Abstract
OBJECTIVE: To identify and define clinical associations and radiologic findings of posterior reversible encephalopathy syndrome (PRES).
PATIENTS AND METHODS: Patients prospectively diagnosed as having PRES from October 1, 2005, through April 30, 2009, were pooled with retrospectively identified patients admitted from August 1, 1999, through September 30, 2005. We performed a detailed review of clinical information, including demographics, presenting symptoms, medical history, and risk factors. All patients underwent computed tomography of the brain or magnetic resonance imaging. Findings on magnetic resonance imaging were analyzed independently by 2 neuroradiologists.
RESULTS: We identified 120 cases of PRES in 113 patients (mean age, 48 years). Mean peak systolic blood pressure was 199 mm Hg (minimum-maximum, 160-268 mm Hg), and mean peak diastolic blood pressure was 109 mm Hg (minimum-maximum, 60-144 mm Hg). Etiologies of PRES included hypertension (n=69 [61%]), cytotoxic medications (n=21 [19%]), sepsis (n=8 [7%]), preeclampsia or eclampsia (n=7 [6%]), and multiple organ dysfunction (n=1 [1%]). Autoimmune disease was present in 51 patients (45%). Clinical presentations included seizures (n=84 [74%]), encephalopathy (n=32 [28%]), headache (n=29 [26%]), and visual disturbances (n=23 [20%]). In the 115 cases (109 patients) for which magnetic resonance imaging findings were available, the parieto-occipital regions were the most commonly involved (n=108 [94%]), followed by the frontal lobe (n=88 [77%]), temporal lobe (n=74 [64%]), and cerebellum (n=61 [53%]). Cerebellar involvement was significantly more frequent in patients with a history of autoimmunity (P=.008), and patients with sepsis were more likely to have cortical involvement (P<.001).
CONCLUSION: A substantial proportion of patients with PRES have underlying autoimmune conditions that may support endothelial dysfunction as a pathophysiologic mechanism. On brain imaging, the location and severity of vasogenic edema were mostly similar for the different clinical subgroups.
This study involved 120 cases of posterior reversible encephalopathy syndrome in 113 patients; a substantial proportion of these patients have underlying autoimmune conditions that may support endothelial dysfunction as a pathophysiologic mechanism. On brain imaging, the location and severity of vasogenic edema were mostly similar for the different clinical subgroups.
CT = computed tomography; min-max = minimum-maximum; MRI = magnetic resonance imaging; PRES = posterior reversible encephalopathy syndrome; SE = status epilepticus
Although posterior reversible encephalopathy syndrome (PRES) has gained substantial recognition since its initial description by Hinchey et al1 in 1996, both its clinical spectrum and underlying pathophysiology remain poorly defined. A clinical diagnosis of PRES includes the presence of headache, seizures, encephalopathy, and visual disturbances, as well as radiologic findings of focal reversible vasogenic edema, best seen on magnetic resonance imaging (MRI) of the brain. The syndrome is most commonly encountered in association with acute hypertension, preeclampsia or eclampsia, renal disease, sepsis, and exposure to immunosuppressants.2-8 It has been less commonly described in the setting of autoimmune disease.9-13 The distinctive role of autoimmune disease in the pathophysiology of PRES is often clouded by concurrent hypertension, renal disease, and/or the use of immunosuppressants.
Despite the syndrome's name, radiographic lesions in PRES are rarely isolated to the “posterior” parieto-occipital white matter and instead often involve the cortex, frontal lobes, basal ganglia, and brainstem.14,15 No conclusive evidence supports a clear relationship between clinical conditions and specific imaging findings of severity or location of edema,16 although some studies have suggested correlations such as greater vasogenic edema in normotensive patients5 and a trend for basal ganglia involvement in patients with preeclampsia or eclampsia.16
The underlying pathophysiology of PRES remains elusive. Several theories have been proposed, the most widely accepted of which states that rapidly developing hypertension leads to a breakdown in cerebral autoregulation, particularly in the posterior head region (where there is a relative lack of sympathetic innervation). Hyperperfusion ensues with protein and fluid extravasation, producing focal vasogenic edema.1,10,17 An alternative theory, which has been best characterized in preeclampsia, eclampsia, and sepsis, implicates endothelial dysfunction.18,19 A third theory proposes that vasospasm with subsequent ischemia may be responsible.20-22
Early recognition of PRES is important for timely institution of therapy, which typically consists of gradual blood pressure control and withdrawal of potentially offending agents. Although reversible by definition, secondary complications, such as status epilepticus (SE), intracranial hemorrhage, and massive ischemic infarction, can cause substantial morbidity and mortality.23-26
Our understanding of PRES is derived primarily from small retrospective studies in which the definition of the syndrome varies. The range of presentations and clinical associations is still being recognized. Our study aims to further characterize the syndrome both clinically and radiologically in an attempt to better understand its pathophysiology.
PATIENTS AND METHODS
Patients with clinically diagnosed PRES were identified prospectively during admission to Saint Marys Hospital, Mayo Clinic, Rochester, MN from October 1, 2005, through April 30, 2009. These patients were pooled with a database of retrospectively identified patients admitted from August 1, 1999, through September 30, 2005, by means of a text-retrieval system that searches the final diagnosis in electronic clinic notes. The study was approved by the Mayo Clinic Institutional Review Board.
The presence of all 3 of the following criteria were mandatory for inclusion: (1) clinical history of acute neurologic change including headache, encephalopathy, seizure, visual disturbance, or focal deficit; (2) brain imaging findings of focal vasogenic edema; and (3) clinical or radiologic proof of reversibility. Hypertension was defined as a systolic blood pressure of 140 mm Hg or greater or a diastolic blood pressure of 90 mm Hg or greater.
Patient records were assessed for demographic data, clinical presentation, peak systolic and diastolic blood pressure measurements, comorbid and predisposing conditions, time to neuroimaging, time to follow-up imaging, and recurrence. The primary etiology of PRES was determined for each case on the basis of the diagnosis of the attending neurologist.
The first 100 brain MRIs were reviewed independently by 2 neuroradiologists who were blinded to the clinical details of the cases (H.J.C., D.F.K.). Fifteen additional MRIs were reviewed by an experienced neurologist (A.A.R.). Each reviewer assessed lesion location, distribution, severity of signal abnormality, presence of hemorrhage, and presence of restricted diffusion. Location was defined as frontal lobe, parieto-occipital region, temporal lobe, basal ganglia, cerebellum, brainstem, deep white matter, cortical zone, subcortical zone, and watershed zone. Episodes with multiple MRIs were analyzed for resolution of abnormalities. Conflicts between reviewers' assessments were resolved by consensus.
Demographic and clinical findings are reported as means ± SD. Statistical comparison between clinical and radiologic characteristics was performed using a 2-tailed Fisher exact test or a χ2 test. For patients with recurrent episodes of PRES, each episode was considered independently in the analysis and is referred to as a case throughout the article. Radiologic characteristics used for statistical analysis included location of edema, severity of edema, cortical vs subcortical location, and presence of hemorrhage. Clinical variables included seizures at presentation, SE, hypertension, and history of autoimmunity. P<.05 was considered statistically significant.
RESULTS
Clinical Findings
We identified 120 cases of PRES in 113 patients (73 women and 40 men). Mean ± SD age at presentation was 48±19 years (minimum-maximum [min-max], 9-82 years). Of the study patients, 105 (93) were aged 18 years or older. Primary etiologies of PRES included hypertension (n=69 [61%]), cytotoxic medications (n=21 [19%]), sepsis (n=8 [7%]), thrombotic thrombocytopenic purpura (n=6 [5%]), preeclampsia or eclampsia (n=7 [6%]), and multiple organ dysfunction (n=1 [1%]). Seven patients (6%) had recurrent PRES.
Acute hypertension was present in 97 patients (86%). Of these, 45 (46% of hypertensive patients) had no known preexisting hypertension. Mean peak systolic blood pressure was 191 mm Hg (min-max, 99-268 mm Hg), and mean peak diastolic blood pressure at presentation was 104 mm Hg (min-max, 50-190 mm Hg). Eleven patients (10%) had a systolic blood pressure of 140 mm Hg or less.
Mean ± SD creatinine level at presentation was 2.5±2.7 mg/dL (min-max, 0.3-18.3 mg/dL; to convert to μmol/L, multipy by 88.4). Thirty-five patients (31%) had preexisting chronic kidney disease. Renal failure (either acute or chronic) was present in 64 patients (57%) at presentation. Among these, the mean ± SD creatinine level was 3.8±2.9 mg/dL (min-max, 1.4-18.3 mg/dL). Thirty-two patients (28%) had new-onset acute renal failure, 16 (14%) had chronic kidney disease complicated by acute kidney injury, and 16 (14%) had chronic renal failure determined on the basis of their known baseline creatinine level.
The most common clinical presentation was seizures, seen in 84 patients (74%), including 20 (18%) with SE, 61 (54%) with generalized tonic-clonic seizures, and 3 (3%) with partial seizures. Seven of these patients had a history of epilepsy. Other clinical presentations included encephalopathy (32 [28%]), headache (29 [26%]), and visual disturbances (23 [20%]).
Of the study patients, 47 (42%) were being treated with immunosuppressive medication. Of these patients, 9 were taking cyclophosphamide; 9, tacrolimus; 6, cyclosporine; 6, mycophenolate; 2 each, bevacizumab, rituximab, vincristine, methotrexate, or hydroxychloroquine; and 1 each, 5-fluorouracil, sirolimus, thalidomide, gemcitabine, paclitaxel, carboplatin, sorafenib, infliximab, or hydroxyurea.
A history of autoimmune disease was present in 51 patients (45%), of whom three-fourths were women. Autoimmune diseases in these patients included thrombotic thrombocytopenic purpura (14 [27%]), systemic lupus erythematosus (9 [18%]), hypothyroidism (5 [10%]), scleroderma (3 [6%]), Crohn disease (3 [6%]), ulcerative colitis and/or primary sclerosing cholangitis (2 [4%]), rheumatoid arthritis (2 [4%]), diabetes mellitus type 1 (2 [4%]), and 1 patient each (2%) with Grave disease, Hashimoto thyroiditis, antiphospholipid syndrome, antiglomerular basement membrane antibody disease, autoimmune hepatitis, polyarteritis nodosa, thromboangiitis obliterans, polyglandular autoimmune syndrome, Sjögren syndrome, granulomatous interstitial nephritis, and neuromyelitis optica.
Radiologic Findings
All 120 cases had initial neuroimaging with either MRI or computed tomography (CT) of the head. Magnetic resonance imaging was available for 115 of 120 cases. Mean time to MRI from clinical presentation was 2 days (min-max, 1-11 days).
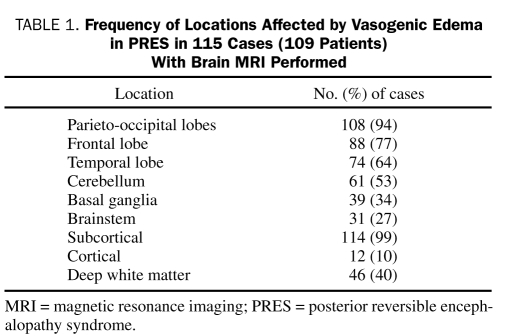
The most commonly involved location was the parietooccipital brain region, which was seen in 108 (94%) of the 115 cases. This was followed by the frontal lobe in 88 cases (77%), the temporal lobe in 74 (64%), the cerebellum in 61 (53%), the basal ganglia in 39 (34%), and the brainstem in 31 (27%) (Table 1). Of the cases, 114 (99%) had subcortical involvement and 12 (10%) had cortical involvement. Lesions were located in the deep white matter in 46 cases (40%), showed an external watershed pattern in 50 (43%), and showed an internal watershed pattern in 6 (5%). Lesions were asymmetric in nearly half of the cases (n=55 [48%]) and unilateral in 1 case. Restricted diffusion was present in 30 cases (26%), and hemorrhage was present in 10 (9%). Contrast enhancement was evident in 16 (15% of overall population and 21% of those given contrast medium).
TABLE 1.
Frequency of locations Affected by Vasogenic Edema in PRES in 115 Cases (109 Patients) With Brain MRI Performed
When analyzed according to clinical subgroups, imaging characteristics, including lesion location and severity, were largely similar (Table 2). Representative images demonstrating severity of edema are seen in Figure 1. Only 2 (11%) of the 19 patients with SE had cortical involvement, and only slightly over half (10 [53%]) had deep white matter involvement. There was limited correlation with severity of edema for patients with SE, with 8 (42%) demonstrating mild edema and 11 (58%) showing moderate to severe edema. The parieto-occipital region was involved in almost all of these patients (18 [95%]). Similarly, most patients with seizures of any type showed no cortical involvement (76 [88%]). Involvement of the classic parieto-occipital brain region was noted in 51 (59%) of the 86 patients with seizures.
TABLE 2.
Radiologic Features of 115 Cases (109 Patients) With PRES, Visualized With MRI, According to Clinical Subset
FIGURE 1.
Axial fluid-attenuated inversion recovery magnetic resonance images depicting examples of mild (A), moderate (b), and severe (C) vasogenic edema in posterior reversible encephalopathy syndrome.
Of 54 patients with autoimmune disease, 35 (65%) had cerebellar involvement (Figure 2), a statistically significant increase compared with those without autoimmunity (P=.008). Half of these had mild edema and half had moderate to severe edema. Asymmetry was also seen in half (27 [50%]). Immunosuppressed patients (n=49) also showed a fairly even combination of mild (24 [49%]) and moderate to severe (25 [51%]) edema.
FIGURE 2.
Axial fluid-attenuated inversion recovery magnetic resonance images of posterior reversible encephalopathy syndrome demonstrating cerebellar involvement in patients with autoimmune disease (A and b), cortical involvement in sepsis (C and D), and atypical patterns of asymmetric involvement (E), hemorrhage (F), brainstem involvement (g), and basal ganglia involvement (H).
A significantly higher proportion of patients with sepsis or active infection (n=11 [58%]) had cortical involvement (Figure 2) compared with patients without infection (P<.001). All patients with sepsis (19 [100%]) had parieto-occipital involvement. Almost three-fourths of normotensive patients had mild edema (8 [73%]), whereas only 3 (27%) had moderate to severe edema. None of these patients demonstrated hemorrhage. In 66 patients with hypertension as the etiology of PRES, 38 (58%) had moderate to severe edema, and 28 (42%) had mild edema. Four (6%) showed evidence of hemorrhage. All patients with preeclampsia or eclampsia (7 [100%]) had involvement of the parieto-occipital region, and all but 1 also had frontal lobe involvement.
The frequency of agreement on imaging characteristics between 2 neuroradiologists independently reviewing images is shown in Table 3. The most consistently agreed upon characteristics were subcortical involvement and presence of hemorrhage (in agreement for 98% of cases in each). The presence of restricted diffusion was agreed upon in 79% of cases, and edema severity and asymmetry were both agreed upon in 63% of cases.
TABLE 3.
Frequency of Agreement Between 2 Independent neuroradiologists on Radiologic Characteristic on Brain MRI in PRES for 100 Cases (93 Patients)
Cerebral vascular imaging with magnetic resonance angiography, CT angiography, or digital subtraction catheter angiography was performed in 37 cases (32 with magnetic resonance angiography, 1 with CT angiography, and 4 with digital subtraction catheter angiography). Vasospasm was noted in 4 cases, distal vasculature pruning in 1, focal posterior cerebral artery stenosis in 1, vertebral artery dissection in 1, and an incidental right internal carotid aneurysm in 1. Findings on the remaining angiographic studies were unremarkable.
Follow-up neuroimaging was performed in 74 of 120 cases; the overall median time to follow-up imaging was 20 days (min-max, 1-390 days). Follow-up imaging showed evidence of radiologic improvement in 65 cases (88%), with complete or near-complete resolution of abnormalities in 52 (70%). Median time to follow-up imaging for this group was 26.5 days (min-max, 4-390 days). Thirteen (18%) had partial resolution with a median time to follow-up imaging of 7 days (min-max, 4-30 days). Nine (12%) had no change, with a median time to follow-up imaging of 5 days (min-max, 1-92 days); 7 of them probably had follow-up imaging too early (≤11 days from symptom onset) and the other 2 were imaged at the time of a recurrent episode of PRES.
DISCUSSION
In this large clinical series, we found many of the classic etiologies and predisposing factors known to be associated with PRES, including abrupt hypertension, immunosuppressant use, infection, preeclampsia or eclampsia, and renal disease. In addition, we found a substantial proportion of patients affected by underlying autoimmune and inflammatory conditions. The most common clinical presentations were new-onset seizures, encephalopathy, headache, and visual disturbances. Status epilepticus as a clinical presentation of PRES was not uncommon. Brain MRIs reviewed independently by 2 neuroradiologists revealed the parieto-occipital head region to be the region most consistently involved, followed by the frontal lobe, temporal lobe, and cerebellum. Unexpectedly, patients with seizures and SE rarely had involvement of cortical matter. Patients with autoimmune disease were more likely to have cerebellar involvement, and patients with sepsis or active infection were more likely to have cortical involvement.
The high percentage of patients with PRES who have autoimmune disorders may support the theory that PRES is in part caused by endothelial dysfunction, a process in which the host autoimmune response is essential. Endothelial cells become activated and damaged by an inflammatory cytokine response stemming from monocytes and lymphocytes, which can lead to leakage of fluid and protein into the interstitium.18,19 Autoimmunity has been recently implicated in PRES in association with neuromyelitis optica spectrum disorders.27 This raises the possibility of autoimmune-mediated disruption of the endothelial aquaporin 4 water channels, which may predispose to PRES.
Abrupt hypertension undoubtedly contributes to the development of PRES, and the hyperperfusion theory is supported by the frequent presence of substantial hypertension in patients with PRES and subsequent resolution of clinical symptoms and radiologic edema with prompt treatment of hypertension. Hyperperfusion has also been shown radiologically on single-photon emission CT in case reports of patients with PRES.7,17 However, this theory is not comprehensive because PRES can affect normotensive patients23 and does not uniformly occur in patients with hypertensive surges above the normal upper limits of cerebral autoregulation.
We found no correlation between clinical characteristics and the extent of vasogenic edema seen on brain imaging in PRES. This is consistent with previous findings,16 yet contradicts other reports suggesting that the extent of vasogenic edema may be inversely related to blood pressure at the time of symptom onset.5 We also found no overall difference in lesion location on the basis of suspected PRES etiology. However, we found strong associations with a history of autoimmune disease, involvement of the cerebellum, and cortical involvement with infection. Although the relevance of the last association is uncertain, it is interesting given the tendency of patients with sepsis to have epileptogenic abnormalities on electroencephalography.28
Status epilepticus is a not uncommon presentation of PRES. Its occurrence is not associated with the severity of radiologic edema, and most of these patients actually lack cortical involvement. Recognition that PRES may present with SE is important because, in addition to anticonvulsants, appropriate treatment requires identifying and treating the underlying cause of PRES.
This study is limited because the transplant and chemotherapy patient population is underrepresented as a result of the organization of our medical center. In addition, the study shares the limitations of all retrospective studies, particularly in that the timing of follow-up imaging was not uniform. There may be a bias toward more benign cases because patients who died of acute critical illness had to be excluded to ensure reversibility. However, requiring proof of reversibility is actually a strength of our study because it preserves the homogeneity of the population. Additional strengths of the study are that it is one of the largest clinical series of PRES patients and that radiologic evaluations were completed by 2 independent neuroradiologists blinded to the clinical details of the cases.
CONCLUSION
The most novel finding in our clinical series of PRES patients is the high prevalence of autoimmune disorders. Although we do not consider PRES an autoimmune condition per se, this association with autoimmune disorders suggests that endothelial dysfunction may lie at the core of its pathophysiology. Further research is needed to assess the merit of this hypothesis.
Supplementary Material
REFERENCES
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500 [DOI] [PubMed] [Google Scholar]
- 2.Hauser RA, Lacey DM, Knight MR. Hypertensive encephalopathy: magnetic resonance imaging demonstration of reversible cortical and white matter lesions. Arch Neurol. 1988;45(10):1078-1083 [DOI] [PubMed] [Google Scholar]
- 3.Schwaighofer BW, Hesselink JR, Healy ME. MR demonstration of reversible brain abnormalities in eclampsia. J Comput Assist Tomogr. 1989;13(2):310-312 [DOI] [PubMed] [Google Scholar]
- 4.Raroque HG, Jr, Orrison WW, Rosenberg GA. Neurologic involvement in toxemia of pregnancy: reversible MRI lesions. Neurology 1990;40(1):167-169 [DOI] [PubMed] [Google Scholar]
- 5.Bartynski WS, Boardman JF, Zeigler ZR, et al. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27(10):2179-2190 [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa M, Terae S, Chu BC, et al. MRI in seven cases of tacrolimus (FK-506) encephalopathy: utility of flair and diffusion-weighted imaging. Neuroradiology 2001;43(8):615-621 [DOI] [PubMed] [Google Scholar]
- 7.Small SL, Fukui MB, Bramblett GT, et al. Immunosuppression-induced leukoencephalopathy from tacrolimus (FK506). Ann Neurol. 1996;40(4):575-580 [DOI] [PubMed] [Google Scholar]
- 8.Appignani BA, Bhadelia RA, Blacklow SC, et al. Neuroimaging findings in patients on immunosuppressive therapy: experience with tacrolimus toxicity. AJR Am J Roentgenol. 1996;166(3):683-688 [DOI] [PubMed] [Google Scholar]
- 9.Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome–an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183 [PubMed] [Google Scholar]
- 10.Primavera A, Audenino D, Mavilio N, et al. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pozo-Rosich P, Villoslada P, Canton A, et al. Reversible white matter alterations in encephalopathy associated with autoimmune thyroid disease. J Neurol. 2002;249(8):1063-1065 [DOI] [PubMed] [Google Scholar]
- 12.Tateishi Y, Iguchi Y, Kimura K, et al. A case of autoimmune thyroid disease presenting posterior reversible encephalopathy syndrome. J Neurol Sci. 2008;271(1-2):203-206 [DOI] [PubMed] [Google Scholar]
- 13.Bohnen NI, Parnell KJ, Harper CM. Reversible MRI findings in a patient with Hashimoto's encephalopathy. Neurology 1997;49(1):246-247 [DOI] [PubMed] [Google Scholar]
- 14.Lee VH, Wijdicks EF, Manno EM, et al. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210 [DOI] [PubMed] [Google Scholar]
- 15.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28(7):1320-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller-Mang C, Mang T, Pirker A, et al. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology 2009;51(6):373-383 [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RB, Jones KM, Kalina P, et al. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159(2):379-383 [DOI] [PubMed] [Google Scholar]
- 18.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359-1375 [DOI] [PubMed] [Google Scholar]
- 19.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003;101(10):3765-3777 [DOI] [PubMed] [Google Scholar]
- 20.Trommer BL, Homer D, Mikhael MA. Cerebral vasospasm and eclampsia. Stroke 1988;19(3):326-329 [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Sakai T, Inagawa S, et al. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346 [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JT, Wang SJ, Fuh JL, et al. Prolonged reversible vasospasm in cyclosporin A-induced encephalopathy. AJNR Am J Neuroradiol. 2003;24(1):102-104 [PMC free article] [PubMed] [Google Scholar]
- 23.Ay H, Buonanno FS, Schaefer PW, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology 1998;51(6):1369-1376 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz RB. A reversible posterior leukoencephalopathy syndrome [letter]. N Engl J Med. 1996;334(26):1743 [DOI] [PubMed] [Google Scholar]
- 25.Koch S, Rabinstein A, Falcone S, et al. A. Diffusion-weighted imaging shows cytotoxic and vasogenic edema in eclampsia. AJNR Am J Neuroradiol. 2001;22(6):1068-1070 [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer PW, Buonanno FS, Gonzalez RG, et al. Diffusion-weighted imaging discriminates between cytotoxic and vasogenic edema in a patient with eclampsia. Stroke 1997;28(5):1082-1085 [DOI] [PubMed] [Google Scholar]
- 27.Magana SM, Matiello M, Pittock SJ, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology 2009;72(8):712-717 [DOI] [PubMed] [Google Scholar]
- 28.Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37(6):2051-2056 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.