Abstract
Williams syndrome (WS) is a genetic neurodevelopmental disorder with a distinctive phenotype including cognitive-linguistic features, non-social anxiety, and a strong attraction to music. We performed functional MRI studies examining brain responses to musical and other types of auditory stimuli in young adults with WS and typically-developing controls. In Study 1, the WS group exhibited unforeseen activations of the visual cortex to musical stimuli, and it was this novel finding that became the focus of two subsequent studies. Using retinotopy, color localizers and additional sound conditions, we identified specific visual areas in WS subjects that were activated by both musical and non-musical auditory stimuli. The results, similar to synesthetic-like experiences, have implications for cross-modal sensory processing in typical and atypical neurodevelopment.
INTRODUCTION
Williams syndrome (WS; OMIM#194050) is a rare neurodevelopmental disorder caused by a hemizygous microdeletion on chromosome 7 (7q11.23), which contains approximately 28 genes(Stromme et al., 2002; Bayes et al., 2003). Hypersociable and unusually empathetic, individuals with WS exhibit relative strengths in expressive language and face processing, and their IQ scores tend to fall in the mild range of intellectual disability(Dykens & Rosner, 1999; Klein-Tasman & Mervis, 2003; Reilly et al., 2004; Bellugi et al., 1999). Their performance on tasks involving visuospatial cognition is often impaired relative to age-matched typically-developing (TD) controls, and some show abnormal sensitivity to loud sounds, aversion to innocuous sounds and attraction to other sounds (Klein et al., 1990; Nigam & Samuel, 1994; Levitin et al., 2005). These auditory symptoms are indicative of a more general heightened sensitivity or reactivity that might be related to increased anxiety, fear and arousal in some persons with WS (Blomberg S et al., 2006; Dykens, 2003).
In addition to the characteristics summarized above, individuals with WS often exhibit a distinct musical phenotype. Individuals with WS show interest in music at an earlier age, spend more time listening to music, and are more emotionally responsive to music compared to comparison groups of chronological age-matched subjects with TD, autism or Down syndrome (Levitin et al., 2004). Individuals with WS are also more likely to play a musical instrument and to take music lessons compared to chronological age-matched subjects with Prader-Willi syndrome or Down syndrome (Dykens et al., 2005). One study showed enhanced skill for rhythmic production, in particular, which is consistent with our observations as well (Levitin, 2005). While engaged in musical activities, individuals with WS experience unusually high levels of emotion (Levitin, 2005), and caregivers report, they seem to use music instinctively in a therapeutic manner to reduce anxiety and to increase positive affect (Dykens et al., 2005). In fact, among WS subjects, those who spent more time listening to music had fewer Externalizing symptoms related to aggression and impulsivity, and those who had played a musical instrument for longer had fewer Internalizing symptoms, related to anxiety (as measured by Child Behavior Checklist (Achenbach, 1991)) (Dykens et al., 2005).
Intrigued by this strong attraction to music evidenced by people with WS, we set out to measure brain responses of WS individuals and those of matched controls in response to musical passages. From earlier work using brain imaging techniques, we knew that WS tends to be associated with abnormalities in the corpus callosum (Schmitt et al., 2001; Wang et al., 1992) and the hippocampal formation (Meyer-Lindenberg et al., 2005b), as well as reduced cortical brain volume (Thompson et al., 2005; Reiss et al., 2000) and altered gyral patterns (Gaser et al., 2006; Kippenhan et al., 2005). Moreover, studies using functional MRI (fMRI) have found differential activation patterns between persons with WS and TD controls during the performance of tasks involving visuospatial processing (Meyer-Lindenberg et al., 2004), response inhibition (Mobbs et al., 2007), face processing (Mobbs et al., 2004), and social cognition (Meyer-Lindenberg et al., 2005a).
However, only one previous study examined brain responses in individuals with WS evoked by auditory stimuli including classical music (Levitin et al., 2003). Levitin et al. (2003) found that, in WS subjects relative to TD controls, temporal lobe activations were decreased and right amygdala activations were increased in response to musical stimuli. While results from our initial study reported herein showed a similar trend, our measurements also revealed a remarkable pattern of auditory activations in areas of the brain conventionally associated with visual perception. Those intriguing, unanticipated results were suggestive to us of synesthesia, in which a person sees colors when hearing musical notes (Rizzo & Eslinger, 1989; Ward et al., 2006). Thus, we were motivated to perform two more sets of measurements to more specifically localize the responses within each subject’s visual cortex and to test whether these responses were restricted to music or also extended to other kinds of musical and non-musical auditory stimuli. The results of these subsequent studies confirm the presence of strong auditory activations within extrastriate visual areas. These synesthesia-like activations may well be related to the vivid visual imagery our participants with WS describe when listening to music‥
STUDY 1: Between-groups analysis of brain responses to musical stimuli
Methods
Subjects
Participants in Study 1 included 13 individuals with Williams Syndrome (5 females), ranging in age from 16 to 33, and 13 TD controls (6 females), ranging in age from 17 to 27 (Table 1). TD controls were recruited from the local community using flyers and website postings with IRB-approved language. WS participants were recruited through the Williams Syndrome Music Camp sponsored by the Vanderbilt Kennedy Center for Research on Human Development, with the assistance of the Vanderbilt Blair School of Music and the National Williams Syndrome Association. Hence, the WS sample was biased for individuals with a talent for and/or interest in music. Therefore, TD controls were ascertained to have some, but not an extensive, musical background. Specifically, 10 of 13 WS subjects and all TD control subjects played one or more musical instruments (Table 1). As assessed by the individually-administered Kaufman Brief Intelligence Test (K-BIT, Kaufman & Kaufman, 1990), individuals with WS had composite IQ scores that ranged from 49 to 91, with a mean of 69 and a standard deviation of 14, indicating mild levels of intellectual disability. (See Table 1 for verbal and non-verbal K-BIT summary statistics.) All WS participants exhibited the physical, cognitive and behavioral profile of WS and previously had received a clinical diagnosis of WS and confirmatory genetic testing.
Table 1. Study 1 Subject Demographics, IQ and Musical Experience.
| Age | Sex | Verbal IQ | Non-Verbal IQ | % Subjects Who Have Played | ||||
|---|---|---|---|---|---|---|---|---|
| Sample Size |
Median (Min-Max) |
M | F | Mean ± SD | Mean ± SD | At Least 1 Instrument |
2 or More Instruments |
|
| WS | 13 | 25 (16–33) |
8 | 5 | 80 ± 11 | 65 (19) | 77% | 46% |
| TD | 13 | 23 (17–33) |
7 | 6 | 114 ± 15 | 103 ± 16 | 100% | 54% |
To optimize success and minimize anxiety with the MRI procedures, we mailed each WS participant an audio CD of the sounds an MRI machine makes while scanning so that he/she could listen to them prior to attending music camp. While TD control subjects were not provided such CDs, all subjects would have been exposed to the actual MRI scanner sounds prior to the acquisition of functional scans (since several structural scans precede the first functional scan). Participants also visited the scanner and interacted with imaging staff prior to their scan, and we employed a research assistant with WS who had successfully completed previous scans with us and could talk to his peers about his experiences. The study protocol was approved by the Vanderbilt University Medical Center Institutional Review Board. Each participant gave his/her informed assent, and the participant’s parent or guardian gave informed consent prior to the experiment.
Functional Neuroimaging
MRI Data Acquisition
The same data acquisition parameters were used in all three studies. A Philips Achieva 3-Tesla MRI scanner (Philips Healthcare, Inc., Best, The Netherlands) was used to acquire T1-weighted anatomical volume images (TR = 4.6 msec, TE = 9 msec, 1 × 1 × 1 mm voxels, 170 sagittal slices, FOV=256mm) and functional MR images. A total of 31 axial slices were acquired parallel to the anterior-posterior (AC–PC) commissural line with an image matrix of 80 × 80 pixels, reconstructed to 128 × 128 pixels, and a FOV of 240mm. Slice thickness was 3.5mm with a 0.35mm gap, resulting in a voxel size of 1.875 × 1.875 × 3.85mm. Functional MR images were recorded using a single-shot T2*-weighted gradient-echo echo planar sequence that was sensitive to changes in blood oxygen level-dependent (BOLD) contrast with a TR of 2000ms and a TE of 35ms with a SENSE factor of 1.5. In addition, a set of high-resolution T1-weighted images, which were used for coregistration and alignment, were acquired at the same location and with the same slice thickness as the functional scans.
Stimuli and fMRI Experimental Design
For Study 1, we were interested in investigating how subjects responded to music with differing emotional valence. Participants passively listened to blocks of silence and blocks of instrumental music categorized as upbeat or downbeat or from an over-rehearsed song (‘Happy Birthday’; see Figure 1) Upbeat music included three 20 sec clips from polka, jazz and new-age genres considered to elicit a positive affect. Downbeat music included three 20 sec clips from the modern classical genre considered to elicit a negative affect. A larger set of song clips was initially selected for each category and was rated by research staff; those found to elicit the desired affect most consistently were chosen for inclusion. The ‘Happy Birthday’ (HB) song was 20 sec long and was played in a big-band jazz style. The rest condition consisted of 10 seconds of silence. During all conditions, visual stimuli consisted of a black background with a white cross, on which subjects were instructed to fixate throughout the functional runs. Two block design runs (230 sec each) were conducted. The upbeat and downbeat blocks each consisted of three distinct song clips (3 × 20 sec = 60 sec total), whereas the HB block consisted of just one 20 sec presentation of the over-rehearsed song. Therefore, each run consisted of one upbeat block (60 sec), one downbeat block (60 sec) and one HB block (20 sec), and each of these song blocks was followed by a resting block (10 sec). The presentation order of the song blocks was randomized.
Figure 1. Study 1 fMRI experimental design for a single run.
Actual order of presentation of music blocks was randomized.
During scanning (in all three studies) the room lights were off and the fixation cross was projected via a rear projection system onto a translucent screen placed on the top of the head coil. Subjects viewed the screen through a double mirror attached to the head coil. Stimuli were controlled using E-Prime (Psychological Software Tools, Pittsburgh, PA). Stimulus presentation was synchronized with the data acquisition by a trigger pulse delivered by the scanner console. We used an MR-compatible pneumatic auditory stimulation system incorporated into standard Philips headphones for binaural stimulus delivery.
Statistical Analysis
Functional MRI data were preprocessed using slice time correction, 3D motion correction, 3D spatial smoothing (6mm FWHM Gaussian kernel; for Study 1 only), linear trend removal, and a high pass filter (3 cycles/time course). Scans with excessive head motion (>3mm of translation or 3 deg of rotation) were removed from analysis. (Only one run from one subject was removed for excessive motion). Functional images were co-registered with structural images from the same subject, and all images were transformed to Talairach space. Brain Voyager QX (Brain Innovation, Maastricht, The Netherlands) was used to perform data preprocessing, as well as GLM and ROI analyses in all three studies (version 1.9) and, in Study 2, to perform color localizer and retinotopy analyses (version 1.6 and 1.9).
In Study 1, the random-effects general linear model (GLM) was applied to functional MRI data, which measured changes in BOLD response. Within and between group contrasts were conducted for each of the musical stimuli conditions (upbeat; downbeat; HB) versus the silent condition, for the combined conditions (upbeat + downbeat + HB) versus the silent condition, and for upbeat versus downbeat conditions. A priori anatomical regions of interest were specified based on previous research (Levitin et al., 2003) and analyzed using the Talairach-Tournoux Atlas (TTatlas+tlrc) dataset from AFNI (Cox RW, 1996). Whole brain analysis focused on areas identified from between-groups statistical maps using a voxel-wise significance threshold of 0.005 and a cluster-size threshold of 50 mm3.
Results
We conducted a whole-brain analysis looking for areas of differential activation to music listening between groups (n=13 per group) at a voxel-wise significance threshold of 0.005. A cluster size threshold of 50 mm3 was employed to reduce false positive rates while maintaining power to discover moderately sized clusters of activation (Loring et al., 2002; Hayasaka & Nichols, 2003). Within groups, activation patterns were very similar across music conditions; therefore, we report results from the contrast of combined (upbeat+downbeat+HB) music conditions versus silent fixation. There were 19 significant clusters of differential between-group activation for the combined music conditions versus silent fixation (Table 2). Sixteen of these differential activations were the result of increased activation in the WS group, and three were the result of increased activation in the TD group. None involved significant deactivations in the respective contrast group.
Table 2. WS group shows increased activation to music listening.
Significant clusters of differential activation by group to combined (Upbeat + Downbeat + HB) music conditions versus silent fixation. Positive t-test values indicate activation was greater in the WS group versus TD group; negative t-test values indicate activation was greater in the TD group versus WS group. A cluster threshold of 50 mm3 and a voxel-wise α of 0.005 were used to create the statistical map and resulting regions of interest.
| Hemi- sphere |
Region(s) | Subregion(s) | Brodmann Area |
Cluster Size (mm3) |
Peak Activation | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | p | |||||
| R | Frontal Lobe | Precentral Gyrus | 6 | 108 | 39 | −3 | 44 | 3.85 | 0.0008 |
| R | Frontal Lobe | WM, Precentral Gyrus |
6 | 135 | 32 | 6 | 36 | 3.74 | 0.002 |
| R | Limbic Lobe | Insula | 13 | 1053 | 45 | −35 | 19 | 6.01 | 0.000003 |
| R | Limbic Lobe | Posterior Cingulate | 30 | 135 | 12 | −47 | 6 | 4.30 | 0.0003 |
| R | Temporal Lobe | Superior Temporal Gyrus | 13 | 108 | 56 | −41 | 21 | 3.90 | 0.0007 |
| R | Temporal Lobe | WM, Superior Temporal Gyrus |
13 | 108 | 55 | −42 | 15 | 3.32 | 0.003 |
| R | Temporal Lobe | Inferior Temporal Gyrus | 37 | 297 | 45 | −65 | −8 | 4.84 | 0.00007 |
| L | Temporal Lobe | Superior Temporal Gyrus | 38 | 108 | −43 | 9 | −16 | 3.52 | 0.002 |
| L | Parietal Lobe | WM, Inferior Parietal Lobe |
135 | −32 | −37 | 26 | 3.70 | 0.002 | |
| R | Occipital Lobe | Cuneus | 18 | 108 | 14 | −68 | 15 | 4.05 | 0.0005 |
| R | Occipital Lobe | Cuneus | 18 | 108 | 1 | −80 | 21 | 3.76 | 0.001 |
| L | Occipital Lobe | WM, Middle Occipital Gyrus |
243 | −34 | −82 | 2 | 4.32 | 0.0003 | |
| R | Cerebellum, Occipital Lobe |
Declive, Lingual Gyrus |
108 | 11 | −76 | −12 | 3.80 | 0.0009 | |
| R | Cerebellum | Declive | 9 | 108 | 31 | −54 | −13 | 3.33 | 0.003 |
| R | Cerebellum | Culmen | 270 | 21 | −35 | −11 | 5.36 | 0.00002 | |
| L | Limbic Lobe | Posterior Cingulate | 23 | 135 | −5 | −50 | 23 | −4.24 | 0.0003 |
| L | Thalamus | Medial Dorsal Nucleus | 135 | −4 | −10 | 13 | −4.51 | 0.0002 | |
| R | Temporal Lobe | Inferior Temporal Gyrus | 20 | 108 | 62 | −25 | −15 | −3.87 | 0.0008 |
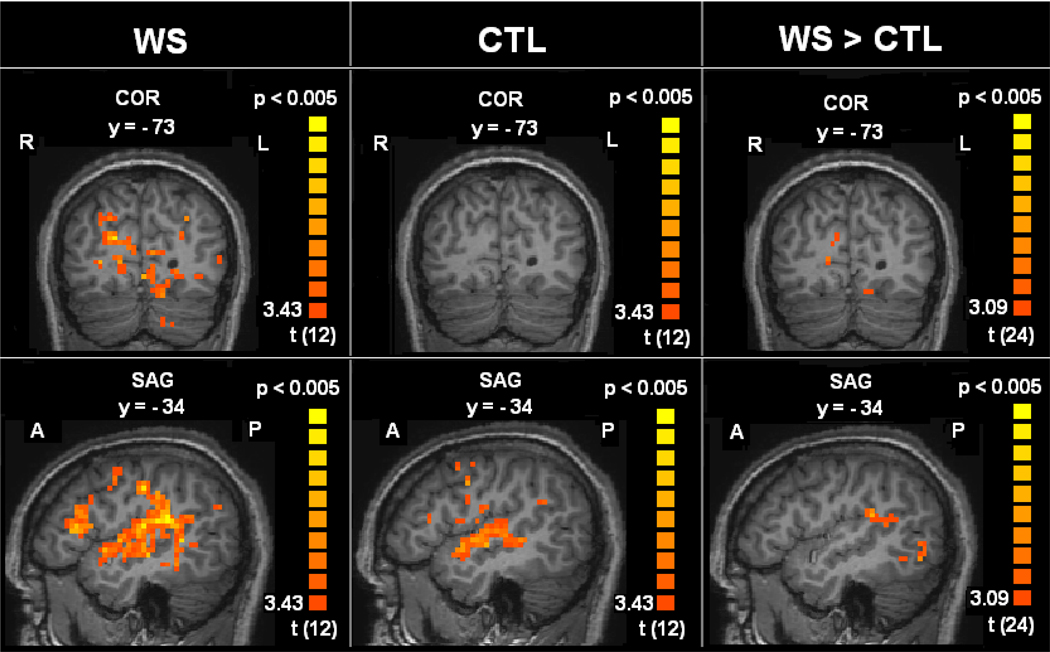
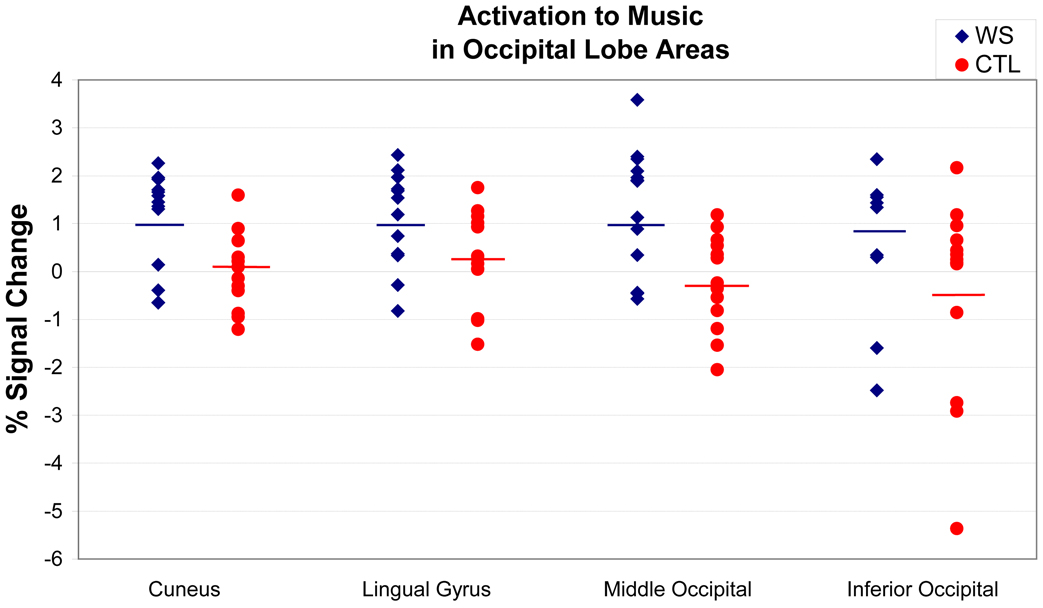
Previous studies suggest that attending to auditory stimuli results in hypoactivation of the visual cortex (Laurienti et al., 2002). Therefore, the differential activations in the occipital lobe areas that are associated with visual processing—cuneus, middle occipital gyrus and lingual gyrus—were highly unexpected. At the within-group level of analysis, increased activation in these occipital lobe areas was significant only for the WS group (Figure 2). Inspection of the underlying distributions of these occipital activations reveals a shift in the mean percent signal change from near or below zero (+0.27 to −0.42) for the TD group to approximately one percent (+0.87 to +1.00) in the WS group, with comparable within group variability (Figure 3). Notably, posthoc correlational analyses showed no relationship between measures of musicality and occipital lobe activations in either group. These highly unusual responses to music listening in areas of the brain known to be involved in vision motivated our subsequent two studies in WS subjects, in which we aimed to better localize and characterize these activations.
Figure 2. Occipital lobe and temporal lobe / insula activations to music listening in WS > TD.
Contrast of combined musical conditions (Upbeat+Downbeat+HappyBirthday) versus silent fixation. Within-group GLM of WS group (left) and TD control group (center). Between-groups GLM showing activations greater in the WS group versus TD control group (right). Statistical group maps are rendered on a representative single subject anatomical image. The top row of images shows activations in the occipital lobe. The bottom row of images shows activations in the left hemisphere.
Figure 3. Distribution of occipital lobe activations to music listening by group.
Contrast of combined musical conditions (Upbeat+Downbeat+HappyBirthday) versus silent fixation. Vertical bars indicate group means.
In addition, as seen in a previous report (Levitin et al., 2003), the cerebellum showed increased bilateral activation in the WS group. There were also a number of other activations in regions not previously reported to be associated with auditory perception in the WS group (see Table 2). Some of these were in areas related to emotion processing—insula, parahippocampal gyrus and posterior cingulate gyrus—which are consistent with increased emotional experience with music for individuals with WS.
We had identified the amygdala as an a priori region of interest (ROI) based on previous fMRI findings (Levitin et al., 2003). While we did find amygdala activation to music listening, we found no significant difference in this activation between the WS and TD groups using a between-groups ROI general linear model (GLM). Two regions involved in audition, the bilateral superior and middle temporal gyri (STG and MTG, respectively), were also selected as a priori ROIs based on previous findings (Levitin et al., 2003). The STG was activated bilaterally in both WS (t = 6.95, p < 2 × e−5) and TD (t = 9.34, p < 5 × e−6), and the between-groups ROI GLM was not significant (see Figure 2). Activations in the MTG were more isolated, primarily to the posterior portions of the gyrus, and only the WS activations were significant (t = 5.90, p < 8 × e−5). Once again, using a between-groups ROI GLM, we did not find significant differences between the WS and TD groups.
In summary, in Study 1, we investigated differences in brain responses to music in individuals with WS versus TD controls. Novel activations in occipital lobe areas related to visual processing were found in the WS group. We also found increased activation in areas related to emotion processing in the WS group.
STUDY 2: Localization of WS occipital lobe responses to musical stimuli using color localizer and retinotopy
Methods
Subjects
Ten WS participants in Study 1 who showed activations to musical stimuli in occipital lobe regions were asked to participate in a follow-up study. Of these, six participants (five males) were available for follow-up scans. After their scans, we interviewed participants about how they experienced the musical stimuli they heard, as well as other music. Because individuals with WS are highly suggestive and are very eager to please, we used open-ended, non-leading questions regarding how participants experience music (e.g., When you listen to music, what happens to you? When you hear that song, how do you feel? What do you think about or imagine when you are listening to music?).
Functional Neuroimaging
Music Stimuli and fMRI Experimental Design
Musical stimuli used in Study 2 consisted of (1) songs and (2) musical notes and chords. Although brain responses to upbeat versus downbeat music were not consistent across subjects in Study 1, we were still interested in whether familiarity or preference for the stimuli might be important, or whether auditory stimuli devoid of emotional valence might give similar brain responses. Thus, we had each participant choose songs he/she liked, and then we, the researchers, picked songs that all participants would hear. Song stimuli consisted of three 30 sec song clips selected by the participant (PS songs) and three 30 sec song clips selected by the researchers (RS songs). All RS songs were instrumental, but for the PS songs, participants were allowed to select any songs, some of which contained vocals. We also wanted to test some more basic musical stimuli generally devoid of emotional valence, so we chose single musical notes and chords. The notes and chords were two-second clips of a single note (A, C or E) or chord (A, C or E major), generated electronically to simulate either a piano or a guitar, with an equal number of clips from each instrument. There was also a silent rest condition. During all conditions, visual stimuli consisted of a black background with a white cross, on which subjects were instructed to fixate throughout the functional runs.
Two block design runs (270 sec each) of song stimuli were conducted (see Figure 4). Each run consisted of three blocks of PS songs (3 × 30sec = 90 sec total), three blocks of RS songs (3 × 30sec = 90 sec total), and three blocks of rest (3 × 30sec = 90 sec total). Each song block consisted of only one song clip. The presentation order of the song blocks was randomized, and a silent block followed every song block.
Figure 4. Study 2 block design for Song stimuli runs.
Actual order of presentation of sound blocks was randomized.
Two block design runs (360 sec each) of notes and chords were also conducted in Study 2 (see Figure 5). Each music block consisted of 20 clips of the 2 sec long stimuli (40 sec per block). Each run consisted of three blocks of notes (3 × 40 sec = 120 sec), three blocks of chords (3 × 40 sec = 120 sec), and three blocks of silent rest (3 × 40 sec = 120 sec). The presentation order of the music blocks and the presentation order of the stimuli (notes/chords) within a block were randomized. A silent block followed every music block.
Figure 5. Study 2 fMRI experimental design for Notes and Chords runs.
Actual order of presentation of musical sound blocks was randomized.
Retinotopy and Color Localizer Experiments
In Study 2, retinotopically organized visual areas V1, V2, V3, and V4v were identified using conventional phase-encoded retinotopic mapping methods (DeYoe et al., 1996; Engel et al., 1997; Sereno et al., 1994). Functional MRI images were acquired while participants viewed a slowly rotating, contrast-reversing checkerboard wedge subtending 22.5 deg in polar angle while fixating on the center cross. The wedge started at the lower right visual field and slowly rotated counter-clockwise. After 8 sec, the wedge was at the lower vertical meridian and kept rotating counter-clockwise for a full cycle of 360 deg thereafter (within 64 sec). Each retinotopic mapping run consisted of 4 repetitions of this rotation. Each of two color localizer scans lasted 4 min 32 sec, the initial 8 sec (4 volumes) of which were discarded prior to analysis to allow MR stabilization.
Color-selective areas were also defined by a conventional color area localizer that contrasts viewing of partially overlapping chromatic rectangles (i.e., chromatic Mondrians) with viewing of achromatic, luminance-varied Mondrians (Howard et al., 1998). Each of two color localizer scans lasted 5 min 4 sec, the initial 8 sec (4 volumes) of which were discarded prior to analysis to allow for MR stabilization. The scan was divided into 6 blocks of chromatic Mondrians and 6 blocks of achromatic Mondrians, with interspersed fixation baseline blocks.
Functional MRI data from the retinotopic mapping and color localizer scans were registered with the subject’s high-resolution anatomical images, providing a subject-specific mapping of visual area regions of interest (ROIs) at the voxel level. These ROIs were used for analyzing the functional MR images corresponding to the music listening conditions.
Statistical Analysis
A fixed-effects GLM with separate study predictors (for multiple runs) was applied to each subject’s functional MRI data from the music stimuli experiments. Statistical maps of BOLD activation were generated for music conditions, and due to the highly constrained nature of our hypothesis to determine whether activations were present within visual cortex, we used a slightly more liberal significance threshold of 0.01. Retinotopically defined visual areas were identified using cross correlation analysis on the retinotopic mapping data. Data from two separate functional runs were averaged for statistical analysis. We used the predicted hemodynamic signal time course for the first half of a stimulation cycle (corresponding to 180° visual angle in the polar mapping experiment) and shifted this reference function successively in time. Sites activated at particular polar angles were identified through selection of the lag value that resulted in the highest cross-correlation value for a particular voxel (Muckli et al., 2005). Color-selective regions of interest (ROIs) were defined as the clusters of voxels that showed significantly higher BOLD response (multi-study GLM on two scans; p < 0.05, FDR corrected) to colored Mondrians than to achromatic Mondrians. In subjects whose color localizer or retinotopy data did not permit adequate localization, extra efforts were made to identify early visual, including color-selective, areas from each subject’s anatomy. For example, the calcarine sulcus (whose banks are V1 areas) of a subject was examined to see if any of voxels activated by auditory stimulation fell on or around this region. It should be noted that each subject had good localization on one or both of the color localizer and retinotopy scans. For Subjects 2 and 5, functional runs using the PS and RS songs were incomplete and could not be analyzed.
Results
Since individuals with WS are known to have decreased cortical volume in posterior occipital and parietal areas, it was important to test whether such differences in anatomy across groups, which can result in poor coregistration of images, was responsible for the novel occipital lobe activations found in Study 1. Study 2 assessed whether within-subject occipital lobe activations to music were located in areas related to visual processing, as initially expected from Study 1 based on anatomy, and how areas of activation to music related spatially to visual cortical areas.
Within-subject functional ROIs were identified based on color localizer and retinotopy scans. All six subjects with WS showed activation (p < 0.01) to one or more musical conditions in areas that were identified by color localizer, retinotopy and/or anatomy as being visual areas, including V1, V1 and V4v (Table 3). The contrasts involving simple chords consistently activated early visual areas, including color-selective areas, across all six subjects. Contrasts involving the participant-selected (PS) or researcher-selected (RS) songs showed activation in visual area ROIs in three of the four subjects for which there were complete data.
Table 3. Visual areas are activated by musical conditions in all six WS subjects in Study 2.
BOLD activations (p < 0.01) to contrasts of musical condition versus silent fixation that overlap with color localizer ROIs in each of six subjects in Study 2. “Color-Selective” areas were areas that showed greater response to colored Mondrians than to achromatic Mondrians. These areas included V4v. “Non-Color-Selective” areas were V1 and V2 that were not identified by the color localizer.
| Early Visual Areas | ||||
|---|---|---|---|---|
| Color-Selective | Non-Color-Selective | |||
| Subject | Left H | Right H | Left H | Right H |
| 1 | N, C†, RS | N, C†, RS | N, C† | N, C† |
| 2 | N†, C† | N, C | --- | N, C† |
| 3 | C† | C | C† | C† |
| 4 | N†, C†, PS† | N | N, C†, PS†, RS | N, C† |
| 5 | N | C | N, C† | C† |
| 6 | C | C†, RS | RS | |
Right H = Right Hemisphere; Left H = Left Hemisphere
N=notes; C=chords; PS=participant-selected song; RS=researcher-selected song
Activation significant (p<0.01) according to ROI GLM using subject-specific color localizer ROIs.
--- No early visual ROIs could be identified in this area for Subject 2.
We also used more stringent subject-specific ROI GLM analyses to determine whether the activation across the entire volume of a given visual ROI was significantly different for a music condition versus silent fixation. All six subjects had at least one color localizer ROI that was significantly activated by either the notes or the chords condition versus silent fixation contrast. In contrast, only one subject had activation to the PS song that was significant by ROI GLM analysis. Figure 6 shows intra-subject activations to the chords condition in visual areas identified using their respective color localizer ROIs. Table 3 summarizes data from color localizer and retinotopy analyses, indicating which visual areas (denoted by †) were activated significantly (by ROI GLM analysis) to which conditions in which subjects. It should be noted that hypoactivations, indicating lower BOLD response than during silent fixation, were also observed in visual areas to some conditions in five of six subjects, as would be more typical of (inhibitory) brain responses to auditory perception tasks (Laurienti et al., 2002).
Figure 6. Listening to musical and non-musical sounds activates early visual areas.
Intra-subject activations to chords (Study 2), human non-word vocalizations (Study 3) and white noise conditions (Study 3) versus silent fixation in early visual areas identified by color localizer runs. Subjects 2 and 5 did not participate in Study 3.
In summary, Study 2 demonstrated that early visual areas, including color-selective areas, were being activated to music in individuals with WS. Notably, visual cortex responses were more consistent across subjects for the simple musical stimuli of notes and chords than to songs.
STUDY 3: Characterization and localization of WS occipital lobe responses to non-musical stimuli
Methods
Subjects
All six participants from Study 2 were asked to participate in a third MRI scan for Study 3; of these, four (all males) were available to participate. After their scans, participants were re-interviewed with open-ended questions regarding how they experienced the same songs involved in Study 2, as well as notes, chords and other music.
Functional Neuroimaging
Sound Stimuli and fMRI Experimental Design
We were interested in whether brain responses observed in Studies 1 and 2 were specific to musical stimuli or whether other non-musical auditory stimuli could elicit similar responses. Stimuli in Study 3 consisted of (1) musical notes and chords similar to those in Study 2, (2) human non-word vocalizations, and (3) different frequency bands of white noise. All sound stimuli were 2 sec clips, and there were six unique clips per sound condition. The human non-word vocalizations were selected from a library of such sounds used in previous brain imaging work on voice perception (Belin et al., 2000). The white noise clips were created using commercially available sound-editing software (Sound Studio 3.0©) running on a Macintosh© G4 Power PC computer. Each sound was created from white noise that was bandpass-filtered at three different center frequencies: 256, 512 or 1024 Hz. Three of the six sounds consisted of these three bandpass noise samples, and the other three consisted of weighted combinations of these three components. Each sound clip was audibly distinct from the others, none were judged aversive among researchers involved, and all were adjusted to be equal in loudness. The rest condition consisted of 24 sec of silence. During all conditions, visual stimuli consisted of a black background with a white cross, on which subjects were instructed to fixate throughout the functional runs.
Two block design runs (360 sec each) were conducted (see Figure 7). Each sound block consisted of 12 clips of the two-second stimuli (12 × 2 sec = 24 sec per block). Each run consisted of three blocks of notes (3 × 24 sec = 72 sec), three blocks of chords (3 × 24 sec = 72 sec), three blocks of human non-word vocalizations (3 × 24 sec = 72 sec), three blocks of white noise (3 × 24 sec = 72 sec), and three blocks of silent rest (3 × 24 sec = 72 sec). The presentation order of the sound blocks and the presentation order of the stimuli within a block were randomized. A silent rest block followed each repetition of the four sound blocks.
Figure 7. Study 3 block design for a sing run.
Actual order of presentation of sound blocks was randomized.
Statistical Analysis
A fixed-effects GLM with separate study predictors was applied to each subject’s functional MRI data from the sound stimuli experiments. Statistical maps of BOLD activation were generated for each sound condition, and due to the highly constrained nature of our hypothesis to determine whether activations were present within visual cortex, we used a slightly more liberal significance threshold of 0.01. Each subject’s anatomical images from Study 3 were co-registered with those from Study 2, and then functional data were registered with the subject’s high-resolution anatomical images, providing a subject-specific mapping of visual area regions of interest (ROIs) at the voxel level. These ROIs were used for analyzing the auditory condition functional MR images.
Results
In a final study, we assessed whether non-musical auditory stimuli could also elicit activation in areas of the brain involved in visual processing. The same within-subject functional ROIs identified using color localizer and retinotopy scans in Study 2 were used in Study 3. All four subjects showed activation (p < 0.01) to one or more sound conditions in areas that were identified by color localizer, retinotopy and/or anatomy as being visual areas (Table 4). Brain responses to notes and chords were not as consistent as they were in Study 2, perhaps due to lower power (fewer data points) per condition; however, each subject showed activation in visual areas to one or both of these conditions versus silent fixation. Three of four subjects showed activation to the human non-word vocalization condition versus silent fixation (Figure 6). All four subjects showed activation to the white noise condition versus silent fixation (Figure 6). As in Study 2, hypoactivations, indicating lower BOLD response than during silent fixation, were also observed in early visual areas to some auditory conditions in each of the four subjects, as would be more typical of (inhibitory) brain responses to auditory perception tasks (Laurienti et al., 2002).
Table 4. Visual areas are activated by sound conditions in all four WS subjects in Study 3.
BOLD activations (p < 0.01) to contrasts of sound condition versus silent fixation that overlap with color localizer ROIs in each of four subjects in Study 3. “Color-Selective” areas were areas that showed greater response to colored Mondrians than to achromatic Mondrians. These areas included V4v. “Non-Color-Selective” areas were V1 and V2 that were not identified by the color localizer.
| Early Visual Areas | ||||
|---|---|---|---|---|
| Color-Selective | Non-Color-Selective | |||
| Subject | Left H | Right H | Left H | Right H |
| 1 | C | N, C, H, W | N, C, W | N, C, H, W |
| 3 | N†, C†, H†, W† | N†, C, H†, W† | N†, H†, W† | N†, C†, W† |
| 4 | W† | N, W | W | N, W |
| 6 | W | H†, W† | H | H |
Left H = Left Hemisphere; Right H = Right Hemisphere
N=notes; C=chords; H=human non-word vocalizations; W=white noise
Activations significant (p<0.01) according to ROI GLM using intra-subject color localizer ROIs.
DISCUSSION
In this three-part study we examined the neural correlates of the unique auditory and musical phenotype of individuals with WS. Previous work focused on differential activation of the amygdala and temporal lobes in music perception in subjects with WS versus TD controls (Levitin et al., 2003). While we did observe a trend toward weaker but more widespread activation of temporal lobe areas in WS versus TD, these results were not significant in our larger sample using more conservative ROI GLM analyses. Instead, we found compelling evidence that some persons with WS activate occipital and early visual areas in response to musical and other auditory stimuli. These novel findings have implications for current views of cross-modal processing in the general population, and they may help explain the unusually strong attraction to music and sounds often seen in people with WS.
At first glance, these cross-sensory activations of visual cortical areas are reminiscent of brain activations in synesthetic individuals who report seeing colors when listening to musical notes (Rizzo & Eslinger, 1989) or spoken words (Nunn et al., 2002). Indeed, upon repeated interviews, our participants with WS reported vivid, detailed, colorful imagery in response to listening to music, including favorite songs, and these images often contained strong affective connotation (see sample excerpt in Appendix). However, unlike classical synesthesia, subjects with WS did not describe experiencing the exact same visual sensations in response to specific notes, chords, or songs; consistent, repeatable sensory experience is another defining characteristic of synesthesia. In this respect, the visual experiences of the subjects with WS depart from that of the classic synesthesia syndrome.
Nonetheless, the finding that simple notes and chords and two types of non-musical stimuli (chosen because they seemed unlikely to produce visual imagery) activated early visual areas strongly suggests that the responses in WS participants might be stimulus-driven and not—at least not exclusively—the result of top-down feedback or association pathways. Likewise, the fact that all types of auditory stimuli we presented elicited these responses, albeit to varying degrees in different individuals, suggests that they were likely not being filtered for salience and, therefore, might be the result of bottom-up, or automatic, processes. Thus, it perhaps is worthwhile to consider possible common mechanisms that might underlie both synesthesia and the phenomenon we observe in individuals with WS. One mechanism posited by the “cross-activation” theory of synesthesia states that functionally distinct brain regions, such as the auditory and visual cortices, possess aberrant neural connections in individuals with synesthesia, as the result of failure of pruning at some point in development (Maurer, 1993; Baron-Cohen et al., 1993). A second theory posits “disinhibited feedback,” whereby functional segregation of brain regions (due to top-down inhibitory processes that strengthen during the course of development) is unusually weak in individuals with synesthesia (Grossenbacher & Lovelace, 2001). While these theories are often treated as competing, they need not be mutually exclusive; indeed, both of these processes could be at work in the same individual or in a group that shares similar sensory phenomena.
In this vein, growing evidence indicates that cross-modal integration of information from two or more sensory modalities is the norm rather than the exception and that sensory cortical areas are not as isolated from each other as previously thought. Both animal and human studies suggest that we all have, to some degree, feedback pathways from areas of the brain that respond to multiple sensory modalities (e.g., auditory and visual) (Calvert et al., 1999; Driver & Spence, 2000; Macaluso et al., 2001). At least one study found that synesthetic experience exploits the same neural connections that enable cross-modal mechanisms, suggesting that these pathways are not privileged to synesthetes (Ward et al., 2006). These shared neural connections lend support for the disinhibited feedback (reduced top-down inhibition) theory of synesthesia playing a role in the unique auditory-visual phenomenon observed in participants with WS.
Our analysis of the underlying distribution of visual activations to sound (Figure 3) lends further support to the idea that cross-modal processing might not be unique to synesthetes but that there is a wide distribution of ability and that individuals with synesthesia are at one end of the spectrum and that, on average, individuals with WS are also near the end of that spectrum but to a lesser degree than synesthetes. Activations that extended beyond the boundaries of visual areas mapped by retinotopy and color localizer might also implicate association pathways. While the literature on auditory processing in TD does not include reports of visual activation to auditory stimuli, most studies simply do not look for them and possibly dismiss them as spurious when they are found, since they do not conform to the dogma that sensory systems are “hard-wired” and functionally distinct. The literature on auditory processing in persons with congenital blindness, however, shows extensive plasticity of sensory cortices and the recruitment of occipital lobe areas for the processing of auditory stimuli (Collignon et al., 2009; Hertrich et al., 2009).
Although under some debate, a developmental approach suggests that multisensory processing, and perhaps synesthesia, might be the norm in infancy, with perceptual systems becoming more specialized throughout development (Maurer, 1993; Baron-Cohen et al., 1993; Baron-Cohen et al., 1996; Harrison, 2001). It is not clear what developmental features in WS might contribute to altered sensory processing, but one study implicates aberrant neurotrophin nerve growth factor (NGF) levels. Calamandrei et al. (2000) examined hyperacusis and other auditory abnormalities in WS and found that while NGF levels were elevated in typically-developing children from 2 to 6 years of age, individuals with WS had high NGF levels from 2 to 20 years of age, more than 4 times as long as in controls (Calamandrei et al., 2000). This extended window of prolonged, high NGF could be responsible, in part, for the abnormal development of cortical regions and/or the white matter tracts that connect them. This would lend support for the cross-activation (reduced pruning) theory of synesthesia playing a role in the unique visual experience to sound in individuals with WS.
Preliminary findings from diffusion tensor imaging (DTI) studies shed some light on altered fiber tracts in WS. Marenco et al. recently used DTI to examine white matter architecture in high-functioning WS adults and IQ-matched typical controls (Marenco et al., 2007). Subjects with WS showed increases in longitudinal tracts, coursing along the anterior-posterior axis, and decreases in transverse fibers, coursing right-to-left. Further, compared to controls, the longitudinal fiber tracts in those with WS, including the inferior longitudinal fasciculus (ILF) that connects the temporal and occipital lobes, had increased fiber coherence (anisotropy), skewness and lattice index values. Also, fiber tracts of WS subjects diverged from those of controls at the junction between the medial temporal and occipital lobes. Hoeft et al. (2007) also found evidence of increased fractional anisotropy (FA) in the ILF, particularly in the right ILF, which the authors suggest might be related to relative strengths in face recognition (Hoeft et al., 2007). Marenco et al. speculate that these findings might be related to the relatively spared verbal abilities in WS (Marenco et al., 2007). Although it is unclear whether these findings will generalize to a WS cohort with more variable IQs, DTI findings may also relate to the unique visual activations to auditory stimuli we are reporting here.
Future studies also should investigate the genetic basis of this unusual cross-sensory ‘hyperconnectivity’ in WS. Almost all persons with WS have the same chromosomal microdeletion, with the same breakpoints; however, the identification of atypical cases of WS with shorter or longer deletions, translocations, or inversions has aided in the ongoing attempts to map specific genes to particular parts of the WS phenotype, including cardiac defects, visuospatial deficits, hypersociability and intellectual disability, in general (Bellugi et al., 1999; Tassabehji et al., 1999; Borg et al., 1995; Frangiskakis et al., 1996; Gray et al., 2006; Morris et al., 2003; Tassabehji et al., 2005; Morris et al., 2003; Doyle et al., 2004; Young et al., 2007). Thus far, no genes in the chromosome 7 deletion region have been associated with differences in auditory perception, musicality or affective response to music. Within group variability in the cross-modal connectivity or musical phenotype of persons with WS could be due to other genetic factors or environmental exposures, such as musical training, neither of which has been fully explored in the literature.
Despite the challenge of recruiting adequate numbers of participants with rare disorders such as WS, Study 1 included a sample size (13 with WS; 13 controls) that represents the largest fMRI study to date on auditory processing in WS. Future studies should strive to increase these numbers further and should include a wider range of auditory stimuli, including sounds with positive or negative emotional valence or those are encountered in everyday life.
The choice of an appropriate control group and matching criteria is very important and often controversial. In the current study, we were primarily interested in understanding how individuals with WS differ from those with TD. However, it would also be interesting to now investigate these same phenomena in other neurodevelopmental groups, such as Down syndrome or autism, whose neuropsychological profiles are very different from that of WS. Given the wide range of intellectual disability in our WS group and our choice to use TD controls, we did not try match subjects on mental age. Hence, intellectual ability is a potential confounder in this study. Finally, although we did not find evidence for a relationship between musicality and cross-modal activity, perhaps other musically-enriched samples such as professional musicians or individuals with perfect pitch would show evidence of cross-modal processing similar to that seen in WS. Ideally, future studies would try to control for musical interest (regardless of talent), as well as for musical talent.
This study is among the first to examine brain responses to music and auditory stimuli in WS. Musicality remains a less-well understood but prominent feature of the WS behavioral phenotype, and findings from this three-part study have led to new hypotheses regarding multi-sensory processing in this population. Not all persons with WS have a passion for music, and it is unclear if only certain subsets of those with WS respond to auditory stimuli with visual activation. Our ongoing analyses of structural and functional connectivity, especially connections between auditory and visual cortical regions are promising ways to shed light on this question.
ACKNOWLEDGEMENTS
The authors thank the many individuals with Williams Syndrome and their families for their participation in this research. The authors gratefully acknowledge the efforts of Elizabeth Roof, Rebecca Kossler and Elizabeth Pantino in organizing and helping conduct the fMRI scans for this project, the statistical consultation provided by Drs. Baxter Rogers and Jennifer Blackford and the careful reading of an earlier draft of the manuscript by Drs. Blackford, Nicole Davis and Pat Levitt. This work has been funded in part by the National Institutes of Health through the NIH Roadmap for Medical Research (T32 MH075883), the National Institute of Child Health and Development (P30 HD15052), the National Eye Institute (R03 EY014437), and a Vanderbilt University Discovery Grant.
Appendix
Sample excerpt from participant interview after Study 2
The following is a sample excerpt from one of the WS participant interviews about their experience listening to music. I: interviewer. P: participant.
I: ‘She’s gonna make it’ by Garth Brooks. Did you see anything when you heard that song?
P: Mountain…with stairs on the mountain.
I: So tell me about the mountain. Did it have a color?
P: Orange.
I: All right. So the stairs, what did the stairs look like?
P: Like an escalator.
I: Anything else beside the mountain and the stairs?
P: It had roses on the steps.
I: What color were they?
P: Red.
I: They were red? Anything else can you tell me?
P: It was a wedding type thing.
I: Any people?
P: Just the bride and the bridesmaids were going upstairs.
I: What was the bride wearing?
P: She was wearing a white gown with a silver necklace with a diamond shape
I: What was the diamond shape?
P: The necklace. And she was wearing sandals.
I: Sandals?
P: Yeah, this is kinda weird.
I: So tell me about her sandals.
P: They were crocs.
I: Did the crocs have a color?
P: White, so they could match
I: At least she was matching.
P: Yeah.
References
- Achenbach TM. Manual for the Child Behavior Checklist. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. Ref Type: Generic. [Google Scholar]
- Baron-Cohen S, Burt L, Smith-Laittan F, Harrison J, Bolton P. Synaesthesia: Prevalence and familiarity. Perception. 1996;25:1073–1079. doi: 10.1068/p251073. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Harrison J, Goldstein LH, Wyke M. Coloured speech perception: Is synaesthesia what happens when modularity breaks down? Perception. 1993;22:419–426. doi: 10.1068/p220419. [DOI] [PubMed] [Google Scholar]
- Bayes M, Magano LF, Rivera N, Flores R, Perez Jurado LA. Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum.Genet. 2003;73:131–151. doi: 10.1086/376565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P, Zatorre R, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Mills D, Galaburda A, Korenberg JR. Bridging cognition, the brain and molecular genetics: evidence from Williams syndrome. Trends Neurosci. 1999;22:197–207. doi: 10.1016/s0166-2236(99)01397-1. [DOI] [PubMed] [Google Scholar]
- Blomberg S, Rosander M, Andersson G. Fears, hyperacusis and musicality in Williams syndrome. Research in Developmental Disabilities. 2006;27:668–680. doi: 10.1016/j.ridd.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Borg I, Delhanty JD, Baraitser M. Detection of hemizygosity at the elastin locus by FISH analysis as a diagnostic test in both classical and atypical cases of Williams syndrome. J.Med.Genet. 1995;32:692–696. doi: 10.1136/jmg.32.9.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamandrei G, Alleva E, Cirulli F, Queyras A, Volterra V, Capirci O, et al. Serum NGF levels in children and adolescents with either Williams syndrome or Down syndrome. Dev.Med.Child Neurol. 2000;42:746–750. doi: 10.1017/s0012162200001389. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Brammer MJ, Bullmore ET, Campbell R, Iversen SD, David AS. Response amplification in sensory-specific cortices during crossmodal binding. Neuroreport. 1999;10:2619–2623. doi: 10.1097/00001756-199908200-00033. [DOI] [PubMed] [Google Scholar]
- Collignon O, Voss P, Lassonde M, Lepore F. Cross-modal plasticity for the spatial processing of sounds in visually deprived subjects. Exp.Brain Res. 2009;192:343–358. doi: 10.1007/s00221-008-1553-z. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software tools for analysis and visualization of FMRI Data. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, et al. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc.Natl.Acad Sci.U.S.A. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TF, Bellugi U, Korenberg JR, Graham J. "Everybody in the world is my friend" hypersociability in young children with Williams syndrome. Am.J.Med.Genet.A. 2004;124:263–273. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- Driver J, Spence C. Multisensory perception: beyond modularity and convergence. Curr.Biol. 2000;10:R731–R735. doi: 10.1016/s0960-9822(00)00740-5. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Anxiety, fears, and phobias in persons with Williams syndrome. Dev.Neuropsychol. 2003;23:291–316. doi: 10.1080/87565641.2003.9651896. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Rosner BA. Refining behavioral phenotypes: personality-motivation in Williams and Prader-Willi syndromes. Am.J.Ment.Retard. 1999;104:158–169. doi: 10.1352/0895-8017(1999)104<0158:RBPPIW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Rosner BA, Ly T, Sagun J. Music and anxiety in Williams syndrome: a harmonious or discordant relationship? Am J Ment.Retard. 2005;110:346–358. doi: 10.1352/0895-8017(2005)110[346:MAAIWS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb.Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Frangiskakis JM, Ewart AK, Morris CA, Mervis CB, Bertrand J, Robinson BF, et al. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell. 1996;86:59–69. doi: 10.1016/s0092-8674(00)80077-x. [DOI] [PubMed] [Google Scholar]
- Gaser C, Luders E, Thompson PM, Lee AD, Dutton RA, Geaga JA, et al. Increased local gyrification mapped in Williams syndrome. Neuroimage. 2006;33:46–54. doi: 10.1016/j.neuroimage.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Gray V, Karmiloff-Smith A, Funnell E, Tassabehji M. In-depth analysis of spatial cognition in Williams syndrome: A critical assessment of the role of the LIMK1 gene. Neuropsychologia. 2006;44:679–685. doi: 10.1016/j.neuropsychologia.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Grossenbacher PG, Lovelace CT. Mechanisms of synesthesia: cognitive and physiological constraints. Trends Cogn Sci. 2001;5:36–41. doi: 10.1016/s1364-6613(00)01571-0. [DOI] [PubMed] [Google Scholar]
- Harrison J. Synaesthesia: The Strangest Thing. Oxford: Oxford University Press; 2001. [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, Moos A, Trouvain J, Ackermann H. Enhanced speech perception capabilities in a blind listener are associated with activation of fusiform gyrus and primary visual cortex. Neurocase. 2009;15:163–170. doi: 10.1080/13554790802709054. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, et al. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci. 2007;27:11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, ffytche DH, Barnes J, McKeefry D, Ha Y, Woodruff PW, et al. The functional anatomy of imagining and perceiving colour. Neuroreport. 1998;9:1019–1023. doi: 10.1097/00001756-199804200-00012. [DOI] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, Morris CA, Kohn P, Meyer-Lindenberg A, et al. Genetic contributions to human gyrification: sulcal morphometry in Williams syndrome. J.Neurosci. 2005;25:7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AJ, Armstrong BL, Greer MK, Brown FR., III Hyperacusis and otitis media in individuals with Williams syndrome. J Speech Hear.Disord. 1990;55:339–344. doi: 10.1044/jshd.5502.339. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman BP, Mervis CB. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev.Neuropsychol. 2003;23:269–290. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Wallace MT, Yen YF, Field AS, Stein BE. Deactivation of sensory-specific cortex by cross-modal stimuli. J Cogn Neurosci. 2002;14:420–429. doi: 10.1162/089892902317361930. [DOI] [PubMed] [Google Scholar]
- Levitin DJ. Musical behavior in a neurogenetic developmental disorder: evidence from Williams Syndrome. Ann.N.Y.Acad.Sci. 2005;1060:325–334. doi: 10.1196/annals.1360.027. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Cole K, Chiles M, Lai Z, Lincoln A, Bellugi U. Characterizing the musical phenotype in individuals with Williams Syndrome. Child Neuropsychol. 2004;10:223–247. doi: 10.1080/09297040490909288. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Cole K, Lincoln A, Bellugi U. Aversion, awareness, and attraction: investigating claims of hyperacusis in the Williams syndrome phenotype. J.Child Psychol.Psychiatry. 2005;46:514–523. doi: 10.1111/j.1469-7610.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Menon V, Schmitt JE, Eliez S, White CD, Glover GH, et al. Neural correlates of auditory perception in Williams syndrome: an fMRI study. Neuroimage. 2003;18:74–82. doi: 10.1006/nimg.2002.1297. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Allison JD, Pillai JJ, Lavin T, Lee GP, et al. Now you see it, now you don't: statistical and methodological considerations in fMRI. Epilepsy Behav. 2002;3:539–547. doi: 10.1016/s1525-5050(02)00558-9. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science. 2001;289:1206–1208. doi: 10.1126/science.289.5482.1206. [DOI] [PubMed] [Google Scholar]
- Marenco S, Siuta MA, Kippenhan JS, Grodofsky S, Chang WL, Kohn P, et al. Genetic contributions to white matter architecture revealed by diffusion tensor imaging in Williams syndrome. Proc.Natl.Acad Sci.U.S.A. 2007;104:15117–15122. doi: 10.1073/pnas.0704311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D. Neonatal synaesthesia: Implications for the processing of speech and faces. In: de Boysson-Bardies B, de Schonen S, Jusczyk P, McNeilage P, Morton J, editors. Developmental neurocognition: Speech and face processing in the first year of life. Dordrecht: Kluwer; 1993. [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat.Neurosci. 2005a;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, et al. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Sarpal D, Koch P, Steele S, Kohn P, et al. Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. J.Clin.Invest. 2005b;115:1888–1895. doi: 10.1172/JCI24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Eckert MA, Mills D, Korenberg J, Bellugi U, Galaburda AM, et al. Frontostriatal Dysfunction During Response Inhibition in Williams Syndrome. Biol.Psychiatry. 2007;62:256–261. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Garrett AS, Menon V, Rose FE, Bellugi U, Reiss AL. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;62:2070–2076. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- Morris CA, Mervis CB, Hobart HH, Gregg RG, Bertrand J, Ensing GJ, et al. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am.J.Med.Genet.A. 2003;123:45–59. doi: 10.1002/ajmg.a.20496. [DOI] [PubMed] [Google Scholar]
- Muckli L, Kohler A, Kriegeskorte N, Singer W. Primary visual cortex activity along the apparent-motion trace reflects illusory perception. PLoS.Biol. 2005;3:e265. doi: 10.1371/journal.pbio.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam A, Samuel PR. Hyperacusis and Williams syndrome. J Laryngol.Otol. 1994;108:494–496. doi: 10.1017/s0022215100127203. [DOI] [PubMed] [Google Scholar]
- Nunn JA, Gregory LJ, Brammer M, Williams SC, Parslow DM, Morgan MJ, et al. Functional magnetic resonance imaging of synesthesia: activation of V4/V8 by spoken words. Nat.Neurosci. 2002;5:371–375. doi: 10.1038/nn818. [DOI] [PubMed] [Google Scholar]
- Reilly J, Losh M, Bellugi U, Wulfeck B. "Frog, where are you?" Narratives in children with specific language impairment, early focal brain injury, and Williams syndrome. Brain Lang. 2004;88:229–247. doi: 10.1016/S0093-934X(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, et al. IV. Neuroanatomy of Williams syndrome: a high-resolution MRI study. J.Cogn Neurosci. 2000;12 Suppl 1:65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Eslinger PJ. Colored hearing synesthesia: an investigation of neural factors. Neurology. 1989;39:781–784. doi: 10.1212/wnl.39.6.781. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Eliez S, Warsofsky IS, Bellugi U, Reiss AL. Corpus callosum morphology of Williams syndrome: relation to genetics and behavior. Dev.Med.Child Neurol. 2001;43:155–159. [PubMed] [Google Scholar]
- Sereno MI, McDonald CT, Allman JM. Analysis of retinotopic maps in extrastriate cortex. Cereb.Cortex. 1994;4:601–620. doi: 10.1093/cercor/4.6.601. [DOI] [PubMed] [Google Scholar]
- Stromme P, Bjornstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J.Child Neurol. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Hammond P, Karmiloff-Smith A, Thompson P, Thorgeirsson SS, Durkin ME, et al. GTF2IRD1 in craniofacial development of humans and mice. Science. 2005;310:1184–1187. doi: 10.1126/science.1116142. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Karmiloff-Smith A, Carette MJ, Grant J, Dennis N, et al. Williams syndrome: use of chromosomal microdeletions as a tool to dissect cognitive and physical phenotypes. Am.J.Hum.Genet. 1999;64:118–125. doi: 10.1086/302214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Eckert MA, et al. Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J.Neurosci. 2005;25:4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PP, Doherty S, Hesselink JR, Bellugi U. Callosal morphology concurs with neurobehavioral and neuropathological findings in two neurodevelopmental disorders. Arch.Neurol. 1992;49:407–411. doi: 10.1001/archneur.1992.00530280101029. [DOI] [PubMed] [Google Scholar]
- Ward J, Huckstep B, Tsakanikos E. Sound-colour synaesthesia: to what extent does it use cross-modal mechanisms common to us all? Cortex. 2006;42:264–280. doi: 10.1016/s0010-9452(08)70352-6. [DOI] [PubMed] [Google Scholar]
- Young EJ, Lipina T, Tam E, Mandel A, Clapcote SJ, Bechard AR, et al. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 2007;7:224–234. doi: 10.1111/j.1601-183X.2007.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]