Abstract
The native extracellular matrix (ECM) of elastic tissues is strong and flexible and supports cell adhesion and enzymatic matrix remodeling. In an attempt to convey these ECM properties to a synthetic scaffold appropriate for soft tissue engineering applications, a biodegradable, elastomeric poly(ester urethane)urea (PEUU) was combined with type I collagen at various ratios (2.5, 5, 10, 20, 50, 60, 70, 80, and 90 wt% collagen) and electrospun to construct elastic matrices. Randomly orientated fibers in the electrospun matrices ranged in diameter from 100–900 nm, dependent on initial polymer concentration. Picrosirius red staining of matrices and CD spectroscopy of released collagen confirmed collagen incorporation and preservation of collagen structure at the higher collagen mass fractions. Matrices were strong and distensible possessing strengths of 2–13 MPa with breaking strains of 160–280% even with low PEUU content. Collagen incorporation significantly enhanced smooth muscle cell adhesion onto electrospun scaffolds. An approach has been demonstrated that mimics elastic extracellular matrices by using a synthetic component to provide mechanical function together with a biomacromolecule, collagen. Such matrices may find application in engineering soft tissue.
Keywords: biodegradable, elastomer, scaffold, poly(ester urethane) urea, electrospinning, collagen
INTRODUCTION
Biodegradable elastomers offer attractive mechanical properties for many soft-tissue engineering applications where nonelastomeric biodegradable polymers are currently used. In order to develop functionally compliant and mechanically robust tissue, evidence suggests that scaffolds should be designed to effectively transmit mechanical signals to the developing tissue in the dynamic in vitro or in vivo environment.1-6 To date, the majority of tissue engineering reports in the literature have focused on a narrow series of biodegradable polyesters that are stiff with limited or no elastomeric properties. Therefore, several groups are actively pursuing the development of degradable and cytocompatible elastomeric materials that could find application in engineering soft tissues.7-13 Our laboratory has developed a family of biodegradable polyurethanes since this material class allows for great latitude in design through the choice of hard and soft segments and also due to its potential to serve as a processable thermoplastic elastomer.12,13
Several types of biodegradable elastomers have been reported in the literature; however, there are fewer reports wherein these materials are fabricated into three-dimensional scaffolds compatible with cell growth. Techniques to fabricate porous structures for tissue engineering include particulate leaching, solvent casting, melt molding, fiber casting, membrane lamination, thermally induced phase separation, ink-jet printing, fused deposition modeling, and others.14-16 Another technique, electrospinning, offers a means to process a polymer into sub-micron diameter fibers.17 While this method was originally studied in 1914 and patented in the 1930s,18,19 recent interest exists in its application towards development of tissue engineering scaffolds in order to more closely mimic the size and scale of the natural extracellular matrix.20-22 Briefly, electrospinning occurs when a polymer solution or melt is charged with a high voltage generating an electrical force that can overcome the surface tension of a pendant drop of the solution by first forming a conical shape called the Taylor cone23 and then ejecting a polymer jet. The ejected jet experiences a complicated bending and whipping instability combined with fiber splaying and rapid solvent evaporation to yield fibers of very narrow diameters that can be collected on a grounded or charged collection surface. The processing variables of electrospinning can greatly influence the resulting morphology and size of the fibers. These variables include voltage magnitude, polymer feed rate, pendant drop-collector distance, solution viscosity, solution concentration, and polymer molecular weight.
Since polymers can be electrospun from solution, the potential exists to blend biodegradable elastomers with proteins to introduce biomacromolecules into the resulting scaffold. Elastic scaffolds might be modified to incorporate biological activity from proteins found in the extracellular matrix such as growth factors, adhesive proteins, and structural proteins as well as to introduce enzyme sensitivity for remodeling. Collagen is an obvious candidate given its support of cell adhesion, relatively resilient conformation, and sensitivity to collagenase.24 However, fabrication of scaffolds composed of collagen alone can lead to poor mechanical strength and the absence of the appropriate flexibility to undergo cyclic mechanical loading.15,25 By blending with a polyurethane, the combined biofunctionality of collagen with the mechanical properties of the polyurethane might result in an elastic matrix with potential to impact tissue engineering approaches to soft tissue replacement.
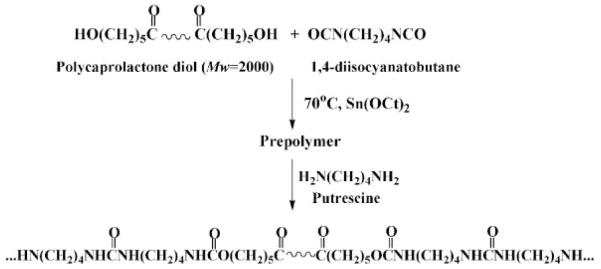
The objective in this study was to process and characterize biodegradable, elastomeric scaffolds with sub-micron scale fibrillar morphologies using an electrospinning technique. A previously reported biodegradable and cytocompatible poly(ester urethane)urea (PEUU) from our laboratory based on polycaprolactone diol (PCL) and 1,4-diisocyanatobutane (BDI) was used as the base material.12 Biofunctionality was introduced into scaffolds by blending the polyurethane with type I collagen prior to electrospinning. We report here on the impact of polymer concentration in the spinning solution on scaffold morphology as well as scaffold collagen content and collagen structure preservation determined using Fourier transform infrared spectroscopy (FTIR), picrosirius red staining, and circular dichroism (CD) spectroscopy. Tensile mechanical properties were measured of both the cast polymer film and the scaffolds. Furthermore, smooth muscle cell adhesion was evaluated on the electrospun PEUU/collagen scaffolds.
MATERIALS AND METHODS
Polymer synthesis and film fabrication
Stannous octoate (Sigma, St. Louis, MO) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) (Oakwood Products) were used as received. 1,4-diisocyanatobutane (Fluka, Milwaukee, WI) and putrescine (Fluka) were distilled under vacuum. Polycaprolactone diol (MW = 2000, Aldrich, Milwaukee, WI) was dried under vacuum for 48 h to remove residual water. Solvents dimethyl sulfoxide (DMSO) and N,N-dimethylformamide (DMF) were dried over 4-Å molecular sieves.
PEUU was synthesized as reported previously (Scheme 1).12 Briefly, a 2:1:1 molar ratio of BDI:PCL:putrescine was used. First, BDI and PCL were reacted in DMSO with stannous octoate as catalyst for3hat 75°C. The prepolymer solution was allowed to cool to room temperature and then putrescine was added dropwise while stirring. After 18 h of reaction at room temperature, the polymer solution was precipitated in distilled water and then wet polymer was incubated in 2-propanol for 48 h to remove unreacted monomer. The polymer was then dried under vacuum. PEUU transparent films were prepared from a 3-wt% solution in DMF by casting onto Teflon dishes. Films were dried under vacuum for 48 h.
Scheme 1.
PEUU synthesis.
Polymer characterization
Polymer molecular weight was determined by gel permeation chromatography (GPC, Waters Breeze V3.2, Waters 1515 isocratic HPLC pump, Waters 2414 refractive index detector) using monodisperse polystyrene standards for calibration. Measurements were made at 35°C with 1-methyl-2-pyrrolidinone (NMP) as solvent. FTIR spectra were obtained at room temperature using a Genesis II FTIR spectrometer.
Differential scanning calorimetry (DSC) was performed on a differential scanning calorimeter (Shimadzu; DSC 60) under helium purge. Scanning rates of 20°C/min were used over a temperature range of −100°C to 250°C. Tensile properties were measured on an MTS Tytron™ 250 MicroForce Testing Workstation (10 mm/min crosshead speed) according to ASTM D638-98. Five samples were tested at each condition studied.
Electrospinning
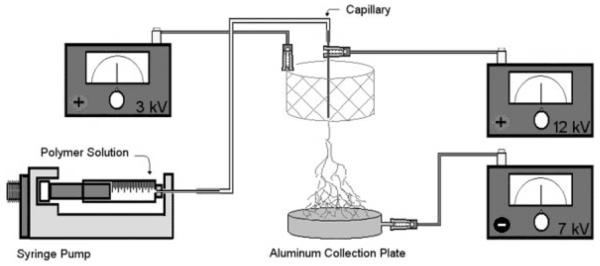
PEUU and type I acid soluble bovine collagen (Sigma) (at 0, 2.5, 5, 10, 20, 50, 60, 70, 80, and 90 final wt% of collagen) were blended at 5 wt% in HFIP under mechanical stirring at 25°C. The polymer solution was fed by syringe pump (Harvard Apparatus) into a steel capillary (I.D. = 0.047′) suspended vertically over the center of a cylindrical steel mesh focusing screen and aluminum collector plate (Fig. 1). A combination of three high-voltage generators (Gamma High Voltage Research) was employed with a high positive voltage (12 kV) to charge the steel capillary containing the polymer solution, a negative voltage (−7 kV) to charge the aluminum collector plate, and a slightly lower positive voltage (3 kV) to charge a steel mesh screen. The screen acted to control the area of fiber deposition onto the aluminum plate. Preliminary investigations without the control mesh found evidence of unwanted fiber deposition on the outer surfaces of the fume hood containing the apparatus. This setup was similar to the multiple field electrospinning apparatus constructed by Deitzel et al.26 By adjusting the voltage magnitude charged to the control mesh, it was possible to minimize outside electrical disturbances as well as to electrospin thicker scaffolds more rapidly. The component spacing and applied voltages were optimized to provide controlled deposition of scaffolds up to 500 μm thick. PEUU was electrospun at various concentrations in HFIP (1–8 wt%) to assess the effect of polymer concentration on scaffold morphology. Deposited scaffolds were allowed to dry overnight at room temperature and then placed under vacuum for 48 h at 30°C.
Figure 1.
Electrospinning setup consisting of a syringe pump to feed polymer solution along with a combination of 3 high-voltage generators and a steel mesh screen to control the area of fiber deposition.
Scaffold characterization
Samples were sputter coated with Pd/Au and imaged with scanning electron microscopy (SEM, JEOL JSM6330F). Fiber diameter as a function of PEUU wt% in HFIP was quantified using digital image processing. For picrosirius red staining,27 samples were cryosectioned and fixed on glass slides with 2% formaldehyde for 20 min. Samples were rinsed with water and then picrosirius red solution (0.3 g Sirius red F3B and 500 mL saturated aqueous picric acid) was added for 1 h. Next, the samples were washed twice with acetic acid solution, dehydrated with ethanol (50, 70, 90, and 100%) and mounted. To quantify absorbance, 6-mm-diameter scaffold discs were weighed and stained with picrosirius red as above. Next, stained scaffolds were incubated in 0.1 N NaOH at 37°C for 30 min to transfer the stain from the scaffold to solution. The absorbance of the solutions was read at 540 nm and normalized by dividing by scaffold mass.
CD spectroscopy was performed to evaluate the preservation of collagen secondary structure in the electrospun biohybrid scaffolds. Collagen was removed from the scaffolds by incubation in 0.1M acetic acid for 48 h at 25°C and then lyophilizing the resulting solutions. CD spectra were run on an Aviv 62A DS Circular Dichroism Spectrometer using 1 mg/mL collagen solutions in 0.1M acetic acid at 25°C. Wavelength scans (n = 3) were performed from 280–185 nm sampling every 1 nm with a 1-s averaging time. Scaffold mechanical properties were measured similar to those of the cast polymer films described above.
Cellular adhesion on PEUU/collagen scaffolds
PEUU/collagen samples were punched into 6-mm-diameter discs and sterilized by immersion in 90% alcohol for 7 h, rinsed multiple times with PBS, and then placed in media in an incubator overnight. Media consisted of Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum supplemented with 1% penicillin-streptomycin. Vascular smooth muscle cells isolated from a rat aorta28 (rSMCs, 8th passage) were statically seeded on scaffolds at a density of 2 × 106 cells/mL. Cellular adhesion 1 day after cell seeding was evaluated using the MTT mitochondrial activity assay (n = 5 per sample studied).29 Data were normalized to tissue culture polystyrene (TCPS). Samples were also rinsed with PBS, fixed with 2.5% glutaraldehyde and 1% osmium tetraoxide in PBS, and subjected to graded ethanol dehydrations before being critical point dried, sputter-coated, and then imaged by SEM to observe cellular morphology.
Statistics
Results are displayed as the mean ± standard deviation. Pearson’s correlation was used to evaluate the linearity of the fiber diameter versus concentration plot. One-way ANOVA testing was carried out on picrosirius absorbance, mechanical properties, and rSMC adhesion using the Neuman-Keuls test for post hoc assessments of the differences between samples.
RESULTS
Polymer characterization
PEUU weight average molecular weight and number average molecular weight as determined by GPC were 228,700 and 87,600, respectively, yielding a poly-dispersity index of 2.61. FTIR spectra of PEUU indicated urethane, urea, and ester groups as well as the absence of any unreacted isocyanate peaks (at approximately 2267 cm−1) or residual solvent. DSC results indicated a glass transition temperature of −54.6°C and soft segment melt temperature of 41.0°C. PEUU was flexible and found to have a tensile strength of 27 ± 4 MPa and a breaking strain of 820 ± 70 %.
Electrospinning
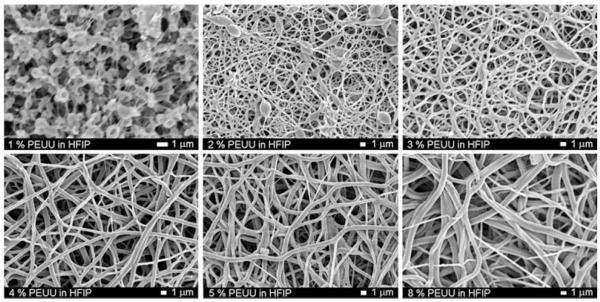
In order to fabricate scaffolds with continuous fibers of sub-micron dimensions, the feed polymer concentration was varied and electrospun PEUU morphology was investigated by SEM (Fig. 2). At concentrations below 2%, a “beads on a string” morphology was observed. At polymer concentrations greater than 2%, continuous fibers were spun with diameters in the hundreds of nanometers. A trend of increasing fiber diameter with increasing polymer concentration was observed (Fig. 3). Pearson’s correlation for linearity revealed r = 0.87 at p < 0.05. Scaffolds possessed fiber diameters ranging from 100 to 900 nm as a function of polymer concentration ranging from 1 to 8%.
Figure 2.
Scanning electron micrographs of electrospun PEUU as a function of polymer concentration in HFIP (scale bars = 1 μm)
Figure 3.
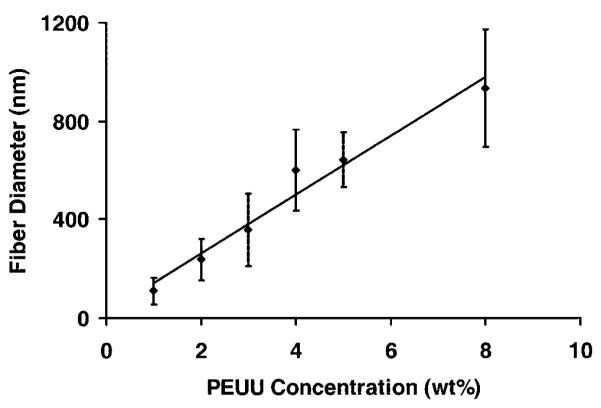
Electrospun fiber diameter as a function of PEUU concentration in the feed solution (r = 0.87; p < 0.05).
Scaffold characterization
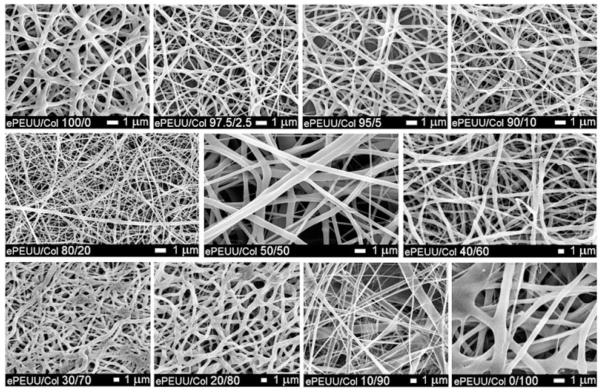
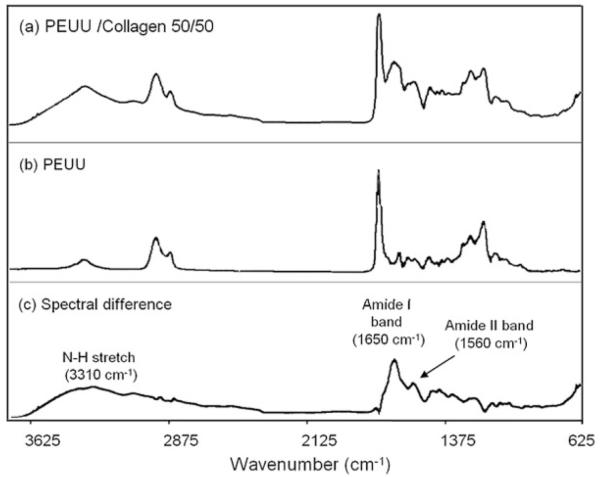
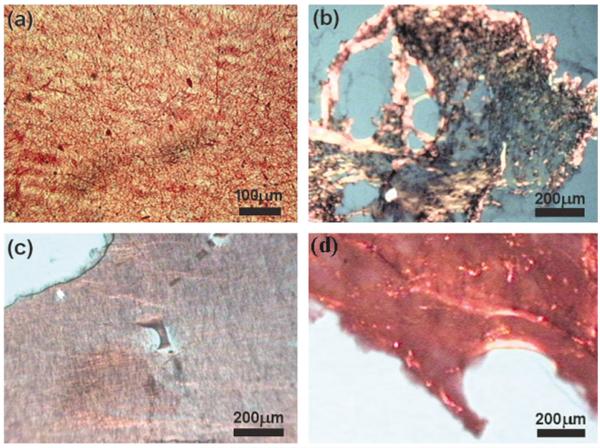
Scanning electron micrographs revealed continuous fiber morphologies spun at all ratios (0, 2.5, 5, 10, 20, 50, 60, 70, 80, and 90% type I collagen) examined (Fig. 4). Different diameters and morphologies were observed at the various PEUU/collagen ratios studied. FTIR of PEUU/collagen samples revealed peaks characteristic of type I collagen. For example, Figure 5 shows the FTIR spectrum of PEUU alone subtracted from the spectrum for a 50/50 PEUU/collagen blend to yield a N—H stretch at 3310 cm−1, an amide I band at 1650 cm−1, and an amide II band at 1560 cm−1. Picrosirius red stained PEUU/collagen electrospun scaffolds positive and polarization microscopy revealed the presence of birefringence in the electrospun scaffolds containing collagen. In general, more prominent staining and birefringence were observed in samples containing at least 20% electrospun collagen. Figure 6(a) shows picrosirius staining of electrospun PEUU/collagen (50/50) and also compares birefringence in pre-processed acid soluble type I collagen [Fig. 6(b)], an electrospun blend of 50/50 PEUU/collagen [Fig. 6(c)], and electrospun collagen [Fig. 6(d)]. Stain leached from scaffolds after the picrosirius staining procedure displayed a trend of increasing absorbance with % type I collagen (Fig. 7).
Figure 4.
Scanning electron micrographs of electrospun scaffolds composed of varying ratios of PEUU and Type I collagen.
Figure 5.
FTIR spectra of (a) blend of 50/50 PEUU/collagen, (b) PEUU, and (c) difference spectrum resulting from subtraction of (b) from (a). Peaks attributable to collagen are noted in the difference spectrum.
Figure 6.
Picrosirius red staining of electrospun PEUU/collagen blends: (a) optical micrograph of 50/50 PEUU/collagen, polarized light micrographs of (b) collagen (c) 50/50 electrospun PEUU/collagen, and (d) 0/100 electrospun PEUU/collagen.
Figure 7.
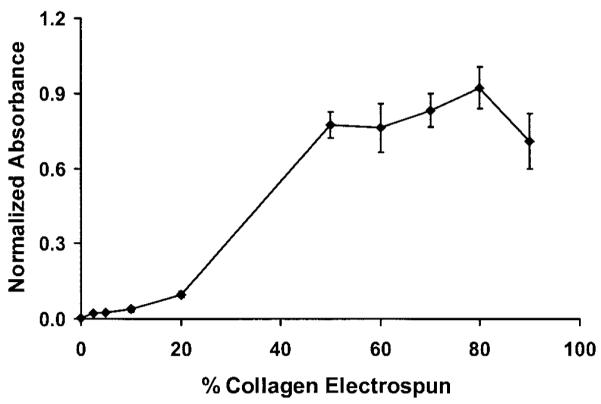
Absorbance normalized to scaffold mass as a function of collagen concentration for picrosirius red stain removed by NaOH.
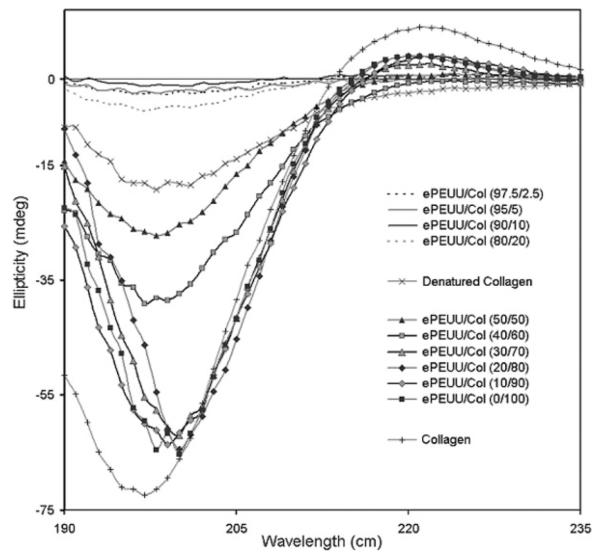
CD spectra of collagen removed from the electrospun scaffolds into 0.1M acetic acid were compared with spectra of type I collagen before processing and also type I collagen that was thermally denatured at 50°C. Representative spectra of electrospun PEUU/collagen samples studied are displayed in Figure 8. Table I shows the difference in ellipticities between electrospun collagen samples relative to the collagen control at different blending ratios. The spectrum of the collagen control exhibits a maxima peak at 221 nm and a minima peak of 197 nm. Upon thermal denaturation at 50°C, the collagen spectrum exhibited loss of the peak at 221 nm along with a difference in ellipticity of 54.36 mdeg from the non-denatured collagen. In general, a trend of decreasing difference in ellipticity between the collagen control was exhibited at increasing collagen ratios. This trend indicated more triple helix retention in electrospun samples containing 50% or more collagen. Electrospun samples containing 2.5, 5, 10, and 20% collagen did not show a peak at 221 nm and had differences in minima ellipticity >66 mdeg. Samples containing 50 and 60% collagen gave small or no peaks at 221 nm with minima ellipticity differences from the collagen control at 45.23 and 33.26 mdeg, respectively. Electrospun samples containing 70–100% collagen exhibited peaks at 221 nm and minima ellipticity differences at 10.33 mdeg or less indicating greater collagen helix retention. All electrospun samples including electrospun collagen alone possessed some secondary structure modification as a result of the electrospinning process and exposure to the HFIP solvent. CD signal error consisted of a few hundredths of a mdeg for all samples except near 185 nm and is not shown for sake of clarity.
Figure 8.
CD spectra of 1 mg/mL collagen removed from scaffolds in 0.1M acetic acid compared with spectra of type I collagen control and thermally denatured type I collagen.
TABLE I.
Comparison of Ellipticities of ππ* (Minima) and nπ* (Maxima) Transitions Between Type I Collagen Control and Collagen Processed Into ePEUU/Col Scaffoldsa
| ππ* |
nπ* |
|||
|---|---|---|---|---|
| Sample | Ellipticity (mdeg) |
Collagen ellipticity difference (mdeg) |
Ellipticity (mdeg) |
Collagen ellipticity difference (mdeg) |
| Collagen | −72.43 | — | 9.02 | — |
| Denatured Collagen | −18.07 | 54.36 | −2.18 | 11.20 |
| ePEUU/Col (0/100) | −65.29 | 7.14 | 3.90 | 5.12 |
| ePEUU/Col (10/90) | −63.62 | 8.81 | 3.82 | 5.20 |
| ePEUU/Col (20/80) | −64.46 | 7.97 | 3.96 | 5.06 |
| ePEUU/Col (30/70) | −62.10 | 10.33 | 2.54 | 6.48 |
| ePEUU/Col (40/60) | −39.17 | 33.26 | −0.55 | 9.57 |
| ePEUU/Col (50/50) | −27.20 | 45.23 | 0.91 | 8.11 |
| ePEUU/Col (80/20) | −5.62 | 66.81 | 0.80 | 8.22 |
| ePEUU/Col (90/10) | −1.32 | 71.11 | 0.60 | 8.42 |
| ePEUU/Col (95/5) | −2.54 | 69.89 | −0.54 | 9.56 |
| ePEUU/Col (97.5/2.5) | −2.75 | 69.68 | 0.48 | 8.54 |
| ePEUU/Col (100/0) | — | — | — | — |
e = electrospun scaffold; Col = collagen.
Mechanical properties
Mechanical properties of electrospun biohybrid scaffolds are summarized in Table II. Tensile strengths ranged from 2 MPa to 13 MPa and breaking strains from 160 to 280%. Electrospun PEUU had a tensile strength of 13 ± 4 MPa and a breaking strain of 220 ± 80%. Figure 9 displays typical stress-strain curves for PEUU film along with electrospun PEUU and electrospun PEUU/collagen (50/50) scaffolds. Incorporation of collagen lead to a significant decrease in tensile strength for samples containing 2.5, 10, 50, 60, 70, 80, and 90% collagen (p < 0.05) as well as reductions in initial and 100% modulus for samples containing 5, 10, 20, 50, 60, 70, 80, and 90% collagen (p < 0.05). No significant correlation between increasing collagen content and breaking strain were observed for electrospun scaffolds.
TABLE II.
Tensile Properties of Electrospun Scaffolds Compared with Cast PEUU Filma
| Sample | Initial modulus (MPa) |
100% modulus (MPa) |
Tensile strength (MPa) |
Breaking strain (%) |
|---|---|---|---|---|
| PEUU (film) | 60 ± 10 | 2 ± 0.4 | 27 ± 4 | 820 ± 70 |
| e-PEUU | 8 ± 2 | 8 ± 2 | 13 ± 4 | 220 ± 80 |
| e-PEUU/Col (97.5/2.5) | 7 ± 1 | 7 ± 1 | 8 ± 2 | 160 ± 40 |
| e-PEUU/Col (95/5) | 6 ± 2 | 6 ± 2 | 10 ± 4 | 210 ± 70 |
| e-PEUU/Col (90/10) | 6 ± 2 | 4 ± 2 | 7 ± 3 | 160 ± 60 |
| e-PEUU/Col (80/20) | 9 ± 2 | 6 ± 3 | 11 ± 2 | 170 ± 40 |
| e-PEUU/Col (50/50) | 3 ± 1 | 3 ± 1 | 6 ± 1 | 240 ± 40 |
| e-PEUU/Col (40/60) | 1 ± 0.1 | 2 ± 0.3 | 3 ± 1 | 280 ± 10 |
| e-PEUU/Col (30/70) | 1 ± 0.3 | 1 ± 0.2 | 2 ± 1 | 240 ± 30 |
| e-PEUU/Col (20/80) | 1 ± 0.3 | 1 ± 0.1 | 3 ± 0.4 | 250 ± 30 |
| e-PEUU/Col (10/90) | 2 ± 0.3 | 1 ± 0.1 | 2 ± 0.1 | 270 ± 50 |
e=electrospun scaffold; Col = collagen.
Figure 9.
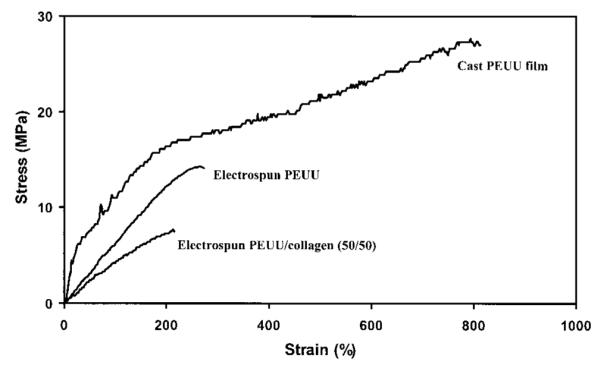
Typical stress-strain curves of PEUU cast film, electrospun PEUU scaffold, and electrospun PEUU/collagen (50/50).
Smooth muscle cell adhesion on PEUU/collagen scaffolds
Table III summarizes rat smooth muscle cell adhesion relative to TCPS after 1 day in culture on electrospun PEUU and electrospun PEUU/collagen scaffolds. Electrospun PEUU exhibited increased rSMC adhesion as reflected by an MTT absorbance value 118% of TCPS. Collagen or gelatin presence led to significant increases in rSMC adhesion onto electrospun PEUU/collagen for samples containing 5, 10, 20, 50, and 70% collagen relative to both electrospun PEUU alone and TCPS with values ranging from 160–200% of TCPS (p < 0.05). Electron micrographs (not shown) qualitatively supported this result showing surfaces more confluent and with more spread cells for samples containing collagen versus PEUU alone. A typical image representing rSMC morphology 1 day after cell seeding onto electrospun PEUU is shown in Figure 10. The cells appeared spread and attached to the electrospun scaffolds. No differences were observed in the cell morphologies between electrospun PEUU and the various electrospun PEUU/collagen samples aside from the relative amount of adherent cells. It is of note that rSMC adhesion was greater even on samples containing primarily gelatin as indicated by CD results.
TABLE III.
Cellular Adhesion 1 Day After rSMC Seeding Onto Biohybrid Scaffoldsa
| Sample | % TCPS |
|---|---|
| e-PEUU | 117.9 ± 12.8 |
| e-PEUU/Col (95/5)* | 174.3 ± 16.4 |
| e-PEUU/Col (90/10)* | 158.6 ± 8.8 |
| e-PEUU/Col (80/20)* | 175.5 ± 16.9 |
| e-PEUU/Col (50/50)* | 196.9 ± 63.0 |
| e-PEUU/Col (40/60) | 140.7 ± 18.2 |
| e-PEUU/Col (30/70)* | 161.0 ± 20.7 |
| e-PEUU/Col (20/80) | 123.5 ± 8.5 |
| e-PEUU/Col (10/90) | 107.2 ± 16.2 |
e = electrospun scaffold; Col = collagen.
p < 0.05.
Figure 10.
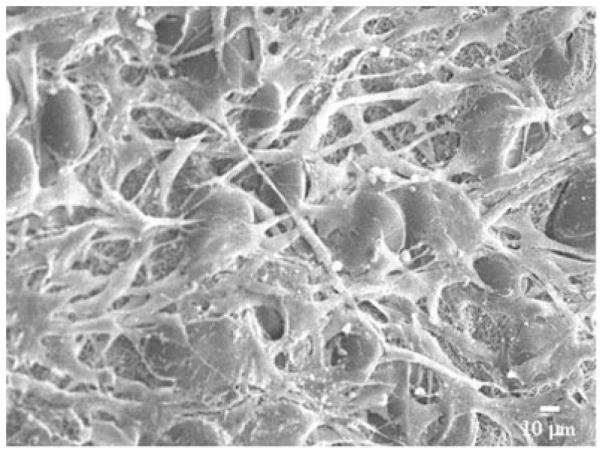
Representative rSMC morphology on electrospun PEUU.
DISCUSSION
Biodegradable polyesters have been the most commonly investigated materials in developing tissue engineering scaffolds. For example, poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and their copolymers (PLGA) are relatively stiff, nonelastic materials and are not ideally suited for engineering of soft flexible tissues under a mechanically demanding environment such as cardiovascular, urological, or gastrointestinal tissue. Mechanical signals are thought necessary to develop cell alignment leading to tissue structure exhibiting the correct biomechanical properties and function.1,2 Kim et al. showed that cyclic straining upregulated extracellular matrix production in developing smooth muscle tissue resulting in increased mechanical properties of the tissue constructs.3 They also demonstrated that for longer culture periods under cyclic strain, a more elastic scaffold than PLLA-bonded PGA fibers would be necessary.4 Reports have also shown that mechanical stimulation can lead to increased matrix production and mechanical strength in tissue engineered cardiac muscle grafts.5 In addition, tissue engineered blood vessels cultured under pulsatile flow were found to more closely resemble native vessels in both histological appearance and function than vessels cultured under nonpulsed conditions.6
Electrospinning offers a means to generate polymeric fibers with diameters in the 100–1000 nm range. This technique, first patented in the 1930s by Formhals,19 has seen recent interest in the tissue engineering community and for other applications. Several groups have reported on the electrospinning of PLGA.20,21 Annis et al.30 have electrospun nonbiodegradable polyurethane for vascular graft applications and Demir et al.31 investigated the results of various solution properties on electrospinning non-biodegradable polyurethane. Furthermore, investigators have demonstrated spinning of natural materials such as collagen22,32 or engineered proteins such as elastin.33,34 However, to date no groups have reported on the electrospinning of an elastomeric biodegradable polyurethane that might be suitable for biomedical applications.
Electrospinning is influenced by a multitude of process variables. In general, these variables can affect the resulting size and morphology of electrospun fibers.21,35 The most notable effect is the transition from electrospraying (i.e., spraying droplets of polymer in contrast to continuous nanofibers) to electrospinning. We observed a trend of increasing fiber diameter with increasing PEUU concentration. This trend was consistent with reports in the literature for other electrospun polymers such as poly(ethylene-co-vinyl alcohol).36 Preliminary investigations into the effect of capillary-to-collector distance on electrospun PEUU revealed lower fiber density at distances greater than 30 cm. Varying polymer feed rate did not appear to have a significant effect on fiber diameter or density. HFIP was found to be an ideal electrospinning solvent for PEUU because it evaporates rapidly, is nonflammable, and is applicable for solubilization of polyesters or proteins such as collagen. At PEUU concentrations <2 wt% and holding the voltage magnitudes constant (+12 kV to polymer solution, +3 kV to steel cage, and −7 kV to Al collector plate), more of a “beads on a string” morphology37 was observed than continuous fibers. As the polymer concentration was increased above 2%, almost entirely continuous fibers were observed. This trend was thought to be due to the increasing polymer concentration resulting in increased solution viscosity and surface tension more appropriate for spinning continuous fibers.
It was desired to incorporate bioactivity to develop scaffolds capable of increased cellular interactions as well as enzyme sensitive degradation. By incorporating these properties into polymer scaffolds, scaffolds that degrade at least in part, due to cellular infiltration and enzyme secretion, might be produced. To accomplish this design, we blended PEUU with type I bovine collagen in HFIP. It was previously shown by Matthews et al.22 that this type of collagen can be electrospun in HFIP and still retain its characteristic banding. Continuous fibers of PEUU/collagen blends were spun at all of the polymer/protein ratios examined. To achieve this, it was necessary to modify some electrospinning process variables to attain continuous fibers and avoid transitioning to electrospraying. The most common modification was a slight increase in voltage magnitude charged to the polymer solutions. For some of the higher collagen concentrations studied, it was necessary to increase the solution feedrate. This increase in feedrate may have contributed to the changes in fiber diameter qualitatively observed in Figure 4 at these concentrations.
To illustrate that collagen was present after treatment with HFIP, the FTIR spectrum of PEUU was subtracted from the spectrum for a PEUU/collagen (50/50) blend. The resulting spectrum yielded peaks characteristic of collagen as reported previously by other groups.38,39 The presence of collagen in the electrospun scaffolds was visualized by staining with picrosirius red. This stain, specific for collagen, reacts through its sulphonic acid groups with the basic groups of collagen. The extended dye molecule aligns parallel with structurally intact collagen fibers resulting in increased birefringence under polarized light.27 The scaffolds containing blended type I collagen stained positive for picrosirius red with increased absorbance as a function of increased collagen content (p < 0.05 for 20, 50, and 80%). The slight decrease of the 90% collagen sample may have been a result of the lower mechanical stability and slight shrinkage of the high collagen sample after staining and NaOH treatment. The picrosirius absorbance trend provides some insight into the morphology of the blends indicating the presence of collagen at the fiber surface. Samples at lower collagen contents such as 2.5, 5, and 10% collagen may have more PEUU present on the fiber surface compared with 20% and greater collagen samples. Water in air contact angles were found to decrease as a function of the amount of collagen blended with PEUU to give values ranging from 75° for PEUU alone to less than 40° with 50% collagen blended (data not shown). In addition to the trend of total picrosirius red binding, increased birefringence under polarized light was observed with samples containing at least 20% collagen, further suggesting the presence of non-denatured collagen in the scaffolds.
In processing a triple helical structural biomacromolecule such as collagen in solution, it is important to characterize the retention of the triple-helical structure in the resulting scaffold. Structure retention is important in providing maximal bioactivity to the scaffold in terms of its ability to influence cell adhesion or enzymatic degradation. Furthermore, structure information may also provide insight into the mechanical property contribution from the electrospun collagen. CD spectroscopy is a technique that gives a relative measure of collagen triple-helical content through comparison of the minima (ππ* amide transition) at 197 nm and maxima (nπ*) at 221 nm of the collagen spectrum.40 From the CD results on collagen released from electrospun PEUU/collagen blends, all samples, including electrospun collagen alone, showed signs of some secondary structure modification. This slight modification likely resulted from exposure to the HFIP solvent. These results are consistent with those reported by Doillon et al.41 that HFIP does modify the secondary structure of collagen. This report also found that cell growth was not impaired on the post-HFIP-treated collagen.41 Samples with less than 50% collagen appeared to consist mostly of denatured collagen or gelatin and PEUU. This greater loss of triple helix structure was perhaps due to the presence of larger amounts of PEUU disrupting the collagen hydrogen bonding and resulting secondary structure. In general, samples with higher collagen content (collagen >50%) possessed greater evidence of triple helical retention with ellipticity difference values lower than that of denatured collagen, indicating at least partial collagen structure retention at these concentrations.
While most porous scaffold processing techniques greatly reduce the mechanical properties relative to the bulk polymer, electrospinning retains a great deal of the strength and distensibility of the bulk PEUU. This trend is evident when comparing the stress–strain curves of PEUU film and electrospun PEUU in Figure 9. The fibrillar microstructure of the electrospun scaffolds may be responsible for the robust mechanical properties observed. Single electrospun PEUU fibers would be expected to possess higher tensile strengths and lower breaking strains compared with the cast PEUU film. However, because the network of fibers is porous, the overall scaffold tensile strength would be expected to decrease in comparison to the PEUU film. The attractive distension observed in the scaffolds may result from randomly orientated fibers aligning themselves in the direction of stress. Distension would be expected to approximate the breaking strains measured once fibers are aligned. In general, the incorporation of collagen led to slight decreases in mechanical properties such as tensile strength in the samples containing 2.5, 10, and 50% collagen. Larger decreases in tensile strength were observed in samples containing 60% and greater collagen. The collagen molecules may act to reduce hydrogen bonding interactions between the polyurethane molecules, thus eliminating some of the physical crosslinking that give polyurethanes their impressive mechanical properties. It was unexpected that the high collagen content samples would still be distensible with elongations of at least 240% since the electrospun collagen sample (without PEUU) was extremely brittle and could not be removed from the collection plate for testing.
In order to develop scaffolds that mimic the function of the native extracellular matrix, the incorporation of a biomacromolecule such as collagen would be ideal to guide and support cell adhesion. The data show increased smooth muscle cell adhesion to samples containing collagen and gelatin. High adhesion values resulted even from samples containing primarily gelatin. This trend may result simply from increased fibronectin from the culture medium binding to gelatin. It is well known that denatured collagen possesses greater binding affinity for fibronectin than structurally intact collagen because the binding sites on the interior of the triple-helical collagen may be inaccessible.24 Further, another factor that may contribute to larger cell adhesion may be the greater mechanical stability of the samples with 50% or less collagen incorporated. Samples containing at least 50% collagen experienced some slight contraction with time after cell seeding. This effect was most prominent in the samples with 80 and 90% collagen.
CONCLUSION
Electrospinning was utilized to process a previously characterized biodegradable PEUU into porous yet strong and flexible matrices with sub-micron scale fibers. Furthermore, collagen was introduced into the matrix by blending with PEUU to result in increased cell adhesion and mechanical properties still attractive for soft tissue engineering applications. These scaffolds were found to possess evidence of preserved collagen structure at high collagen ratios. These results demonstrate an approach to mimic elastic extracellular matrices by relying upon synthetic components to provide mechanical function and augmenting this function with native matrix proteins to provide bioactivity for cell growth and putative enzymatic degradation.
Acknowledgments
This work was supported by the Commonwealth of Pennsylvania and the National Institutes of Health (HL069368). We thank the Center for Biologic Imaging at the University of Pittsburgh for use of the SEM and Dr. Kazuro Fujimoto for isolation of the rSMCs. We also acknowledge Jim Culhane, Lorenza Draghi, Erin Pekarcik, Hann-Chung E. Wong, and Dr. Michael Sacks and laboratory for their assistance with this project.
Contract grant sponsor: Commonwealth of Pennsylvania
Contract grant sponsor: National Institutes of Health; contract grant number: HL069368
References
- 1.Simpson DG, Carver W, Borg TK, Terracio L. Role of mechanical stimulation in the establishment and maintenance of muscle cell differentiation. Int Rev Cytol. 1994;150:69–92. doi: 10.1016/s0074-7696(08)61537-5. [DOI] [PubMed] [Google Scholar]
- 2.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 3.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 1999;17:979–983. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 4.Kim BS, Mooney DJ. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J Biomech Eng. 2000;122:210–215. doi: 10.1115/1.429651. [DOI] [PubMed] [Google Scholar]
- 5.Akhyari P, Fedak PWM, Weisel RD, Lee TYJ, Verma S, Mickle DAG, Li RK. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002;106:I–337–I-142. [PubMed] [Google Scholar]
- 6.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 7.Skarja GA, Woodhouse KA. Synthesis and characterization of degradable polyurethane elastomers containing an amino acid-based chain extender. J Biomater Sci, Polym Edn. 1998;9:271–295. doi: 10.1163/156856298x00659. [DOI] [PubMed] [Google Scholar]
- 8.Skarja GA, Woodhouse KA. Structure–property relationships of degradable polyurethane elastomers containing an amino acid-based chain extender. J Appl Polym Sci. 2000;75:1522–1534. [Google Scholar]
- 9.Saad B, Hirt TD, Welti M, Uhlschmid GK, Neuenschwander P, Suter UW. Development of degradable polyesterurethanes for medical applications: in vitro and in vivo evaluations. J Biomed Mater Res. 1997;36:65–74. doi: 10.1002/(sici)1097-4636(199707)36:1<65::aid-jbm8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Kylma J, Seppala JV. Synthesis and characterization of a biodegradable thermoplastic poly(ester-urethane) elastomer. Macromolecules. 1997;30:2876–2882. [Google Scholar]
- 11.Spaans CJ, de Groot JH, Dekens FG, Pennings AJ. High molecular weight polyurethane urea based on 1,4 butanediisocyanate. Polym Bull. 1998;41:131–138. [Google Scholar]
- 12.Guan J, Sacks MM, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 13.Guan J, Sacks MM, Beckman EJ, Wagner WR. Biodegradable poly(ether ester urethane)urea elastomers based on poly(ether ester) triblock copolymers and putrescine: synthesis, characterization and cytocompatibility. Biomaterials. 2004;25:85–96. doi: 10.1016/s0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 14.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 15.Yang S, Leong K, Du Z, Chua C. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 16.Yang SF, Leong KF, Du ZH, Chua CK. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002;8:1–11. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 17.Reneker D, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. [Google Scholar]
- 18.Zeleny J. The electrical discharge from liquid points, and a hydrostatic method of measuring the electric intensity at their surfaces. J Phys Rev. 1914;3:69–91. [Google Scholar]
- 19.Formhals A. Apparatus for producing artificial filaments from material such as cellulose acetate. 1975504 US Patent. 1934
- 20.Li W, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 21.Zong X, Kim K, Fang D, Ran S, Hsiao BS. Structue and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43:4403–4412. [Google Scholar]
- 22.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 23.Taylor GI. Disintegration of water drops in an electric field. Proc R Soc Lond. 1964;280:383–397. [Google Scholar]
- 24.Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981;88:473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G, Ushida T, Tateishi T. Development of biodegradable porous scaffolds for tissue engineering. Mater Sci Eng. 2001;17:63–69. [Google Scholar]
- 26.Deitzel JM, Kleinmeyer JD, Hirvonen JK, Tan NC Beck. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer. 2001;42:8163–8170. [Google Scholar]
- 27.Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 28.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2002;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 29.Ohno M, Abe T. Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6) J Immunol Methods. 1991;145:199–203. doi: 10.1016/0022-1759(91)90327-c. [DOI] [PubMed] [Google Scholar]
- 30.Annis D, Bornat A, Edwards RO, Higham A, Loveday B, Wilson JT. An elastomeric vascular prothesis. Am Soc Art Int Org. 1978;24:209–214. [PubMed] [Google Scholar]
- 31.Demir MM, Yilgor I, Yilgor E, Erman B. Electrospinning of polyurethane fibers. Polymer. 2002;43:3303–3309. [Google Scholar]
- 32.Huang L, Nagapudi K, Apkarian RP. Engineered collagen-PEO nanofibers and fabrics. J Biomat Sci Polym Edn. 2001;12:979–993. doi: 10.1163/156856201753252516. [DOI] [PubMed] [Google Scholar]
- 33.Nagapudi K, Brinkman WT, Leisen JE, Huang L, McMillan RA, Apkarian RP, Conticello VP, Chaikof EL. Photomediated solid-state cross-linking of an elastin-mimetic recombinant protein polymer. Macromolecules. 2002;35:1730–1737. [Google Scholar]
- 34.Huang L, McMillan RA, Apkarian RP, Pourdeyhimi B, Conticello VP, Chaikof EL. Generation of synthetic elastin-mimetic small diameter fibers and fiber networks. Macromolecules. 2000;33:2989–2997. [Google Scholar]
- 35.Deitzel JM, Kleinmeyer J, Harris D, Tan NC Beck. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261–272. [Google Scholar]
- 36.Kenawy ER, Layman JM, Watkins JR, Bowlin GL, Matthews JA, Simpson DG, Wnek GE. Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials. 2003;24:907–913. doi: 10.1016/s0142-9612(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 37.Fong H, Chun I, Reneker DH. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585–4592. [Google Scholar]
- 38.Sachlos E, Reis N, Ainsley C, Derby B, Czernuszka JT. Novel collagen scaffolds with predefined internal morphology made by solid freeform fabrication. Biomaterials. 2003;24:1487–1497. doi: 10.1016/s0142-9612(02)00528-8. [DOI] [PubMed] [Google Scholar]
- 39.Camacho NP, West P, Torzilli PA, Mendelsohn R. FTIR spectroscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers. 2001;62:1–8. doi: 10.1002/1097-0282(2001)62:1<1::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 40.Bhatnagar RS, Gough CA. Circular dichroism of collagen and related polypeptides. In: Fasman GD, editor. Circular dichroism and the conformational analysis of biomacromolecules. Plenum; New York: 1996. pp. 183–199. [Google Scholar]
- 41.Doillon CJ, Drouin R, Cote M, Dallaire N, Pageau J, Laroche G. Chemical inactivators as sterilization agents for bovine collagen materials. J Biomed Mater Res. 1997;37:212–221. doi: 10.1002/(sici)1097-4636(199711)37:2<212::aid-jbm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]