Abstract
Prostaglandin E2 (PGE2 ) EP1 receptors (EP1R) may contribute to hypertension and related end-organ damage. Because of the key role of angiotensin II (AngII) in hypertension, we investigated the role of EP1R in the cerebrovascular alterations induced by AngII. Mice were equipped with a cranial window and cerebral blood flow (CBF) was monitored by laser-Doppler flowmetry. The attenuation in CBF responses to whisker stimulation (−46±4%), and the endothelium-dependent vasodilator acetylcholine (−40±4%) induced by acute administration of AngII (250 ng/kg/min; i.v. for 30–40 min) was not observed after cyclooxygenase-1 or EP1R inhibition, and in cyclooxygenase-1 or EP1-null mice. In contrast, cyclooxygenase-2 inhibition or genetic inactivation did not prevent the attenuation. AngII-induced oxidative stress was not observed after cyclooxygenase-1 or EP1R inhibition, and in EP1R-null mice. PGE2 reinstated the AngII-induced cerebrovascular dysfunction and oxidative stress after cyclooxygenase-1 inhibition. Brain PGE2 levels were not increased by AngII and were attenuated by cyclooxygenase-1, but not cyclooxygenase-2 inhibition. The cerebrovascular dysfunction induced by 14 day administration of “slow pressor” doses of AngII (600ng/kg/min) was attenuated by neocortical application of SC51089. Cyclooxygenase-1 immunoreactivity was observed in microglia and EP1R in endothelial cells. We conclude that the cerebrovascular dysfunction induced by AngII requires activation of EP1R by constitutive production of PGE2 derived from cyclooxygenase-1. The findings provide the first evidence that EP1R are involved in the deleterious cerebrovascular effects of AngII and suggest new therapeutic approaches to counteract them.
Keywords: Hypertension, NOX2, functional hyperemia, endothelium-dependent relaxation, amyloid-beta, PGE2, microglia
INTRODUCTION
Hypertension is an independent risk factor for major neurological diseases, including stroke, vascular cognitive impairment and Alzheimer’s dementia1. Cerebral blood vessels are the main target of the deleterious effects of hypertension on the brain2. In addition to inducing structural changes in cerebral vessels, hypertension alters cerebrovascular regulatory mechanisms that are critical for the structural and functional integrity of the brain. For example, hypertension suppresses the increase in cerebral blood flow (CBF) induced by neural activity, a vital response that matches local energy demands with the delivery of nutrients through blood flow, and disrupts the endothelial regulation of cerebral blood vessels3–6. These alterations compromise cerebrovascular reserves and render the brain more vulnerable to ischemia, setting the stage for devastating diseases such as stroke and dementia2. In models of hypertension induced by angiotensin-II (AngII), an octapeptide thought to play a central role in essential hypertension7, the cerebrovascular alterations are mediated by reactive oxygen species (ROS) produced by the enzyme NADPH oxidase3. However, the factors modulating the ROS production induced by AngII in cerebral blood vessels and contributing to the vascular dysfunction are poorly understood.
Prostanoids are powerful lipid mediators that are synthesized from arachidonic acid by cyclooxygenase (COX) 1 and 2, and exert a wide variety of biological effects by acting on multiple G-coupled membrane receptors8. In the cardiovascular system, prostanoids have long been implicated both in the normal regulation of vascular tone9, and in the pathobiology of cardiovascular diseases including hypertension8. Recent evidence suggests that prostaglandin E2 (PGE2), a prostanoid that acts on 4 receptors (EP1–EP4), participates in the mechanisms of hypertension and related complications10–12. In particular, EP1 receptors have been implicated in the vasoconstriction and blood pressure elevation induced by AngII11, 13. Furthermore, EP1 receptor antagonists lower blood pressure in spontaneously hypertensive rats and in db/db diabetic mice11, 13. However, it is not known whether PGE2 and EP1 receptors also play a role in the deleterious effects of AngII on cerebral blood vessels, a key determinant of the end-organ damage to the brain2.
In this study, we investigated the role of EP1 receptors in the cerebrovascular dysfunction induced by AngII. Using a mouse model of acute AngII hypertension, we examined whether PGE2 and EP1 receptors contribute to the cerebrovascular alterations induced by AngII, and whether these effects involve AngII-dependent ROS production. In addition, we sought to define the enzymatic sources of PGE2 and the brain cells involved in PGE2 synthesis.
METHODS
Please see the online data supplement at http://hyper.ahajournals.org for a more detailed description of the methods.
General surgical procedures
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Studies were conducted in 3-month-old male mice (weight: 27–30g). EP1−/−, COX-1, COX-2 and NOX2−/− mice were obtained from in house colonies14–17. Mice were congenic with the C57Bl/6 strain and C57Bl/6 mice (The Jackson Laboratory) were used as wild type controls. Mice were anesthetized with isoflurane intubated, and artificially ventilated (SAR-830; CWE Inc.)4, 5. Mean arterial pressure (MAP), rectal temperature and blood gases were monitored and controlled4, 5. After surgery, anesthesia was maintained with urethane (750 mg/kg; i.p.) and chloralose (50 mg/kg; i.p.)4, 5. In some studies, mice were anesthetized with isoflurane and implanted with osmotic minipumps delivering saline or AngII (600 ng/kg/min)3, 4. CBF was tested 14 days after pump implantation.
Monitoring of CBF
CBF was monitored with a laser-Doppler probe (Periflux System 5010, Perimed A.B.) in a cranial window overlying the somatosensory cortex5. CBF was expressed as percentage increases relative to the resting level.
Pharmacological agents
The COX-1 inhibitor SC-560, the COX-2 inhibitor NS398, PGE2, PGF2α (all from Cayman Chemical) or the EP1 antagonist SC-51089 (Biomol) were dissolved in dimethylsulfoxide (DMSO) and superfused on the somatosensory cortex16.
Experimental protocol
MAP and blood gases are presented in Table S1 at http://hyper.ahajournals.org. The cranial window was first superfused with Ringer’s solution (vehicle), and CBF responses were recorded3–6, 18, 19. The whisker-barrel cortex was activated for 60 seconds by stroking the contralateral vibrissae4, and the evoked changes in CBF were recorded. The endothelium-dependent vasodilator acetylcholine (ACh; 10μM; Sigma, St. Louis, MO) or the smooth muscle relaxant adenosine (400μM; Sigma, St. Louis, MO) was superfused on the exposed neocortex for 5 min. After testing baseline CBF responses, saline or AngII were infused intravenously at a rate of 0.25±0.02μg/kg per minute4. CBF responses were tested again after 30–40 minutes of AngII or saline infusion in wild type, EP1, COX-1, COX-2 or NOX2-null mice. In experiments using pharmacological inhibitors, CBF responses were first tested with vehicle superfusion and then after 30 min of superfusion with SC51089 (10μM), SC560 (25μM), or NS398 (100μM). Then, the infusion of Ang II was started and CBF responses were tested again 30–40 minutes later. In some experiments, the effect of 30 min neocortical application of PGE2 (1μM) or PGF2α (1μM) on the CBF responses was evaluated after AngII or saline infusion in mice treated with SC560 or in EP1 and NOX2-null mice14, 16, 17. In experiments with AngII administration for 14 days, CBF responses were tested before and after neocortical application of SC51089.
Immunohistochemistry
Coronal brain sections were cut through the somatosensory cortex using a cryostat and incubated with primary antibodies against COX-1, NeuN, CD31, or EP1 receptors. After incubation with a secondary antibody, sections were mounted on slides and examined using a Leica confocal microscope.
ROS detection
ROS production was assessed by hydroethidine (HE) microfluorography5, 6, 15. HE was topically superfused on the somatosensory cortex for 60 min. Ringer’s solution containing HE or HE plus SC51089, SC560, NS398, was superfused and, 30 min later, the i.v. infusion of AngII or vehicle was started. Mice were killed 30–40 min later, coronal brain sections were cut through cortex underlying the cranial window, and ROS determined as previously described5, 6, 15. In some experiments the effect of neocortical application of PGE2 or PGF2α on ROS production after COX-1 or EP1 receptor inhibition was also studied after AngII or saline infusion.
PGE2 assay
Mice were surgically prepared as described above and killed after 30–40 min of i.v. AngII infusion. Samples were immediately collected from the somatosensory cortex and the renal medulla, weighed, homogenized and prostanoid concentration was determined using an enzyme immunoassay kit14.
Data Analysis
Data are expressed as mean±SEM. Two group comparisons were evaluated using the Student’s t-test. Multiple comparisons were evaluated by the analysis of variance and Tukey’s test. Differences were considered statistically significant for p<0.05.
RESULTS
EP1 receptors are required for the cerebrovascular effects of AngII
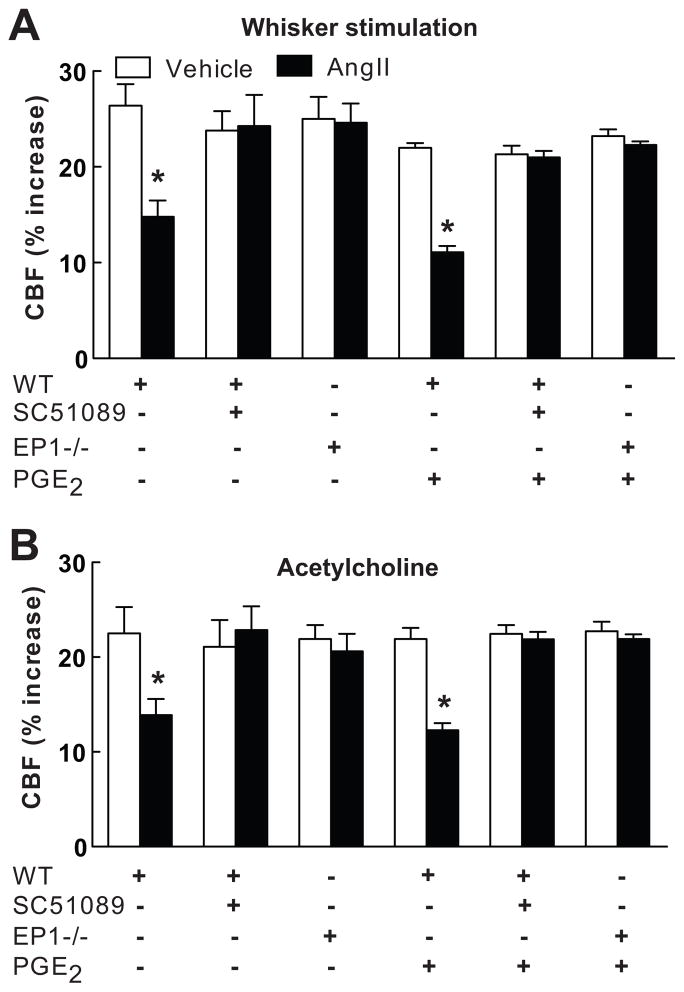
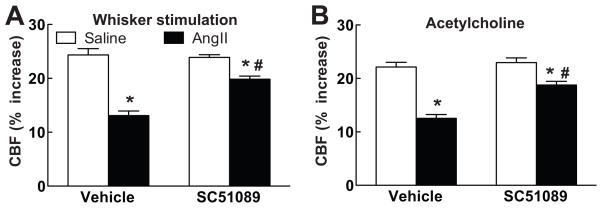
In agreement with previous studies3–6, 18, 19, administration of AngII to wild type mice (n=5/group) elevated MAP (see Table S1 at http://hyper.ahajournals.org) and attenuated the increase in CBF evoked by whisker stimulation (−46±4%) or by ACh superfusion (−40±4%) (p<0.05, ANOVA and Tukey’s test), but not adenosine (Fig. 1A,B; p<0.05; see Figure S1 at http://hyper.ahajournals.org). In wild type mice, neocortical superfusion with the EP1 receptor antagonist SC51089 did not alter AngII hypertension or resting CBF (see Tables S1 and S2 at http://hyper.ahajournals.org), but it prevented the attenuation of the CBF responses to whisker stimulation and ACh (Fig. 1A,B; n=5/group). Similarly, in EP1-null mice AngII elevated MAP (see Table S1 at http://hyper.ahajournals.org), but failed to attenuate the increased in CBF induced by whisker stimulation or ACh (Fig. 1A, B; n=5/group). In contrast, topical application of Aβ1–40 (5μM for 40 min), a peptide that induces cerebrovascular dysfunction through NOX2-derived ROS15, 20, attenuated the CBF responses in EP1 nulls (whisker stimulation: −43±5%; ACh: −44±7; p<0.05; n=5/group), indicating that NOX2 is functional in EP1 null mice. Administration of “slow pressor” doses of AngII for 14 days elevated MAP and attenuated the increase in CBF induced by whisker stimulation or ACh (Fig. 2A,B; see Table S1 at http://hyper.ahajournals.org). The cerebrovascular dysfunction was attenuated by neocortical application of SC51089 (Fig. 2A,B; n=5/group; p<0.05 from vehicle).
Figure 1.
Effect of AngII on the CBF increase produced by whisker stimulation (A) or acetylcholine (B) in wild type (WT) mice with neocortical superfusion of SC51089 or in EP1-null mice, with or without PGE2. WT (+ or −) and EP1−/− (+ or −) refer to the genotype of the mice. Mean±SEM. *p<0.05 from vehicle; analysis of variance and Tukey’s test; n=5/group.
Figure 2.
Effect of 14 day administration of AngII via osmotic minipumps on the increase in CBF induced by whisker stimulation (A) or acetylcholine (B) in mice with neocortical superfusion of vehicle or SC51089. *p<0.05 from vehicle; #p<0.05 from AngII vehicle; n=5/group.
COX-1 but not COX-2 inhibition attenuates the cerebrovascular effects of AngII
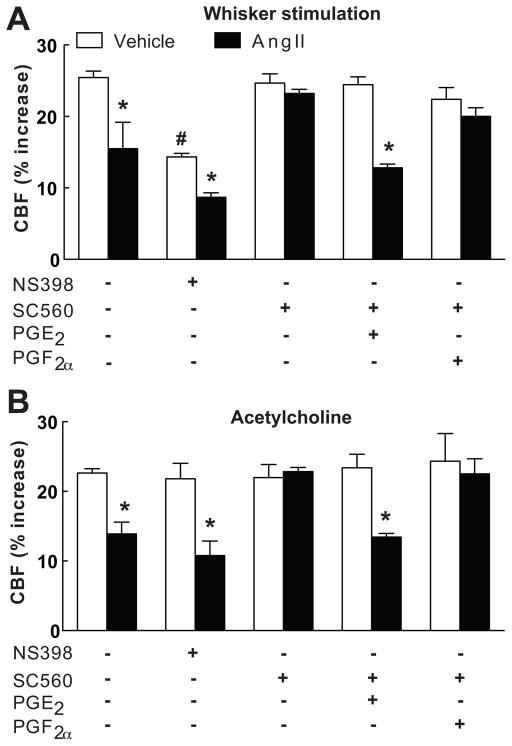
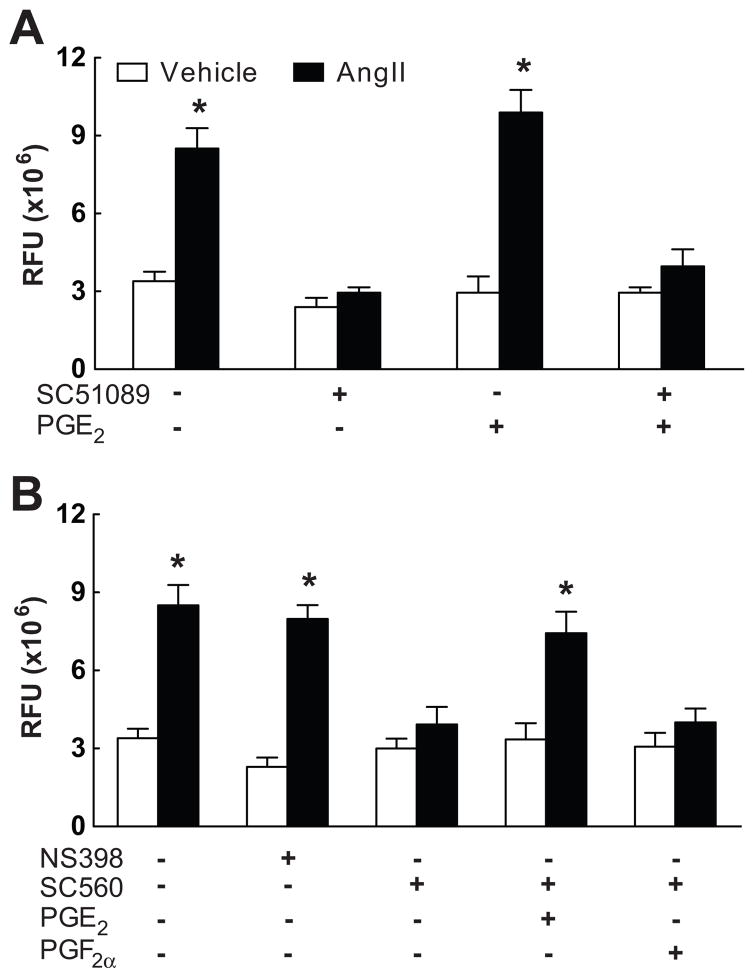
The observation that EP1 receptors are required for the cerebrovascular effects of AngII points to the involvement of PGE2, a reaction product of the COX pathway8. Therefore, we sought to determine whether the source of PGE2 was COX-1 or COX-2. Topical superfusion with the COX-2 inhibitor NS398 did not alter resting CBF (see Table S2 at http://hyper.ahajournals.org), and attenuated the increase in CBF induced by whisker stimulation, but not ACh (Fig. 3A,B), as previously described17. However, NS398 did not affect the attenuation of CBF responses to whisker stimulation or ACh induced by acute AngII administration (p<0.05 from control; n=5/group; Fig. 3A,B). Similarly, AngII attenuated the CBF response to whisker stimulation (Vehicle: 15±1; Ang: 10±1%) and ACh (Vehicle: 22±1; AngII: 13±1%) in COX-2 null mice (p<0.05 from wild type; n=5/group). Superfusion with the COX-1 inhibitor SC560 lowered resting CBF, but not the increase in CBF evoked by whisker stimulation or ACh (Fig. 3A,B; n=5/group)16. However, SC560 prevented the attenuation of these responses induced by AngII without altering the CBF response to adenosine (Fig. 3A,B; see Figure S1 and Table S1 at http://hyper.ahajournals.org; n=5/group). Similarly, AngII failed to attenuate the increase in CBF induced by whisker stimulation (Vehicle: 25±1; AngII: 24±1%) ACh (Vehicle: 23±1; AngII: 23±1) in COX-1 null mice (p>0.05; n=5/group).
Figure 3.
Effect of AngII on the CBF increase produced by whisker stimulation (A) or acetylcholine (B) in mice with neocortical superfusion of NS398, SC560, PGE2 and PGF2α. *p<0.05 from vehicle; #p<0.05 from NS398 vehicle; n=5/group.
PGE2 but not PGF2a counteracts the effect of COX-1 inhibition by acting on EP1 receptors
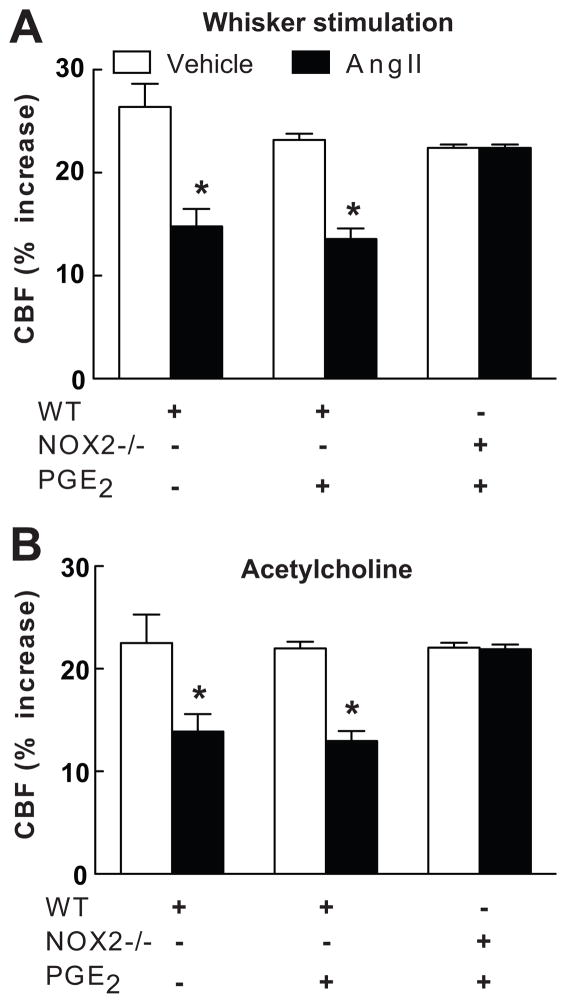
The findings presented above implicate COX-1-derived PGE2 in the cerebrovascular alterations induced by AngII. To test this hypothesis we examined whether exogenous PGE2 could re-establish the cerebrovascular effects of AngII after COX-1 inhibition. Based on a dose-response study, we chose a concentration of PGE2 (1μM) that does not alter resting CBF, or the increase in CBF induced by whisker stimulation, ACh or adenosine (Fig. 1A,B; n=5/group; see Table S2 and Figure S2 at http://hyper.ahajournals.org). Superfusion with this concentration of PGE2 re-established the attenuation of the CBF response to whisker stimulation or ACh in mice treated with SC560, while PGF2a was not effective (Fig. 3A,B; n=5/group). In contrast, PGE2 failed to reestablish the cerebrovascular effects of AngII in wild type mice treated with the EP1 antagonist SC51089 or in EP1-null mice (Fig. 1A,B; n=5/group), attesting to the fact that the effect of PGE2 requires EP1 receptors. NOX2-null mice are protected from the cerebrovascular dysfunction induced by AngII3. However, PGE2 did not re-establish the vascular dysfunction induced by AngII in NOX2-null mice (Fig. 4A,B; n=5/group), indicating that PGE2 is upstream of NOX2 in the signaling pathway mediating the cerebrovascular effects of AngII.
Figure 4.
Effect of AngII on the CBF increase produced by whisker stimulation (A) or acetylcholine (B) in wild type (WT) or NOX2−/− mice with or without neocortical superfusion with PGE2. WT (+ or −) and NOX2−/− (+ or −) refer to the genotype of the mice. *p<0.05 from vehicle; n=5/group.
EP1 receptors and COX-1-derived PGE2 contribute to AngII-induced ROS production
In wild type mice AngII increased ROS production in neocortical cerebral blood vessels, as previously reported5(Fig. 5A; n=5/group). The increase in ROS evoked by AngII was markedly attenuated by SC51089 superfusion (Fig. 5A). Furthermore, the ROS increase was attenuated by the COX-1 inhibitor SC560, but not by the COX-2 inhibitor NS398 (Fig. 5B; n=5/group). In vehicle-treated mice, PGE2 superfusion did not affect baseline ROS production, but, in mice treated with SC560, PGE2 re-established the increase in ROS induced by AngII, while PGF2a had no effect (Fig. 5A,B; n=5/group).
Figure 5.
Effect of Ang II on ROS production assessed by in situ HE microfluorography. A: Mice treated with neocortical superfusion of SC51089 and/or PGE2. B: Mice treated with NS398, SC560 and PGE2 or PGF2α. *p<0.05 from vehicle; n=5/group.
Cellular localization of COX-1 and EP1 receptors
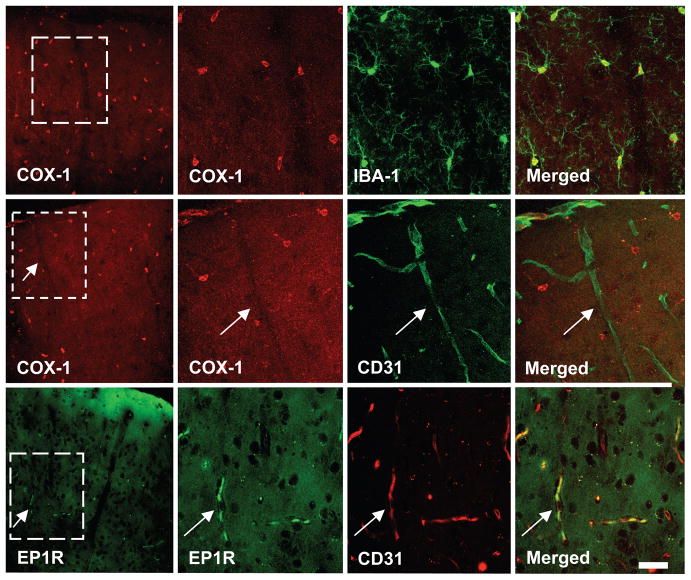
In the somatosensory cortex, COX1 immunoreactivity was localized to small cells with fine ramified processes that were also immunopositive for the microglial marker Iba-1 (Fig. 6). COX-1 immunoreactivity was not observed in cerebral blood vessels, identified by the endothelial cell marker CD31 (Fig. 6), neurons (NeuN) or astrocytes (GFAP)(see Figure S3 at http://hyper.ahajournals.org). In contrast, EP1 receptor immunoreactivity was observed in CD31 positive vascular profiles and neurons (Fig. 6; see Figure S3 at http://hyper.ahajournals.org), but did not co-localize with microglial and astrocytic markers (see Figure S3 at http://hyper.ahajournals.org). Therefore, COX-1 is present in microglia, while EP1 receptors are present in neurons and endothelial cells.
Figure 6.
In somatosensory cortex, COX-1 immunoreactivity (red) is co-localized with the microglial marker Iba-1 (green)(top row), but not with the endothelial marker CD31 (green) (middle row, arrows). EP1 receptor (EP1R) immunoreactivity (green) is co-localized with CD31 (red) (lower row, arrows). The cortical surface is at the top of the panels. The second, third and fourth panels in each row represent enlargements of the square in the first panel. Calibration bar: 10μm.
Effect of AngII, NS398 and SC560 on PGE2 in somatosensory cortex
To determine whether AngII increases PGE2 production in brain we studied the effect of acute AngII infusion on PGE2 concentration in the somatosensory cortex and the renal cortex. AngII infusion increased PGE2 in the kidney (Saline: 0.53±0.09; AngII: 1.43±0.03 ng/mg tissue; p<0.05; n=5/group), but not in brain (Saline: 3.49±0.34; AngII: 3.54±0.4 ng/mg tissue; p>0.05; n=5/group). In separate mice, superfusion with SC560, but not NS398, markedly attenuated PGE2 concentration (vehicle: 4.32±0.76; SC560: 0.23±0.04, p<0.05; NS398: 4.52±0.29 ng/mg tissue; p>0.05; n=5/group), attesting to the dominant role of COX-1 in constitutive PGE2 production in the neocortex.
DISCUSSION
We have demonstrated that the cerebrovascular dysfunction induced by acute or chronic AngII is prevented by the EP1 receptor antagonist SC51089. The COX-1 inhibitor SC560 prevented the cerebrovascular effects of acute AngII, whereas the COX-2 inhibitor NS398 did not, implicating COX-1 as the enzymatic source of PGE2. Measurements of PGE2 documented that COX1 is the major source of this prostaglandin in the somatosensory cortex. Neocortical superfusion with PGE2 reinstated the cerebrovascular effects of AngII in mice treated with SC560. However, PGE2 was not effective in mice treated with SC51089 or in EP1-null mice, ruling out the participation of other prostanoid receptors in the effect of PGE2. AngII-induced ROS production was attenuated after inhibition of COX-1 or EP1 receptors, and PGE2 counteracted the attenuation observed with COX1 inhibition. Using immunocytochemistry to determine the cellular source(s) and targets of PGE2, we observed COX-1 in microglia and EP1 receptors in endothelial cells and neurons. These novel findings provide the first evidence that the deleterious cerebrovascular effects of AngII require COX-1 derived PGE2 and EP1 receptors.
The observation that PGE2 reinstates the cerebrovascular dysfunction induced by AngII after COX-1 inhibition cannot be due to a deleterious cerebrovascular effect of PGE2 because PGE2 did not alter resting CBF or its response to whisker stimulation, ACh or adenosine. SC51089 and NS398 did not alter resting CBF, but SC560, as anticipated16, produced a small CBF reduction that does not affect the interpretation of the data. in Although the concentration of PGE2 applied to the cerebral cortex (1μM) is larger than the endogenous concentration, we anticipate that the effective concentration reaching the volume of tissue in which CBF was recorded (approximately 1 mm3) is lower because the bioavailability of PGE2 is reduced in biological fluids8. In addition, the observation that PGE2 does not counteract the cerebrovascular dysfunction in mice treated with SC51089 or in EP1-null mice, and that PGF2a does not mimic the effects of PGE2 rules out non-specific effects.
We have shown here that in the cerebral cortex, unlike the renal cortex, circulating AngII does not increase PGE2 production. This observation is consistent with the well-established finding that COX-1, the source of the PGE2 precursor PGH2 in our model, is present in microglial cells21, which reside inside the blood-brain barrier and are not accessible to circulating AngII. It is therefore likely that constitutive PGE2 production by COX-1, rather than AngII-stimulated PGE2 production, is required for the expression of the cerebrovascular dysregulation induced by AngII. Our finding that SC560, but not NS398, reduces baseline PGE2 production in neocortex supports this hypothesis. Collectively, our data raise the possibility that microglial COX-1-dependent PGE2 production plays a role in cerebrovascular dysregulation induced by AngII. However, we cannot rule out that endothelial COX-1 is expressed below immunocytochemistry detection levels, or that COX1-derived PGH2 from microglia is converted to PGE2 by PGE2 synthase present in the vessel wall22.
We found that the increase in ROS induced by AngII requires COX-1 derived PGE2 and EP1 receptors. We have previously demonstrated that the increase in ROS induced by circulating AngII is restricted to cerebrovascular cells, mainly endothelial cells, and is mediated by a NOX-2-containing NADPH oxidase3, 5. NOX-2-derived superoxide reacts with NO derived from endothelial NO synthase to form peroxynitrite, which, in turn, is responsible for the attenuation of endothelium dependent responses and functional hyperemia6. The findings of the present study suggest that constitutive PGE2 -induced activation of EP1 receptors is needed for AngII to trigger ROS production from NOX-2. The observation that PGE2 by itself does not increase ROS indicates that PGE2 does not affect NADPH oxidase activity directly, but that baseline levels of this prostanoid are needed to enable the NADPH oxidase activation induced by AngII. This scenario is reminiscent of the effect of PGE2 on Ca2+ in neurons, in which PGE2 does not increase intracellular Ca2+ but is required for the Ca2+ rise induced by NMDA14. The finding that PGE2 does not increase ROS if EP1 receptors are inhibited provides evidence that only EP1 receptors, and not other prostanoids receptors, are involved in this process. Considering that EP1 receptors can increase intracellular Ca2+ through the Na+/Ca2+ exchanger14, their activation may be needed to facilitate the intracellular Ca2+ increase required to activate NADPH oxidase. This possibility is supported by evidence that the Na+/Ca2+ exchanger contributes to the increase in cytosolic Ca2+ induced by AngII in renal arterioles23.
EP1-null mice have previously been reported to have small reductions in resting blood pressure10, a finding confirmed here, although the MAP change did not reach statistical significance. An EP1-null mouse developed more recently exhibited reduced resting MAP and an attenuation of the increase in blood pressure induced by AngII11. In our study, we did not observe a reduction in the acute hypertensive effects of AngII, despite the attenuation of the cerebrovascular effects. The reasons for this discrepancy are not clear, although confounding effects of anesthesia could play a role. Furthermore, the mice used by Guan et al.11 were generated using a different gene targeting strategy and cannot be directly compared to the EP1-null mice used in the present study. Irrespective of the role of EP1 receptors in the elevation in blood pressure, our findings clearly establish their involvement in the cerebrovascular effects of AngII.
Essential hypertension attenuates the increase in CBF induced by neural activity24, but, to our knowledge, the role of COX inhibitors in such attenuation has not been examined. However, there is evidence that the COX pathway participates in the systemic endothelial dysfunction observed in hypertensive patients25. We found that EP1 receptors are essential in the cerebrovascular dysfunction induced by acute or chronic AngII administration. With chronic AngII administration, the rescue of the cerebrovascular dysfunction is not complete, suggesting that a component of the dysfunction is mediated by mechanisms independent of EP1 receptors.
Perspective
We have demonstrated that the vascular dysregulation induced by AngII requires COX-1-derived PGE2 acting on EP1 receptors. PGE2 and EP1 receptors do not increase ROS directly, but they are necessary for the NADPH oxidase-dependent ROS production induced by AngII. AngII does not increase PGE2 in the cerebral cortex, indicating that COX-1-dependent constitutive PGE2 production is involved in the effect. Based on the localization of COX-1 in microglia and of EP1 receptors in cerebral arterioles, we speculate that PGE2 could originate from microglia and acts on vascular EP1 receptors to enable AngII-induced vascular oxidative stress. These observations provide evidence for a previously unrecognized permissive role of EP1 receptors in the cerebrovascular dysfunction induced by AngII and raise the possibility that microglial cells are capable of modulating cerebrovascular responses through COX-1 derived PGE2.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
Supported by NIH grants HL18974, HL96571 and NS35806
Footnotes
DISCLOSURES
None.
References
- 1.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH-oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 4.Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2003;285:H1890–1899. doi: 10.1152/ajpheart.00464.2003. [DOI] [PubMed] [Google Scholar]
- 5.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 6.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 7.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R893–912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 8.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50 (Suppl):S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickard JD. Role of prostaglandins and arachidonic acid derivatives in the coupling of cerebral blood flow to cerebral metabolism. J Cereb Blood Flow Metabol. 1981;1:361–384. doi: 10.1038/jcbfm.1981.41. [DOI] [PubMed] [Google Scholar]
- 10.Stock JL, Shinjo K, Burkhardt J, Roach M, Taniguchi K, Ishikawa T, Kim HS, Flannery PJ, Coffman TM, McNeish JD, Audoly LP. The prostaglandin E2 EP1 receptor mediates pain perception and regulates blood pressure. J Clin Invest. 2001;107:325–331. doi: 10.1172/JCI6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, Wei M, Fan X, Carmosino M, Hao C, Imig JD, Breyer RM, Breyer MD. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suganami T, Mori K, Tanaka I, Mukoyama M, Sugawara A, Makino H, Muro S, Yahata K, Ohuchida S, Maruyama T, Narumiya S, Nakao K. Role of prostaglandin E receptor EP1 subtype in the development of renal injury in genetically hypertensive rats. Hypertension. 2003;42:1183–1190. doi: 10.1161/01.HYP.0000101689.64849.97. [DOI] [PubMed] [Google Scholar]
- 13.Rutkai I, Feher A, Erdei N, Henrion D, Papp Z, Edes I, Koller A, Kaley G, Bagi Z. Activation of prostaglandin E2 EP1 receptor increases arteriolar tone and blood pressure in mice with type 2 diabetes. Cardiovasc Res. 2009;83:148–154. doi: 10.1093/cvr/cvp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E(2) EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 15.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- 17.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am J Physiol Heart Circ Physiol. 2008;294:H156–163. doi: 10.1152/ajpheart.01137.2007. [DOI] [PubMed] [Google Scholar]
- 19.Capone C, Anrather J, Milner TA, Iadecola C. Estrous Cycle-Dependent Neurovascular Dysfunction Induced by Angiotensin II in the Mouse Neocortex. Hypertension. 2009;54:302–307. doi: 10.1161/HYPERTENSIONAHA.109.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Bueno B, Serrats J, Sawchenko PE. Cerebrovascular cyclooxygenase-1 expression, regulation, and role in hypothalamic-pituitary-adrenal axis activation by inflammatory stimuli. J Neurosci. 2009;29:12970–12981. doi: 10.1523/JNEUROSCI.2373-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadhav V, Jabre A, Chen MF, Lee TJ. Presynaptic prostaglandin E2 EP1-receptor facilitation of cerebral nitrergic neurogenic vasodilation. Stroke. 2009;40:261–269. doi: 10.1161/STROKEAHA.108.516104. [DOI] [PubMed] [Google Scholar]
- 23.Fellner SK, Arendshorst WJ. Angiotensin II-stimulated Ca2+ entry mechanisms in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am J Physiol Renal Physiol. 2008;294:F212–F219. doi: 10.1152/ajprenal.00244.2007. [DOI] [PubMed] [Google Scholar]
- 24.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47:914–921. doi: 10.1016/j.neuroimage.2009.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.