Abstract
Pain management in patients with cirrhosis is a difficult clinical challenge for health care professionals, and few prospective studies have offered an evidence-based approach. In patients with end-stage liver disease, adverse events from analgesics are frequent, potentially fatal, and often avoidable. Severe complications from analgesia in these patients include hepatic encephalopathy, hepatorenal syndrome, and gastrointestinal bleeding, which can result in substantial morbidity and even death. In general, acetaminophen at reduced dosing is a safe option. In patients with cirrhosis, nonsteroidal anti-inflammatory drugs should be avoided to avert renal failure, and opiates should be avoided or used sparingly, with low and infrequent dosing, to prevent encephalopathy. For this review, we searched the available literature using PubMed and MEDLINE with no limits.
OTCA = over-the-counter analgesic; COX-2 = cyclooxygenase 2; CYP = cytochrome P450; FDA = Food and Drug Administration; GFR = glomerular filtration rate; HCV = hepatitis C virus; NAPQI = N-acetyl-p-benzoquinone imine; NSAID = nonsteroidal anti-inflammatory drug; TCA = tricyclic antidepressant
Cirrhosis is a substantial public health problem, accounting for approximately 770,000 deaths annually and, according to autopsy studies, affecting 4.5% to 9.5% of the global population.1 Pain management in patients with cirrhosis generates considerable misconception and apprehension among health care professionals. In patients with end-stage liver disease, adverse events from analgesics are frequent and can be severe. The most important and concerning complications include hepatic encephalopathy, acute renal failure, and gastrointestinal bleeding, which can lead to death in some patients. This article is a review (not a systematic review) of the available literature (using PubMed and MEDLINE with no search limits).
The greater the progression of liver dysfunction, the greater the impairment in drug metabolism.2,3 Patients with asymptomatic chronic liver disease without cirrhosis do not have liver dysfunction, and thus analgesic metabolism is similar to that in the general population. In patients with severe liver disease but not cirrhosis (eg, severe hepatitis), drug metabolism may be altered, and thus concerns and dose reductions, as discussed in this article, may be warranted.2-4 A patient with well-compensated cirrhosis and near-normal synthetic function will have impaired drug metabolism, but to a lesser extent than will patients with abnormal synthetic function or decompensated cirrhosis. Decompensated cirrhosis can be a result of progressive liver dysfunction, worsened portal hypertension, or both. Such patients may have even greater restrictions on analgesic choice. This article pertains to all patients with cirrhosis (compensated or decompensated) and to patients with liver dysfunction (with elevated bilirubin levels and prothrombin time), whether they do or do not have cirrhosis.
The efficiency of drug removal by the liver relies on hepatic blood flow, hepatic enzyme capacity, and plasma protein binding. Cirrhosis affects all these processes and may also lead to formation of portosystemic shunts by which a drug can circumnavigate hepatic elimination.2,4 Advanced liver disease and cirrhosis alter the metabolism and effects of many drugs through a variety of mechanisms, including changes in pharmacokinetic behavior, altered accumulation of free drug in plasma, and end-organ response.
Major categories of pain medications, including over-the-counter analgesics (OTCAs) such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), as well as cyclooxygenase 2 (COX-2) inhibitors, anticonvulsants, antidepressants, and opioids, are largely metabolized by the liver. Unfortunately, there are no endogenous markers for hepatic clearance that can be used as a guide for drug dosing, nor are there readily available tests to accurately estimate the extent of residual liver function. Moreover, there is a paucity of high-quality, prospective data that examine the pharmacology and adverse effect profile of many analgesics in patients with advanced liver dysfunction.
Drug metabolism in general occurs in the liver via 3 mechanisms: (1) oxidation, reduction, or hydrolysis reactions of the hepatic cytochrome P450 (CYP) enzyme system; (2) conjugation to glucuronic acid, sulfate, acetate, glycine, glutathione, or a methyl group; and (3) biliary excretion and elimination.3,5 The pharmacokinetics of analgesic medications rely heavily on liver and renal function. Drugs with high hepatic extraction (or first-pass metabolism), such as morphine or fentanyl, have low bioavailability in healthy people but higher bioavailability in cirrhotic patients. For drugs with a low hepatic extraction, such as methadone, liver disease does not impact bioavailability, but hepatic clearance may be altered substantially. The ability to clear drug metabolites decreases with liver dysfunction, resulting in altered parent drug or metabolite bioavailability and increased toxicity in cirrhotic patients. Thus, if such drugs are administered to cirrhotic patients, the dose should be reduced and/or the drug used less frequently.2 Cirrhotic patients often have low serum protein and albumin concentrations. If a drug is highly protein bound, a low albumin level can result in increased levels of free drug and consequent increased adverse effects and toxicity.2 In patients with severe cholestasis, the clearance of drugs with high biliary elimination, such as buprenorphine, may also be compromised because of dysfunction of basolateral and/or apical transmembrane transport systems in hepatocytes, requiring dose reduction or avoidance of use of the drug.4,6,7
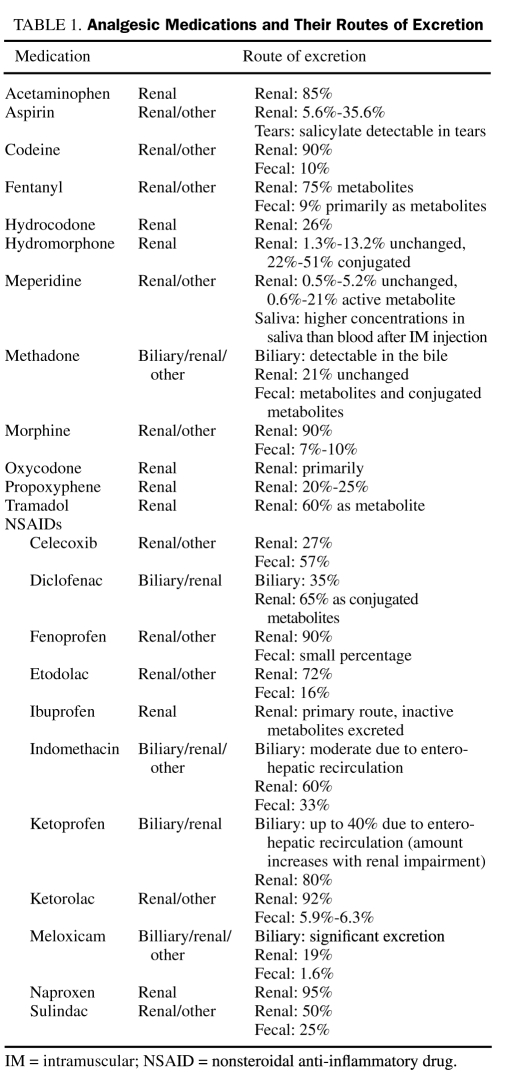
Dosing of analgesic drugs with a predominant renal elimination may require adjustment in patients with liver disease (Table 1). Cirrhotic patients often have impaired renal function despite a normal serum creatinine level because of poor nutrition and reduced muscle mass resulting in less creatinine production. Therefore, in cirrhotic patients, creatinine clearance should be measured or calculated using the Cockcroft and Gault equation to better estimate the dosing of analgesic drugs that have preponderant renal elimination.2,8 Because the creatinine clearance tends to overestimate the glomerular filtration rate (GFR) in cirrhotic patients, the dose of a given drug may still need to be reduced. Unfortunately, criterion standard tests to estimate GFR, such as inulin or iothalamate clearance, are not widely available, and their accuracy in this patient population is unknown.
TABLE 1.
Analgesic Medications and Their Routes of Excretion
OTCA MEDICATIONS
Over-the-counter analgesics, principally acetaminophen and NSAIDs, are commonly used medications worldwide. Guidelines for the use of OTCAs in patients with chronic liver disease are not readily available despite the possibility that such patients may be more susceptible to adverse reactions. Patients are often counseled to modify use of these drugs. Health care professionals frequently recommend avoidance of the use of acetaminophen in patients with liver disease or cirrhosis, whereas NSAIDs are more commonly endorsed.9 Variability and misconception regarding the safety of OTCAs for patients with hepatic dysfunction are widespread among health care professionals.
Acetaminophen
Acetaminophen is the most common cause of fulminant hepatic failure in the United States, creating the perception that it may be dangerous in patients with chronic liver disease.9-11 Moreover, concern is increasing regarding the safety of acetaminophen at a maximal dosage of 4 g/d in the general population. Surveillance data from the United States from 1990 to 1998 estimated 56,000 emergency department visits, 26,000 hospitalizations, and 458 deaths per annum because of acetaminophen overdoses.12 When one considers that 28 billion doses of products containing acetaminophen were consumed in 2005 alone,13 the probability of an individual patient without preexisting liver disease or concomitant alcohol consumption developing clinically important hepatotoxicity or nephrotoxicity when acetaminophen dosing is limited to less than 4 g/d is exceedingly rare.13-16 However, liver failure can occur with a 1-time ingestion of high doses of acetaminophen (>12 g in an adult or 250 mg/kg in a child).17,18 Case reports have demonstrated that long-term ingestion (often accidental) of supratherapeutic doses (>4 g/d) of acetaminophen in patients without known liver disease, and therapeutic doses in alcoholic patients without cirrhosis, resulted in acute liver failure.19-21 To address the fact that approximately half of all cases of acetaminophen-induced acute liver failure are due to unintentional overdosing,11 advisory committees to the Food and Drug Administration (FDA) endorse relabeling of acetaminophen-containing products to better inform the consumer of the potential for liver injury with supratherapeutic doses, and while concurrently consuming 3 or more alcoholic drinks per day. In addition, the advisory committees support lowering the maximal dosage of acetaminophen to 2600 mg/d and eliminating or reducing the availability of combination analgesics, most commonly combinations of opioid with acetaminophen.13 Opiates can be addictive, and patients may develop tolerance to these agents, necessitating dose escalation and thereby increasing the risk of acetaminophen toxicity. These recommendations have not yet been instituted.
Unfortunately, no prospective, long-term studies have assessed the safety of long-term use of acetaminophen in patients with cirrhosis. In such patients, the half-life of oral acetaminophen is double that in healthy controls, but hepatic injury and renal injury are rare when the dosage is limited to less than 4 g/d.5,22 This assumption is supported by a double-blind, 2-period crossover study of 20 patients with chronic stable liver disease (8 with cirrhosis), who tolerated acetaminophen at a dosage of 4 g/d for 13 days without adverse effects.23
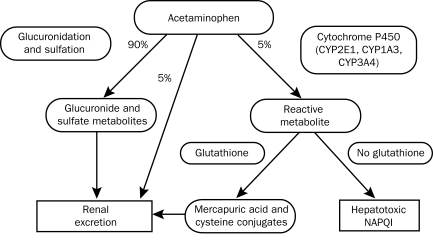
The prevailing mechanism of acetaminophen-induced hepatotoxicity includes altered metabolism via CYP activity in combination with depleted glutathione stores that cause accumulation of a hepatotoxic intermediate, N-acetyl-p-benzoquinone imine (NAPQI) (Figure 1). Studies in patients with cirrhosis have shown that CYP activity is not increased and glutathione stores are not depleted to critical levels in those taking recommended doses of acetaminophen. Glutathione stores are variable in patients with and without underlying liver disease but generally have not been found to be depleted in cirrhotic patients.14 On the basis of these data, the longer half-life, and very limited clinical studies, our recommendation (expert opinion) for long-term acetaminophen use (>14 days) in cirrhotic patients (not actively drinking alcohol) is for reduced dosing at 2 to 3 g/d.14 For short-term use or 1-time dosing, 3 to 4 g appears safe; however, with the new FDA guidelines in mind, a maximum dosage of 2 to 3 g/d is recommended.
FIGURE 1.
Acetaminophen metabolism. At therapeutic doses, 90% of acetaminophen is metabolized to glucuronide and sulfate compounds and ultimately excreted via the renal system. Of the remaining acetaminophen, 50% is excreted unchanged in the urine, and the remainder is metabolized by the cytochrome P450 system; a hepatotoxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI), is subsequently produced. Hepatic glutathione conjugates with NAPQI to produce nontoxic metabolites that are renally excreted. With a toxic ingestion of acetaminophen, the glucuronidation and sulfation pathways become overwhelmed, and glutathione stores diminish, resulting in hepatocyte necrosis due to NAPQI.
Glutathione is predictably depleted in the setting of long-term alcohol consumption or malnutrition.14,15,21 Alcohol itself, its main metabolite acetaldehyde, and a malnourished state also deplete the antioxidant reserve, rendering alcoholic patients more susceptible to drug-induced liver injury. In addition, long-term alcohol ingestion induces CYP2E1, the major enzyme responsible for the metabolism of acetaminophen to its toxic metabolite NAPQI24 (Figure 1). Hence, the population at modestly higher risk of toxicity with long-term supratherapeutic dosing of acetaminophen is the chronic alcoholic or malnourished patient, but no prospective studies exist in these patient populations. Toxicity has been seen in alcoholic patients taking greater than 4 g/d of acetaminophen.25,26 A randomized controlled trial of 4 g/d of acetaminophen for 10 days in patients consuming daily alcohol (defined as 1-3 drinks per day) suggested no significant toxicity after up to 10 days of use, but a small increase in liver enzymes (8 IU/mL) was observed, the clinical importance of which is unclear.27 A study of alcohol-dependent patients (defined as >6 drinks per day for >6 weeks) admitted to a chemical detoxification unit who were receiving 4 g/d of acetaminophen for 3 days during the immediate withdrawal period showed no evidence of toxicity.28 A systematic review of methodologically sound short-term studies suggested that the use of therapeutic dosing of acetaminophen in patients with chronic alcoholism has not been associated with liver injury, but no studies of longer-term therapy have been performed.15 Thus, less than 4 g/d of acetaminophen appears safe for short-term dosing in patients with mild to moderate alcohol intake, but most hepatologists (written communication, expert opinion: see end of article for list of sources) advocate for lower dosing at 2 g or less per day, given the small margin for error in a nonstudy population. Data do not exist for long-term acetaminophen use in patients with active alcohol use. Multiple hepatologists agree that 2 g or less per day of acetaminophen would be recommended for these patients (written communication, expert opinion). Careful follow-up of these patients is recommended. Patients who have underlying alcohol-related liver disease but have prolonged abstinence and are nutritionally replete can be treated similarly to other cirrhotic patients.
Nonsteroidal Anti-inflammatory Drugs
NSAIDs as a class are largely metabolized by CYPs, and most are heavily protein bound. As such, altered metabolism and bioavailability that result in increased serum levels can be anticipated in the cirrhotic patient.29 NSAID-induced (and idiosyncratic) hepatotoxicity has also been well described.9 However, in cirrhotic patients with portal hypertension, the greater concern with NSAID use is the associated renal impairment, in particular hepatorenal syndrome. This is thought to be due to the inhibition of prostaglandins, which leads to a profound decrease in renal perfusion, reduction in GFR, and marked sodium retention. Cirrhotic patients require prostaglandins to counteract the renin-angiotensin-aldosterone and sympathetic systems that reduce perfusion to the kidneys.30 Hepatorenal syndrome is a dreaded and frequently fatal complication of advanced liver disease.
NSAIDs can cause mucosal bleeding in patients at increased risk of bleeding as a result of thrombocytopenia and coagulopathy associated with advanced liver disease. This risk is even greater in patients with portal hypertension–related complications, such as esophageal/gastric varices and portal hypertensive gastropathy or gastric antral vacular ectasias.31 NSAIDs may be tolerated in patients with mild chronic liver disease, but they should be avoided in all patients with cirrhosis because of the increased risk of hepatorenal syndrome and the dire consequences relating to this complication.30 Preventive medicine, including avoidance of NSAIDs, is exceedingly important in maintaining the clinical stability of patients with well-compensated cirrhosis.
No prospective studies have assessed the safety and efficacy of COX-2 inhibitors in the management of chronic pain in patients with cirrhosis. Studies comparing NSAIDs with COX-2 inhibitors in patients without underlying liver disease have demonstrated similar effectiveness in the treatment of musculoskeletal pain.32,33 Although some COX-2 inhibitors may protect against gastrointestinal hemorrhage compared with NSAIDs, an increased risk of cardiovascular adverse events has been observed. Cyclooxygenases are highly regulated in response to changes in intravascular volume, and COX-2 is implicated in the mediation of renin release, sodium regulation, and the maintenance of renal blood flow. COX-2 inhibitors may reduce portal pressure in cirrhotic patients, but pilot data suggest a decreased GFR in patients with cirrhosis and ascites treated with celecoxib.34 The safety of COX-2 inhibitors needs further study in patients with cirrhosis.
Opioid Analgesics
Like anti-inflammatory medications, opioids can have deleterious effects in patients with cirrhosis. Although large epidemiological studies are lacking, sedatives and opioids are common precipitants of hepatic encephalopathy and hospitalization, and thus they should be avoided in patients with cirrhosis, especially in those with portal hypertension and encephalopathy.22 The liver is the main site of metabolism for most opioids. The major metabolic pathways for most opioids are oxidation via the CYP system (CYP2D6 and 3A4) or glucuronidation; both processes can be impaired in the setting of end-stage liver disease, although the CYP more so.2,3 not only is the CYP system affected by liver dysfunction, but also it can be affected by malnutrition and suboptimal protein consumption, common issues in cirrhotic patients. Although there are phenotypic variations of CYP2D6 in the general population, data are inconclusive on how CYP isoenzymes are affected by cirrhosis.4 As a result, patients with cirrhosis have decreased drug clearance and/or increased oral bioavailability, leading to drug accumulation in the body, especially with repeated administration.
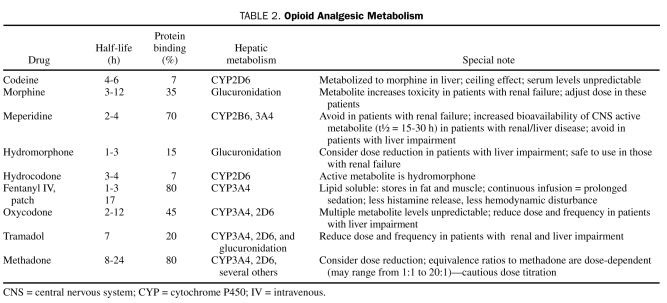
Glucuronidation is thought to be less affected by cirrhosis,2 but studies have consistently shown that the half-life of morphine, for instance, is prolonged in patients with cirrhosis. Compared with healthy controls, in cirrhotic patients the half-life of morphine is approximately double (3 to 4 vs 1.5 to 2 hours), which is attributable to a reduction in total body clearance4,35 (Table 2). Similarly, other opioids have also been shown to have increased bioavailability and prolonged half-life.36 Codeine is another frequently prescribed opioid in patients with cirrhosis. The analgesic effect from codeine is presumed to be secondary to its conversion to morphine via CYP2D6; thus, in patients with cirrhosis, serum levels can be even more variable. Similarly, hydrocodone and oxycodone are metabolized to hydromorphone and oxymorphone via CYP2D6 and CYP3A4, which may also result in variability in serum levels. Ineffective drug metabolism in this patient population can also lead to decreased analgesic action of these medications.2,4 Meperidine is metabolized largely by CYP2B6 and CYP3A4 to normeperidine, a metabolite with serious central nervous system toxicity, particularly in the setting of concomitant renal dysfunction.36 Meperidine should be avoided in patients with liver dysfunction because of the increased bioavailability (heavily protein bound) and prolonged half-life of its toxic metabolite.36 Although methadone and fentanyl are also heavily protein bound and as such require reduced dosing in patients with cirrhosis, the metabolism of these agents does not yield toxic metabolites, and hence they, along with hydromorphone, may be better tolerated37,38 (Table 2).
TABLE 2.
Opioid Analgesic Metabolism
Patients with cirrhosis have a high prevalence of and increased likelihood for renal dysfunction. This is relevant to the risk of adverse drug events in these patients because renal function has a significant impact on the toxicity of several opioids. Most opioids require adjustment of dose based on GFR. With morphine, although metabolized largely by glucuronidation, the resulting metabolite has central nervous system toxicity and is poorly excreted in the setting of renal insufficiency. Hence, dose reduction or avoidance of morphine in cirrhotic patients would be prudent. Hydromorphone and fentanyl appear to be the least affected by renal dysfunction, and fentanyl has less hemodynamic disturbance (due to lack of histamine release associated with other opioids).39
Tramadol is another opiate occasionally used in low doses in patients with cirrhosis who are experiencing intractable pain because of its impact on peripheral pain pathways, partial inhibition of serotonin reuptake, and low affinity for opioid receptors, thought to result in less sedation, respiratory depression, and potential for tolerance; however, constipation can still be problematic because of anticholinergic adverse effects.40 Caution should be exercised in administering tramadol to epileptic patients because this drug is known to lower the seizure threshold. In addition, tramadol should not be combined with drugs such as morphine, selective serotonin reuptake inhibitors, tricyclic antidepressants (TCAs), or anticonvulsants because it can precipitate serotonin syndrome.41 Doses may need to be reduced in patients with renal failure.
If opiates are required for pain control, lower doses and/or longer intervals between doses are needed to minimize risks.4,40 Hydromorphone and fentanyl may be the better choices. Careful follow-up is required to check for signs of sedation, constipation (a risk for precipitating encephalopathy), and early encephalopathy. Any sign of these complications necessitates immediate discontinuation of the opiate.
Health care practitioners should be prudent in not using potentially addictive substances like opioids in cirrhotic patients with a history of alcoholism because of increased risk of cross-addiction.42 For many transplant programs, ongoing opioid use in such patients may be a contraindication to liver transplant because opioid dependency is widely believed to predict alcohol recidivism, and discussion with the transplant program is advised before initiating these drugs.42
Polysubstance abuse treated with methadone maintenance is particularly common among patients with chronic liver disease from hepatitis C virus (HCV). The prevalence of anti-HCV antibodies is as high as 67% to 96% among patients in methadone programs.43 Methadone is well-absorbed from the gastrointestinal tract and has high oral bioavailability, corresponding with low hepatic extraction. It undergoes considerable biotransformation in the liver. Although no prospective studies have assessed the safety of methadone in patients with hepatic dysfunction, 11 patients with alcoholic cirrhosis were noted to have disposition parameters similar to those in healthy participants, suggesting that usual methadone maintenance dosages are likely safe in patients with advanced liver disease.4,38 Nevertheless, avoidance of methadone in patients actively consuming alcohol is advisable because alcohol inhibits the metabolism of methadone, leading to elevated plasma methadone concentrations.38 Patients who take methadone for heroin addiction have a similar rate of treatment response to anti-HCV therapy and, more importantly, a decreased likelihood of heroin use. For patients with chronic liver disease in the absence of active alcoholism, no absolute contraindications exist, and the benefits of methadone maintenance to achieve abstinence from heroin would likely outweigh the potential risks.43,44
Other Analgesics
Not infrequently, patients with cirrhosis experience neuropathic pain due to neuropathies from a variety of causes, including diabetes, alcoholism, nutrient deficiencies, and cryoglobulinemia. Tricyclic antidepressants such as amitriptyline and imipramine have been the mainstay treatment of neuropathic pain for decades, although their use in this capacity is off-label.45,46 The exact mechanism of antineuralgic action of these agents is unknown, but they may diminish chronic pain by blocking presynaptic serotonin and/or noradrenalin reuptake in neurons involved in pain transmission or dampened endogenous opioid systems.46 Tricyclic antidepressants rely on hepatic biotransformation with first-pass effects (via CYP2D6 largely) and renal elimination. Health care professionals should start a TCA at a low dose because these agents are sedating, and patients may be more susceptible to the anticholinergic adverse effects, including dry mouth, blurry vision, drowsiness, tachycardia, and orthostatic hypotension due to altered metabolism in the setting of liver dysfunction. The clinician and patient must be particularly watchful for intestinal stasis as an adverse effect of a TCA because this can precipitate hepatic encephalopathy. If TCAs are deemed necessary, nortriptyline and desipramine are less potent and appear to be less sedating than other TCAs. Additionally, nortriptyline and desipramine may have less tachycardia and hypotension associated with their use than older and more potent TCAs, particularly amitriptyline and doxepin.45
Anticonvulsants such as carbamazepine or gabapentin also have an established role in neuropathic pain management. The rationale for their use is that neuropathic pain presumably involves an imbalance of excitatory and inhibitory neurotransmitters, and anticonvulsants may modulate peripheral and central components of neurotransmission to correct this imbalance and thus diminish pain.46 Most anticonvulsants are metabolized by the liver (via CYPs) and excreted by the renal system, once again necessitating lower and less frequent dosing in cirrhotic patients. Carbamazepine has been reported to cause hepatotoxicity in the general population; it may precipitate a rapid deterioration in cirrhotic patients and thus should be avoided.46 Gabapentin is unique among many anticonvulsants because it is not metabolized by the liver or bound to plasma proteins, making it a preferred anticonvulsant in patients with cirrhosis. However, the general use of gabapentin in patients with cirrhosis may be limited by other potential adverse effects, including sedation, nausea, and dizziness. Doses should be adjusted for renal failure because gabapentin is renally excreted.
Pregabalin is another anticonvulsant shown to be effective for neuropathic pain; its mechanism of action is as a potent ligand for the α-2-δ subunit of voltage-gated calcium channels in the central nervous system.46 like gabapentin, it is not subject to hepatic metabolism and hence may be an appealing agent of choice in cirrhotic patients with neuropathic pain. A recent case report from Sweden determined that pregabalin was a probable cause of acute liver failure in a 61-year-old healthy man with no previous liver disease.47 Although this may have been an idiosyncratic event because no further case reports have been published in the literature, clinicians must be mindful of the increased risk of drug-induced liver injury in patients with underlying liver disease.
SUMMARY
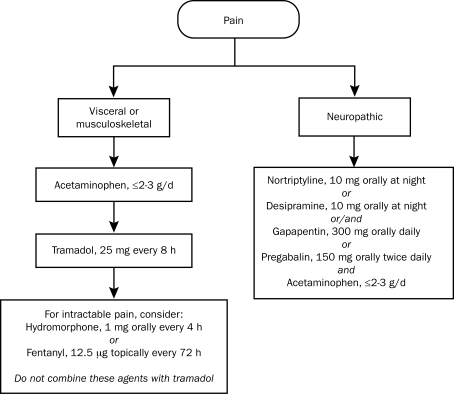
No evidence-based guidelines exist on the use of analgesics in patients with liver disease and cirrhosis. This review underscores the paucity of prospective studies that have assessed the safety of various analgesics in patients with advanced hepatic dysfunction. It has been an unspoken standard of practice by hepatologists alike to err on the side of caution, recommending 2 to 3 g/d of acetaminophen. Because the FDA may recommend limiting acetaminophen to a maximum daily dosage of 2.6 g, we have provided a uniform recommendation and a practical guide to approaching analgesia in the cirrhotic patient (Figure 2), which has been reviewed and agreed on by hepatologists within our group practices (unpublished survey of 10 hepatologists). An important caveat is that the care of patients with cirrhosis must be individualized, and analgesic options may vary depending on a number of factors, such as nutritional status, adherence, renal function, and liver transplant candidacy.
FIGURE 2.
A pharmacological approach to analgesia in patients with cirrhosis who have no renal failure, active alcoholism, or active substance abuse. Starting doses are used unless otherwise indicated. Doses should be carefully titrated as tolerated. Minimize total acetaminophen to less than or equal to 2 to 3 g/d. Avoid polypharmacy and monitor for adverse drug events.
CONCLUSION
In general, our recommendation (expert opinion) for long-term acetaminophen use in cirrhotic patients (not actively drinking alcohol) is for reduced dosing at 2 to 3 g/d.14 For short-term use or 1-time dosing, 3 to 4 g/d appears to be safe; however, with the new FDA recommendations, a maximum dosage of 2 to 3 g/d is recommended. NSAIDs and opioids may be used at reduced doses in patients with chronic liver disease without cirrhosis. Patients with cirrhosis have fewer analgesic options. NSAIDs should be avoided in those with both compensated and decompensated cirrhosis, primarily because of the risk of acute renal failure due to prostaglandin inhibition. Opiates should be avoided or used sparingly at low and infrequent doses because of the risk of precipitating hepatic encephalopathy. Patients with a history of encephalopathy or substance abuse should not take opioids. When appropriate, anticonvulsants and antidepressants are options worthy of exploration in chronic neuropathic pain management in patients with advanced liver disease. Diligent follow-up for toxicity, adverse effects, and complications is necessary.
Supplementary Material
Acknowledgments
We thank Laura J. Myhre, PharmD, RPh, for her assistance in preparing Table 1.
Footnotes
An earlier version of this article appeared Online First.
List of sources of written communication, expert opinion, is as follows: John J. Poterucha, MD; Michael R. Charlton, MD; J. E. Hay, MD; John B. Gross Jr, MD; Russell H. Wiesner, MD; Patrick S. Kamath, MD; William Sanchez, MD; W. Ray Kim, MD; Gerry M. Minuk, MD; William M. Lee, MD; Timothy M. McCashland, MD; Michael F. Sorrell, MD; Marie Laryea, MD; and Josh Levitsky, MD.
REFERENCES
- 1.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12(4):733-746, vii [DOI] [PubMed] [Google Scholar]
- 2.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147-1161 [DOI] [PubMed] [Google Scholar]
- 3.Elbekai R, Korashy H, El-Kadi A. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;5(2):157-167 [DOI] [PubMed] [Google Scholar]
- 4.Tegeder I, Lotsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet 1999;37(1):17-40 [DOI] [PubMed] [Google Scholar]
- 5.Villeneuve JP, Raymond G, Bruneau J, Colpron L, Pomier-Layrargues G. Pharmacokinetics and metabolism of acetaminophen in normal, alcoholic and cirrhotic subjects. Gastroenterol Clin Biol. 1983;7(11):898-902 [PubMed] [Google Scholar]
- 6.Bohan A, Boyer JL. Mechanisms of hepatic transport of drugs: implications for cholestatic drug reactions. Semin Liver Dis. 2002;22(2):123-136 [DOI] [PubMed] [Google Scholar]
- 7.Delco F, Tchambaz L, Schlienger R, Drewe J, Krahenbuhl S. Dose adjustment in patients with liver disease. Drug Saf. 2005;28(6):529-545 [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16(1):31-41 [DOI] [PubMed] [Google Scholar]
- 9.Rossi S, Assis DN, Awsare M, et al. Use of over-the-counter analgesics in patients with chronic liver disease: physicians' recommendations. Drug Saf. 2008;31(3):261-270 [DOI] [PubMed] [Google Scholar]
- 10.Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28(2):142-152 [DOI] [PubMed] [Google Scholar]
- 11.Larson A, Polson J, Fontana RJ, et al. Acute Liver Failure Study Group Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42(6):1364-1372 [DOI] [PubMed] [Google Scholar]
- 12.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetamol)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15(6):398-405 [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. US Food and Drug Administration (FDA) Drugs: acetaminophen information. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm Accessed February 19, 2010.
- 14.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133-141 [DOI] [PubMed] [Google Scholar]
- 15.Dart R, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy 2007;27(9):1219-1230 [DOI] [PubMed] [Google Scholar]
- 16.Temple AR, Lynch JM, Vena J, Auiler JF, Gelotte CK. Aminotransferase activities in healthy subjects receiving three-day dosing of 4, 6, or 8 grams per day of acetaminophen. Clin Toxicol (Phila) 2007;45(1):36-44 [DOI] [PubMed] [Google Scholar]
- 17.Prescott LF. Paracetamol overdosage: pharmacological considerations and clinical management. Drugs 1983;25(3):290-314 [DOI] [PubMed] [Google Scholar]
- 18.Makin AJ, Wendon J, Williams R. A 7-year experience of severe acetaminophen-induced hepatotoxicity (1987-1993). Gastroenterology 1995;109(6):1907-1916 [DOI] [PubMed] [Google Scholar]
- 19.Pezzano M, Richard C, Lampl E, et al. Hepatic and renal toxicity of paracetamol in chronic alcoholic patient. Presse Med. 1988;17(1):21-24 [PubMed] [Google Scholar]
- 20.Mofredj A, Cadranel JF, Darchy B, et al. Hepatotoxicity caused by therapeutic doses of paracetamol in alcoholics: report of 2 cases of fatal hepatitis in cirrhosis. Ann Med Interne (Paris) 1999;150(6):507-511 [PubMed] [Google Scholar]
- 21.Bolesta S, Haber SL. Hepatotoxicity associated with chronic acetaminophen administration in patients without risk factors. Ann Pharmacother. 2002;36(2):331-333 [DOI] [PubMed] [Google Scholar]
- 22.Hirschfield GM, Kumagi T, Heathcote EJ. Preventative hepatology: minimising symptoms and optimising care. Liver Int. 2008;28(7):922-934 [DOI] [PubMed] [Google Scholar]
- 23.Benson GD. Acetaminophen in chronic liver disease. Clin Pharmacol Ther. 1983;33(1):95-101 [DOI] [PubMed] [Google Scholar]
- 24.Villeneuve JP, Pichette V. Cytochrome P450 and liver diseases. Curr Drug Metab. 2004;5(3):273-282 [DOI] [PubMed] [Google Scholar]
- 25.Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994;272(23):1845-1850 [DOI] [PubMed] [Google Scholar]
- 26.Prescott LF. Paracetamol, alcohol and the liver. Br J Clin Pharmacol. 2000;49(4):291-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuffner EK, Green JL, Bogdan GM, et al. The effect of acetaminophen (four grams a day for three consecutive days) on hepatic tests in alcoholic patients: a multicenter randomized study. BMC Med. 2007;5:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26(2):283-290 [DOI] [PubMed] [Google Scholar]
- 29.Williams RL, Upton RA, Cello JP, et al. Naproxen disposition in patients with alcoholic cirrhosis. Eur J Clin Pharmacol. 1984;27(3):291-296 [DOI] [PubMed] [Google Scholar]
- 30.Laffi G, La Villa G, Pinzani M, Marra F, Gentilini P. Arachidonic acid derivatives and renal function in liver cirrhosis. Semin Nephrol. 1997;17(6):530-548 [PubMed] [Google Scholar]
- 31.Castro-Fernandez M, Sanchez-Munoz D, Galan-Jurado MV, et al. Influence of nonsteroidal antiinflammatory drugs in gastrointestinal bleeding due to gastroduodenal ulcers or erosions in patients with liver cirrhosis. Gastroenterol Hepatol. 2006;29(1):11-14 [DOI] [PubMed] [Google Scholar]
- 32.Hur C, Chan AT, Tramontano AC, Gazelle GS. Coxibs versus combination NSAID and PPI therapy for chronic pain: an exploration of the risks, benefits, and costs. Ann Pharmacother. 2006;40(6):1052-1063 [DOI] [PubMed] [Google Scholar]
- 33.Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 2008;12(11):1-278, iii [DOI] [PubMed] [Google Scholar]
- 34.Guevara M, Abecasis R, Jimenez W, et al. Effect of celecoxib on renal function in cirrhotic patients with ascites: a pilot study [abstract]. J Hepatol. 2002;36(suppl 1):203 [DOI] [PubMed] [Google Scholar]
- 35.Hasselstrom J, Eriksson S, Persson A, Rane A, Svensson JO, Sawe J. The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br J Clin Pharmacol. 1990;29(3):289-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haberer JP, Schoeffler P, Couderc E, Duvaldestin P. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. Br J Anaesth. 1982;54(12):1267-1270 [DOI] [PubMed] [Google Scholar]
- 38.Novick DM, Kreek MJ, Fanizza AM, Yancovitz SR, Gelb AM, Stenger RJ. Methadone disposition in patients with chronic liver disease. Clin Pharmacol Ther. 1981;30(3):353-362 [DOI] [PubMed] [Google Scholar]
- 39.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119-141 [DOI] [PubMed] [Google Scholar]
- 40.Kotb HI, Fouad IA, Fares KM, Mostafa MG, Abd El-Rahman AM. Pharmacokinetics of oral tramadol in patients with liver cancer. J Opioid Manag. 2008;4(2):99-104 [DOI] [PubMed] [Google Scholar]
- 41.Vizcaychipi MP, Walker S, Palazzo M. Serotonin syndrome triggered by tramadol. Br J Anaesth. 2007;99(6):919 [DOI] [PubMed] [Google Scholar]
- 42.DiMartini A, Day N, Dew MA, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12(5):813-820 [DOI] [PubMed] [Google Scholar]
- 43.Sylvestre DL. Treating hepatitis C in methadone maintenance patients: an interim analysis. Drug Alcohol Depend 2002;67(2):117-123 [DOI] [PubMed] [Google Scholar]
- 44.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev. 2005;24(3):205-214 [DOI] [PubMed] [Google Scholar]
- 46.Harvey JN. Update on treatments for neuropathic pain. J Pain Palliat Care Pharmacother. 2008;22(1):54-57 [DOI] [PubMed] [Google Scholar]
- 47.Einarsdottir S, Bjornsson E. Pregabalin as a probable cause of acute liver injury. Eur J Gastroenterol Hepatol. 2008;20(10):1049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.