Abstract
A fascinating mechanistic study of ynamido-palladium-π-allyl complexes is described that features isolation of a unique silyl-ketenimine via aza-Claisen rearrangement, which can be accompanied by an unusual thermal N-to-C 1,3-Ts shift in the formation of tertiary nitriles, and a novel cyclopentenimine formation via a palladium catalyzed aza-Rautenstrauch-type cyclization pathway.
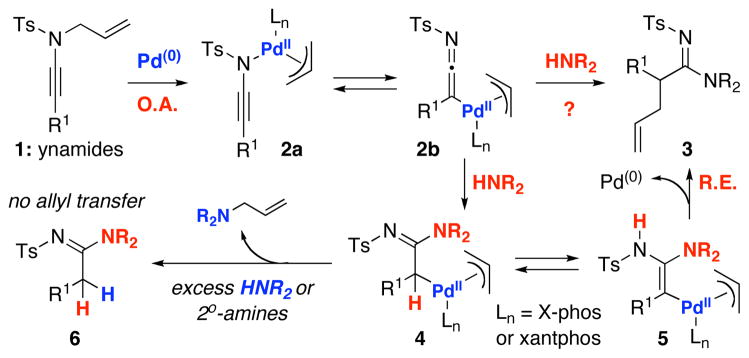
We recently reported an account on the synthesis of pharmacologically useful amidines1–4 from ynamides5,6 via a Pd(0)-catalyzed N-to-C allyl transfer.7 As shown in Scheme 1, the formation of amidine 3 was proposed to proceed through ynamido-π-allyl complexes 2a or 2b after the initial oxidative addition [O.A.] of N-allyl-ynamides 1. Subsequently, after the addition of an amine, the reaction pathway would diverge from 4 or 5 depending upon the concentration of the amine HNR2 and the nature of the palladium catalyst and ligands. Excess amount of amines or more nucleophilic secondary amines8 tend to attack the ynamido-π-allyl complexes 4 [or 5], leading to deallylated-amidines 6, whereas Pd(0) catalysts such as Pd2(dba)3 [instead of starting from Pd(II) species], and more bulky ligands such as X-phos9 and/or bidentate ligands with unique bite angles such as xantphos10,11 that presumably promote reductive elimination [R.E.] favored the formation of allyl transferred amidines 3. Given the novelty of these ynamido-metal complexes and the potential of harvesting new reactivities, we examined this reaction in greater details mechanistically and uncovered a unique ketenimine intermediate, a rare 1,3-Ts shift, and an unusual and formally a Nazarov-type pathway leading to cyclopentenimine formation. We report here these findings.
Scheme 1.
Ynamido-Pd-π-Allyl Complexes.
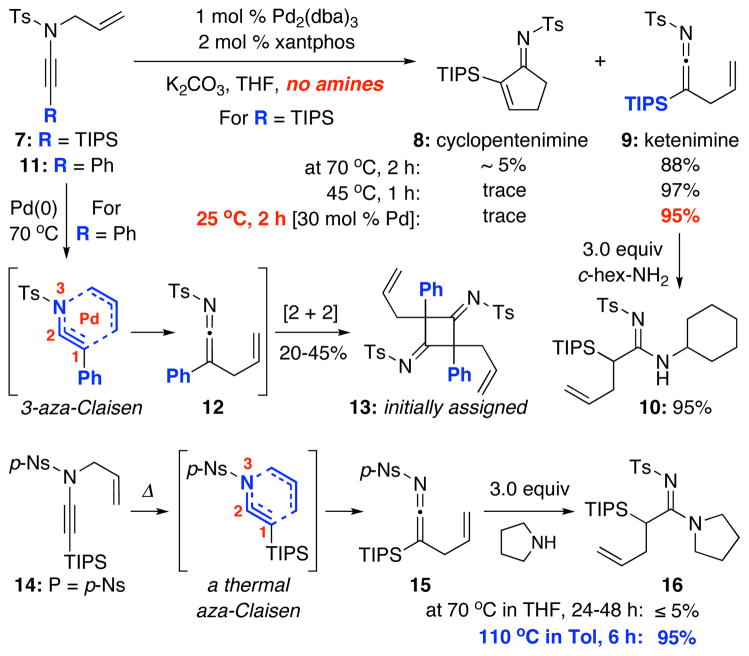
Our initial experiments involved removing the amine nucleophile to suppress amidine formation in an attempt to isolate and/or observe key intermediates. As shown in Scheme 2, in the presence of 1 mol % of Pd2(dba)3 and 2 mol % of xantphos, heating of N-allyl ynamide 7 at 70 °C afforded two interesting products: Cyclopentenimine 8 and silyl-ketenimine 9 in ~ 5% and 88% yield, respectively.12 The yield of 9 was improved with the formation of 8 completely impeded when the reaction was run at lower temperatures. While characterizations of 9 were unambiguous given its stability, the formation of amidine 10 in 95% yield via treatment of 9 with c-hex-NH2 solidifies the identification of this novel intermediate.13
Scheme 2.
Isolation of a Stable Silyl-Ketenimine.
Despite the potential reactivity of N-sulfonyl-ketenimines, the surprising stability of ketenimine 9 is likely unique to the silyl substitution.14,15 Under similar reaction conditions, ynamide 11 containing a Ph substituent led to a very different product, although in low yields. The product was initially assigned based on literature report16 as cyclobutane bis-imine 13, presumably attained through a facile dimerization or [2 + 2] cycloaddition of the less stable ketenimine 12.
The formation of ketenimines from N-allyl ynamides invokes an aza-Claisen rearrangement,17 specifically 3-az-Claisen, although those involving a C1-C2 acetylenic motif are very rare if not unprecedented.17–19 However, the involvement of the palladium metal in the formation of 9 is distinctly clear, as a non-palladium involved aza-Claisen pathway required higher temperatures and longer reaction times [see 14→ 15→ 16 in Scheme 3]. When the aza-Claisen rearrangement was carried out at 70 °C, the reaction was sluggish as evident in yield of the trapping of the ketenimine 15 with pyrrolidine, and the usage of Ts or p-Ns group was not critical to the reactivity.
Scheme 3.
An Unusual N-to-C 1,3-Ts Shift.
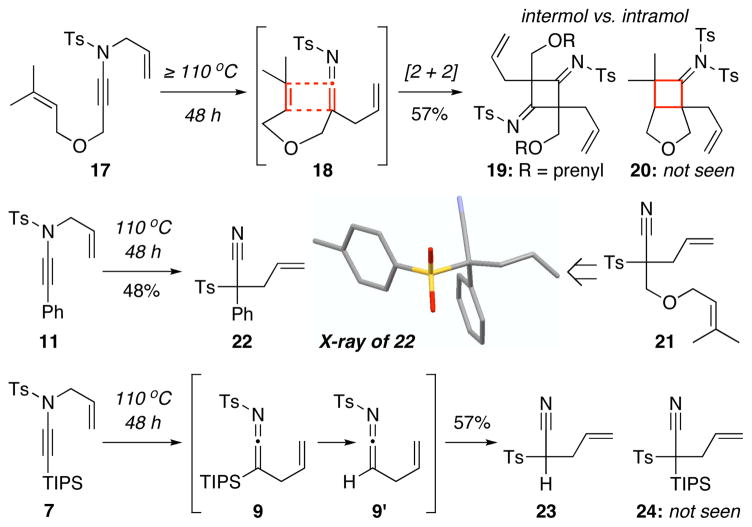
When heating ynamide 17 at 110 °C led to again the dimer 19 and not the intended intramolecular cycloadduct 20 [Scheme 3], we sensed a possible mis-assignment because an intermolecular process had just dominated over an intramolecular one. We attained X-ray structure of the aza-Claisen product from 11 and were surprised that it was not the dimer 12 but a tertiary nitrile 22. Aza-Claisen rearrangements of ynamides in fact can occur in tandem with a rare N-to-C 1,3-Ts shift at 110 °C,20 leading to nitriles with a quaternary carbon formation.
It is noteworthy that these results further accentuate the stability of silyl-ketenimine 9. While thermal rearrangement of 7 took place at 110 °C, 1,3-Ts shift to give TIPS-less nitrile 23 only proceeded after desilylation [9→9′], thereby suggesting sterics could also be at play. While this unusual 1,3-Ts shift in the formation of tertiary nitriles holds significant merit in synthesis,21 and that our finding cautions the assignment of possible homo-dimeric products from ketenimines, details of this shift will be examined in another study.
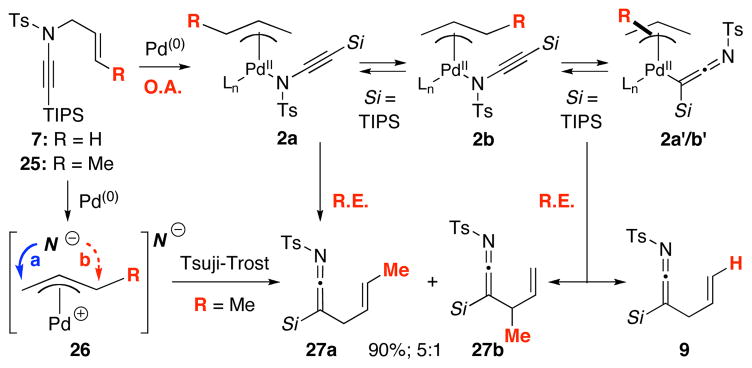
Having established the significance of the palladium metal in this aza-Claisen rearrangement, we believe ynamido-π-allyl complexes 2 derived from the oxidative addition of ynamide 7 should be responsible for the formation of ketenimines [Scheme 4]. The question is whether it proceeds through a reductive elimination process via an intramolecular pathway, or a Tsuji-Trost pathway. To addressing this question, we pursued two experiments.
Scheme 4.
A Pd-Model for the Ketenimine Formation.
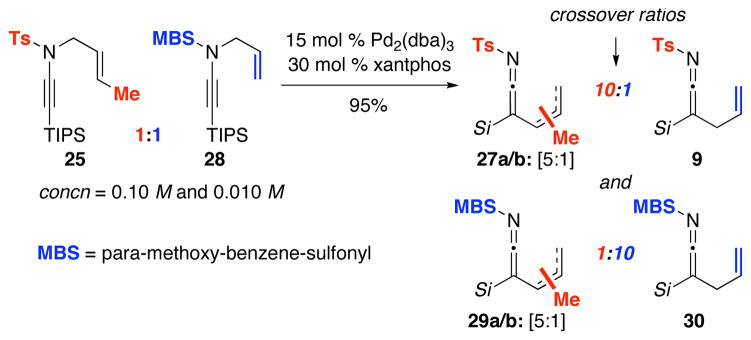
The first experiment involved the use of ynamide 25 containing a crotyl group as shown in Scheme 4. While the reaction temperature had to be higher, new ketenimine 27 was isolated in 90% yield with a regiochemical ratio of 5:1. While this outcome concisely suggests that the crotyl group is scrambled through the oxidative addition, the resulting regiochemical ratio reflects that both pathways are possible with the major isomer 27a being derived from either reductive elimination of 2a [or 2a′: a ketenimine palladium complex], or a favored addition of ynamido anion [No−] to 26 at the less hindered site [blue arrow].
The second study is a revealing crossover experiment as shown in Scheme 5. With a 1:1 mixture of ynamides 25 and 28 containing a crotyl and allyl group, respectively, we were able to concisely assign 4 sets of ketenimines: 27a/b, 9, 29a/b, and 30. While the regiochemical ratio of 27a/b or 29a/b is the same at 5:1, the ratios of crossover of 10:1 even when the reaction was run at 0.10 M suggest that the ketenimine formation is likely an intramolecular process, and that these are likely tightly bound ynamido-π-allyl complexes shown as 2a and 2b [or 2a′/2b′].
Scheme 5.
A Crossover Experiment.
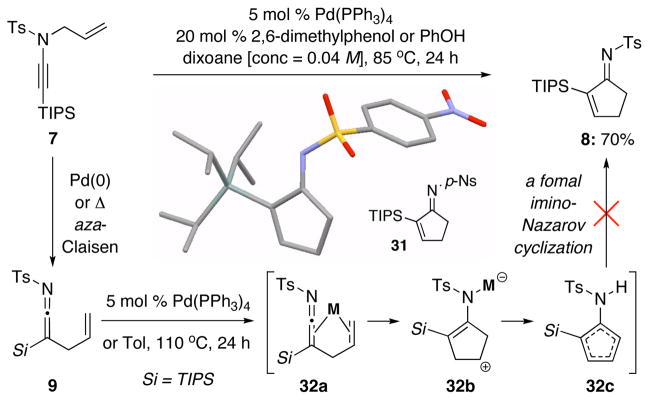
While literature precedents15 suggest that ketenimines represent highly reactive entities for developing useful synthetic methods, the observation of trace amounts of cyclopentenimine 8 and its mechanistic course captured our attention. Although it took much effort to optimize the formation of 8,22 it was found that conditions which relatively disfavored reductive elimination [5 mol % of Pd(PPh3)4] as well as the use of a phenolic substrate as an additive led to cyclopentenimine 8 in 70% yield. Preparation of 31 led to an X-ray structure, allowing an unambiguous assignment.
The difference in conditions implied that cyclopentenimine 8 is likely derived from a different mechanistic course for which silyl-ketenimine 9 is not an intermediate.23 This assertion is consistent with the fact that treatment of 9 with either 5 mol % of Pd(PPh3)4, or by using thermal conditions, did not provide any identifiable amount of 8, thereby ruling out the possibility of a formal imino-Nazarov type cyclization24,25 involving 32a–c [Scheme 6].
Scheme 6.
An Effective Cyclopentenimine Formation.
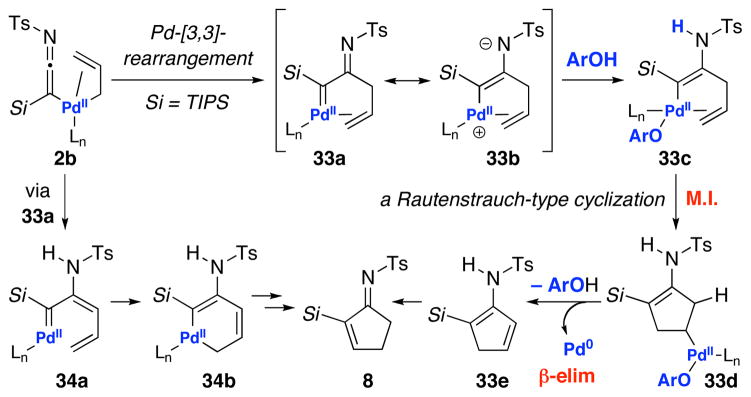
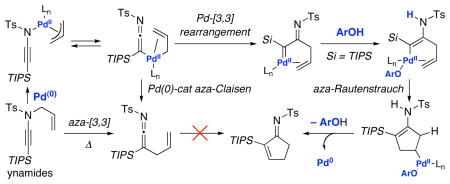
Instead, a likely mechanistic course would involve an aza-variant of a Rautenstrauch-type cyclization [or also formally an aza-Nazarov-type cyclization]26,27 as shown in Scheme 7. While the Pd-complex 2b could readily reductive eliminate to give ketenimine 9, under conditions in which the reductive elimination is slowed, a Pd-[3,3] sigmatropic rearrangement could occur to give α-imino palladium carbenoid 33a. While a number of possibilities could take place from thereon, one possibility that is consistent with the use of PhOH would entail the formation of enamido-Pd-Complex 33c could undergo migratory insertion [M.I.] followed by β-elimination to afford cyclopentenimine 8 after tautomerization of cyclopentadienamide 33e. An alternative pathway would proceed through dienyl palladium carbenoid 34a derived from tautomerization of 33a.
Scheme 7.
An Aza-Rautenstrauch-Type Cyclization Pathway.
We have uncovered here a fascinating divergent mechanistic pathway consisting of a Pd(0)-catalyzed aza-Claisen rearrangement of N-allyl ynamides, which can also be accompanied with an N-to-C 1,3-Ts shift through the ketenimine intermediate, and an aza-Rautenstrauch cyclization. These studies provide insight into the nature of ynamido-π-allyl complexes as well as new reactivities with synthetic potential. Efforts are underway in pursuing synthetic methods involving ketenimines and the N-to-C 1,3-Ts shift as well as applications using cyclopentenimines.
Supplementary Material
Acknowledgments
We thank NIH [GM066055] for funding. We thank Dr. Vic Young of the University of Minnesota for providing X-ray structural analysis.
Footnotes
Supporting Information Available: Experimental procedures as well as NMR spectra, and characterizations are available for all new compounds and free of charge via Internet http://pubs.acs.org.
References
- 1.Greenhill JV, Lue P. Prog Med Chem. 1993;30:203. doi: 10.1016/s0079-6468(08)70378-3. [DOI] [PubMed] [Google Scholar]
- 2.For a leading review on amidine derivatives serving as selective muscarinic agonists in the treatment of Alzheimer’s diseases, see: Messer WS, Jr, Dunbar PG. Muscarinic Agonists and the Treatment of Alzheimer’s Disease. 1996:131–153.
- 3.Dunn PJ. In: Compreh Org Funct Group Transform II, Amidines and N-Substituted Amidines. Katritzky Alan R, Taylor Richard JK, editors. Vol. 5. Pfizer Global Research and Development; Sandwich, UK: 2005. pp. 655–699. [Google Scholar]
- 4.For recent examples of amidine synthesis, see: Shang Y, He X, Hu J, Wu J, Zhang M, Yu S, Zhang Q. Adv Synth Catal. 2009;351:2709.She J, Jiang Z, Wang Y. Synlett. 2009;12:2023.Yu RT, Rovis T. J Am Chem Soc. 2008;130:3262. doi: 10.1021/ja710065h.
- 5.For reviews, see: Katritzky AR, Jiang R, Singh SK. Heterocycles. 2004;63:1455.Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379.Zificsak CA, Mulder JA, Hsung RP, Rameshkumar C, Wei LL. Tetrahedron. 2001;57:7575.
- 6.For efforts in 2009 alone, see: Yao B, Liang Z, Niu T, Zhang Y. J Org Chem. 2009;74:4630. doi: 10.1021/jo900595c.Coste A, Karthikeyan G, Couty F, Evano G. Angew Chem Int Ed. 2009;48:4381. doi: 10.1002/anie.200901099.Coste A, Couty F, Evano G. Org Lett. 2009;11:4454. doi: 10.1021/ol901831s.Laroche C, Kerwin MS. Tetrahedron Lett. 2009;50:5194.Kramer S, Dooleweerdt K, Lindhardt AT, Rottländer M, Skrydstrup T. Org Lett. 2009;11:4208. doi: 10.1021/ol901565p.Das JP, Chechik H, Marek I. Nature Chem. 2009;1:128. doi: 10.1038/nchem.131.Koester DC, Werz DB. Angew Chem Int Ed. 2009;48:7971. doi: 10.1002/anie.200903773.Gourdet B, Lam HW. J Am Chem Soc. 2009;131:3802. doi: 10.1021/ja900946h.Gourdet B, Rudkin ME, Watts CA, Lam HW. J Org Chem. 2009;74:7849. doi: 10.1021/jo901658v.Sato A, Yorimitsu H, Oshima K. Synlett. 2009:28.Cockburn N, Karimi E, Tam W. J Org Chem. 2009;74:5762. doi: 10.1021/jo9010206.Riddell N, Villeneuve K, Tam W.J Org Chem 200974576219572573Shindoh N, Takemoto Y, Takasu K. Chem Eur J. 2009;15:7026. doi: 10.1002/chem.200901103.Buzas A, Istrate F, Le Goff XF, Odabachiam Y, Gagosz F. J Organomet Chem. 2009;694:515.Garcia P, Moulin S, Miclo Y, Leboeuf D, Gandon V, Aubert C, Malacria M. Chem Eur J. 2009;15:2129. doi: 10.1002/chem.200802301.Friedman RK, Rovis T. J Am Chem Soc. 2009;131:10775. doi: 10.1021/ja903899c.Buzas AK, Istrate FM, Gagosz F. Tetrahedron. 2009;65:1889.Dooleweerdt K, Ruhland T, Skrydstrup T. Org Lett. 2009;11:221. doi: 10.1021/ol802477d.Couty S, Meyer C, Cossy J. Tetrahedron. 2009;65:1809.Alayrac C, Schollmeyer D, Witulski B. Chem Commun. 2009:1464. doi: 10.1039/b820291e.For our own recent efforts in 2009, see: Li H, Hsung RP. Org Lett. 2009;11:4462. doi: 10.1021/ol901860b.Oppenheimer J, Johnson WL, Figueroa R, Hayashi R, Hsung RP. Tetrahedron. 2009;65:5001. doi: 10.1016/j.tet.2009.03.078.
- 7.Zhang Y, DeKorver KA, Lohse AG, Zhang YS, Huang J, Hsung RP. Org Lett. 2009;11:899. doi: 10.1021/ol802844z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For a leading reference on relative nucleophilicity of amines, see: Brotzel F, Chu YC, Mayr H. J Org Chem. 2007;72:3679. doi: 10.1021/jo062586z.
- 9.For leading references on X-phos, see: Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J Am Chem Soc. 2003;125:6653. doi: 10.1021/ja035483w.Barder TE, Buchwald SL. J Am Chem Soc. 2007;129:12003. doi: 10.1021/ja073747z.
- 10.For a leading reference on xantphos, see: Kranenburg M, van der Burgt YEM, Kamer PCJ, van Leeuwen PWNM. Organometallics. 1995;14:3081.
- 11.For a rationale on the usage of bidentate xantphos in promoting reductive elimination with its unique bite angle, see: Fujita KI, Yamashita M, Puschmann F, Alvarez-Falcon MM, Incarvito CD, Hartwig JF. J Am Chem Soc. 2006;128:9044. doi: 10.1021/ja062333n.
- 12.See Supporting Information.
- 13.Although attempts were made, these reactions were too fast to allow NMR studies to be revealing of possible ynamido-π-allyl complexes even at 25 °C. Only the starting ynamide 7 and silyl-ketenimine 9 [and amidine 10 if the reaction was run in the presence of an amine] were clearly in display spectroscopically.
- 14.For documentation of silicon-stabilization of ketenes and ketenimines, see: Brady WT, Saidi K. J Org Chem. 1990;55:4215.
- 15.For reviews on chemistry of ketenimines, see: Krow GR. Angew Chem, Int Ed Engl. 1971;10:435.Gambaryan NP. Usp Khim. 1976;45:1251.Dondoni A. Heterocycles. 1980;14:1547.Barker MW, McHenry WE. In: The Chemistry of Ketenes, Allenes and Related Compounds. Part 2. Patai S, editor. Wiley-Interscience; Chichester, UK: 1980. pp. 701–720.Alajarin M, Vidal A, Tovar F. Targets Heterocycl Syst. 2000;4:293.
- 16.Whiting M, Fokin VV. Angew Chem Int Ed. 2006;45:3157. doi: 10.1002/anie.200503936. [DOI] [PubMed] [Google Scholar]
- 17.For leading reviews on aza-Claisen rearrangements, see: Majumdar KC, Bhayyacharyya T, Chattopadhyay B, Nandi RK. Synthesis. 2009:2117.Nubbemeyer U. Top Curr Chem. 2005;244:149.
- 18.For general reviews on Claisen rearrangements, see: Castro AMM. Chem Rev. 2004;104:2939. doi: 10.1021/cr020703u.Ito H, Taguchi T. Chem Soc Rev. 1999;28:43.Enders D, Knopp M, Schiffers R. Tetrahedron Asym. 1996;7:1847.Wipf P. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 5. Pergamon Press; Oxford: 1991. p. 827.
- 19.For some examples of aza-Claisen rearrangements, see: Majumdar KC, Samanta S, Chattopadhyay B, Nandi RK. Synthesis. 2010:863.Cheung LLW, Yudin AK. Org Lett. 2009;11:1281. doi: 10.1021/ol900118d.Cant AA, Bertrand GHV, Henderson JL, Roberts L, Greaney MF. Angew Chem Int Ed. 2009;48:5199. doi: 10.1002/anie.200901410.Yasui H, Yorimitsu H, Oshima K. Chem Lett. 2008;37:40.Walters MA. J Am Chem Soc. 1994;116:11618.Jolidon S, Hansen HJ. Helv Chim Acta. 1977;60:978.For some earlier work of palladium promoted aza-Claisen-type rearrangements using N-allylthioamide, allylimidates, or allyl carbamates, see: Overman LE. Acc Chem Res. 1980;13:218.Overman LE. Angew Chem, Int Ed Engl. 1984;23:579.Tamaru Y, Kagotani M, Yoshida Z. J Org Chem. 1985;50:764.Overman LE, Donde Y. J Am Chem Soc. 1999;121:2933.Lei A, Lu X. Org Lett. 2000;2:2357. doi: 10.1021/ol006130u.
- 20.For a related 1,3-shift, see: Bendikov M, Duong HM, Bolanos E, Wudl F. Org Lett. Vol. 7. 2005. p. 783.It is noteworthy that our work distinctly indicates that the N-to-C 1,3-Ts shift took place after the aza-Claisen rearrangement. Monitoring the thermal aza-Claisen rearrangement of 11 using NMR did not reveal any ketenimine 12, thereby suggesting 1,3-Ts shift was very fast at 110 °C.
- 21.For leading references, see: Fleming FF, Liu W. Eur J Org Chem. 2009:699.Fleming FF, Wei G, Steward OW. J Org Chem. 2008;73:3674. doi: 10.1021/jo8001062.Mermerian AH, Fu GC. Angew Chem Int Ed. 2005;44:949. doi: 10.1002/anie.200461886.
- 22.For some of the conditions explored, see: Pd(0) source: Pd2(dba)3, Pd(PPh3)4; ligand: xantphos, X-Phos, (C6F5)3P; solvent: THF, Dioxane; concn = 0.04–0.10 M; Lewis acid: Zn(OTf)2, Cu(OTf)2,Sc(OTf)3, Ln(OTf)3; base: K2CO3; additive: t-BuOH, PhOH, 4-NO2C6H4OH, 2,6-dimethylphenol, 2,3-dimethylphenol.
- 23.Sosa JR, Tudjarian AA, Minehan TG. Org Lett. 2008;10:5091. doi: 10.1021/ol802147h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.For reviews, see: Habermas KL, Denmark SE, Jones TK. In: Organic Reactions. Paquette LA, editor. Vol. 45. John Wiley & Sons; New York: 1994. pp. 1–158.Denmark SE. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 5. Pergamon; Oxford, U.K: 1991. pp. 751–784.Frontier A, Collison C. Tetrahedron. 2005;61:7577.Tius MA. Eur J Org Chem. 2005:2193.Pellissier H. Tetrahedron. 2005;61:6479.Tius MA. Acc Chem Res. 2003;36:284. doi: 10.1021/ar0200394.Harmata M. Chemtracts. 2004;17:416.
- 25.For examples of imino-Nazarov cyclizations, see: Tius MA, Chu CC, Nieves-Colberg R. Tetrahedron Lett. 2001;42:2419.Kim SH, Cha JK. Synthesis. 2000:2113.Bow WF, Basak AK, Jolit A, Vicic DA, Tius MA. Org Lett. 2010;12:440. doi: 10.1021/ol9025765.
- 26.Rautenstrauch V. J Org Chem. 1984;49:950. [Google Scholar]
- 27.For a leading reference on Rautenstrauch rearrangement, see: Shi X, Gorin DJ, Toste FD. J Am Chem Soc. 2005;127:5802. doi: 10.1021/ja051689g.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.