Abstract
Context
Imaging and post-mortem studies provide converging evidence that patients with schizophrenia have a dysregulated developmental trajectory of frontal lobe myelination. The hypothesis that typical and atypical medications may differentially impact brain myelination in adults with schizophrenia was previously assessed with inversion recovery (IR) images. Increased white matter (WM) volume suggestive of increased myelination was detected in the patient group treated with an atypical antipsychotic compared to a typical one.
Objective
In a follow-up reanalysis of MRI images from the original study, we used a novel method to assess whether the difference in WM volumes could be caused by a differential effect of medications on the intracortical myelination process.
Design, setting, and participants
Two different male cohorts of healthy controls ranging in age from 18–35 years were compared to cohorts of subjects with schizophrenia who were treated with either oral risperidone (Ris) or fluphenazine decanoate (Fd).
Main outcome measure
A novel MRI method that combines the distinct tissue contrasts provided by IR and proton density (PD) images was used to estimate intracortical myelin (ICM) volume.
Results
When compared with their pooled healthy control comparison group, the two groups of schizophrenic patients differed in the frontal lobe ICM measure with the Ris group having significantly higher volume.
Conclusions
The data suggest that in adults with schizophrenia antipsychotic treatment choice may be specifically and differentially impacting later-myelinating intracortical circuitry. In vivo MRI can be used to dissect subtle differences in brain tissue characteristics and thus help clarify the effect of pharmacologic treatments on developmental and pathologic processes.
Keywords: Schizophrenia, second generation antipsychotic medication, atypical, intracortical myelin, white matter, gray matter, oligodendrocyte, trajectory, development, lipid, age, prevention
INTRODUCTION
The extensive process of myelination increases the human brain’s capacity to process information and underlies many of our unique behavioral and cognitive capabilities such as language (Bartzokis, 2004a; Bartzokis, 2005). The human brain is unique in its disproportionately high myelin content and long developmental (myelination) phase that extends until middle age when maximal myelinated white matter (WM) volumes are reached in frontal lobes and association areas (Allen et al, 2005; Bartzokis et al, 2001; Benes et al, 1994; Ge et al, 2002; Jernigan and Gamst, 2005; Kemper, 1994; Miller et al, 1980; Sowell et al, 2003; Walhovd et al, 2005; Yakovlev and Lecours, 1967; reviewed in Bartzokis, 2009) (Figure 1).
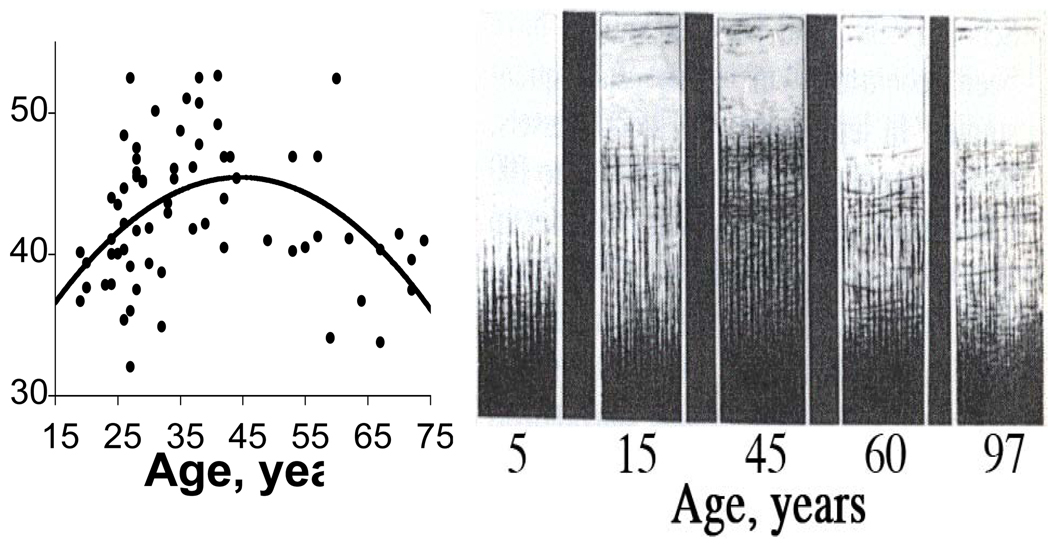
Figure 1. Quadratic (inverted U) trajectories of human brain myelination over the lifespan.
Myelination (Y axis) versus age (X axis) in frontal lobes of normal individuals. Left panel is in vivo data from Bartzokis et al (2001). Right panel shows post-mortem intracortical myelin stain data from Kaes (1907) adapted and reproduced in Kemper (1994) depicting the heavy myelination of the lower cortical layers. Used with permission. The data were acquired 100 years apart yet the two samples of normal individuals show remarkably similar frontal lobe myelination trajectories, both reaching a peak at age 45.
The vulnerability of the myelination process likely also contributes to the unique susceptibility of the human brain to highly prevalent disorders of development (e.g., schizophrenia, autism, ADHD, bipolar disorder) (Bartzokis, 2002; Bartzokis, 2004b; Bartzokis, 2005). A dysregulation in this developmental process is hypothesized to result in an insufficient capacity to maintain temporal synchrony of the brain’s widely distributed functional neural networks and manifests in the heterogeneity of symptoms and cognitive impairments that characterize disorders such as schizophrenia (Bartzokis, 2002; Bartzokis, 2005; Dwork et al, 2007; Spencer et al, 2004; van der Stelt et al, 2004)
Myelin has the highest cholesterol content of any brain tissue (O'Brien and Sampson, 1965; Rouser et al, 1972; Saher et al, 2005). Inversion-recovery (IR) MRI images are most sensitive to the high cholesterol concentrations in myelin (Koenig, 1991), and are optimal for quantifying myelination (Barkovich et al, 1992; Valk and van der Knaap, 1989; van der Knaap and Valk, 1990). There is excellent agreement between the lifetime myelination trajectory of normal individuals observed in vivo with IR sequences and published post-mortem data (Bartzokis, 2005; Bartzokis, 2009; Bartzokis et al, 2001). In frontal lobes peak myelination is reached at age 45 as measured by both this in vivo and post-mortem myelin stain data (Bartzokis et al, 2001; Kemper, 1994) (Figure 1). This close agreement with post-mortem data validates in vivo IR volume measures and suggests that IR sequences likely track what may be better referred to as “myelinated WM volume” that includes the heavily myelinated lower layers of cortex (Bartzokis et al, 2001; Bartzokis et al, 2007; Bartzokis et al, 2003b) (Figure 1). For simplicity this measure will herein be referred to as WM.
Atypical (also referred to as second generation) antipsychotics have been shown to be particularly efficacious in some treatment-resistant cases of schizophrenia and show a wide spectrum of efficacy across multiple psychiatric diseases. We hypothesized that in adults with schizophrenia, exposure to atypical antipsychotics may differentially promote brain myelination compared to exposure to typical antipsychotics (Bartzokis, 2002; Bartzokis, 2005; Bartzokis and Altshuler, 2003; Bartzokis et al, 2003b). Using available data, we previously tested and reported on the possibility that when compared to Fd, treatment with Ris was associated with increased myelination that on IR images manifests as increased WM and decreased gray matter (GM) volumes (Bartzokis et al, 2007).
As depicted in Figure 1 (left panel), the human frontal lobe undergoes extensive intracortical myelination that does not peak until middle age. In the current study we used the different contrasts available from our imaging protocol to measure whether the medication effect on WM observed in our prior study (Bartzokis et al, 2007) was driven by an effect on this later-developing intracortical myelination process.
METHODS
Subjects
Two male cohorts between age 18 and 35 were recruited from longitudinal studies of schizophrenia conducted at the UCLA Aftercare Research Program (Nuechterlein et al, 1992). All subjects received written and oral information about the study and signed written informed consents approved by the local Institutional Review Board prior to study participation.
All subjects with schizophrenia had a DSM-IV diagnosis of schizophrenia and 13% of each cohort had a diagnosis of schizoaffective (depressed type) disorder, which was established using structured diagnostic interviews by diagnosticians with demonstrated inter-rater reliability (Nuechterlein et al, 1994; Nuechterlein et al, 2001). Patients with significant substance abuse or history of neurological disorders or significant head trauma were excluded (see Nuechterlein et al, 1994).
At a minimum, each healthy subject (all males) completed a clinical interview based on written standardized questions and administered by an experienced clinician-investigator (GB) to assess the history of medical, psychiatric, and substance dependence disorders. The MRI scans were obtained after diagnosis, early in the course of treatment (Table 1), and at variable times in relation to clinical assessments. Selection criteria were as follows: no evidence of significant current or past psychiatric diagnosis or substance dependence based on DSM-IV criteria; no significant use of drugs or alcohol in the past year (amount of use did not meet DSM-IV criteria for alcohol/substance dependence or abuse); no history or gross evidence of central nervous system impairment or any history of neurological disorder (head trauma with loss of consciousness for greater than 15 minutes), no history of chronic medical conditions that are likely to result in structural brain abnormalities (i.e., stroke, transient ischemic attack, seizures, hypertension, diabetes, etc.); and self-report that no first-degree relatives have been treated for a major psychiatric disorder.
Table 1.
Characteristics for Schizophrenic and Healthy Control Cohorts
| Schizophrenia | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| First Cohort (N=34) |
Second Cohort (N=16) |
First Cohort (N=17) |
Second Cohort (N=26) |
|||||
| Demographics | M (sd) | M (sd) | M (sd) | M (sd) | F or χ2 |
p | ||
| Age | 27.1 (5.1) | 23.6 (4.3) | 24.9 (4.1) | 27.2 (4.5) | 2.97 | .036 | ||
| Education | 12.6 (1.5) | 13.3 (1.7) | 14.2 (2.4) | 15.9 (2.1) | 15.55 | <.0001 | ||
| Race, white/non-white | 26/8 | 7/9 | 15/2 | 17/9 | 8.86 | .031 | ||
| Illness Information | t | p | ||||||
| Age at onset (yrs.) | 23.3 (4.1) | 22.7 (4.3) | 0.44 | .66 | ||||
| Medication exposure (yrs.) | 3.5 (3.6) | 1.1 (0.8) | 3.64 | .0008 | ||||
The first cohort of schizophrenic and healthy control subjects was originally imaged before the introduction of atypical antipsychotics. This sample consisted of 51 schizophrenic subjects treated only with fluphenazine decanoate (Fd) (Nuechterlein et al, 1992) and 23 healthy controls. The dosage was adjusted based on clinical need with the target dose of 12.5 mg IM every two weeks; this was achieved by over 50% of subjects with the vast majority of the remainder receiving half that dose. The second cohort of similarly recruited groups was imaged after the introduction of risperidone (Ris), which then became the first line antipsychotic in the Aftercare Research Program protocol. This second sample consisted of 20 schizophrenic subjects treated with Ris and 38 healthy controls. Their dosage was also adjusted based on clinical need with the 3–6 mg per day target range achieved by the vast majority. Of these 132 subjects, 38 did not show a sufficiently bimodal histogram on the PD image to reliably use the histogram-based gray/white matter separation protocol and were eliminated from further analysis. Another subject had marked movement on MRI and was also removed from analysis. Demographics and clinical information for the remaining evaluable subjects are presented in Table 1.
Even though an ANOVA revealed an overall group difference in age, post-hoc analysis using the Scheffe test did not detect any significant differences between groups. However, post-hoc analysis did indicate that the healthy control group from the second cohort had a significantly greater mean number of years of education than the control group from the first cohort and the schizophrenic cohorts. The second cohort of schizophrenia patients also had significantly fewer years of medication exposure at the time of the MRI scan than the first cohort.
Imaging
The two cohorts were scanned using the same MRI techniques, MRI instrument type, and field strength (1.5 Tesla) but each cohort was scanned with a different instrument (Bartzokis et al, 2007).
MRI Protocol
The MRI examination used our previously published methods (Bartzokis et al, 2001; Bartzokis et al, 2007; Bartzokis et al, 1993). In brief, a coronal pilot sequence was used to align a sagittal MRI pilot sequence. The sagittal pilot sequence was then used to specify the position of the coronal image acquisition grid. The sagittal image containing the left hippocampus was used to define an oblique coronal acquisition plane perpendicular to the hippocampus. Two coronal sequences of the same brain slices were acquired: a transverse asymmetric dual spin-echo Carr-Purcell-Meiboom-Gill sequence (TR=2500, TE=30,90) and an inversion-recovery (IR) sequence (TR=2500, TI=600, TE=30).Both sequences have 256 × 192 view matrix, 25 cm field of view, 2 repetitions, and produce co-registered 3 mm thick contiguous slices. Inversion recovery sequences provide the best gray/white contrast available with MRI, especially compared to proton density (PD) sequences (see Figure 2).
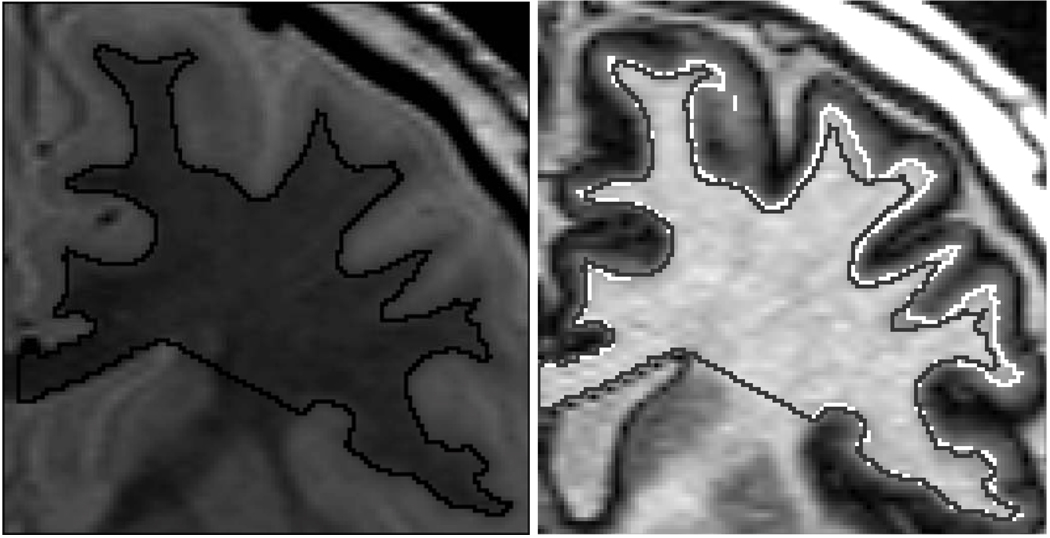
Figure 2. In Vivo Measure of Frontal Lobe Intracortical Myelin Volume.
Left: Proton density (PD) image that is not sensitive to the cholesterol in myelin. The black region of interest (ROI) line depicts the border between the gray and white matter (WM). This same gray/white separation line is depicted in the image on the right as the gray line inside the white line.
Right: Inversion recovery (IR) Image of the same slice of brain as in the PD image on the left (both images obtained sequentially in the same imaging session). The IR image optimally detects the high cholesterol in myelin and is used to obtain the “myelinated WM volume” that includes heavily myelinated parts of the deeper portions of gray matter (see Figure 1). The white ROI line separates myelinated WM and unmyelinated portion of gray matter. The difference between the gray and white lines is the measure of intracortical myelination.
Image Analysis
All images were initially assessed and blindly rated for whether the protocol could be carried out using both the IR and PD contrast. The 93 scans that qualified were analyzed in random order by a single rater who was blind to clinical and demographic characteristics of subjects. The brain/CSF boundary in the frontal lobe was traced using the contrast of a calculated T2 image (Bartzokis et al, 1993). The resultant brain ROI was pasted onto both the inversion recovery (IR) image and PD image. Using the IR image, myelinated WM volume in the frontal lobe was measured using a histogram-based separation of the bimodal distribution of pixel intensities of white and gray matter (Bartzokis et al, 2007; Bartzokis et al, 1993). On the PD image, the gray matter histogram peak was eliminated using the shrink function to produce an ROI containing only WM intensity pixels. The resulting PD ROI was pasted onto the co-registered TE=90 image to better visualize the boundary of the brain’s gyri and sulci. The PD ROI was then manually corrected so that the only permitted difference between the IR and PD ROIs was along the cortical white/gray matter boundary (Figure 2)
A contiguous seven-slice volume centered on the anterior commissure was used for data quantification (Bartzokis et al, 1993). Volumes were computed by multiplying each cross-sectional area by the slice thickness and summing these products. Previously published test-retest reliabilities for the regions of interest had an intraclass reliability coefficient (rxx) of .86 for total frontal lobe volume and .90 for WM (Bartzokis et al, 1993).
Data analyses
It is known that age has a nonlinear (quadratic) relationship with WM of healthy males in variables spanning a wider age range (Bartzokis et al, 2001) (Figure 1), but the age range of our sample made a linear aging model appropriate for all the variables studied as the peak of WM development in males are well above (by a decade) the maximum 35 years of age of our study samples. Different MRI instruments were used (one for each cohort) in the study and therefore it is possible that group differences can be generated by the difference in the instruments. We statistically adjusted for any such global instrument differences by using the respective healthy control group that was scanned with that patient cohort. MRI instrument effects were estimated by regressing the variables of interest on Study (a dummy coded dichotomous variable) in the samples of healthy subjects in the two projects (N=17 and 26). The model assumed parallel age slopes of the healthy cohorts on the two instruments. Age, education, and ethnicity (white versus non-white) were also included as covariates in the regression models to control for possible differences in the samples attributable to those variables. The covariate-adjusted instrument means were then subtracted from the respective raw scores. Finally, the scores were divided by the within-study standard deviation, again calculated using the healthy controls. The procedure yielded standard scores in the healthy control samples with mean of zero and standard deviation of one for all variables. We then evaluated differences between the two Study samples of schizophrenia patients (Study 1: fluphenazine, N=34; Study 2: risperidone, N=16) in separate analyses of covariance on the four residual scores (total frontal, WM, gray matter, and intracortical myelination (ICM), defined as the volume difference between IR and PD images). The analysis used Study as the independent variable, and controlled for age, ethnicity (coded as above), education, and years of medication exposure, using a square root transformation on years of medication exposure to correct positive skew. In addition to comparing the two Study groups (the notable difference of interest between studies was the typical versus atypical antipsychotic medication used), we also tested the group mean scores against zero (the value in the study normal controls).
RESULTS
The results are summarized in Table 2 and Figure 3. The table presents covariate-adjusted means and standard deviations (SD) on which the statistical tests are based. Note that a similar table for healthy controls would have mean=0 and SD=1 in each cell.
Table 2.
Covariate-adjusted mean (SD) scores in subjects with schizophreniaa
| Region | Study 1 (Fluphenazine) N=34 |
Study 2 (Risperidone) N=16 |
t | p |
|---|---|---|---|---|
| Frontal lobe | −0.49 (0.89)** | −0.17 (1.11) | −1.03 | 0.308 |
| Frontal white volume – IR | −0.07 (1.19) | 0.76 (1.15) | −2.40 | 0.021 |
| Frontal gray volume – IR | −0.70 (0.80)*** | −0.90 (1.07)** | 0.64 | 0.526 |
| Frontal white volume – PD | −0.23 (1.13) | 0.09 (1.04) | −0.96 | 0.342 |
| Frontal gray volume – PD | −0.54 (0.92)** | −0.34 (0.97) | −0.55 | 0.584 |
| Frontal ICM | 0.26 (0.96)* | 0.92 (0.84)* | −2.14 | 0.038 |
The t-test results are between-group comparisons; all df = 44. Within-cell t-tests to contrast with healthy controls were based on the covariance-adjusted means.
p<.05;
p<.01;
p<.001
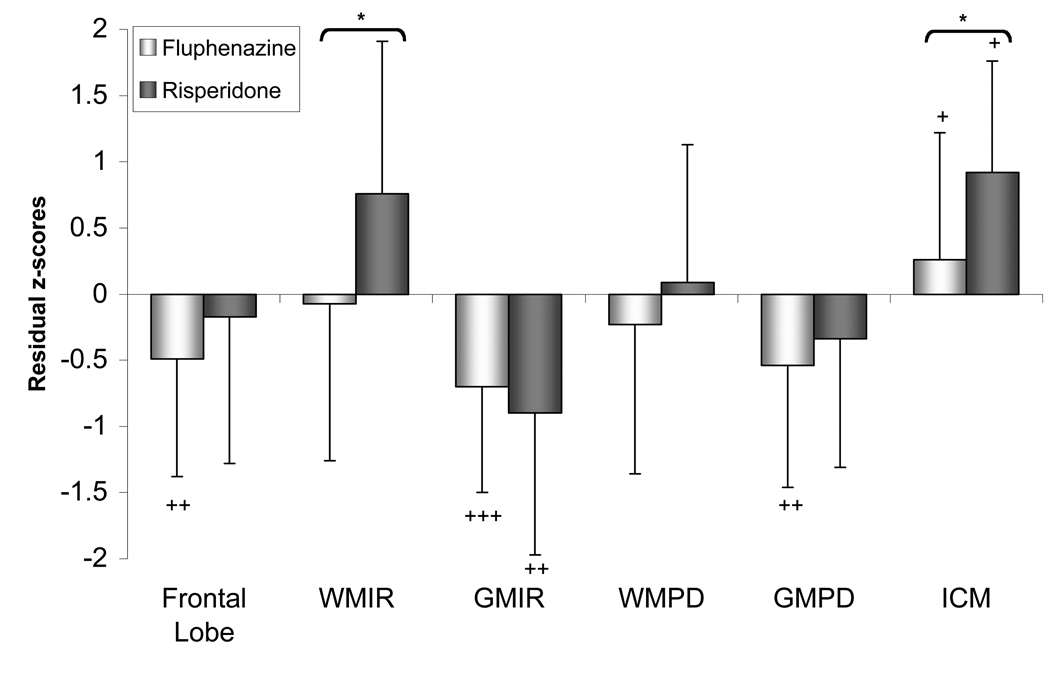
Figure 3. Frontal Lobe Volume Residual z-scores in Male Subjects with Schizophrenia Receiving Either a Typical or Atypical Antipsychotic Medication.
Frontal lobe = total frontal lobe (white plus gray matter), WMIR = frontal lobe white matter volume measured on IR images, GMIR = frontal lobe gray matter volume measured on IR images, WMPD = frontal lobe white matter volume measured on PD images, GMPD = frontal lobe gray matter volume measured on PD images; ICM = intracortical myelination.
Between group tests (risperidone vs. fluphenazine): *p<0.05.
Within group tests (schizophrenic vs. healthy controls, standardized to mean = 0 and SD = 1): +p < 0.05, ++p < 0.01, +++p < 0.001.
The data resulting from the IR images are essentially the same as those published in our prior study (Bartzokis et al, 2007) despite the reduced samples of the current analysis. The IR-derived myelinated WM volume was significantly different between the two medication groups with the Ris group having significantly more WM than the Fd group. The two groups of patients differed from healthy controls in opposite directions: the Ris group had increased WM volume while the Fd group had decreased WM volume relative to the control group, although the differences did not reach statistical significance (Figure 3). The overall size of the frontal lobes did not differ between the Ris- and Fd-treated groups although the total frontal lobe measure of the Fd group was significantly smaller than the normal controls. Both groups of subjects with schizophrenia had significantly lower GM volumes than the healthy control group.
The PD image analysis could not differentiate between the two medication groups in either white or gray matter volume. The GM volume of the Fd-treated schizophrenic group was significantly smaller when compared to normal controls (Figure 3).
The ICM measure differentiated the two treatment groups with the Ris-treated group having significantly more ICM than the Fd group, and both patient groups had higher ICM volume compared to the control group (Figure 3).
DISCUSSION
To our knowledge this is the first study to report a differential effect of type of medication on ICM volume. We observed that in the frontal lobe of schizophrenic subjects the atypical antipsychotic Ris is associated with greater ICM volume compared to treatment with the typical antipsychotic Fd (Table 2 and Figure 3). Furthermore, the ICM data (Figure 3) suggest that, as hypothesized (Bartzokis et al, 2007), the medication effect may primarily impact later-developing intracortical myelin.
The data also demonstrate that different image acquisition protocols produce different image contrasts (Figure 2), track different underlying cellular substrates, and thus detect different aspects of underlying physiology (Figure 3). We previously proposed that the high sensitivity of IR sequences to cholesterol levels (Koenig, 1991) and the very high concentration of cholesterol in myelin (O'Brien and Sampson, 1965; Rouser et al, 1972; Saher et al, 2005) make IR sequences optimal for assessing myelinated WM volume, a measure which includes the heavily myelinated lower layers of cortex (Bartzokis et al, 2001; Bartzokis et al, 2007; Bartzokis et al, 2003b) (Figure 1 and Figure 2). The data support this assertion and show that unlike IR images, PD images do not detect a significant treatment effect.
Depending on the contrast produced by different imaging sequences the white/gray matter boundary shifts (Bartzokis et al, 2007) (Figure 2) changing gray/white matter segmentation characteristics. The GMPD data best exemplify how such differences could help explain some of the inconsistencies in the literature (Walterfang et al, 2008; Witthaus et al, 2008). Unlike IR, PD images are less impacted by the myelination process and thus produce data that may more closely reflect gray matter volume. The GMPD measure shows than when compared to the control sample, the Fd-treated group has significant smaller gray matter (Figure 3) while the Ris-treated subjects do not differ significantly from the healthy controls. Both the GMPD measures and total frontal lobe (left column Figure 3) follow the same diagnosis and non-significant medication effect pattern. Studies that acquire images whose contrast characteristics are influenced by similar underlying physiology as our PD images, could be interpreted to suggest that the “gray matter” is driving frontal lobe volume differences. On the other hand, the IR and ICM measures suggest that, as depicted in Figure 1 and predicted by our initial analysis (Bartzokis et al, 2007), the principal medication effect may be on myelination (Table 2 and Figure 3).
The differential sensitivity of imaging sequences to underlying physiology may help explain inconsistent antipsychotic medication effects on brain volumes of patients with schizophrenia (Garver et al, 2005; Lieberman et al, 2005; McCormick et al, 2005; Molina et al, 2005; Thompson et al, 2008). Chronic atypical antipsychotic treatments have been associated with increased (Molina et al, 2005) as well as decreased (McCormick et al, 2005) cortical volumes. Similarly, chronic typical medications have been associated with increased (McCormick et al, 2005) as well as decreased (Lieberman et al, 2005; Thompson et al, 2008) cortical volumes.
Human post-mortem data suggest that a glial deficit exists in the frontal cortex of patients with schizophrenia (Cotter et al, 2002; Stark et al, 2004) and that this deficit is primarily due to lower oligodendrocyte numbers (Hamidi et al, 2004; Hof et al, 2003; Uranova et al, 2005; Uranova et al, 2004). A deficit in intracortical myelination is also suggested by decreased expression of myelin genes (Aston et al, 2004; Hakak et al, 2001; Peirce et al, 2006; Tkachev et al, 2003), and decreased intracortical myelin markers (Chambers and Perrone-Bizzozero, 2004; Flynn et al, 2003) in post-mortem samples. The reported absence of a myelin deficit in subcortical WM (Marner and Pakkenberg, 2003) suggests that a myelination deficit may be limited to late-myelinating structures such as the cortex and adjacent WM (Bartzokis, 2005; Uranova et al, 2005). We have previously suggested (Bartzokis and Altshuler, 2005) that reduced levels of intracortical oligodendrocytes and myelin may underlie the reduced intracortical neuropil that results in increased neuronal density observed in post-mortem studies (Selemon and Goldman-Rakic, 1999; Selemon et al, 2003; Selemon et al, 1995).
By their very nature however, most post-mortem data are limited to samples from subjects that were chronically treated and/or in later stages of life when degenerative processes may predominate especially in vulnerable late-myelinating regions such as the frontal lobes (Bartzokis et al, 2003a; Salat et al, 2005). Myelination trajectories over the lifespan suggest that great changes occur during normal aging (Figure 1) and between early- and late-disease stages (Bartzokis, 2002; Bartzokis et al, 2003b). This wider lifespan perspective has been developed into a “myelin model” of the human brain which suggests that deviations from the normal trajectory of myelination (Figure 1) is a key contributor to the derangements observed in schizophrenia as well as other neuropsychiatric disorders (Bartzokis, 2002; Bartzokis, 2004b; Bartzokis, 2005; Bartzokis, 2009).
In treated subjects with schizophrenia, their WM trajectory is flat and crosses the upward trajectory of healthy individuals (Figure 1) in the third decade. Before that age, treated subjects with schizophrenia have higher WM while afterwards and beyond they have significantly lower WM compared to controls (Bartzokis et al, 2003b; Okugawa et al, 2007; Tanskanen et al, 2008; reviewed in Bartzokis, 2002). In samples such as the one in this study that are early in their illness (Table 1), compensatory processes may predominate, resulting in increased myelination that, later in the disease process, can turn into deficits (Bartzokis et al, 2003b; Ho et al, 2003; Molina et al, 2005; Whitford et al, 2007) and may contribute to the common occurrence of treatment resistance (Bartzokis and Altshuler, 2003). Brain changes associated with different disease and treatment stages have been observed in gene expression studies (Narayan et al, 2008), primate models with different levels of medication exposure (Konopaske et al, 2008; Selemon et al, 1999), and imaging studies demonstrating progressive changes (Garver et al, 2005; Ho et al, 2003; Lappin et al, 2007; Lieberman et al, 2005; Thompson et al, 2008; Whitford et al, 2007).
It is possible that early in treatment atypical antipsychotics have a greater “promyelinating” effect than typical antipsychotics (Figure 3). Evidence of initial promyelinating effects of treatments has emerged from animal models. Electroconvulsive (ECT) (Madsen et al, 2005) and atypical antipsychotics treatments have been shown to increase the genesis of glia (gliogenesis) (Kodama et al, 2004; Selemon et al, 1999; Wang et al, 2004a; Xiao et al, 2008). A primate model that compared multiple typical and atypical antipsychotics showed increased glial (but not neuron) density in the frontal cortex of chronically treated animals; however, only Ris treatment was associated with a decline in neuronal density (Selemon et al, 1999). The greater intracortical myelination in the Ris group suggested by our data (Figure 3) could reduce fixation-associated cortical shrinkage (Bartzokis and Altshuler, 2005; Bartzokis and Altshuler, 2003) and thus explain the Ris-associated decline in neuronal density observed in this primate model (Selemon et al, 1999). Unfortunately this primate study did not provide direct evidence of a medication effect on intracortical myelination because glial cell subtypes (e.g., astrocytes versus oligodendrocytes) and myelin stains were not assessed (Selemon et al, 1999).
The current cross-sectional study cannot determine if the greater WM of the Ris-treated group, compared to the Fd-treated group was induced by the treatments themselves or represents maintenance of preexisting higher WM that preceded treatment (Molina et al, 2005). Similarly this study cannot determine whether the lower ICM of the Fd group was related to smaller treatment-related increase or a larger decline from higher pretreatment levels. Prospective studies of unmedicated first episode subjects are needed to address such questions. Several additional important limitations must also be acknowledged before further discussion. First, the sample was composed of males of a restricted age-range (18–35 years of age), thus limiting the generalizability of the results to females and older or younger cohorts (Chambers and Perrone-Bizzozero, 2004). Second, the two cohorts of schizophrenia subjects were not randomly assigned to the treatment arms. Although this could result in a biased group assignment or cohort effects, the cohorts were selected with parallel criteria from the same psychiatric facilities by the same research team and were treated with either typical or atypical medications. Finally, the groups were not matched in age, race, education, medication exposure, or duration of illness; however, statistically controlling for these variables did not meaningfully alter the results. Replication of these findings using randomized matched samples is necessary to fully rule out these variables as contributing factors and prospective design that includes healthy controls is needed to definitively define treatment effect on disease course.
Multiple non-exclusive molecular mechanisms could underlie promyelinating effects of atypical antipsychotic medications. Recent data suggests that atypical antipsychotic medications change lipid metabolism (Ferno et al, 2005; La et al, 2007; Le Hellard et al, 2008; Raeder et al, 2006; Smith et al, 2008), an effect that may also be related to the weight gain and peripheral lipid abnormalities associated with their use (Bartzokis, 2002; Le Hellard et al, 2008; Meyer et al, 2008). Similarly, elevation in prolactin is associated with both typical antipsychotic and Ris treatment (Baptista et al, 2001; Friberg et al, 2008), and prolactin has been shown to have substantial promyelinating effects (Gregg et al, 2007). Other mechanisms could involve neurotransmitters that may act on glia and serve as a mode of neuron-glial communication and coordination (Bartzokis, 2007). Many key neurotransmitters such as dopamine, acetylcholine, and serotonin are released non-synaptically (Aznavour et al, 2005; Descarries and Mechawar, 2000; Umbriaco et al, 1995) and could fulfill such a role. Increased prefrontal cortex dopaminergic neurotransmission underlies some of the beneficial effects of atypical antipsychotics (Barch and Carter, 2005; Eltayb et al, 2005; Li et al, 2005; Roy et al, 2007). Consistent with these possibilities in vitro and in vivo models have suggested that dopamine and cholinergic stimulation may be protective of oligodendrocytes (Altar et al, 2008; Belachew et al, 1999; Rosin et al, 2005; Roy et al, 2007) and promote the genesis of new cells (Van Kampen et al, 2004) and/or myelination (Bartzokis, 2007). Finally, recent studies suggest that the Neuregulin 1 gene, one of the genetic loci most strongly linked to schizophrenia (Li et al, 2004; Liu et al, 2005; Stefansson et al, 2003; for review see Tosato et al, 2005), may be particularly important for adequate myelination of smaller diameter axons (Michailov et al, 2004). In late-myelinating regions such as the frontal cortex (Figure 1), these smaller diameter axons with thinner sheaths are being myelinated during the teens and early adulthood when onset prevalence of schizophrenia and bipolar disorder reach their highest levels. By disrupting the later stages of brain myelination, abnormalities in myelination signaling and repair processes may therefore contribute to the pathophysiology of these diseases and could help explain the high comorbidity of several neuropsychiatric disorders (Bartzokis, 2005).
In addition to schizophrenia, severe depression, bipolar disorder, and other neuropsychiatric disorders have also been associated with abnormal myelination (Aston et al, 2005; Bartzokis, 2005; Hamidi et al, 2004; Jones et al, 2008; Le-Niculescu et al, 2008; McDonald et al, 2004; Regenold et al, 2007; Tkachev et al, 2003; Uranova et al, 2005; Uranova et al, 2004; Wang et al, 2004b; Zai et al, 2004). If treatment with atypical antipsychotics alters myelination, then such treatment could be effective in these other psychiatric conditions (Jones et al, 2008). Shared abnormalities in the myelination process could thus offer a parsimonious explanation for the wide spectrum of efficacy of atypical antipsychotic medications such as Ris on multiple diseases such as schizophrenia, autism, bipolar disorder, severe depression, and post traumatic stress disorder, as well as their associated cognitive deficits (Bartzokis, 2002; Bartzokis, 2005; Bartzokis et al, 2005; Harvey et al, 2003; McDougle et al, 2008; Mori et al, 2004; Sachs and Gardner-Schuster, 2007).
In vivo MRI can be used to dissect subtle differences in brain tissue characteristics and thus help clarify the effect of pharmacologic treatments on developmental and pathologic processes (Bartzokis, 2004a; Woo and Crowell, 2005) and may have applications in medication development (Bartzokis, 2004a; Bartzokis et al, 2007; Bartzokis et al, 2004; Garver et al, 2008; Molina et al, 2005; Thompson et al, 2008). The myelin model of the human brain suggests that interceding early in dysregulated developmental trajectories may increase the effectiveness of treatments and decrease the need for more aggressive later interventions (Bartzokis, 2002; Bartzokis, 2004a). Improved long-term outcome however, will likely depend on targeting treatments to specific disease stages, genetic markers, and environmental circumstances that likely impacts the continual process of brain development and repair across the human lifespan (Bartzokis, 2009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 47th ACNP Annual Meeting in Scottsdale, Arizona, December 10, 2008.
REFERENCES
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Altar CA, Hunt RA, Jurata LW, Webster MJ, Derby E, Gallagher P, et al. Insulin, IGF-1, and muscarinic agonists modulate schizophrenia-associated genes in human neuroblastoma cells. Biol Psychiatry. 2008;64:1077–1087. doi: 10.1016/j.biopsych.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Aznavour N, Watkins KC, Descarries L. Postnatal development of the cholinergic innervation in the dorsal hippocampus of rat: Quantitative light and electron microscopic immunocytochemical study. J Comp Neurol. 2005;486:61–75. doi: 10.1002/cne.20501. [DOI] [PubMed] [Google Scholar]
- Baptista T, Lacruz A, Meza T, Contreras Q, Delgado C, Mejias MA, et al. Antipsychotic drugs and obesity: is prolactin involved? Can J Psychiatry. 2001;46:829–834. doi: 10.1177/070674370104600906. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Lyon G, Evrard P. Formation, maturation, and disorders of white matter. AJNR.Am.J.Neuroradiol. 1992;13:447–461. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27:672–683. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004a;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiol Aging. 2004b;25:49–62. [Google Scholar]
- Bartzokis G. Brain myelination in prevalent neuropsychiatric developmental disorders: Primary and comorbid addiction. Adolescent Psychiatry. 2005;29:55–96. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Acetylcholinesterase inhibitors may improve myelin integrity. Biological Psychiatry. 2007;62:294–301. doi: 10.1016/j.biopsych.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Brain Volume: Age-Related Changes. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol 2. Oxford: Academic Press; 2009. pp. 417–447. [Google Scholar]
- Bartzokis G, Altshuler L. Reduced intracortical myelination in schizophrenia. Am J Psychiatry. 2005;162:1229–1230. doi: 10.1176/appi.ajp.162.6.1229-a. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Altshuler LL. Biological Underpinnings of Treatment Resistance in Schizophrenia: An Hypothesis. Psychopharmacol Bull. 2003;37:5–7. [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003a;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, et al. Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophr Res. 2007;93:13–22. doi: 10.1016/j.schres.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Turner J, Mintz J, Saunders CS. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biological Psychiatry. 2005;57:474–479. doi: 10.1016/j.biopsych.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, et al. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magn.Reson.Imaging. 1993;11:993–1006. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: A magnetic resonance imaging study. Biol Psychiatry. 2003b;53:412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings J. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical "disconnection" in aging and Alzheimer's disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Belachew S, Rogister B, Rigo JM, Malgrange B, Moonen G. Neurotransmitter-mediated regulation of CNS myelination: a review. Acta Neurol Belg. 1999;99:21–31. [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochem Res. 2004;29:2293–2302. doi: 10.1007/s11064-004-7039-x. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Descarries L, Mechawar N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog Brain Res. 2000;125:27–47. doi: 10.1016/S0079-6123(00)25005-X. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. Int J Neuropsychopharmacol. 2007:1–24. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Eltayb A, Wadenberg ML, Svensson TH. Enhanced cortical dopamine output and antipsychotic-like effect of raclopride with adjunctive low-dose L-dopa. Biol Psychiatry. 2005;58:337–343. doi: 10.1016/j.biopsych.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Ferno J, Raeder MB, Vik-Mo AO, Skrede S, Glambek M, Tronstad KJ, et al. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 2005;5:298–304. doi: 10.1038/sj.tpj.6500323. [DOI] [PubMed] [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- Friberg L, Vermeulen A, Petersson K, Karlsson M. An Agonist-Antagonist Interaction Model for Prolactin Release Following Risperidone and Paliperidone Treatment. Clin Pharmacol Ther. 2008 doi: 10.1038/clpt.2008.234. [DOI] [PubMed] [Google Scholar]
- Garver DL, Holcomb JA, Christensen JD. Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biol Psychiatry. 2005;58:62–66. doi: 10.1016/j.biopsych.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacol. 2008;11:49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-Related Total Gray Matter and White Matter Changes in Normal Adult Brain. Part I: Volumetric MR Imaging Analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. 2007;27:1812–1823. doi: 10.1523/JNEUROSCI.4441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. PNAS. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Green MF, McGurk SR, Meltzer HY. Changes in cognitive functioning with risperidone and olanzapine treatment: a large-scale, double-blind, randomized study. Psychopharmacology (Berl) 2003;169:404–411. doi: 10.1007/s00213-002-1342-5. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age-consistency and interpretation of observed effects. Neurobiol Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Jones LD, Payne ME, Messer DF, Beyer JL, Macfall JR, Krishnan KR, et al. Temporal lobe volume in bipolar disorder: Relationship with diagnosis and antipsychotic medication use. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaes T. Die Grosshirnrinde des Menschen in ihren Massen und in ihrem Fasergehalt. Jena: Gustav Fisher; 1907. [Google Scholar]
- Kemper T. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert M, Knoefel J, editors. Clinical Neurology of Aging. 2nd ed. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry. 2004;56:570–580. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Koenig SH. Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn Reson Med. 1991;20:285–291. doi: 10.1002/mrm.1910200210. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La YJ, Wan CL, Zhu H, Yang YF, Chen YS, Pan YX, et al. Decreased levels of apolipoprotein A-I in plasma of schizophrenic patients. J Neural Transm. 2007;114:657–663. doi: 10.1007/s00702-006-0607-2. [DOI] [PubMed] [Google Scholar]
- Lappin JM, Dazzan P, Morgan K, Morgan C, Chitnis X, Suckling J, et al. Duration of prodromal phase and severity of volumetric abnormalities in first-episode psychosis. Br J Psychiatry Suppl. 2007;51:s123–s127. doi: 10.1192/bjp.191.51.s123. [DOI] [PubMed] [Google Scholar]
- Le Hellard S, Muhleisen TW, Djurovic S, Ferno J, Ouriaghi Z, Mattheisen M, et al. Polymorphisms in SREBF1 and SREBF2, two antipsychotic-activated transcription factors controlling cellular lipogenesis, are associated with schizophrenia in German and Scandinavian samples. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.110. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Kurian SM, Yehyawi N, Dike C, Patel SD, Edenberg HJ, et al. Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.11. [DOI] [PubMed] [Google Scholar]
- Li T, Stefansson H, Gudfinnsson E, Cai G, Liu X, Murray RM, et al. Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol Psychiatry. 2004:1–7. doi: 10.1038/sj.mp.4001485. [DOI] [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Huang M, Prus AJ, Dai J, Meltzer HY. ACP-103, a 5-HT(2A/2C) inverse agonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Psychopharmacology (Berl) 2005;183:144–153. doi: 10.1007/s00213-005-0170-9. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Liu CM, Hwu HG, Fann CS, Lin CY, Liu YL, Ou-Yang WC, et al. Linkage evidence of schizophrenia to loci near neuregulin 1 gene on chromosome 8p21 in Taiwanese families. Am J Med Genet B Neuropsychiatr Genet. 2005;134:79–83. doi: 10.1002/ajmg.b.20161. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Yeh DD, Valentine GW, Duman RS. Electroconvulsive seizure treatment increases cell proliferation in rat frontal cortex. Neuropsychopharmacology. 2005;30:27–34. doi: 10.1038/sj.npp.1300565. [DOI] [PubMed] [Google Scholar]
- Marner L, Pakkenberg B. Total length of nerve fibers in prefrontal and global white matter of chronic schizophrenics. J Psychiatr Res. 2003;37:539–547. doi: 10.1016/s0022-3956(03)00069-4. [DOI] [PubMed] [Google Scholar]
- McCormick L, Decker L, Nopoulos P, Ho BC, Andreasen N. Effects of atypical and typical neuroleptics on anterior cingulate volume in schizophrenia. Schizophr Res. 2005;80:73–84. doi: 10.1016/j.schres.2005.06.022. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Stigler KA, Erickson CA, Posey DJ. Atypical antipsychotics in children and adolescents with autistic and other pervasive developmental disorders. J Clin Psychiatry. 2008;69 Suppl 4:15–20. [PubMed] [Google Scholar]
- Meyer JM, Davis VG, McEvoy JP, Goff DC, Nasrallah HA, Davis SM, et al. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE Schizophrenia Trial phase 1. Schizophr Res. 2008;103:104–109. doi: 10.1016/j.schres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Miller AK, Alston RL, Corsellis JA. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyser. Neuropathol Appl Neurobiol. 1980;6:119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sanz J, Palomo T, Benito C, Sanchez J, et al. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res. 2005;80:61–71. doi: 10.1016/j.schres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao M, Yamashita H, Morinobu S, Yamawaki S. Effect of switching to atypical antipsychotics on memory in patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:659–665. doi: 10.1016/j.pnpbp.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008 doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, et al. Developmental Processes in Schizophrenic Disorders: longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Ventura J, Gitlin M, Subotnik KL, Snyder KS, et al. The vulnerability/stress model of schizophrenic relapse: a longitudinal study. Acta Psychiatr.Scand.Suppl. 1994;382:58–64. doi: 10.1111/j.1600-0447.1994.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Gitlin MJ, Siegel BV, Bartzokis G, Fogelson DL, et al. Functional and cognitive outcome of first episode psychosis patients treated with fluphenazine decanoate or risperidone. American College of Neuropsychopharmacology 39th Annual Meeting; San Juan, Puerto Rico. 2001. Abstracts of Panels and Posters:56. [Google Scholar]
- O'Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- Okugawa G, Tamagaki C, Agartz I. Frontal and temporal volume size of grey and white matter in patients with schizophrenia: an MRI parcellation study. Eur Arch Psychiatry Clin Neurosci. 2007;257:304–307. doi: 10.1007/s00406-007-0721-7. [DOI] [PubMed] [Google Scholar]
- Peirce TR, Bray NJ, Williams NM, Norton N, Moskvina V, Preece A, et al. Convergent evidence for 2',3'-cyclic nucleotide 3'-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch Gen Psychiatry. 2006;63:18–24. doi: 10.1001/archpsyc.63.1.18. [DOI] [PubMed] [Google Scholar]
- Raeder MB, Ferno J, Vik-Mo AO, Steen VM. SREBP activation by antipsychotic- and antidepressant-drugs in cultured human liver cells: relevance for metabolic side-effects? Mol Cell Biochem. 2006;289:167–173. doi: 10.1007/s11010-006-9160-4. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007;151:179–188. doi: 10.1016/j.psychres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Rosin C, Colombo S, Calver AA, Bates TE, Skaper SD. Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation. Glia. 2005;52:336–343. doi: 10.1002/glia.20250. [DOI] [PubMed] [Google Scholar]
- Rouser G, Kritchevsky G, Yamamoto A, Baxter CF. Lipids in the nervous system of different species as a function of age: brain, spinal cord, peripheral nerve, purified whole cell preparations, and subcellular particulates: regulatory mechanisms and membrane structure. Adv Lipid Res. 1972;10:261–360. doi: 10.1016/b978-0-12-024910-7.50013-0. [DOI] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GS, Gardner-Schuster EE. Adjunctive treatment of acute mania: a clinical overview. Acta Psychiatr Scand Suppl. 2007:27–34. doi: 10.1111/j.1600-0447.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- Saher G, Brugger B, Lappe-Siefke C, Mobius W, Tozawa R, Wehr MC, et al. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Lidow MS, Goldman-Rakic PS. Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry. 1999;46:161–172. doi: 10.1016/s0006-3223(99)00113-4. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Mrzljak J, Kleinman JE, Herman MM, Goldman-Rakic PS. Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca's area 44 and area 9. Arch Gen Psychiatry. 2003;60:69–77. doi: 10.1001/archpsyc.60.1.69. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819-20. [DOI] [PubMed] [Google Scholar]
- Smith RC, Segman RH, Golcer-Dubner T, Pavlov V, Lerer B. Allelic variation in ApoC3, ApoA5 and LPL genes and first and second generation antipsychotic effects on serum lipids in patients with schizophrenia. Pharmacogenomics J. 2008;8:228–236. doi: 10.1038/sj.tpj.6500474. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B. Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry. 2004;161:882–888. doi: 10.1176/appi.ajp.161.5.882. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen P, Ridler K, Murray GK, Haapea M, Veijola JM, Jaaskelainen E, et al. Morphometric Brain Abnormalities in Schizophrenia in a Population-Based Sample: Relationship to Duration of Illness. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Bartzokis G, Hayashi KM, Klunder AD, Lu PH, Edwards N, et al. Time-Lapse Mapping of Cortical Changes in Schizophrenia with Different Treatments. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Tosato S, Dazzan P, Collier D. Association between the neuregulin 1 gene and schizophrenia: a systematic review. Schizophr Bull. 2005;31:613–617. doi: 10.1093/schbul/sbi043. [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Garcia S, Beaulieu C, Descarries L. Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1) Hippocampus. 1995;5:605–620. doi: 10.1002/hipo.450050611. [DOI] [PubMed] [Google Scholar]
- Uranova N, Vostrikov V, Vichreva O, Zimina I. World Federation of Societies of Biological Psychiatry, 8th World Congress of Biological Psychiatry. Vol 6. Vienna, Austria: The World Journal of Biological Psych; 2005. Oligodendroglia in Schizophrenia: Findings from postmortem studies; p. 74. [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Valk J, van der Knaap MS. Magnetic Resonance of Myelin, Myelination, and Myelin Disorders. New York: Spring-Verlag; 1989. [Google Scholar]
- van der Knaap MS, Valk J. MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology. 1990;31:459–470. doi: 10.1007/BF00340123. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:237–248. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D receptor stimulation. Eur J Neurosci. 2004;19:2377–2387. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Walterfang M, McGuire PK, Yung AR, Phillips LJ, Velakoulis D, Wood SJ, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193:210–215. doi: 10.1192/bjp.bp.107.043463. [DOI] [PubMed] [Google Scholar]
- Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004a;29:1230–1238. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang Z, Zhu P, Jiang J. Alterations of myelin basic protein and ultrastructure in the limbic system at the early stage of trauma-related stress disorder in dogs. J Trauma. 2004b;56:604–610. doi: 10.1097/01.ta.0000058122.57737.0e. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, et al. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. Am J Psychiatry. 2007;164:1082–1089. doi: 10.1176/ajp.2007.164.7.1082. [DOI] [PubMed] [Google Scholar]
- Witthaus H, Brune M, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, et al. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophr Res. 2008;102:141–149. doi: 10.1016/j.schres.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Woo TU, Crowell AL. Targeting synapses and myelin in the prevention of schizophrenia. Schizophr Res. 2005;73:193–207. doi: 10.1016/j.schres.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry. 2008;13:697–708. doi: 10.1038/sj.mp.4002064. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. Boston: Blackwell Scientific Publications; 1967. [Google Scholar]
- Zai G, Bezchlibnyk YB, Richter MA, Arnold P, Burroughs E, Barr CL, et al. Myelin oligodendrocyte glycoprotein (MOG) gene is associated with obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2004;129:64–68. doi: 10.1002/ajmg.b.30077. [DOI] [PubMed] [Google Scholar]