Abstract
Flies, like all animals that depend on vision to navigate through the world, must integrate the optic flow created by self-motion with the images generated by prominent features in their environment. Although much is known about the responses of Drosophila melanogaster to rotating flow fields, their reactions to the more complex patterns of motion that occur as they translate through the world are not well understood. In the present study we explore the interactions between two visual reflexes in Drosophila: object fixation and expansion avoidance. As a fly flies forward, it encounters an expanding visual flow field. However, recent results have demonstrated that Drosophila strongly turn away from patterns of expansion. Given the strength of this reflex, it is difficult to explain how flies make forward progress through a visual landscape. This paradox is partially resolved by the finding reported here that when undergoing flight directed towards a conspicuous object, Drosophila will tolerate a level of expansion that would otherwise induce avoidance. This navigation strategy allows flies to fly straight when orienting towards prominent visual features.
Keywords: flight control, Drosophila, vision, optic flow, stripe fixation, expansion avoidance
INTRODUCTION
The tethered flight preparation allows the study of an intact sensorimotor system while permitting a high degree of control over the animal's sensory experience. Such experiments may be operated in either ‘open loop’, in which the flies' reactions to specified patterns of visual motion are simply measured, or ‘closed loop’, in which the flies' turning reactions are configured to control the motion of the visual display. While flying under such rotational closed-loop conditions, tethered Drosophila will vigorously orient towards a prominent vertical stripe (Heisenberg and Wolf, 1979). This behavior, termed fixation, is so robust that in a remarkable experiment flies exhibited sustained object orientation during a nearly continuous 32-hour period (Gotz, 1987). More recently it has been established that tethered Drosophila strongly avoid the focus of expansion (FOE) within a panoramic pattern that coarsely approximates the optic flow seen during translatory flight. This avoidance of visual expansion results in an equally robust closed-loop paradigm wherein flies will actively orient towards the focus of contraction (FOC) (Tammero et al., 2004). These two reflexes probably serve very different functions. In the case of object orientation, the reflex allows the animal to navigate towards conspicuous visual targets whereas expansion avoidance is likely to serve as a rapid response to an imminent collision (Duistermars et al., 2007b) or a compensatory reaction to a strong gust (Reiser et al., 2004). The robustness of this closed-loop expansion avoidance (and contraction fixation) behavior is paradoxical because in order to move forward an animal must tolerate a frontal FOE as it navigates through a visual landscape. If animals robustly turn away from frontal expansion, how do they ever make forward progress? One possibility is that the attractiveness of a visual landmark is able to override the collision-avoidance reflex and thus at least transiently stabilize forward motion. In this paper we present strong evidence for this hypothesis by conducting a series of experiments with tethered Drosophila, using a ‘wingbeat analyzer’ to optically track the wings (Götz, 1987; Lehmann and Dickinson, 1997) and a recently developed light-emitting diode (LED)-based system (Reiser and Dickinson, 2008) to present visual stimuli. Our results, in combination with several recent findings, suggest that much of the straight flight observed in Drosophila may result from sensory-guided control of flight towards attractive targets.
MATERIALS AND METHODS
Animal preparation and flight arena
Details of the fly preparation and visual display are identical to those described previously (Reiser and Dickinson, 2008). We used 3–4-day-old adult female Drosophila melanogaster Meigen from a laboratory culture originated from 200 wild-caught females. Flies were cold-anesthetized and tethered in a hover posture to a 0.1 mm tungsten rod with UV-activated glue. Flies were kept on a 12h:12 h light:dark cycle, and were tested during the last 5 h of their subjective day. The visual stimulus system consisted of a cylinder constructed from 44 modular LED panel displays with a resolution of 32 pixels × 88 pixels, spanning 330 deg. in azimuth and approximately 94 deg. of elevation. The cylindrical display is not uniformly distant from the retina of a fly suspended in the center, so the angular subtense of each LED depends on its elevation. The maximum pixel size for this arena geometry occurs in the coronal plane that runs through the middle of each of the fly's eyes and subtends a visual angle of 3.75 deg. on the fly's retina. This maximum pixel size is below the interommatidial distance of Drosophila (Heisenberg and Wolf, 1984), so pattern motion is effectively simulated as an apparent motion stimulus; the one-pixel jumps between consecutive frames produce the illusion of continuous motion.
The display system supports eight levels of intensity at each pixel, with a maximum level of 72 cd m−2. The maximum relative (Michelson) contrast of the display for the type of stimuli presented here is approximately 93%. This is the maximum measured contrast when the display is set to show a grating pattern with 100% calculated contrast; the reduction is due to light reflected from the opposite side of the curved display. The linearity of the display's intensity output means that the effective contrast for lower contrast settings should be scaled by 0.93. The instantaneous wing positions were monitored via an optical sensor, called a ‘wingbeat analyzer’ (JFI Electronics Laboratory, University of Chicago, Chicago, IL, USA), described previously (Gotz, 1987; Lehmann and Dickinson, 1997). This device provides the instantaneous measurement of the wing stroke amplitude of the right and left wings of the fly; the difference between these signals is taken as the animals' turning response. Data from the wingbeat analyzer and the visual display were sampled at 500Hz by a Digidata 1320A data acquisition system (Molecular Devices, Sunnyvale, CA, USA). All data analysis was performed offline using software written in MATLAB (Mathworks, Natick, MA, USA). All trials were presented using a random block protocol. All trials during which the flies stopped flying were discarded as determined by the wingbeat frequency signal. All flies that were capable of sustaining orientation towards a 30 deg. stripe and were able to complete at least two of all the trials in each experiment were included in the data set (with the exception of the longer protocol in experiment 1, for which flies completing just one repetition were also included).
Experimental protocols
A series of experiments was conducted using several compound stimulus patterns containing combinations of the expansion-rotation (ER) pattern and a moving object. In all experiments the ER patterns consisted of 4 pixel-wide bars of brighter and dimmer pixels, corresponding to a spatial period of 30deg.cycle−1, selected to match that of the previous study (Tammero et al., 2004). In all experiments the stripe object was a 30 deg.-wide bar of inactive pixels and the low-contrast grating had a calculated contrast of 14.3%.
Experiment 1: closed-loop expansion avoidance versus object orientation
A low-contrast ER pattern was created, containing a stripe embedded at the FOE. The closed-loop orientation preference of flies was tested with the ER and the ER plus stripe patterns, with open-loop expansion rates of 37.5 deg. s−1 and 112.5 deg. s−1, corresponding to temporal frequencies of 1.25 Hz and 3.75 Hz, respectively. The experimental series consisted of 30s of closed-loop object orientation trials on a static background (results not shown), followed by 90 s trials of closed-loop control over one of the two patterns at one of the two speeds of expansion. Trials were initiated with either the FOC or the FOE initially positioned in front, according to a random block design. In total, 16 flies completed between one and two repetitions of the eight trial protocol, each repetition required 16 min. The results are presented in Fig. 1 and Fig. 2A–C.
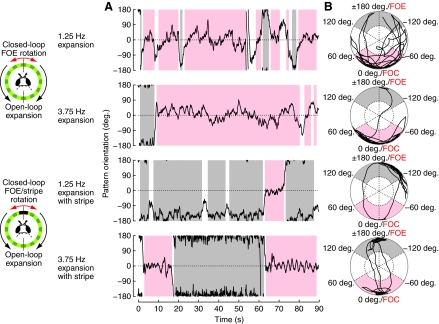
Fig. 1.
A visual paradigm in which flies can preferentially orient towards one of two attractive stimuli – a focus of contraction (FOC) or a dark bar superimposed on the opposing focus of expansion (FOE). (A) Representative trials of orientation data from single flies controlling the position of the foci of an expanding visual pattern (in a behavioral closed loop). All trials shown are initialized with the FOE in front. The trial in the first row consists of low-contrast stripes expanding with a temporal frequency (ft)=1.25 Hz, under these conditions flies preferentially orient towards the FOC but the orientation behavior is weaker than the more robust orientation towards the FOC seen in the second row, corresponding to expansion of low-contrast stripes expanding with a ft=3.75 Hz. In the third row the trial shown consists of low-contrast stripes expanding at ft=1.25 Hz while a high-contrast bar (object) remains fixed at the FOE – in this case the flies preferentially orient towards the FOE with the superimposed stripe. The trial in the fourth row corresponds to a ft=3.75 Hz expansion with a stripe at the FOE, in this case the flies demonstrate a stable but nearly equal preference for these competing stimuli. In this figure (as well as in Fig. 2) black/gray is used to denote FOE orientation, red\pink is used for FOC orientation and white for the case where neither is stably oriented. To quantify the orientation preference in this paradigm, a moving 2 s window of the circular mean and the mean resultant length, r, is computed (B). If r≥0.5, then the pattern position is treated as stable, and if either the FOC is frontal (area shaded pink) or the FOE is frontal (shaded gray), then the window is assigned accordingly.
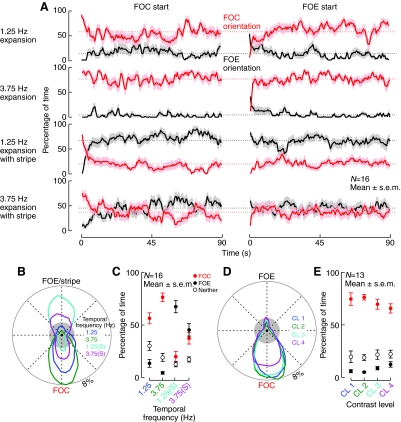
Fig. 2.
Flies will actively orient towards an object fixed to the focus of an expanding pattern, which would otherwise be robustly avoided. (A) The mean (±s.e.m.) percentage of time orienting towards either the focus of expansion (FOE) or the focus of contraction (FOC) during the closed-loop orientation trials described in Fig. 1A. The trials were randomized such that half were initialized with the FOE in front and half with the FOC in front. The broken lines represent the mean orientation percentage combining both initial conditions. Because the long-term orientation behavior is invariant to the initial condition (converging to the average obtained for both starting positions), the summarizing statistics shown in (B) and (C) combine the data from trials with both initial conditions. (B) The mean orientation histograms (plotted in polar coordinates), show the percentage of time that the flies orient towards any single position of the FOE, for each of the two rates of expansion tested alone, as well as with the dark 30 deg. stripe at the FOE, labeled with an (S). The gray circle represents the probability of random orientation. The mean (±s.e.m.) fixation scores, C, reveal the percentage of time that the flies actively regulate the position of either the FOE or the FOC in front (or neither), showing that the strong preference for the FOC is reduced when the stripe is present, and instead, the flies spend most of their time orienting towards the FOE/object even at the higher expansion rate. (D,E) The (polar) mean histograms and mean (±s.e.m.) fixation scores summarizing closed-loop orientation behavior for the four tested contrast levels (CL) of expansion (ft=3.75 Hz), showing that the strong preference for orienting towards the FOC is largely unaffected by the contrast of the pattern.
Experiment 2: contrast dependence of closed-loop expansion avoidance
To test the effect of pattern contrast on the closed-loop expansion avoidance behavior, four patterns were constructed with the same mean luminance but with varying contrast. The bright and dark bars of the patterns were set to intensity levels of 3/4, 2/5, 1/6 and 0/7 (with 0 representing an inactive pixel and 7 corresponding to maximally active), yielding four relative contrast levels (CL 1–4) of 14.3%, 42.9%, 71.4% and 100%, respectively. The rate of open-loop expansion was 112.5 deg. s−1, corresponding to a temporal frequency (ft) of 3.75 Hz. Each pattern (at one of four CL) was presented to tethered flies during 30s closed-loop trials, interspersed with an additional 30 s closed-loop stripe-fixation trial (data not shown in this paper) presented in random block trials. In total, 13 flies completed between two and eight repetitions of this protocol, with each repetition lasting 4 min. The results are shown in Fig. 2D,E.
Experiment 3: open-loop object rotation superimposed on open-loop lateral expansion
The experiment tested two expansion rates (ft of 1.25 Hz and 3.75 Hz), with the FOE positioned laterally and from both sides, with superimposed rotations of the stripe at 120 deg. s−1 in both directions. Additionally, the experimental series included trials where the lateral expansion was presented alone and trials where the stripe was rotated, while the low-contrast (CL 1) striped pattern remained stationary ‘behind’ the stripe. As discussed in the main text, this experiment was designed so that flies were presented with paired symmetrical stimuli that should generate turning responses that were equal in magnitude but opposite in sign, which were then sign-adjusted and averaged first on a per-fly basis and then across flies. This experimental technique provides superior signal conditioning to the typically used normalization strategies [see discussion in Reiser (Reiser, 2006)]. In total, 15 flies completed between three and five repetitions of this protocol, each repetition requiring approximately 4 min. The results are shown in Fig. 3 and further detailed in supplementary material Figs S1 and S2.
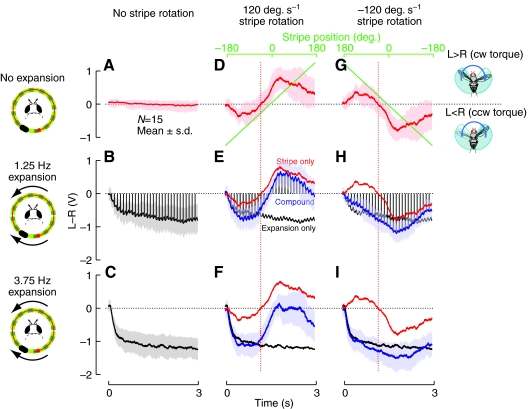
Fig. 3.
The turning response to a compound stimulus depends on the strength and relative configuration of the expansion and object components. The mean (±s.d.) turning response to a non-moving, static grating pattern is shown in A. (B,C) The mean turning response away from a laterally positioned (on the right) focus of expansion (FOE) for the two speeds tested. (D,G) The mean turning response to a dark stripe rotating in both directions over a stationary (low contrast) background is shown; stripe position plotted in green. These stimuli were combined such that the FOE is fixed at the sides while the object is rotated at a constant velocity. In the remaining panels (E,F,H,I) the response to the combined stimulus is shown in blue, while the response to the stripe alone is plotted in red (reproduced from panels D,G) and the response to the expansion-only stimulus is plotted in black (reproduced from panels B,C). The broken red lines show the locations of the zero crossing of the mean stripe response, which precede the time when the stripe actually crosses the midline. The icons depict the conditions with the stripe rotating in the clockwise direction, corresponding to the positive speed trials whose data are shown in the middle column. The vertical black lines in B, E and H mark the times at which the pattern was updated with a one frame advance, to emphasize the repetitive surges in the turning response away from the FOE (downward in this figure), that are a feature of the responses to the slower expansion stimulus. L>R, difference between left and right wingbeat amplitudes; cw torque, clockwise torque; ccw torque, counter-clockwise torque.
Experiment 4: closed-loop object orientation superimposed on open-loop lateral expansion
Closed-loop object orientation was tested in combination with low-contrast, laterally centered expansion, presented in open loop (constant ft). The experiment tested two expansion rates (ft of 1.25 Hz and 3.75 Hz) and a no-expansion case, with the FOE positioned laterally and from both sides, while the fly controlled the position of the stripe in closed loop. All trials begin with the stripe in front of the fly. Each of the five closed-loop trials was presented for 40 s in a random block series interspersed with 20 s open-loop trials whose results are not presented. In total, nine flies completed between two and four repetitions of this protocol, each repetition lasting 5 min. The results are shown in Fig. 4.
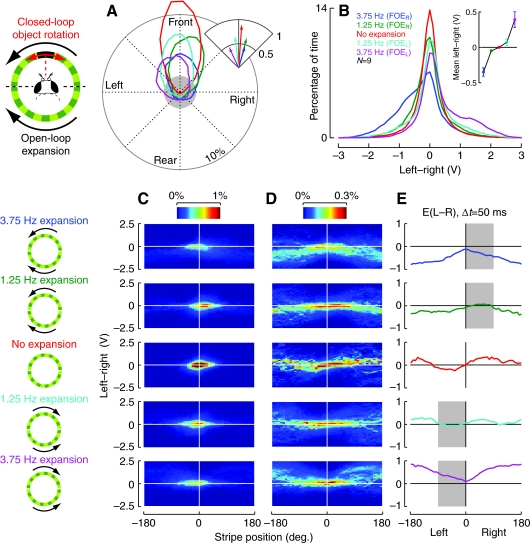
Fig. 4.
Flies are able to orient towards a stripe in the face of constant lateral expansion. (A) The mean polar orientation histograms show that frontal orientation of the object is maintained despite constant expansion. The inset plots the mean of the flies' circular mean orientation direction vector; the length is inversely related to the dispersion in the data. For compactness, only the quadrant centered on the frontal stripe position is shown. (B) The distribution of all instantaneous turning responses (L–R), as measured for all five treatments. The mean (±s.e.m.) turning response is plotted for each condition (inset plot). (C) The relationship between the position of the stripe and the flies' turning response is quantified as a 2-D histogram, showing the distribution of instantaneous turning (L–R) at each position of the stripe. Color is used to represent the percentage of recorded samples that are grouped into each bin; color axis scale bars are at the top for each column. Two versions of the same distributions are shown. In C the histograms represent the true frequency counts of the occurrence of a particular turn amplitude while the pattern is at each position. The histograms in (D) show these same distributions but they have been normalized for each position of the pattern such that each of the 48 columns accounts for (1/48)% of the total probability. In the normalized histograms it is possible to resolve the response of flies to positions of the stripe that are only rarely encountered. (E) The expected value of the turning response at each position of the stripe. To account for sensorimotor conduction delays, the turning response was advanced by 50 ms relative to the object position. The gray bands mark the frontal quadrant on the side of the FOE, the region in which stripe responses are prominent.
RESULTS
In the first experiment we re-examined closed-loop expansion avoidance behavior (Fig. 1). Using the cylindrical electronic display, we created a pattern of vertical stripes with a spatial period of 30 deg. The horizontal motion of the stripes was equal and opposite on two sides of the display, creating a FOE and a FOC separated by 180 deg. The drift speed of the pattern is quantified as the ft, which is equal to the angular velocity of the pattern divided by the spatial period. For the typical trials shown in Fig. 1, ft was either 1.25Hz or 3.75Hz. By adjusting its relative wing stroke amplitudes, a signal that is strongly correlated with yaw torque (Tammero et al., 2004), a fly can change the rotational velocity of the entire pattern, and thus actively steer towards any position within the flow field. The top two trials in Fig. 1A are representative of the orientation behavior observed when the fly is confronted with an expanding pattern at both speeds. At the start of both trials the fly is facing the FOE but within a few seconds turns away from the FOE and prefers to steer towards the FOC (time segments marked in pink). In the top row, corresponding to the 1.25 Hz expansion, the orientation towards the FOC is weak, and the time series shows bouts of FOE orientation (marked in gray) and periods of no orientation. In response to the faster (ft=3.75 Hz) expansion condition, the fly robustly steers towards the FOC. To test the hypothesis that the fly may tolerate a frontal FOE if its flight is directed toward a conspicuous object, we constructed a compound stimulus containing a dark stripe (the object) fixed at the FOE of a low-contrast expanding/contracting flow pattern. When using a slower drift rate (ft=1.25 Hz), flies preferentially orient towards the FOE with an ‘attached’ visual object (third trace of Fig. 1A). At the faster expansion rate (ft=3.75 Hz), this representative trace demonstrates a remarkable bistability in which robust orientation towards both competing stimuli is observed. An alternative representation that facilitates the quantification of this orientation preference is obtained by converting the position time series into a polar plot of the circular mean and the mean resultant length, r, of a sliding 2 s analysis window (Fig. 1B). This transformation is warranted because circular statistics are more appropriate for averaging orientation data (Fisher, 1993). When the data form a single cluster, the magnitude of r is related to the dispersion of the data around the mean heading, with a value closer to one indicating that the data are tightly clustered. We parsed the flies' behavior during each 2s window as either stably towards the FOE or towards the FOC, depending upon whether the circular mean resided within ±60 deg. of the either pole and r>0.5 (represented by the shaded bands in Fig. 1A,B). This classification is similar to the approach introduced recently (Maimon et al., 2008) and was selected to capture our intuition about fixation: it must consist of stable orientation (and thus a large r value) towards one of the pattern foci and it must last for some minimal duration.
The method for scoring orientation data makes it possible to compute the percentage of time that each fly is either fixating the FOE or the FOC. Each plot in Fig. 2A shows two lines corresponding to these percentages over the course of the 90 s trials, with stable orientation towards the FOE in black and orientation towards the FOC in red. To correct for any possible bias resulting from initial conditions, we started each alternate trial with either the FOE or the FOC in front of the fly. Except for the first few seconds of flight, the orientation behavior did not depend on the initial position of the FOE. The flies' behavior quickly converged to the average obtained over the entire experiment, which is represented by the broken lines in Fig. 2A. Because the starting position has only a minor, transient effect, data from the experiments with both initial positions were pooled in the subsequent analysis. Four conditions were tested, corresponding to the expansion pattern with or without the stripe ‘attached’ to the FOE and two temporal frequencies of expansion (1.25 Hz and 3.75 Hz). The means of the distribution of each fly's orientation are shown as histograms plotted in polar coordinates (Fig. 2B). The outer border of the gray circle in Fig. 2B shows the distribution that would result if flies showed no orientation preference. Clearly the behaviors differed from this random orientation for all four treatments. To further quantify orientation preference, the instantaneous behavior of the flies was classified as either orientation towards the FOC, towards the FOE or towards neither, and the percentages of time spent in each of these three categories are presented as fixation scores (Fig. 2C). In the case of the standard expansion avoidance paradigm (i.e. no stripe fixed to the FOE), the flies spent most of their time orienting towards the FOC (as previously demonstrated), and did so with a tighter distribution and greater percentage of time at the higher expansion rate. In the trials in which the stripe was attached to the FOE, the flies preferentially oriented towards the FOE in the slow expansion condition and exhibit a bistable preference in the fast expansion condition with no statistical difference in the time spent orienting towards the FOC versus the FOE [P=0.32, paired t-test, 3.75(S) case; in the other three cases these fixation scores are significantly different with P-values no larger than 2.7 ×10−7]. This apparent bistability is not surprising because when the FOC is directly in front of the flies, the stripe is in the rear blind spot of the flies where it is not visible.
All of the results presented thus far made use of a low-contrast expansion pattern. This was necessary to define the stripe object using luminance, because the background expansion pattern was constructed using two brighter intensity levels. To verify that this manipulation did not interfere with the flies' typical behavior, we tested the effect of pattern contrast on the closed-loop expansion avoidance response. Flies were given active control over the rotational velocity of a pattern with FOE and FOC located 180 deg. apart (ft=3.75 Hz), at one of four contrast levels of the same mean luminance. The orientation histograms and fixation percentages resulting from these experiments (Fig. 2D,E) suggest that the closed-loop expansion avoidance behavior of Drosophila is largely invariant to the contrast of the pattern (CL 1=14.3%; CL 2=42.9%, CL 3=71.4% and CL 4=100%). A balanced one-way analysis of variance (ANOVA) performed on the percentage of time of FOC fixation (Fig. 2E) confirmed that the sample means for all four contrast levels are essentially the same (P=0.36). Similar contrast invariance has been previously reported for closed-loop stripe fixation (Reiser and Dickinson, 2008) and for open-loop avoidance of lateral expansion (Duistermars et al., 2007a). Taken together the results in Figs 1 and 2 reveal that the strong preference for the FOC is reduced when a stripe is present, and instead the flies will actively orient towards the FOE. The magnitude of this effect is dependent on the ft of the expansion but the flies would orient, roughly half the time, towards the FOE even with 3.75 Hz expansion. It is clear from these results that the rate of expansion does contribute to the strength of the closed-loop expansion avoidance behavior. We have examined this effect and it will be the subject of a forthcoming paper.
To further explore the interaction between object fixation and expansion avoidance, we next designed an open-loop experiment in which object movement is decoupled from the FOE. In an open-loop paradigm the flies do not control the stimulus but we record their instantaneous turning responses to repeated presentations of visual stimuli. A laterally positioned, low-contrast expansion pattern was presented, while a dark stripe was rotated around the fly at a constant angular speed. Constant speed rotation of a stripe around the fly is a technique that has been used to determine the position- and direction-dependent response to an attractive stimulus (Geiger, 1981; Heisenberg and Wolf, 1979; Reichardt and Guo, 1986). The compound stimuli used in this experiment allow an analysis of the integration of these two visual stimuli by comparing the response to the combined presentation with the responses to either pattern presented alone. This stimulus might correspond to a case in which the fly attempts to orient towards a prominent object, while simultaneously being buffeted laterally by wind.
The experiment was designed so that flies were presented with paired symmetrical stimuli that should generate turning responses of equal magnitude but opposite sign. For example, responses to expansion on the right paired with clockwise object rotation should be roughly symmetrical to the same fly's responses to expansion from the left paired with counter-clockwise object rotation. We verified the symmetry (see supplementary material Fig. S1) of the turning responses in these trial pairs, and then the results from symmetrical trials were scaled and averaged on a per-fly basis before the data were averaged across flies. The symmetrical data sets have been adjusted to correspond to a right-to-left expansion field by scaling the left-to-right expansion trials by −1. An alternative presentation of these data is contained in supplementary material Fig. S2, in which each trial is plotted without the symmetry transformation.
Fig. 3A shows the mean response of flies during trials in which the display showed the static grating pattern without stripe rotation or lateral expansion – as expected the mean turning response is essentially zero. A laterally positioned FOE was particularly effective at eliciting a strong counter-turn – significantly larger than the turning response to a striped drum rotating at the same speed (Duistermars et al., 2007a; Tammero et al., 2004). The response to a FOE positioned on the right of the flies is shown in Fig. 3B,C. As expected, flies produce a sustained turn away from the source of expansion and turn with larger amplitude away from the faster expansion. At the slower expansion rate, the data show remarkable synchronicity with the single pixel advances of the LED display. The vertical black lines in Fig. 3B (and Fig. 3E,H) mark the times at which the pattern was updated with a one frame advance (10 frames s−1 for the ft=1.25 Hz rate of expansion). The repetitive surges in the turning response away from the FOE (downward in this figure) show a lag of approximately 60ms relative to the single frame advances of the expansion pattern. These surges are not seen in the faster expansion case.
The response to the stripe moving across a static low-contrast grating is shown in Fig. 3D,G. As the stripe rotates from the rear of the display in the clockwise direction (Fig. 3D), the response to the stripe motion is initially small but increases as the flies attempt to turn towards the stripe while it is on their left. As the stripe nears the midline the mean response changes sign as the flies attempt to turn clockwise to follow the stripe. The response is largest when the stripe is in front of the fly and moving progressively (from front to back) on the retina. Note that the mean response changes sign before the stripe actually crosses the midline, suggesting that the flies implement a strategy whereby they ‘anticipate’ the motion of stripe. Due to the data treatment method (and well supported by the stereotypy of the position-dependent turning response to a rotating stripe, see supplementary material Fig. S1), the response to the counter-clockwise rotating stripe (Fig. 3G) is precisely the sign-inverted version of the response to the clockwise stripe rotation in Fig. 3D.
The responses to the combined stripe and expansion motion are shown in Fig. 3E,F, for clockwise stripe rotation, and Fig. 3H,I, for counter-clockwise stripe rotation. To aid the comparison of the individual responses, each plot shows the response to the stripe (red) and expansion (black) alone, along with the averaged response to the combined stimuli (blue). Flies respond to both stimuli in a context-dependent manner. In cases in which the moving stripe and expanding patterns would independently elicit turns in opposite directions, it appears that occasionally one stimulus can trump the other. In Fig. 3E, for example, object orientation dominates the flies' response when the stripe is positioned frontally and is undergoing progressive (front to back) motion. By contrast, the expansion avoidance reflex dominates the flies' response, when the expansion is faster (3.75 Hz) and the stripe is moving regressively (left half of response in Fig. 3F). Later in the same trial (right half of Fig. 3F) when the stripe crosses the midline and moves progressively, the response appears to be a compromise between the two reflexes. When both stimuli would independently elicit a turn in the same direction (as in the right half of Fig. 3H,I), the response is a larger turn than is generated in response to either stimulus presented in isolation, although far less than a linear sum (likely to be due to biomechanical saturation – the flies can only turn so much). Similar to the closed-loop results of Fig. 2B,C, the response towards the stripe is more prominent when the expansion rate is lower. This is most easily seen by comparing the second half of Fig. 3E with the second half of Fig. 3F. The response to the combined stimulus with the slower (ft=1.25Hz) rate of expansion is quite similar to the stripe-alone response (Fig. 3E) whereas the response to the combined stimulus with the faster (ft=3.75 Hz) expansion rate is shifted noticeably towards the expansion response. Note that in all four panels the turning responses suggest that the flies' nervous system performs a complex integration of the two visual cues – rarely does the response to the combined stimulus look like a simple average of the responses to each stimulus alone.
We have shown that when presented with a considerably aversive stimulus (strong lateral expansion), the turning behavior of Drosophila is influenced by the position and direction of motion of a prominent vertical object. Can flies fixate this object in the face of this strong lateral expansion? The results in Fig. 3 suggest that the Drosophila nervous system implements a complex integration of these two stimuli whereby the stripe motion is the dominant cue only in limited circumstances. In our final exploration of the interaction between object fixation and expansion avoidance we devised a paradigm in which flies were given closed-loop control of the rotational velocity of the dark stripe while a lateral expansion stimulus was presented in open loop (Fig. 4). This experiment was organized as the closed-loop analog to the previous one – the conditions tested included the two rates of expansion used throughout (ft of 1.25 Hz and 3.75 Hz) as well as a no-expansion case during which the low-contrast grating was stationary.
For all tested conditions, flies fixate the stripe in front of them in the face of lateral expansion. As with the results shown in Fig. 2B,D, the orientation behavior of the flies in this new paradigm is summarized with a histogram plotted in polar coordinates (Fig. 4A). In addition, for these experiments we also show the corresponding distributions of the flies' steering responses, which were recorded as the instantaneous difference between left and right wingbeat amplitudes (L–R). The distributions plotted in red correspond to data from no-expansion trials. As expected, the mean distribution of stripe positions shows that flies fixate the stripe frontally with high probability (Fig. 4A), and the steering responses are tightly distributed around zero (Fig. 4B). When fixating a stripe flies produce only occasional small turns of alternating sign to keep the stripe in front. For the trials where expansion is presented in combination with closed-loop stripe fixation, the orientation distributions broaden and the steering response distributions shift slightly to one side, suggesting that the lateral expansion acts much like a turning bias. This is further seen in the shift in direction and reduction in length of mean orientation direction vector plotted in the inset; for the lateral expansion cases the shorter vector length corresponds to more dispersed orientation. When the FOE is on the right (blue and green curves in Fig. 4A,B), the flies (on average) fixate the stripe frontally, but the turning distribution shows a shift towards a negative L–R value (seen clearly in the inset plotting mean L–R), indicating a bias to turn away from the expansion. Similarly, when the expansion emanates from the left (cyan and magenta curves in Fig. 4A,B), the distribution of turning response is skewed towards positive L–R, indicating that flies turn away from the left-to-right expansion by generating clockwise torque. The orientation histograms in Fig. 4A show a similarly biased distribution in the presence of lateral expansion. These shifts are in the expected direction – as the flies attempt to turn away from the FOE, the negative feedback causes the stripe to move in the opposite direction, towards the FOE, suggesting that flies fixate the stripe while simultaneously turning away from the superimposed expansion.
A more complete presentation of the data in Fig. 4A,B is provided by constructing a 2-D histogram that tabulates the joint distribution of the flies' turning responses at each position of the pattern. The histograms in Fig. 4C are proper probability distributions; the probability that a sample (of stripe position and turning response) will reside in each bin is assigned a color value that is related to percentage by the scale bar. The histograms in Fig. 4A,B can be obtained from the 2-D histograms in Fig. 4C by integrating over one dimension – the orientation histograms (panel A) are recovered by integrating over L–R, and the L–R distributions are recovered by integrating over position. In these data, it is difficult to see the detailed relationship between stripe position and turning response because the animals are in behavioral closed loop, and, thus, most of the time the stripe is very near the midline and the flies primarily produce only small-amplitude turns. Therefore, the quick corrective responses that are produced in response to stripe positions that are away from the front are obscured. To visualize these rare but informative events, the 2-D histograms in Fig. 4D show the result of normalizing the original data such that the values within each of the 48 columns sum to (1/48)%. This normalization procedure makes visible the large turns made when the stripe is not in front of the animal.
Figs 3 and 4 both quantify the influence of a constant expansion pattern on object orientation but the data were collected under very different conditions. Fig. 3 shows the effect of the translational flow on the open-loop (constant rotational velocity) stripe orientation response whereas Fig. 4A–D shows the effect of the translational flow pattern on closed-loop stripe fixation. If the interaction between the object fixation and expansion avoidance responses results from a simple stereotyped mechanism, then we should be able to reconstruct the open-loop response from the closed-loop data. To test this possibility, we analyzed our closed-loop results as if they taken in an open-loop experiment, i.e. we computed the position-dependent mean turning response (essentially the quantity plotted in Fig. 3) as the expected value of the turning response at each stripe position from the histograms in Fig. 4C. The response of flies to stripe motion is not instantaneous, as there is some delay between visual motion and a motor response. Heisenberg and Wolf (Heisenberg and Wolf, 1988) show that tethered Drosophila respond to the displacement of a bar with a latency of 50 ms, and so Fig. 4E plots the expected value of a 50 ms time-advanced version of the turning response (L–R), relative to the object position. The expected values of these turning response curves confirm the results derived under open-loop conditions. When the stripe is behind the flies they turn away from expansion (L–R<0 for the right FOE, blue and green traces, and L–R>0 for the left FOE, cyan and magenta traces). When the stripe is in the frontal quadrant on the side of the FOE (gray band in Fig. 3E), the area where the open-loop experiments indicate that the behavior is dominated by the stripe response (see Fig. 3E), the responses are near 0 for the faster expansion cases, and toward the stripe and FOE for the slower expansion cases. Also, much of the position dependency of the response seen in the open-loop trials (Fig. 3) is recovered. In contrast to previous attempts (Heisenberg and Wolf, 1988), the experimental protocol and data analysis employed in Fig. 4E allow an explicit comparison of open- and closed-loop experiments. The degree of similarity is surprising, given that the response of a fly to a moving stripe is expected to depend not only on the stripe's current position, but also on the speed and direction of motion, as well as the value of these parameters in the past. The expected value of the turning response should therefore be dependent on more than one variable but there are not enough data in this set to further condition this calculation. This explains why the red curve (no expansion case) does not look exactly like either of the stripe-alone responses or their average (Fig. 3D,G). An additional result is that the turning response values in Fig. 4E are somewhat smaller than those observed in the related open-loop experiment. This finding is consistent with the idea that much of fly steering is actually quite subtle, and flies steer with larger turns in open-loop experiments (in which they are unable to affect the stimulus), than in the counterpart closed-loop trials, which should provide a higher fidelity simulation of free flight.
DISCUSSION
Is goal-oriented behavior required for straight flight?
We have shown with a series of open- and closed-loop experiments that in the presence of a prominent vertical object Drosophila will tolerate a level of image expansion that would otherwise induce strong avoidance. These results suggest that the object orientation response may serve an important role in the control of translatory flight. In agreement with several recent findings, it appears that flies require a goal to drive their flight direction, either towards an object (present study) (Götz, 1987; Heisenberg and Wolf, 1979; Maimon et al., 2008), upwind (Budick and Dickinson, 2006; Budick et al., 2007) or towards an attractive odorant (Budick and Dickinson, 2006; Chow and Frye, 2008). Drosophila flight has been shown to consist of straight segments interspersed with rapid turns (Tammero and Dickinson, 2002). The fact that they can fly straight over a distance of 10–40 cm suggests that visual control must be involved, because the haltere-mediated equilibrium reflex would not correct for the small deviations from straight flight that would accumulate over such a distance (Dickinson, 1999; Sherman and Dickinson, 2003). Even stronger evidence for this comes from experiments in which flies were flown in a large cylindrical environment lined with horizontal stripes (Frye et al., 2003) or a smaller one within a rotating visual panorama (Mronz and Lehmann, 2008). Under these conditions, in which vertical edges are not present, flight trajectories are curved, faster and much closer to the walls. In light of the results presented here, one likely explanation for these curvilinear paths is that these free-flight conditions do not contain any cues that could induce the orientation behavior required to structure straight flight. By inhibiting the expansion avoidance response, orientation towards a stationary object would guarantee segments of straight flight, at least until expanding patterns grow strong as the fly nears an obstacle. Such a strategy would serve to regulate other visually controlled behaviors and enhance information from other sensory systems.
Synchronicity of open-loop turning response to single pixel expansion displacement
The turning responses of flies to the discrete approximation of a slowly expanding (ft=1.25 Hz), laterally positioned stimulus show strong phase-locked ripples. Each single frame advance of the expanding stimulus (1/8 of the spatial wavelength for this pattern) elicits a surge in the turning response away from the FOE, with a lag of approximately 60 ms. This strong phase-locked response to incremental expansion has been noted recently for a slower expansion stimulus (Duistermars et al., 2007a). In their study the phase locking was shown to occur for expanding stimuli (across several spatial periods) but not for patterns rotating at the same angular velocity, suggesting that turning responses to coherent rotation, long studied as the optomotor response, are apparently low-pass filtered relative to the responses to the identical motion stimuli configured to expand laterally. While this response may be viewed as an artifact of an imperfect apparent motion stimulus, it also provides a sensitive read-out for some of the computations carried out by the fly visual system. Further, it should be noted that this discretized expansion presents no ambiguities to the flies – the animals are as sensitive to the direction of the expansion stimulus at the slower rate as they are at the faster 3.75 Hz rate. The flies steer towards the FOC in the closed-loop behavior presented in Figs 1 and 2 and turn strongly away from the FOE in the open-loop experiments presented in Fig. 3. This discretized expansion can be thought of as the motion analog of wide-field flicker, which has long been used to probe the response of motion-sensitive neurons. In particular, the measurement of a visual-to-motor delay in response to a discretized motion stimulus is well defined. The observed delay of approximately 60ms is consistent with previously reported values of 50– 100 ms for tethered flying Drosophila turning in response to a displacing bar (Heisenberg and Wolf, 1988) and 80 ms measured for the body angle corrections of freely flying Drosophila in response to image flow (David, 1985). It is likely that differences in the visual conditions and the motor behavior measured in other studies will contribute significantly to the exact value determined in any one of these measurements – but the agreement between these three independent measurements suggests the general finding that visuomotor delays in flying Drosophila will be no more than 100 ms. If anything, due to lower sampling rates, the previous measurements are likely to have overestimated this delay [and values as low as 20–40ms have been measured in housefly chasing behavior (Land and Collett, 1974)]. It is worth noting that the phase-locked turning reported here is in response to a low-contrast expansion stimulus, and could serve as a motor readout of gain control mechanisms that must be operating as the flies produce large behavioral responses to a very weak signal. Lastly, these phase-locked ripples in the data in Fig. 3 enabled us to directly observe features of the integration of the stripe and the expansion field computations. In response to the compound stimuli in Fig. 3E,H the flies clearly responds to both the stripe and the expansion, as the fine structure of the response demonstrates the phase locking seen in the expansion-alone trial (Fig. 3B). An interesting feature of Fig. 3E, is that when the overall response changes sign from negative (counter-clockwise torque) to positive (clockwise torque), the polarity of the ripples remains the same, that is they are always downwards, in the direction elicited by the expansion stimulus.
Predictive tracking of a moving object
In many dipterans, courtship involves elaborate chases in which males target females in flight. These impressive displays of flight control have inspired many studies on the behavior and physiological correlates of small field processing (Collett and Land, 1975; Egelhaaf et al., 1988; Wagner, 1986). Several previous studies have demonstrated behaviors in which flies produce a torque zero crossing (change in turn direction) that precedes the frontal zero crossing of a rotating object. In examining the chasing behavior of the housefly Fannia, Land and Collet noted that most tracking behavior consists of the pursuing male fly converting the position of the leading fly into an angular velocity, except for instances when the leading fly is within ±35 deg. of the chasing fly's axis, in which case it is the velocity of the leading fly that correlates with the chasing fly's turning velocity (Land and Collet, 1974). They proposed and simulated a chasing rule based on this observation and argued that it is essential for stabilizing the turns during high-speed chases. In examining the torque response of the tethered housefly Musca, Geiger (Geiger, 1981) used a very similar protocol to the one used here to generate the data shown in Fig. 3D,G, in which a dark stripe was rotated around the fly at a constant speed. The time course of the torque response obtained for Musca (Geiger, 1981) shows a striking similarity to the data we collected for Drosophila, remarkable in light of the difference in the fly species, the visual stimulus used, the speed of stripe motion and the different means for measuring the turning behavior. In the Musca experiments, a small lead in the zero crossing of the torque response is observed (approximately 10 deg. or 110 ms), in contrast to the larger lead in the data we present (approximately 38deg. or 320 ms). The specific value of this lead depends on the velocity of the rotating stripe but nonetheless it is remarkable that this large ‘predictive’ turning behavior is observed in D. melanogaster, a species that does not use aerial chasing during courtship and has very little binocular overlap. Although previous studies have observed these behaviors in the context of chasing behavior, in light of the result we present, it is likely that predictive orientation towards a moving object is a fundamental feature of flight stabilization and further supports our hypothesis that the object orientation response may serve an important role in the control of forward flight.
Comparisons with free-flight behavior
In recent years a number of impressive studies have quantified visually guided behaviors of freely flying insects under laboratory conditions (Collett and Land, 1975; David, 1985; Fry et al., 2009; Maimon et al., 2008; Srinivasan et al., 1996; van Hateren and Schilstra, 1999). These studies have provided significant insights into the relationships between visual conditions and flight reactions, and have given support to a more ecological perspective on flight control (Egelhaaf et al., 2002; Zeil et al., 2008), in which the behavior and the visual input to the animal are understood to be tightly coupled. Despite the degree to which these experiments can be controlled under laboratory conditions, there are still questions that are not easily addressed in free-flight experiments, and it is for these questions that the additional stimulus control and measurement precision of the tethered flight preparation become necessary.
In the present study we chose to idealize the visual world such that it consists of an attractive object (a dark stripe) and a simplified expansion stimulus – delivering naturalistic optic flow was not our goal. In a separate series of experiments (results not shown here) designed to examine other possible causes of the paradox we set out to explore (that in a closed-loop experiment flies will preferentially steer towards the FOC of an expanding flow field), we generated more naturalistic optic flow corresponding to what a fly would experience while flying down a corridor patterned with gratings on the walls. These patterns, with the characteristic geometry of expansion (weakest in front, strongest at the sides), when displayed in our flight arena and configured for closed-loop experiments shown in Fig. 1, yielded similar orientation behavior as seen previously (Tammero et al., 2004) and confirmed in this paper – that flies robustly orient towards the FOC. In the set of experiments presented in this paper, we chose to examine questions that would be impossible to study in a free-flight experiment, where the large-field expansion is controlled independently from an attractive object. In most free-flight experiments the occurrence of vertical edges often presents a significant visual confound as all wind tunnels contain them at their ends. Thus, although the mechanism that we study here may be in operation in free flight it is not under the direct control of the experimenters. What we present in this study is a hypothesis about the role of object orientation in the control of forward flight that can only be verified with a suitable free-flight experiment. In addition, it may be technically impossible to ever access the activity of identified visually sensitive neurons in freely flying Drosophila, and so it is also crucial to explore the behavioral repertoire of tethered flies. Free-flight experiments excel in generating detailed observations about how flying insects interact with their visual surround whereas tethered flight approaches allow a finer scale analysis of the reactions of flies to controlled visual stimuli under often purposefully unnatural conditions [and a recent method by Fry et al. (Fry et al., 2009) attempt to fuse the relative merits of both approaches into a free-flight experiment]. These alternative methods are highly complimentary, mirroring trends in the broader field of vision science in which methods dependent on artificial, highly structured visual stimuli, natural scenes and noise-based stimulations all fruitfully coexist.
Neural substrates for complex visually guided behaviors
In addition to the insights into flight control strategies employed by Drosophila and perhaps other insects, the work presented here demonstrates an interesting case of sensory integration within submodalities of the visual system. The computational and physiological mechanisms that generate these behaviors are currently unknown but we expect that the underlying circuits will yield in coming years to the ever-improving toolkit available in Drosophila. The visual behaviors studied in these experiments are almost certainly mediated by the network interactions of motion-sensitive neurons in the optic lobes of Drosophila. In blowflies, a class of neurons in the lobula plate, termed the figure detection or FD cells, have been shown to respond selectively to object motion, are inhibited by large field background motion and have been implicated in figure-ground discrimination and perhaps in object fixation (Egelhaaf, 1985; Kimmerle and Egelhaaf, 2000). Neurons homologous to the large-field lobula plate horizontal and vertical system cells of blowflies have been identified and recently investigated using whole-cell recordings in Drosophila (Joesch et al., 2008; Scott et al., 2002). Perhaps cells homologous to the FD neurons will be identified in Drosophila and may provide a potential substrate for object fixation. The interactions between classes of visual stimuli, as explored in this paper, surely underscore the limitation in our current understanding of the dipteran visual system. A mechanistic description of these behavioral results would require an elucidation of the neural substrate for motion detection [a long-sought prize, see Borst (Borst, 2000)], for object orientation, for expansion avoidance, as well as for the higher-order interaction between these seemingly modular visuomotor responses. It is likely that network interactions among motion-sensitive neurons generate the flow field selectivity mediating these behavioral responses, as has been impressively demonstrated for horizontal rotation responses in blowfly lobula plate tangential cells (Farrow et al., 2006). It is entirely possible that the integrated responses to expansion and object motion might be constructed in this way. A further possibility is that the additional level of organization recently described for the neurons projecting from the optic lobes to the lateral protocerebrum will probably be relevant in orchestrating complex visual behaviors (Otsuna and Ito, 2006; Strausfeld et al., 2007). Truly impressive behavioral screens have been used to identify some circuit elements in the periphery of the fly visual system (Gao et al., 2008; Katsov and Clandinin, 2008; Rister et al., 2007; Zhu et al., 2009); however, we feel that such efforts to understand Drosophila vision at the circuit and molecular levels will benefit from an expanded list of behavioral phenomena of the type presented here.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Mark Frye for constructive discussion during the early stages of this project. This work was supported by the Institute for Collaborative Biotechnologies through grant DAAD 19-03-D-0004 from the US Army Research Office, by NSF award 0623527 to M.H.D. and the CNSE Engineering Research Center at Caltech though NSF award EEC-9402726. We are grateful to the Howard Hughes Medical Institute for supporting M.B.R. as a Janelia Fellow. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/213/10/1771/DC1
- CL
- contrast level
- ER
- expansion-rotation (pattern)
- FD
- figure detection
- FOC
- focus of contraction
- FOE
- focus of expansion
- ft
- temporal frequency
- LED
- light-emitting diode
- L–R
- difference between left and right wingbeat amplitudes
REFERENCES
- Borst A. (2000). Models of motion detection. Nat. Neurosci. 3Suppl., 1168 [DOI] [PubMed] [Google Scholar]
- Budick S. A., Dickinson M. H. (2006). Free-flight responses of Drosophila melanogaster to attractive odors. J. Exp. Biol. 209, 3001-3017 [DOI] [PubMed] [Google Scholar]
- Budick S. A., Reiser M. B., Dickinson M. H. (2007). The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J. Exp. Biol. 210, 4092-4103 [DOI] [PubMed] [Google Scholar]
- Chow D., Frye M. (2008). Context-dependent olfactory enhancement of optomotor flight control in Drosophila. J. Exp. Biol. 211, 2478-2485 [DOI] [PubMed] [Google Scholar]
- Collett T. S., Land M. F. (1975). Visual control of flight behaviour in the hoverfly, Syritta pipiens L. J. Comp. Physiol. A 99, 1-66 [Google Scholar]
- David C. T. (1985). Visual control of the partition of flight force between lift and thrust in free-flying Drosophila. Nature 313, 48-50 [Google Scholar]
- Dickinson M. H. (1999). Haltere-mediated euilibrium reflexes of the fruit fly, Drosophila melanogaster. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 354, 903-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duistermars B. J., Chow D. M., Condro M., Frye M. A. (2007a). The spatial, temporal and contrast properties of expansion and rotation flight optomotor responses in Drosophila. J. Exp. Biol. 210, 3218-3227 [DOI] [PubMed] [Google Scholar]
- Duistermars B. J., Reiser M. B., Zhu Y., Frye M. A. (2007b). Dynamic properties of large-field and small-field optomotor flight responses in Drosophila. J. Comp. Physiol. A 193, 787-799 [DOI] [PubMed] [Google Scholar]
- Egelhaaf M. (1985). On the neuronal basis of figure-ground discrimination by relative motion in the visual-system of the fly. 2. Figure-detection cells, a new class of visual interneurones. Biol. Cybern. 52, 195-209 [Google Scholar]
- Egelhaaf M., Hausen K., Reichardt W., Wehrhahn C. (1988). Visual course control in flies relies on neuronal computation of object and background motion. Trends Neurosci 11, 351-358 [DOI] [PubMed] [Google Scholar]
- Egelhaaf M., Kern R., Krapp H. G., Kretzberg J., Kurtz R., Warzecha A. K. (2002). Neural encoding of behaviourally relevant visual-motion information in the fly. Trends Neurosci. 25, 96-102 [DOI] [PubMed] [Google Scholar]
- Farrow K., Haag J., Borst A. (2006). Nonlinear, binocular interactions underlying flow field selectivity of a motion-sensitive neuron. Nat. Neurosci. 9, 1312-1320 [DOI] [PubMed] [Google Scholar]
- Fisher N. I. (1993). Statistical Analysis of Circular Data Cambridge, UK: Cambridge University Press; [Google Scholar]
- Fry S. N., Rohrseitz N., Straw A. D., Dickinson M. H. (2009). Visual control of flight speed in Drosophila melanogaster. J. Exp. Biol. 212, 1120-1130 [DOI] [PubMed] [Google Scholar]
- Frye M. A., Tarsitano M., Dickinson M. H. (2003). Odor localization requires visual feedback during free flight in Drosophila melanogaster. J. Exp. Biol. 206, 843-855 [DOI] [PubMed] [Google Scholar]
- Gao S., Takemura S.-y., Ting C.-Y., Huang S., Lu Z., Luan H., Rister J., Thum A. S., Yang M., Hong S.-T., et al. (2008). The neural substrate of spectral preference in Drosophila. Neuron 60, 328-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger G. (1981). Is there a motion-independent position computation of an object in the visual system of the housefly? Biol. Cybern. 40, 71-75 [Google Scholar]
- Götz K. G. (1987). Course-control, metabolism and wing interference during ultralong tethered flight in Drosophila melanogaster. J. Exp. Biol. 128, 35-46 [Google Scholar]
- Heisenberg M., Wolf R. (1979). On the fine structure of yaw torque in visual flight orientation of Drosophila melanogaster. J. Comp. Physiol. A 130, 113-130 [Google Scholar]
- Heisenberg M., Wolf R. (1984). Vision in Drosophila: Genetics of Microbehavior Berlin: Springer-Verlag; [Google Scholar]
- Heisenberg M., Wolf R. (1988). Reafferent contol of optomotor yaw torque in Drosophila melanogaster. J. Comp. Physiol. A 163, 373-388 [Google Scholar]
- Joesch M., Plett J., Borst A., Reiff D. F. (2008). Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr. Biol. 18, 368-374 [DOI] [PubMed] [Google Scholar]
- Katsov A. Y., Clandinin T. R. (2008). Motion processing streams in Drosophila are behaviorally specialized. Neuron 59, 322-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerle B., Egelhaaf M. (2000). Performance of fly visual interneurons during object fixation. J. Neurosci. 20, 6256-6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M. F., Collett T. S. (1974). Chasing behaviour of houseflies (Fannia canicularis): a description and analysis. J. Comp. Physiol. A 89, 331-357 [Google Scholar]
- Lehmann F. O., Dickinson M. H. (1997). The changes in power requirements and muscle efficiency during elevated force production in the fruit fly Drosophila melanogaster. J. Exp. Biol. 200, 1133-1143 [DOI] [PubMed] [Google Scholar]
- Maimon G., Straw A. D., Dickinson M. H. (2008). A simple vision-based algorithm for decision making in flying Drosophila. Curr. Biol. 18, 464-470 [DOI] [PubMed] [Google Scholar]
- Mronz M., Lehmann F. O. (2008). The free-flight response of Drosophila to motion of the visual environment. J. Exp. Biol. 211, 2026-2045 [DOI] [PubMed] [Google Scholar]
- Otsuna H., Ito K. (2006). Systematic analysis of the visual projection neurons of Drosophila melanograster. I. Lobula-specific pathways. J. Comp. Neurol. 497, 928-958 [DOI] [PubMed] [Google Scholar]
- Reichardt W., Guo A. (1986). Elementary pattern discrimination. Biol. Cybern. 53, 285-306 [Google Scholar]
- Reiser M. B. (2006). Visually mediated control of flight in Drosophila: not lost in translation PhD Dissertation, California Institute of Technology; [Google Scholar]
- Reiser M. B., Dickinson M. H. (2008). A modular display system for insect behavioral neuroscience. J. Neurosci. Meth. 167, 127-139 [DOI] [PubMed] [Google Scholar]
- Reiser M. B., Humbert J. S., Dunlop M. J., Del Vecchio D., Murray R. M., Dickinson M. H. (2004). Vision as a compensatory mechanism for disturbance rejection in upwind flight. Proceedings of the American Control Conference 1, 311-316 [Google Scholar]
- Rister J., Pauls D., Schnell B., Ting C. Y., Lee C. H., Sinakevitch I., Morante J., Strausfeld N. J., Ito K., Heisenberg M. (2007). Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron 56, 155-170 [DOI] [PubMed] [Google Scholar]
- Scott E. K., Raabe T., Luo L. (2002). Structure of the vertical and horizontal system neurons of the lobula plate in Drosophila. J. Comp. Neurol. 454, 470-481 [DOI] [PubMed] [Google Scholar]
- Sherman A., Dickinson M. H. (2003). A comparison of visual and haltere-mediated equilibrium reflexes in the fruit fly Drosophila melanogaster. J. Exp. Biol. 206, 295-302 [DOI] [PubMed] [Google Scholar]
- Srinivasan M., Zhang S., Lehrer M., Collett T. S. (1996). Honeybee navigation en route to the goal: visual flight control and odometry. J. Exp. Biol. 199, 237-244 [DOI] [PubMed] [Google Scholar]
- Strausfeld N. J., Sinakevitch I., Okamura J. Y. (2007). Organization of local interneurons in optic glomeruli of the dipterous visual system and comparisons with the antennal lobes. Dev. Neurobiol. 67, 1267-1288 [DOI] [PubMed] [Google Scholar]
- Tammero L. F., Dickinson M. H. (2002). The influence of visual landscape on the free flight behavior of the fruit fly Drosophila melanogaster. J. Exp. Biol. 205, 327-343 [DOI] [PubMed] [Google Scholar]
- Tammero L. F., Frye M. A., Dickinson M. H. (2004). Spatial organization of visuomotor reflexes in Drosophila. J. Exp. Biol. 207, 113-122 [DOI] [PubMed] [Google Scholar]
- van Hateren J. H., Schilstra C. (1999). Blowfly flight and optic flow. II. Head movements during flight. J. Exp. Biol. 202, 1491-1500 [DOI] [PubMed] [Google Scholar]
- Wagner H. (1986). Flight performance and visual control of flight of the free-flying housefly (Musca domestica L.). II. Pursuit of targets. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 312, 553-579 [Google Scholar]
- Zeil J., Boeddeker N., Hemmi J. M. (2008). Vision and the organization of behaviour. Curr. Biol. 18, 320-323 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Nern A., Zipursky S. L., Frye M. A. (2009). Peripheral visual circuits functionally segregate motion and phototaxis behaviors in the fly. Curr. Biol. 19, 613-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.