Abstract
Nef is an HIV-1 accessory protein essential for AIDS progression and an attractive target for drug discovery. Lack of a catalytic function makes Nef difficult to assay in chemical library screens. We developed a high-throughput screening assay for inhibitors of Nef function by coupling it to one of its host cell binding partners, the Src-family kinase Hck. Hck activation is dependent upon Nef in this assay, providing a direct readout of Nef activity in vitro. Using this screen, a unique diphenylfuropyrimidine was identified as a strong inhibitor of Nef-dependent Hck activation. This compound also exhibited remarkable antiretroviral effects, blocking Nef-dependent HIV replication in cell culture. Structurally related analogs were synthesized and shown to exhibit similar Nef-dependent anti-viral activity, identifying the diphenylfuropyrimidine substructure as a new lead for antiretroviral drug development. This study demonstrates that coupling non-catalytic HIV accessory factors with host cell target proteins addressable by high-throughput assays may afford new avenues for the discovery of anti-HIV agents.
Nef is one of several accessory proteins encoded by HIV-1, HIV-2, and SIV with essential functions in viral pathogenicity (1,2). Deletions within the SIV nef gene reduce viral replication in vivo and delay the onset of AIDS-like disease in macaques (3). Similarly, HIV isolates from some infected individuals that fail to develop AIDS exhibit defective nef alleles (4–6), supporting a role for Nef in disease progression. Nef has no known catalytic function and targets signaling pathways in infected cells through direct protein:protein interactions (7). Nef binding influences several classes of signaling molecules, including immune receptors, trafficking proteins, guanine nucleotide exchange factors, and protein kinases (7–9). These Nef-mediated interactions enhance viral replication in some cell types and contribute to immune evasion as well as survival of infected cells (10–12).
Members of the Src family of non-receptor protein-tyrosine kinases (SFKs) represent an important class of Nef target proteins. Nef binds to the Src homology 3 (SH3) domains of the Src family members Fyn, Hck, Lck, Lyn and c-Src, all of which are expressed in HIV-1 target cells (13–16). Nef induces constitutive activation of Hck through a mechanism that involves displacement of the SH3 domain from a negative regulatory interaction with the catalytic domain (17,18). Nef activates c-Src and Lyn through a similar mechanism, suggesting that Nef-mediated SFK activation is a common feature of HIV-infected cells (19).
A growing body of evidence suggests that Nef:SFK interaction is important for HIV replication and AIDS progression. Komuro et al. demonstrated a strong positive correlation of macrophage-tropic HIV-1 replication with Hck expression in primary cultures of human macrophages; HIV replication was blocked following suppression of Hck protein levels with anti-sense oligonucleotides (20). In transgenic mice, targeted expression of Nef to T-cells and macrophages induced an AIDS-like syndrome characterized by CD4+ T cell depletion, diarrhea, wasting, and 100% mortality (21). Strikingly, mice expressing a Nef mutant lacking the highly conserved PxxPxR motif essential for SH3 binding showed no evidence of the AIDS-like phenotype (22). When transgenic mice expressing wild-type Nef were crossed into a hck-null background, appearance of the AIDS-like phenotype was delayed and mortality was reduced (22). Taken together, these observations suggest a critical role for Nef:SFK interactions in AIDS and identify complexes of Nef with these host cell protein kinases as attractive targets for anti-HIV drug discovery.
Lack of a catalytic function makes it difficult to design direct high-throughput screening (HTS) approaches to identify small molecule inhibitors of Nef function. As an alternative, we developed an HTS assay that couples Nef to the activation of Hck (15,18,19,23). Using this strategy, we identified three compounds that inhibited Nef-mediated Hck activity and also blocked Nef-dependent HIV-1 replication in vitro. These compounds represent valuable chemical probes for future analyses of Nef biological functions. Moreover, these findings demonstrate that chemical library screens directed against an HIV-1 virulence factor in complex with a host cell signaling partner may provide a new avenue to anti-retroviral drug discovery.
RESULTS AND DISCUSSION
Development of an in vitro kinase assay for Nef-induced Hck activation amenable to HTS
Hck and other SFKs adopt an inactive conformation in vivo as a result of phosphorylation of a conserved tyrosine residue in their C-terminal tails (24). This regulatory phosphorylation event requires an independent kinase known as Csk. To recapitulate this key aspect of SFK regulation in our in vitro kinase assay with Nef, we expressed and purified a form of Hck with a modified C-terminal tail (Hck-YEEI) that drives Hck downregulation independently of Csk (25). Previous studies from our group have shown that Nef activates both native Hck and Hck-YEEI to the same extent in cell-based assays (23), suggesting that Hck-YEEI would provide a useful surrogate for Csk-phosphorylated Hck in our HTS assay. Hck-YEEI was expressed in Sf9 insect cells and purified to homogeneity. Mass spectrometry revealed the presence of a single phosphotyrosine residue in the C-terminal tail, indicating that the kinase was predominantly in the down-regulated conformation (data not shown).
The assay platform used for the screen was the Z’-Lyte method (Invitrogen), an intramolecular FRET-based assay that takes advantage of the differential sensitivity of phosphorylated and non-phosphorylated peptide substrates to proteolytic cleavage. In the first step, the Nef:Hck kinase complex phosphorylates a single tyrosine residue in a synthetic FRET-peptide substrate that is tagged on the N-terminus with coumarin and on the C-terminus with fluorescein. The kinase reactions are then developed with a site-specific protease that selectively cleaves the non-phosphorylated FRET-peptide. Cleavage interrupts FRET between the donor and acceptor fluorophores on the N- and C-termini of the FRET-peptide, whereas the uncleaved phosphopeptide maintains the FRET signal. Activity is expressed as an “Emission Ratio” of donor to acceptor emission after excitation of the donor fluorophore at 400 nm:
The Emission Ratio (ER) remains low if the FRET-peptide is phosphorylated (i.e., no kinase inhibition) and is high if the FRET-peptide is not phosphorylated (i.e., kinase inhibition). By following the reaction progress with this ratio, well-to-well variations in FRET-peptide concentration and signal intensities can be readily controlled.
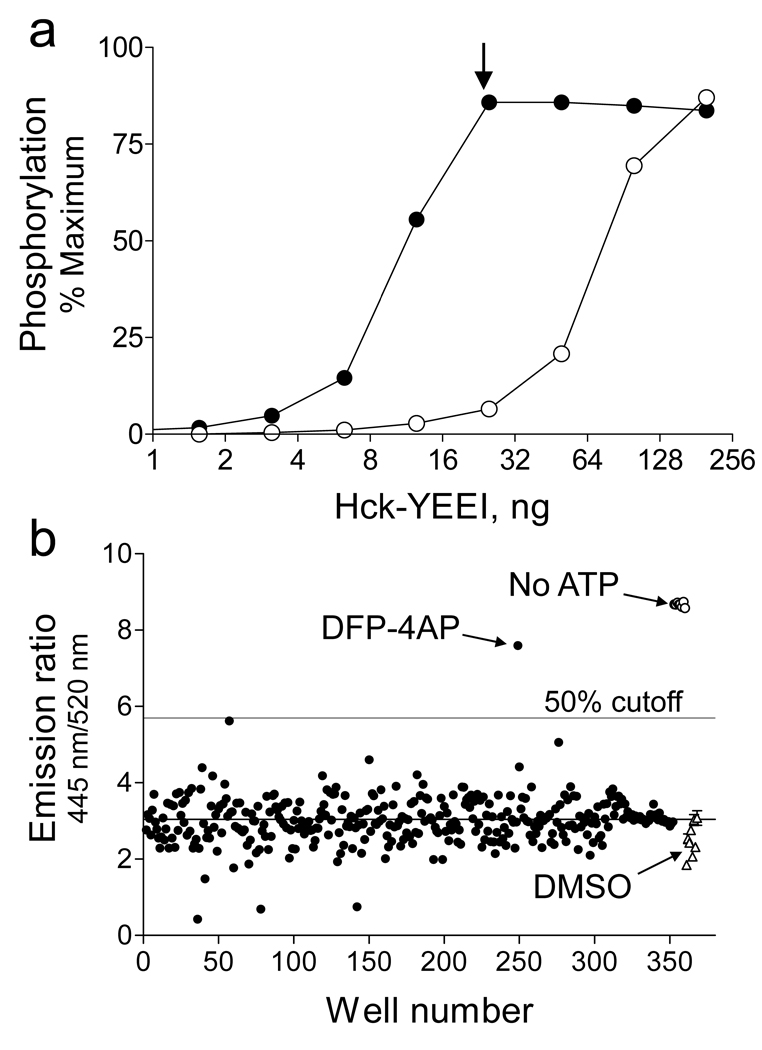
We first established assay conditions under which Hck-YEEI activation was dependent upon the presence of Nef. As shown in Figure 1A, FRET-peptide substrate phosphorylation increased as a function of the amount of Hck-YEEI added to the assay. Under these conditions, kinase activation is likely due to random intermolecular collisions of Hck-YEEI that result in tyrosine phosphorylation of the activation loop. These events increase in frequency as the concentration of Hck-YEEI rises. This experiment was then repeated in the presence of a 10-fold molar excess of HIV-1 Nef at each Hck-YEEI concentration. The presence of Nef markedly shifted the Hck-YEEI activation curve to the left, indicative of its ability to bind to Hck and relieve the inhibitory effect of the SH3 domain on kinase activity as reported previously (17). Importantly, Nef-induced activation of Hck via SH3 domain binding occurs without tail dephosphorylation or release from the SH2 domain (23), suggesting that Nef may induce a novel active conformation of Hck and other SFKs in HIV-infected cells. By including Nef in the assay, we hoped to retain this important aspect of Nef signaling. Subsequent chemical library screens were therefore conducted under conditions where Hck activation was dependent on Nef (Figure 1A; arrow).
Figure 1.
Screening for Nef:Hck inhibitors using the Z’-Lyte assay. (a) Nef stimulates Hck protein-tyrosine kinase activity in vitro. Recombinant Hck was purified from Sf9 insect cells in its downregulated form (Hck-YEEI; see text) and assayed with a FRET-peptide substrate and ATP as described under Materials and Methods. Reactions were run in the presence of increasing amounts of Hck-YEEI either alone (open circles) or in the presence of a 10-fold molar excess of purified recombinant HIV-1 Nef (closed circles). Each condition was repeated in quadruplicate, and the extent of phosphorylation is expressed as mean percent of phosphorylation relative to a control phosphopeptide ± S.D. Chemical library screens were performed under conditions where Hck-YEEI activation is dependent upon Nef (arrow). (b) Chemical libraries (10,000 compounds total) were screened for inhibitors of Nef-induced Hck kinase activity. Shown are representative emission ratios for 350 compounds from one plate which includes a hit (DFP-4AP; see text for details). The average emission ratio and 50% inhibition cutoff are indicated by the horizontal lines. Clusters of control points include wells with no ATP (open circles) and DMSO vehicle control (open triangles).
Identification of Nef:Hck inhibitors by HTS
The Nef:Hck-YEEI assay was then used to screen chemical libraries consisting of approximately 10,000 discrete compounds for inhibitory activity. The libraries were populated with structures biased towards kinase and phosphatase inhibitors as well as more diverse structures. All of the compounds were initially screened in duplicate at 10 µM, with a positive inhibitory compound being defined as one that caused 50% inhibition relative to untreated controls. The assay routinely produced Z’-factors, a measure of HTS statistical robustness (26), in the 0.7 – 0.8 range for each 384-well plate. Results for a representative plate are shown in Figure 1B. The primary screen yielded four candidate inhibitors, three of which were confirmed in subsequent concentration-response assays. All three confirmed inhibitors were obtained from the kinase inhibitor-biased library, and their structures are shown in Supplementary Figure S1A. They include a methoxyphenyl purine derivative [1], an indeno-isoquinolinedione [2] reminiscent of the tyrosine kinase inhibitor staurosporine (27), and a 4-amino substituted diphenylfuropyrimidine [3].
Inhibitors of Nef:Hck activity block HIV replication
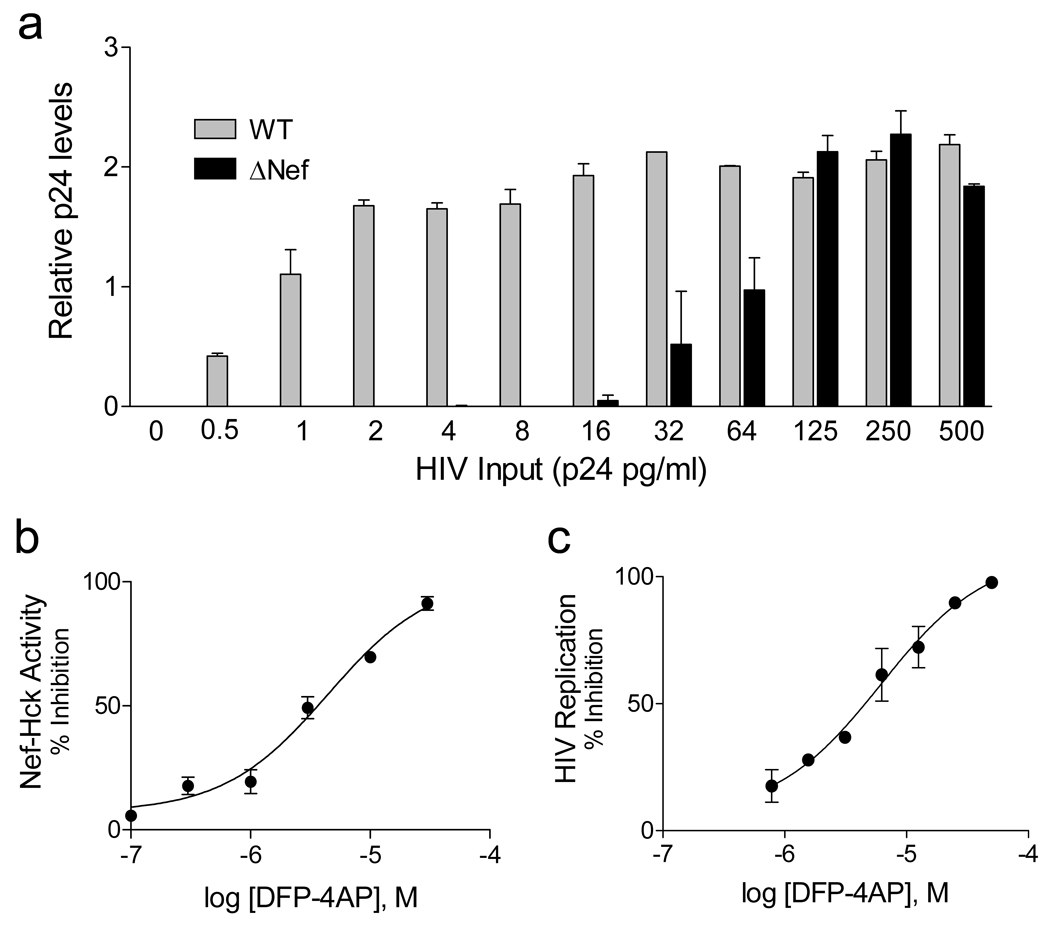
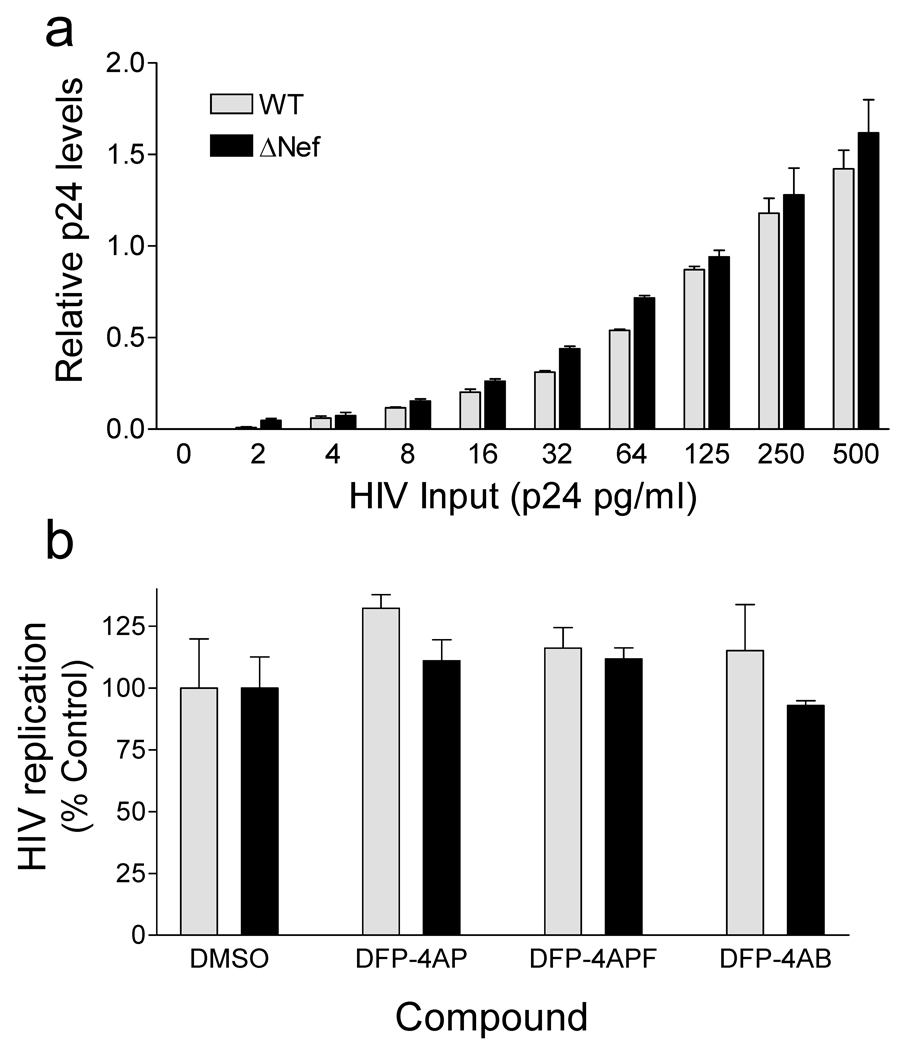
Inhibitors of Nef-mediated Hck activity identified in the library screen were then evaluated for anti-HIV activity. For these experiments we used U87MG astroglioma cells engineered to express the HIV-1 receptors CD4 and CXCR4 (28,29). Importantly, HIV-1 replication is dependent upon Nef in these cells as demonstrated in Figure 2A. In this experiment, U87MG cells were infected with HIV-1 (NL4-3 strain) as well an isogenic variant that fails to express Nef (ΔNef) over a wide range of viral titers. Wild-type HIV replicated about 60-fold more efficiently than the ΔNef mutant in these cells, providing a unique system to evaluate the impact of the compounds on Nef-dependent HIV replication. In addition, Nef expression stimulates endogenous SFK autophosphorylation in this cell line as assessed by immunoblotting with phosphospecific antibodies (Supplementary Figure S1B).
Figure 2.
Inhibition of Nef-induced Hck activation and HIV-1 replication by DFP-4-aminopropanol (DFP-4AP). (a) Nef-dependence of HIV-1 replication in U87MG cells. Cells were infected with wild-type HIV strain NL4-3 (WT) or a mutant that fails to express Nef (ΔNef) over the range of viral titers shown. Relative HIV p24 levels were determined by ELISA 5 days later. (b) Inhibition of Nef-induced Hck activation by DFP-4AP. The Nef:Hck complex was assayed in vitro with a peptide substrate in the presence of DFP-4AP over the range of concentrations shown. Each concentration was assayed in triplicate and data are expressed as percent inhibition relative to control reactions run in the presence of solvent only. The data were best-fit by non-linear regression analysis (GraphPad Prism Software), yielding an IC50 value of 4 µM. (c) Inhibition of HIV-1 replication by DFP. U87MG cells were infected with HIV strain NL4-3 in the presence of DFP-4AP over the range of concentrations shown. Release of viral p24 was determined by ELISA 5 days later. The data were best-fit by non-linear regression analysis, yielding an IC50 value of 6 µM.
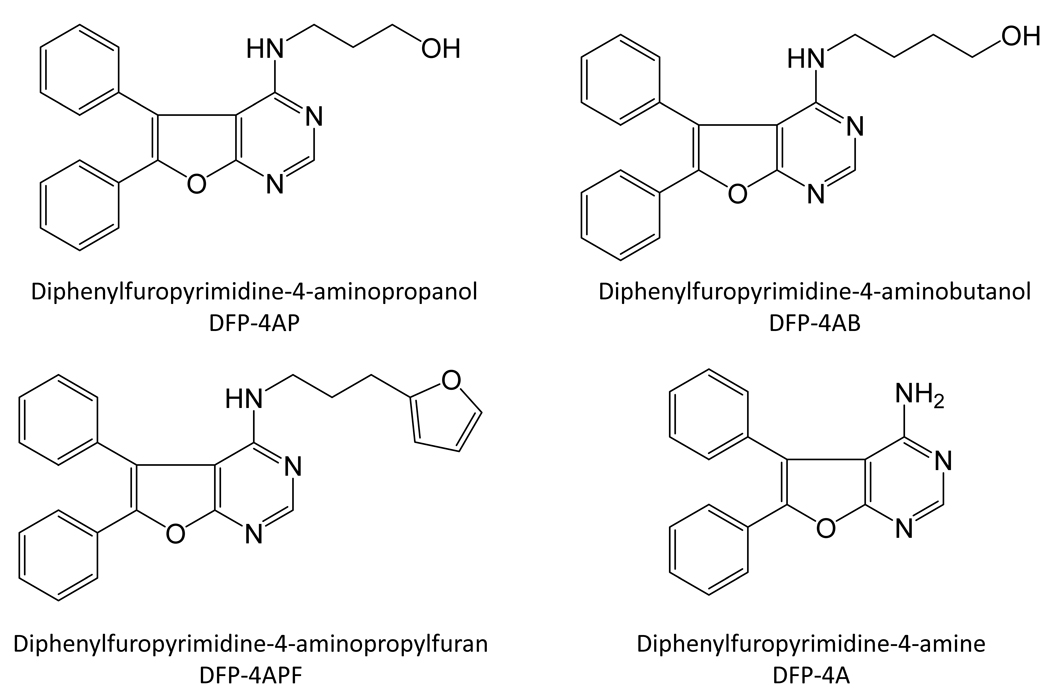
U87MG cells were infected with wild-type HIV-1 in the presence of each compound at 5 µM and viral replication was assessed as HIV p24 antigen release 4 and 5 days later. Supplementary Figure S1C shows that each of these compounds displayed anti-HIV activity; compound [3] was most remarkable, suppressing HIV replication to undetectable levels in this experiment. This compound, 3-(5,6-diphenylfuro[2,3-d]pyrimidin-4-ylamino)propan-1-ol (referred to hereafter as DFP-4AP; Figure 3), is structurally related to a class of recently described protein-tyrosine kinase inhibitors built around a 5,6-biarylfuro[2,3-d]pyrimidine pharmacophore (30). We re-synthesized DFP-4AP and confirmed its structure by NMR and mass spectrometry. We then performed a detailed concentration-response study using the Nef:Hck-YEEI assay and found that DFP-AP blocked Nef-induced kinase activity with an IC50 of about 4 µM (Figure 2B). We next titrated the anti-HIV activity of DFP-4AP in the U87MG system, and found that it blocked HIV replication with an IC50 value of about 6 µM (Figure 2C), which is consistent with the IC50 value for inhibition of Nef-induced Hck activity in vitro. The anti-HIV potency of re-synthesized DFP-4AP was about 10-fold lower than that observed with the compound originally obtained from the chemical library in terms of the maximum extent of viral inhibition observed. This difference may reflect the presence of contaminants or breakdown products present in the commercial preparation.
Figure 3.
Structures of DFP-4-aminopropanol and related 4-amino derivatives.
Anti-retroviral activity of diphenylfuropyrimidines requires Nef
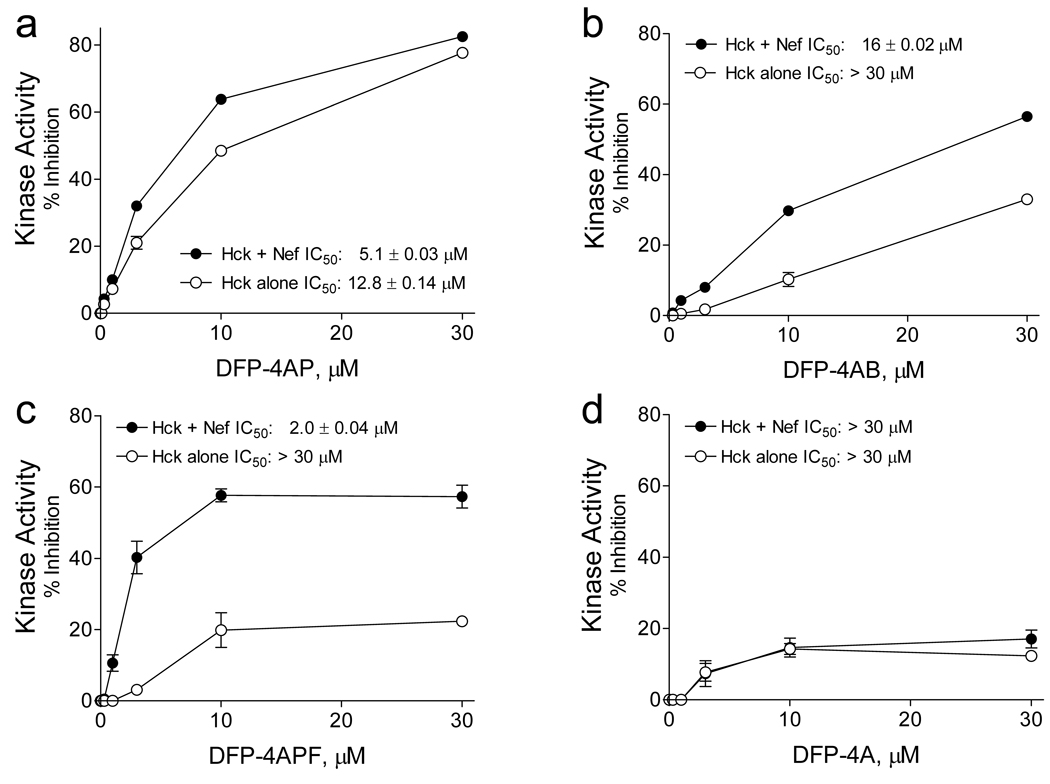
The remarkable anti-HIV efficacy of DFP-4AP led us to investigate whether the presence of Nef affected the inhibitory action of diphenylfuropyrimidines against Hck in vitro and whether Nef was required for their antiretroviral activity. For these studies, we included three additional DFP-4AP analogs: DFP-4AB, which has a slightly longer 4-aminobutanol side chain; DFP-4PF, with a bulkier 4-aminopropylfuran substituent; and DFP-4A, which bears an unsubstituted 4-amino group (Figure 3). Each of these compounds along with DFP-4AP were then tested in concentration-response experiments against Nef-activated Hck vs. Hck alone using the Z’-Lyte assay. As shown in Figure 4, the lead compound (DFP-4AP) showed a modest but highly reproducible 2- to 3-fold increase in potency in the presence of Nef. This effect was magnified with the 4-aminobutanol analog, which also showed less efficacy against Hck alone (Figure 4B). The 4-aminopropylfuranyl derivative showed a remarkable difference in both potency and efficacy for Nef-activated Hck vs. Hck alone (Figure 4C). Finally, the unsubstituted analog (DFP-4A) was virtually inactive against Hck in the presence or absence of Nef, indicating that the 4-amino substitutent is a key activity and specificity determinant (Figure 4D). The presence of Nef also sensitized Lyn, another SFK activated by Nef, to inhibition by DFP-4AP, DFP-4AB, and to a lesser extent DFP-4APF (Supplementary Figure S2). As for Hck, very little inhibition of Lyn was observed with DFP-4A in the presence or absence of Nef. Note that a recent X-ray crystal structure of the Lck kinase domain bound to a related diphenylfuropyrimidine-based inhibitor (30) suggests that the furopyrimidine moiety of DFP-4AP and the active analogs occupy the Hck and Lyn ATP-binding sites (Supplementary Figure S3). Indeed, substitution of the Hck active site “gatekeeper” residue (Thr338) (31), which comes in close contact with the DFP pharmacophore in the Lck crystal structure, with a bulkier methionine dramatically reduced the inhibitory potency of DFP-4AP towards Nef-activated Hck (Supplementary Figure S3). Furthermore, data presented in Figure 4 and Figure S2 suggest that engagement of SFK SH3 domains by Nef may influence the conformation of the active site to favor compound binding. Indeed, experiments with a mutant of Hck in which the SH3 domain is locked to its internal docking site on the back of the kinase domain shows no difference in inhibitor sensitivity in the presence or absence of Nef (Supplementary Figure S4).
Figure 4.
Selective inhibition of Nef-induced Hck activation by diphenylfuropyrimidines. The kinase activities of the Nef:Hck complex and Hck alone were assayed in vitro with a peptide substrate in the presence of DFP-4AP and the three analogs shown in Figure 3 over the range of concentrations shown. Each concentration was assayed in triplicate and data are expressed as percent inhibition relative to control reactions run in the absence of compound. Where possible, non-linear regression analysis was used to estimate IC50 values shown (GraphPad Prism).
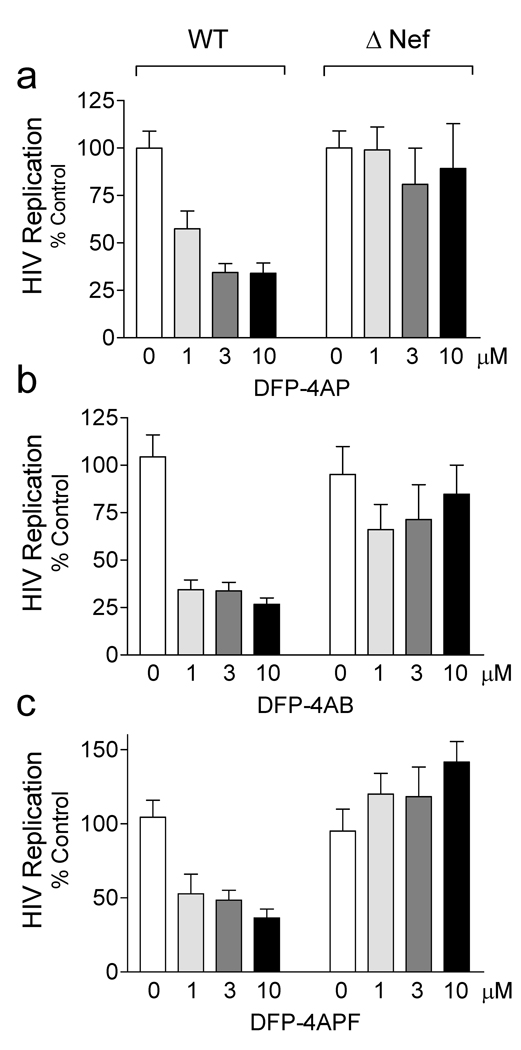
To address whether Nef is required for the anti-retroviral effects of these compounds, we next performed HIV replication assays in U87MG cells infected with either wild-type or Nef-defective HIV. As shown in Figure 5A, the lead compound, DFP-4AP, blocked wild-type HIV replication with an IC50 value in the low µM range, consistent with the results presented in Figure 2C. In contrast, DFP-4AP had no effect on replication of the HIV-ΔNef mutant, even at 10 µM. Similar experiments were then performed with the three DFP analogs. Both of the analogs that showed selectivity for Nef-activated Hck in the in vitro kinase assay also demonstrated potent inhibition of wild-type HIV replication, with IC50 values of 1 µM or less. As with DFP-4AP, however, neither of these analogs inhibited the replication of HIV-ΔNef (Figures 5B and 5C). These data provide strong evidence that 4-amino substituted DFP analogs block HIV replication through a Nef-dependent mechanism. Finally, we examined the activity of DFP-4A, with an unsubstituted 4-amino group. This compound was devoid of anti-retroviral activity (not shown), consistent with its poor activity profile in vitro (Figure 4 and Figure S2). None of these compounds displayed cytotoxicity towards U87MG cells up to 10 µM as assessed by resazurin reduction assay (Supplementary Figure S5).
Figure 5.
Inhibition of HIV-1 replication by diphenylfuropyrimidines requires Nef. U87MG cells were infected in 96-well plates (200 µl final volume) with equal titers (100 pg p24) of wild-type HIV strain NL4-3 (WT) or a mutant that fails to express Nef (ΔNef) in the presence of the DFP analogs (Figure 3) over the range of concentrations shown. HIV p24 levels were determined by ELISA 5 days later. Data are presented as percent of p24 release observed in the absence of compound ± S.D.
To explore the Nef-dependence of the antiretroviral actions of the DFP analogs further, we performed viral replication assays in Jurkat T-cells. Unlike U87MG cells, replication of HIV is independent of Nef in this cell line (Figure 6A). Wild-type and Nef-defective HIV replication was then tested in Jurkat cells in the presence of each compound at 5 µM, a concentration that selectively blocked wild-type HIV replication in U87MG cells (Figure 5). A shown in Figure 6B, none of the DFP analogs showed anti-retroviral activity against wild-type or Nef-defective HIV, providing further support that these compounds act through a novel Nef-dependent mechanism to block HIV replication. No cytotoxicity was observed in Jurkat cells at this concentration (Supplementary Figure S5).
Figure 6.
DFP analogs fail to inhibit HIV-1 replication in Jurkat cells. (a) HIV-1 replication is Nef-independent in Jurkat T-cells. Cells were infected in 96-well plates (200 µl final volume) with wild-type HIV strain NL4-3 (WT) or a mutant that fails to express Nef (ΔNef) over the range of viral titers shown. Relative HIV p24 levels were determined by ELISA 5 days later. (b) Jurkat cells were infected with equal titers of wild-type HIV (WT) or the Nef-defective mutant (ΔNef) in the presence of 5 µM of the DFP analogs (Figure 3). HIV p24 release was determined by ELISA 5 days later. Data are presented as percent of p24 release observed in the absence of compound ± S.D.
Summary and conclusions
The majority of current drug therapies for HIV target either the viral reverse transcriptase and protease enzymes or interfere with virus-host cell fusion (32). While these compounds, especially in combination, have dramatically reduced the morbidity and mortality of HIV-induced disease, the emergence of drug-resistant viruses and the lack of an effective vaccine underscore the need for new anti-HIV agents (33). Work presented in this report supports the concept that Nef, an HIV accessory protein essential for AIDS progression, is a valid target for anti-HIV drug discovery. Coupling Nef to one of its well-known host cell target proteins (Hck) enabled HTS for inhibitors of this critical HIV accessory factor, and identified diphenylfuropyrimidines as potential new leads for anti-HIV drug development. Including Hck in the assay not only provides an enzymatic activity easily adaptable to HTS, but may also induce relevant conformations of both Hck and Nef essential for small molecule inhibitor binding and function. This idea is supported by our observation that DFP-4AP, as well as two active analogs (DFP-4AB; DFP-4APF), all demonstrated enhanced potency and efficacy in the kinase assay when Nef was present. Interestingly, the Nef-dependent antiretroviral effects of diphenylfuropyrimidines may be unique among Src-family kinase inhibitors, as the potent, broad-spectrum SFK inhibitor dasatinib (34) had no effect on HIV replication in the U87MG system (data not shown). The compounds identified in this screen should be valuable tools to dissect the nature of Nef interactions with Src-family kinases and possibly other host cell factors that interact with Nef in HIV-infected cells. Moreover, combining other HIV accessory factors with relevant host cell binding partners may represent a general strategy for enabling anti-retroviral drug discovery.
METHODS
Recombinant protein expression and purification
For the initial screen, recombinant Hck-YEEI and Nef (SF2 allele) were expressed in Sf9 insect cells as N-terminal His-6 fusion proteins and purified as described elsewhere (19). For subsequent experiments, recombinant His-6-tagged Nef was expressed in E. coli (35).
Chemical syntheses
All reactions were conducted in oven-dried (120 °C) glassware under a nitrogen atmosphere. All chemicals were purchased from Aldrich Chemical or Fisher Scientific. Tetrahydrofuran (THF) was distilled over CaH2 prior to use. Dimethylformamide (DMF) was purchased as anhydrous and transferred under dry nitrogen. 5,6-Diphenylfuro[2,3-d]pyrimidin-4-amine (DFP-4-amine) was prepared according to the reported procedure (36). 1H (600 MHz) and 13C (150 MHz) NMR spectra were recorded on a Bruker Avance system in CDCl3 using CHCl3 (1H δ 7.26) and CDCl3 (13C δ 77.00) as internal references. Gas chromatography-mass spectrometry (GC-MS) was carried out on a Hewlett Packard 5890 Series II gas chromatograph equipped with a 30 m HP-5 (5% phenyl methylsilicone) Hewlett Packard capillary column and a Hewlett Packard 5971 mass selective detector in the electron ionization (EI) mode. High resolution mass spectrometry (HRMS) was performed on an Applied Biosystems 4700 MALDI-TOF-MS using α-cyano-4-hydroxycinnamic acid as the matrix.
3-(5,6-Diphenylfuro[2,3-d]pyrimidin-4-ylamino)propan-1-ol (DFP-4-aminopropanol)
Potassium t-butoxide (134 mg, 1.2 mmol) was added to a solution of DFP-4-amine (287 mg, 1.0 mmol) in 4 mL of THF at 0 °C and the mixture stirred for 5 min. 3-Bromopropanol (167 mg, 1.2 mmol) was added and the mixture stirred for 18 h at room temperature. The mixture was concentrated by rotary evaporation, diluted with ethyl acetate, washed with 4M NH4Cl and brine, dried over MgSO4, filtered and concentrated. Purification of the residue by flash column chromatography (silica gel, 2:1 ethyl acetate–hexanes) gave DFP-4-aminopropanol (41.4 mg, 12% yield) as a pale orange solid: mp 152−153 °C; 1H NMR δ 8.39 (1 H, s), 7.57−7.45 (7 H, m), 7.29−7.23 (3 H, m), 4.91 (1 H, s), 3.71 (1 H, br s), 3.61−3.57 (2 H, m), 3.56 (2 H, t, J = 5.6 Hz), 1.67−1.62 (2 H, m); 13C NMR δ 164.7, 158.0, 153.9, 146.9, 132.3, 129.8, 129.7, 129.4, 129.0, 128.5, 126.3, 114.8, 103.0, 58.8, 37.5, 32.6; MS (EI) m/z 345 (M+•), 326, 77; HRMS (MALDI-TOF) calculated for C21H20N3O2 [M+H]+ m/z 346.1556, found 346.1563.
4-(5,6-Diphenylfuro[2,3-d]pyrimidin-4-ylamino)butan-1-ol (DFP-4-aminobutanol)
4-Bromobutan-1-ol (459 mg, 3 mmol) was mixed with dihydropyran (336 mg, 4 mmol) and freshly recrystallized p-toluenesulfonic acid monohydrate (7.1 mg, 0.037 mmol) in 2 mL of dichloromethane and the mixture was stirred at room temperature for 14 h. The resulting mixture was diluted into 20 mL of dichloromethane, then washed with 20 mL of 5% aqueous sodium bicarbonate and 20 mL of brine. The organic layer was dried with MgSO4, filtered and concentrated to give 2-(4-bromobutoxy)tetrahydro-2H-pyran (711 mg, quantitative yield) as a colorless oil.
DFP-4-amine (290.6 mg, 1.01 mmol) in 2 mL of DMF was treated with NaH (48.5 mg, 1.21 mmol) and the mixture was stirred at room temperature for 2 h. The mixture was treated with 2-(4-bromobutoxy)tetrahydro-2H-pyran (450 mg, 1.9 mmol) and stirred at room temperature for 14 h. The mixture was diluted with 20 mL of H2O and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with 60 mL of brine, dried with MgSO4, filtered and concentrated. The residue was purified by flash column chromatography (SiO2, 4:1 hexanes–ethyl acetate) to give 5,6-diphenyl-N-(4-(tetrahydro-2H-pyran-2-yloxy)butyl)furo[2,3-d]pyrimidin-4-amine (274 mg, 99% yield) as a sticky, clear liquid.
5,6-Diphenyl-N-(4-(tetrahydro-2H-pyran-2-yloxy)butyl)furo[2,3-d]pyrimidin-4-amine (150 mg, 0.34 mmol) was dissolved in 10 mL of CH3OH and treated with freshly recrystallized p-toluenesulfonic acid monohydrate (2 mg, 0.01 mmol). The mixture was stirred at 45 °C for 2 h. The mixture was concentrated and purified by flash column chromatography (SiO2, ethyl acetate) and recrystallization from dichloromethane/hexanes to give DFP-4-aminobutanol (122 mg, quantitative yield) as a pale yellow solid: mp 117–119 °C; 1H NMR δ 8.39 (1 H, br), 7.48−7.37 (7 H, m), 7.18 (3 H, br s), 4.75 (1 H (NH), J = 4.9 Hz), 3.67 (2 H, m, app t), 3.46 (2 H, app quintet), 2.08 (1 H, br s), 1.58-1.50 (2 H, m), 1.5-1.46 (2 H, m); 13C δ 164.6, 157.5, 153.9, 146.5, 132.3, 129.7, 129.5, 129.3, 128.8, 128.4, 128.3, 126.2, 114.8, 103.0, 61.9, 40.9, 29.4, 25.8; HRMS (MALDI-TOF) calculated for C22H22N3O2 [M+H]+ m/z 360.1712, found 360.1707.
N-(3-(Furan-2-yl)propyl)-5,6-diphenylfuro[2,3-d]pyrimidin-4-amine (DFP-4-amino-propylfuran)
NaH (48.5 mg, 1.21 mmol) was added to a solution of DFP-4-amine (289 mg, 1.01 mmol) in 2 mL of DMF and the mixture was stirred at room temperature for 2 h. A 6:1 (v/v) mixture of 2-(3-bromopropyl)furan (ca. 350 mg, ca. 1.85 mmol) and 1,2-dibromoethane (ca. 50 mg, ca. 50 mg, ca. 0.25 mmol) in 1 mL of DMF was added, and the mixture was stirred at room temperature for 14 h. The mixture was concentrated on a rotary evaporator, diluted with ethyl acetate, washed with 4M NH4Cl and brine, dried over MgSO4, filtered and concentrated. Purification of the residue by flash column chromatography (silica gel, 10:1 hexanes–ethyl acetate) gave DFP-4-aminopropylfuran (171.6 mg, 68% yield) as a pale yellow oil: 1H δ 8.39 (1 H, br), 7.48−7.37 (7 H, m), 7.25-7.23 (4 H, m), 6.22 (1 H, d, J = 4 Hz), 5.87 (1 H, s), 4.68 (1H, br s, NH), 3.42 (2 H, app t), 2.52 (2 H, app t,), 1.77 (2 H, m), 1.58-1.50 (2 H, m), 1.5-1.46 (2 H, m); 13C δ 164.8, 157.6, 154.8, 154.2, 146.5, 141.0, 132.6, 129.8, 129.7, 129.5, 128.9, 128.5, 128.4, 126.3, 114.8, 110.1, 105,2, 103.2, 39.9, 27.7, 24.9; MS (EI) m/z 395 (M-H), 341, 301 (base peak), 286, 273, 216, 201, 189, 94, 81, 77, 53; HRMS (MALDI-TOF) calculated for C25H22N3O2 [M+H]+ m/z 396.1712, found 396.1718.
In vitro kinase assay and chemical library screening
Protein-tyrosine kinase assays were performed in 384-well plates using the Z’-lyte kinase assay system and Tyr2 peptide substrate (Invitrogen) as described elsewhere (19). Chemical libraries were purchased from ChemDiv, Inc. and included a kinase-directed library (2500 compounds) a phosphatase-directed library (2500 compounds) and a diversity set (5040 compounds). Library screens were conducted in 384-well plates in a final volume of 10 µl per well. Compounds were added to each well (10 µM final), followed by a preformed complex of Hck-YEEI (10 ng/well) and Nef (1:20 molar ratio) plus the substrate peptide (2 µM). Reactions were initiated by the addition of ATP (50 µM final) and incubated at room temperature for 35 min. Reactions were developed and terminated as per the manufacturer’s protocol and fluorescence ratios were calculated as described in the text and elsewhere (19).
HIV replication assays
Virus stocks were prepared by transfection of 293T cells (ATCC) with the wild-type and ΔNef recombinant viral genomes (NL4-3 strain) and amplified in the T-cell line, SupT1 (NIH AIDS Research and Reference Reagent Program) (37). Viral replication was assessed in the U87MG astroglioma cell line (28,29) engineered to express the HIV-1 co-receptors CD4 and CXCR4 or in the Jurkat T-cell line (Clone E6-1; NIH AIDS Research and Reference Reagent Program). Viral replication was monitored by measuring p24 gag protein levels in the culture supernatant by standard ELISA-based techniques. Test compounds were added to the culture 1h prior to infection with HIV, and DMSO was used as the carrier solvent at a final concentration of 0.1%.
Supplementary Material
Acknowledgements
This work was supported by grants CA81398 and AI57083 (to T.E.S.) and MH74411 and CA78039 (to J.S.L.) from the National Institutes of Health. L.E.-S. is supported by a National Institutes of Health Ruth L. Kirschstein National Research Service Award (AI114149). The authors would like to thank the NIH AIDS Research and Reference Reagent Program for providing cell lines and antibodies, as well as R. Trible for generating the immunoblot data shown in Supplementary Figure S1B.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs/org.
REFERENCES
- 1.Fackler OT, Baur AS. Live and let die: Nef functions beyond HIV replication. Immunity. 2002;16:493–497. doi: 10.1016/s1074-7613(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 2.Joseph AM, Kumar M, Mitra D. Nef: "necessary and enforcing factor" in HIV infection. Curr. HIV. Res. 2005;3:87–94. doi: 10.2174/1570162052773013. [DOI] [PubMed] [Google Scholar]
- 3.Kestler H, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high viral loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 4.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 6.Mariani R, Kirchhoff F, Greenough TC, Sullivan JL, Desrosiers RC, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herna RG, Saksela K. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front Biosci. 2000;5:D268–D283. doi: 10.2741/renkema. [DOI] [PubMed] [Google Scholar]
- 8.Arold ST, Baur AS. Dynamic Nef and Nef dynamics: how structure could explain the complex activities of this small HIV protein. Trends Biochem. Sci. 2001;26:356–363. doi: 10.1016/s0968-0004(01)01846-1. [DOI] [PubMed] [Google Scholar]
- 9.Geyer M, Fackler OT, Peterlin BM. Structure--function relationships in HIV-1 Nef. EMBO Rep. 2001;2:580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briggs SD, Scholtz B, Jacque JM, Swingler S, Stevenson M, Smithgall TE. HIV-1 Nef promotes survival of myeloid cells by a Stat3-dependent pathway. J. Biol. Chem. 2001;276:25605–25611. doi: 10.1074/jbc.M103244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi H-J, Smithgall TE. HIV-1 Nef promotes survival of TF-1 macrophages by inducing Bcl-XL expression in an Erk-dependent manner. J. Biol. Chem. 2004;279:51668–51696. doi: 10.1074/jbc.M410068200. [DOI] [PubMed] [Google Scholar]
- 12.Geleziunas R, Xu W, Takeda K, Ichijo H, Greene WC. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature. 2001;410:834–838. doi: 10.1038/35071111. [DOI] [PubMed] [Google Scholar]
- 13.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arold S, Franken P, Strub MP, Hoh F, Benichou S, Benarous R, Dumas C. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure. 1997;5:1361–1372. doi: 10.1016/s0969-2126(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 15.Choi HJ, Smithgall TE. Conserved residues in the HIV-1 Nef hydrophobic pocket are essential for recruitment and activation of the Hck tyrosine kinase. J. Mol. Biol. 2004;343:1255–1268. doi: 10.1016/j.jmb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Arold S, O'Brien R, Franken P, Strub MP, Hoh F, Dumas C, Ladbury JE. RT loop flexibility enhances the specificity of Src family SH3 domains for HIV-1 Nef. Biochemistry. 1998;37:14683–14691. doi: 10.1021/bi980989q. [DOI] [PubMed] [Google Scholar]
- 17.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee C-H, Kuriyan J, Miller WT. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 18.Briggs SD, Sharkey M, Stevenson M, Smithgall TE. SH3-mediated Hck tyrosine kinase activation and fibroblast transformation by the Nef protein of HIV-1. J. Biol. Chem. 1997;272:17899–17902. doi: 10.1074/jbc.272.29.17899. [DOI] [PubMed] [Google Scholar]
- 19.Trible RP, Emert-Sedlak L, Smithgall TE. HIV-1 Nef selectively activates SRC family kinases HCK, LYN, and c-SRC through direct SH3 domain interaction. J. Biol. Chem. 2006;281:27029–27038. doi: 10.1074/jbc.M601128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komuro I, Yokota Y, Yasuda S, Iwamoto A, Kagawa KS. CSF-induced and HIV-1-mediated distinct regulation of Hck and C/EBPbeta represent a heterogeneous susceptibility of monocyte-derived macrophages to M-tropic HIV-1 infection. J. Exp. Med. 2003;198:443–453. doi: 10.1084/jem.20022018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 22.Hanna Z, Weng X, Kay DG, Poudrier J, Lowell C, Jolicoeur P. The pathogenicity of human immunodeficiency virus (HIV) type 1 Nef in CD4C/HIV transgenic mice is abolished by mutation of its SH3-binding domain, and disease development is delayed in the absence of Hck. J. Virol. 2001;75:9378–9392. doi: 10.1128/JVI.75.19.9378-9392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerner EC, Smithgall TE. SH3-dependent stimulation of Src-family kinase autophosphorylation without tail release from the SH2 domain in vivo. Nat. Struct. Biol. 2002;9:365–369. doi: 10.1038/nsb782. [DOI] [PubMed] [Google Scholar]
- 24.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 25.Schindler T, Sicheri F, Pico A, Gazit A, Levitzki A, Kuriyan J. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol. Cell. 1999;3:639–648. doi: 10.1016/s1097-2765(00)80357-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JH, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 27.Ruegg UT, Burgess GM. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 28.Trkola A, Ketas T, Kewalramani VN, Endorf F, Binley JM, Katinger H, Robinson J, Littman DR, Moore JP. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvatori F, Scarlatti G. HIV type 1 chemokine receptor usage in mother-to-child transmission. AIDS Res. Hum. Retroviruses. 2001;17:925–935. doi: 10.1089/088922201750290041. [DOI] [PubMed] [Google Scholar]
- 30.Dimauro EF, Newcomb J, Nunes JJ, Bemis JE, Boucher C, Buchanan JL, Buckner WH, Cheng A, Faust T, Hsieh F, Huang X, Lee JH, Marshall TL, Martin MW, McGowan DC, Schneider S, Turci SM, White RD, Zhu X. Discovery of 4-amino-5,6-biaryl-furo[2,3-d]pyrimidines as inhibitors of Lck: Development of an expedient and divergent synthetic route and preliminary SAR. Bioorg. Med. Chem. Lett. 2007 doi: 10.1016/j.bmcl.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 31.Pene-Dumitrescu T, Peterson LF, Donato NJ, Smithgall TE. An inhibitor-resistant mutant of Hck protects CML cells against the antiproliferative and apoptotic effects of the broad-spectrum Src family kinase inhibitor A-419259. Oncogene. 2008;27:7055–7069. doi: 10.1038/onc.2008.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temesgen Z, Warnke D, Kasten MJ. Current status of antiretroviral therapy. Expert. Opin. Pharmacother. 2006;7:1541–1554. doi: 10.1517/14656566.7.12.1541. [DOI] [PubMed] [Google Scholar]
- 33.Greene WC. The brightening future of HIV therapeutics. Nat. Immunol. 2004;5:867–871. doi: 10.1038/ni0904-867. [DOI] [PubMed] [Google Scholar]
- 34.Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Kocher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 35.Trible RP, Emert-Sedlak L, Wales TE, Ayyavoo V, Engen JR, Smithgall TE. Allosteric loss-of-function mutations in HIV-1 Nef from a long-term non-progressor. J. Mol. Biol. 2007;374:121–129. doi: 10.1016/j.jmb.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gewald K. Heterocycles from CH-acidic nitriles. IX. Reaction of α-hydroxy ketones with malononitrile. Chem. Ber. 1966;99:1002–1007. [Google Scholar]
- 37.Smith SD, Shatsky M, Cohen PS, Warnke R, Link MP, Glader BE. Monoclonal antibody and enzymatic profiles of human malignant T-lymphoid cells and derived cell lines. Cancer Res. 1984;44:5657–5660. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.