-complementary AAV serotypes 2, 3, and 5; VPC = viral particles per cell.
Results are given with standard deviation in parentheses.
A trend (0.05 < p < 0.1) toward significant morphological changes compared with control cells.
A significant (p < 0.05) morphological change compared with control cells.
Last, matrix metalloproteinases (MMPs) and aggrecanase levels are considered a good representation of inflammatory changes in joint tissues (Ishiguro and Kojima, 2004). An increase in these molecules has been noted with adenoviral vectors (Goodrich et al., 2007). Therefore, in an effort to detect any inflammatory response to AAV serotypes, we carried out quantitative RT-PCR assays to monitor the impact of AAV vectors on expression levels of equine MMP-1, MMP-3, MMP-13, and aggrecanase-1, markers of the inflammatory response in joint tissue. Briefly, RNA was extracted from cells on day 7, using a QIAshredder and RNeasy mini kit (Qiagen, Valencia, CA), and complementary DNA was generated using 50 ng of total RNA and oligo(dT) primers with a SuperScript III first-strand synthesis kit (Invitrogen, Carlsbad, CA). mRNA expression of target genes was compared with the expression levels of equine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as control. Equine MMP-1, MMP-3, MMP-13, and GAPDH primer/probes were obtained from the Lucy Whittier Molecular & Diagnostic Core Facility (University of California Davis, Davis, CA). Primer sequences were as follows: MMP-1 forward, 5′-AAGCTGCTTATGAGGTTTCCCA; reverse, 5′-GGGTATCCGTAGAGCACATCCT; probe, 5′-FAM-AGCCCAGTACTTATTACCTTTGAAAAACCGGAC-TAMRA-3′; MMP-3 forward, 5′-AACACTGGACGAAGGATGCAT; reverse, 5′-ACCCAGGGAATGACCAAGTTC; probe, 5′-FAM-AGGGATCAATTTTCTCCTTGTTGCTGCTCA-TAMRA-3′. Aggrecanase sequences were as follows: forward, 5′-GCCTTCACTGCTGCTCATGA; reverse, 5′-CCAACACATGGCTTTGAATTGT; and probe, 5′-FAM-CTGGGCCATGTCTTCAACATGCTCC-TAMRA-3′. Quantitative RT-PCR was performed, with 2.5 ng of RNA per sample (in duplicate) and a 384-well LightCycler 480 (Roche, Indianapolis, IN). Sample cycle threshold data were normalized to standards for each gene and compared with each other by determining the ratio of the gene of interest to GAPDH. No significant trends were noted in the inflammatory response to AAV vectors, with the overall effects being negligible. Although not statistically significant, a slight increase in aggrecanase RNA expression was noted in both chondrocytes and synoviocytes for scAAV3 vectors. These results seem to be consistent with published observations that AAV, when compared with other DNA viruses (e.g., adenovirus), is dramatically different with respect to the acute immune response (Wu et al., 2006).
In conclusion, this study identified four serotypes of scAAV that are effective in transducing primary chondrocytes and synoviocytes ex vivo. This is of significant importance because implantation of primary cells (chondrocytes and synoviocytes) for joint therapy in both human and equine patients is routine for osteochondritis dissecans (OCD) and acute cartilage traumatic lesions and would dramatically benefit from therapeutic gene transfer augmentation (IGF-I, IRAP, and BMP-7) (Marlovits et al., 2004; Fortier and Nixon, 2005). In chondrocytes, scAAV6 and scAAV2 appear to be the most efficient whereas in synoviocytes, scAAV3 and scAAV2 were more robust. With the identification of surface receptors for AAV1, 2, 4, 5, and 6 (Wu et al., 2006), and the high degree of sequence homology between AAV2 and AAV3 (88%), the potential for this information to facilitate the generation and testing of chimeric AAV particles with improved chondrocyte and synovial cell transduction is imminent (Wu et al., 2006). We have successfully transplanted chondrocytes in a lesion in equine joints and tracked cell viability out to 9 weeks (Goodrich et al., 2005). These observations and our findings that self-complementary AAV can efficiently transduce joint tissue ex vivo, resulting in high transgene protein levels over time, have set the stage for evaluating genetically modified therapeutic cells for transplantation in diseased joints.
Acknowledgments
This study was funded by NIH 1K08AR054903-01A2 and by a Colorado State University College Research Council grant.
Author Disclosure Statement
The authors (L.R. Goodrich, V.W. Choi, B.A. Duda Carbone, C.W. McIlwraith, and R.J. Samulski) do not have any conflicts of interest in the publication of this manuscript.
References
- Adriaansen J. Tas S.W. Klarenbeek P.L. Bakker A.C. Apparailly F. Firestein G.S. Jorgensen C. Vervoordeldonk M.J. Tak P.P. Enhanced gene transfer to arthritic joints using adeno-associated virus type: Implications for intra-articular gene therapy. Ann. Rheum. Dis. 2005;64:1677–1684. doi: 10.1136/ard.2004.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J.S. Wilcher R. Samulski R.J. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower-Toland B.D. Saxer R.A. Goodrich L.R. Mi Z. Robbins P.D. Evans C.H. Nixon A.J. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum. Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- Choi V.W. McCarty D.M. Samulski R.J. AAV hybrid serotypes: Improved vectors for gene delivery. Curr. Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiarini M. Madry H. Gene therapy for cartilage defects. J. Gene Med. 2005;7:1495–1509. doi: 10.1002/jgm.824. [DOI] [PubMed] [Google Scholar]
- Dai J. Rabie A.B. Direct AAV-mediated gene delivery to the temporomandibular joint. Front. Biosci. 2007a;12:2212–2220. doi: 10.2741/2224. [DOI] [PubMed] [Google Scholar]
- Dai J. Rabie A.B. Recombinant adeno-associated virus vector hybrids efficiently target different skeletal cells. Front. Biosci. 2007b;12:4280–4287. doi: 10.2741/2387. [DOI] [PubMed] [Google Scholar]
- Duan D. Yue Y. Yan Z. Yang J. Engelhardt J.F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.H. Gene therapies for osteoarthritis. Curr. Rheumatol. Rep. 2004;6:31–40. doi: 10.1007/s11926-004-0081-5. [DOI] [PubMed] [Google Scholar]
- Evans C.H. Robbins P.D. Gene therapy for arthritis. In: Wolff J.A., editor. Gene Therapeutics: Methods and Applications of Direct Gene Transfer. Birkhauser; Cambridge: 1994. pp. 320–343. [Google Scholar]
- Evans C.H. Gouze J.N. Gouze E. Robbins P.D. Ghivizzani S.C. Osteoarthritis gene therapy. Gene Ther. 2004;11:379–389. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- Fortier L.A. Nixon A.J. New surgical treatments for osteochondritis dissecans and subchondral bone cysts. Vet. Clin. North Am. Equine Pract. 2005;21:673–690. doi: 10.1016/j.cveq.2005.07.005. vii. [DOI] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Wilson J.M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Ghivizzani S.C. Lechman E.R. Kang R. Tio C. Kolls J. Evans C.H. Robbins P.D. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor α soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4613–4618. doi: 10.1073/pnas.95.8.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goater J. Muller R. Kollias G. Firestein G. Sanz I. O'Keefe R.J. Schwarz E.M. Empirical advantages of adeno-associated viral vectors in in vivo gene therapy for arthritis. J. Rheumatol. 2000;27:983–989. [PubMed] [Google Scholar]
- Goodrich L.R. Nixon A.J. Hidaka C. Robbins P.D. Evans C.H. Enhanced early repair and cell survival in cartilage defects implanted with chondrocytes over expressing insulin-like growth factor-I. Vet. Surg. 2005;34:E8. [Google Scholar]
- Goodrich L.R. Brower-Toland B.D. Warnick L. Robbins P.D. Evans C.H. Nixon A.J. Direct adenovirus-mediated IGF-I gene transduction of synovium induces persisting synovial fluid IGF-I ligand elevations. Gene Ther. 2006;13:1253–1262. doi: 10.1038/sj.gt.3302757. [DOI] [PubMed] [Google Scholar]
- Goodrich L.R. Hidaka C. Robbins P.D. Evans C.H. Nixon A.J. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J. Bone Joint Surg. Br. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- Gouze E. Pawliuk R. Pilapil C. Gouze J.N. Fleet C. Palmer G.D. Evans C.H. Leboulch P. Ghivizzani S.C. In vivo gene delivery to synovium by lentiviral vectors. Mol. Ther. 2002;5:397–404. doi: 10.1006/mthe.2002.0562. [DOI] [PubMed] [Google Scholar]
- Gouze E. Pawliuk R. Gouze J.N. Pilapil C. Fleet C. Palmer G.D. Evans C.H. Leboulch P. Ghivizzani S.C. Lentiviral-mediated gene delivery to synovium: Potent intra-articular expression with amplification by inflammation. Mol. Ther. 2003;7:460–466. doi: 10.1016/s1525-0016(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Gouze J.N. Gouze E. Palmer G.D. Kaneto H. Ghivizzani S.C. Grodzinsky A.J. Evans C.H. Adenovirus-mediated gene transfer of glutamine: Fructose-6-phosphate amidotransferase antagonizes the effects of interleukin-1β on rat chondrocytes. Osteoarthritis Cartilage. 2004;12:217–224. doi: 10.1016/j.joca.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Grimm D. Kay M.A. From virus evolution to vector revolution: Use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr. Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- Hendrickson D.A. Nixon A.J. Grande D.A. Todhunter R.J. Minor R.R. Erb H. Lust G. Chondrocyte-fibrin matrix transplants for resurfacing extensive articular cartilage defects. J. Orthop. Res. 1994;12:485–497. doi: 10.1002/jor.1100120405. [DOI] [PubMed] [Google Scholar]
- Hidaka C. Goodrich L.R. Chen C.T. Warren R.F. Crystal R.G. Nixon A.J. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J. Orthop. Res. 2003;21:573–583. doi: 10.1016/S0736-0266(02)00264-4. [DOI] [PubMed] [Google Scholar]
- Ishiguro N. Kojima T. [Role of aggrecanase and MMP in cartilage degradation] Clin. Calcium. 2004;14:38–44. [PubMed] [Google Scholar]
- Kafienah W. Al Fayez F. Hollander A.P. Barker M.D. Inhibition of cartilage degradation: A combined tissue engineering and gene therapy approach. Arthritis Rheum. 2003;48:709–718. doi: 10.1002/art.10842. [DOI] [PubMed] [Google Scholar]
- Madry H. Cucchiarini M. Terwilliger E.F. Trippel S.B. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum. Gene Ther. 2003;14:393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- Madry H. Kaul G. Cucchiarini M. Stein U. Zurakowski D. Remberger K. Menger M.D. Kohn D. Trippel S.B. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- Marlovits S. Kutscha-Lissberg F. Aldrian S. Resinger C. Singer P. Zeller P. Vécsei V. Autologous chondrocyte transplantation for the treatment of articular cartilage defects in the knee joint: Techniques and results. Radiologe. 2004;44:763–772. doi: 10.1007/s00117-004-1082-0. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Monahan P.E. Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Nixon A.J. Lust G. Vernier-Singer M. Isolation, propagation and cryopreservation of equine articular chondrocytes. Am. J. Vet. Res. 1992;53:2364–2370. [PubMed] [Google Scholar]
- Rabinowitz J.E. Rolling F. Li C. Conrath H. Xiao W. Xiao X. Samulski R.J. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere C. Danos O. Douar A.M. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- Sams A.E. Nixon A.J. MS thesis. Cornell University; Ithaca, New York: 1993. Studies of chondrocyte laden collagen scaffolds for resurfacing extensive equine articular cartilage defects. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S. Monick M.M. Luleci G. Hunninghake G.W. Engelhardt J.F. Rate limiting steps of AAV transduction and implications for human gene therapy. Curr. Gene Ther. 2001;1:137–147. doi: 10.2174/1566523013348788. [DOI] [PubMed] [Google Scholar]
- Saxer R.A. Bent S.J. Brower-Toland B.D. Mi Z. Robbins P.D. Evans C.H. Nixon A.J. Gene mediated insulin-like growth factor-I delivery to the synovium. J. Orthop. Res. 2001;19:759–767. doi: 10.1016/S0736-0266(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Voutetakis A. Afione S. Zheng C. Mandikian D. Chiorini J.A. Adeno-associated virus type 12 (AAV12): A novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J. Virol. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Vinther M. Gene therapy methods in bone and joint disorders: Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing. Acta Orthop. Suppl. 2007;78:1–64. [PubMed] [Google Scholar]
- Ulrich-Vinther M. Stengaard C. Schwarz E.M. Goldring M.B. Soballe K. Adeno-associated vector mediated gene transfer of transforming growth factor-β1 to normal and osteoarthritic human chondrocytes stimulates cartilage anabolism. Eur. Cell Mater. 2005;10:40–50. doi: 10.22203/ecm.v010a05. [DOI] [PubMed] [Google Scholar]
- Wu Z. Asokan A. Samulski R.J. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol. Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]