Abstract
Extracellular proteolysis mediates tissue homeostasis. In cancer, altered proteolysis leads to unregulated tumor growth, tissue remodeling, inflammation, tissue invasion, and metastasis. The matrix metalloproteinases (MMPs) represent the most prominent family of proteinases associated with tumorigenesis. Recent technological developments have markedly advanced our understanding of MMPs as modulators of the tumor microenvironment. In addition to their role in extracellular matrix turnover and cancer cell migration, MMPs regulate signaling pathways that control cell growth, inflammation, or angiogenesis and may even work in a nonproteolytic manner. These aspects of MMP function are reorienting our approaches to cancer therapy.
Introduction
Cancer originates from mutations in genes that regulate essential pathways of cell function leading to uncontrolled outgrowth of tissue cells (Hanahan and Weinberg, 2000). The resulting tumors are complex structures of malignant cancer cells embedded in vasculature and surrounded by a dynamic tumor stroma consisting of various nonmalignant cells, such as fibroblasts and myeloid cells. The milieu of the tumor microenvironment is akin to the inflammatory response in a healing wound, which promotes angiogenesis, turnover of the extracellular matrix (ECM), and tumor cell motility (Coussens and Werb, 2002). Understanding the molecular mechanisms of this complex interplay between malignant cancer cells and the surrounding nonmalignant stroma represents one of the major challenges in cancer research.
Mounting evidence supports the view that extracellular proteinases, such as the matrix metalloproteinases (MMPs), mediate many of the changes in the microenvironment during tumor progression. These enzymes regulate a variety of physiological processes and signaling events, and thus they represent key players in the molecular communication between tumor and stroma. Here, we review the recent advances in our understanding of MMP-driven regulation of the tumor microenvironment. Regarding the failure of MMP inhibitors as targets for anticancer therapy in clinical trials, we critically discuss the new insights into the functions of these extracellular proteinases in cancer, which, depending on the circumstances, may either suppress or promote tumorigenesis, or even act independently of their proteolytic activity.
Characteristics of the MMP Family
MMPs are a family of zinc-dependent endopeptidases first described almost half a century ago (Gross and Lapiere, 1962). They play a crucial role in various physiological processes including tissue remodeling and organ development (Page-McCaw et al., 2007), in the regulation of inflammatory processes (Parks et al., 2004), and in diseases such as cancer (Egeblad and Werb, 2002). The 23 MMPs expressed in humans are categorized by their architectural features. The general structural blueprint of MMPs shows three domains that are common to almost all MMPs, the pro-peptide, the catalytic domain, and the hemopexin-like C-terminal domain that is linked to the catalytic domain via a flexible hinge region (Figure 1A). MMPs are initially expressed in an enzymatically inactive state due to the interaction of a cysteine residue of the pro-domain with the zinc ion of the catalytic site. Only after disruption of this interaction by a mechanism called cysteine switch, which is usually mediated by proteolytic removal of the pro-domain or chemical modification of the cysteine residue, does the enzyme become proteolytically active. The pro-domain contains a consensus sequence and requires proteolytic cleavage by convertases, which, depending on the sequences, occurs intracellularly by furin or extracellularly by other MMPs or serine proteinases such as plasmin (Sternlicht and Werb, 2001).
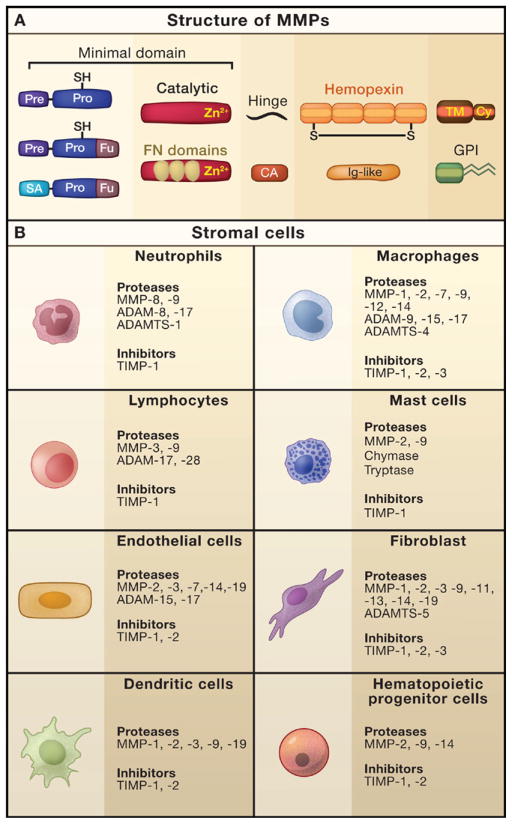
Figure 1. MMP Composition and Expression in the Stroma.
(A) Matrix metalloproteinases (MMPs) are comprised of different subdomains. All MMPs have the “minimal domain” in common, which contains three principal regions: an amino-terminal signal sequence (Pre) to be cleaved by the signal peptidase during entry into the endoplasmic reticulum, a pro-domain (Pro) containing a thiol-group (-SH) and a furin cleavage site, and the catalytic domain with a zinc-binding site (Zn2+). Interaction of the -SH group of the pro-domain with the zinc ion of the catalytic domain keeps the enzyme as an inactive zymogen. Activation of the zymogen is often mediated by intracellular furin-like proteinases that target the furin recognition motif (Fu) between the pro-domain and the catalytic domain. In addition to the minimal domain, most MMPs possess a hemopexin-like region, a domain composed of four repeats that resemble hemopexin and contain a disulfide bond (S-S) between the first and the last subdomain, which is linked to the catalytic domain via a flexible hinge region. Besides their differential domain structure, MMPs can be principally divided into secreted (MMP-1, -2, -3, -7, -8, -9, -10, -11, -12, -13, -19, -20, -21, -22, -27, -28) and membrane-anchored proteinases (MMP-14, -15, -16, -17, -23, -24, -25), the latter of which use either a transmembrane domain (TM) with a cytoplasmic domain (Cy) attached to it, a glycosylphosphatidylinositol (GPI) anchor, or an amino-terminal signal anchor (SA), which is only the case for MMP-23, as it is anchored in the plasma membrane. MMP-23 also contains the unique cysteine array (CA) and an immunoglobulin (Ig)-like domain. The gelatinases MMP-2 and -9 show gelatin-binding repeats that resemble the collagen-binding type II motif of fibronectin (FN).
(B) Expression pattern of proteinases and their physiological inhibitors in non-malignant stromal cells. Cells commonly found in the microenvironment of many cancers include inflammatory cells (such as neutrophils, macrophages, dendritic cells, lymphocytes, and mast cells), endothelial cells, fibroblasts, and hematopoietic progenitor cells. These cells express a plethora of proteinases that are released into the extracellular space and influence multiple events of tumor progression. Selected examples of proteinases and endogenous inhibitors expressed by these cell types are shown.
Closely related to the MMPs are the so-called ADAM (a disintegrin and metalloproteinase) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) families of metzincin proteinases. ADAMs fulfill a broad spectrum of functions with roles in fertilization, development, and cancer (Edwards et al., 2008). Most ADAMs are membrane-anchored and function in the pericellular space. Although all of them have a metalloproteinase domain, only about half of them exhibit proteolytic activity, indicating that ADAMs function by shedding interaction partners or by mediating the biological roles in a nonproteolytic manner. The ADAMTS enzymes have a protease domain, an adjacent disintegrin domain, and one or more thrombospondin domains and are generally secreted and soluble. They play roles in ECM assembly, ovulation, and cancer. The role of these other metzincin proteinases in cancer has recently been extensively discussed elsewhere (Murphy, 2008). This Review will only highlight selected examples of their effects on the tumor microenvironment.
The function of MMPs in vivo depends on the local balance between them and their physiological inhibitors. Substantial energy resources of the human body are allocated for the prevention of unregulated extracellular proteolysis by MMPs and other proteinases. For example, high concentrations of the proteinase inhibitors α2-macroglobulin (α2-MG), α1-proteinase inhibitor (α1-PI), and α1-chymotrypsin (α1-CT) are produced in the liver and released into the plasma, where these molecules efficiently bind to the active site of a variety of proteinases (reviewed in Woessner and Nagase, 2000). The resulting proteinase-inhibitor complexes are then recognized by a scavenger receptor and swiftly engulfed by macrophages. The most important physiological inhibitors of MMP function are the tissue inhibitors of metalloproteinases (TIMPs), which are also commonly expressed in tumor sites (Deryugina and Quigley, 2006). TIMP-1, -2, -3, and 4 form 1:1 stochiometric complexes with active MMPs leading to inhibition of proteolytic activity. Similar to MMPs, the proteolytic ADAM and ADAMTS family members are inhibited by specific TIMPs (Murphy, 2008).
The expression of metalloproteinases and their inhibitors in the tumor microenvironment is quite diverse. Although cancer cells from various tissues can express members of the MMP and ADAM families as well as TIMPs, the major source of these proteinases is from stromal cells infiltrating the tumor (Egeblad and Werb, 2002). The different types of stromal cells produce a specific set of proteinases and proteinase inhibitors (Figure 1B), which are released into the extracellular space and specifically alter the milieu around the tumor. The cellular source of MMPs can therefore have important consequences on their function and activity; for example, neutrophil-derived MMP-9, which does not have a bound TIMP-1 molecule, is more readily activatable (Ardi et al., 2007).
Regulation of MMP Activity
The complexity of the tumor microenvironment allows for a variety of regulatory cascades that determine the functions of the diverse MMPs expressed. Proteolytic activity of MMPs can be regulated at different levels: gene expression, compartmentalization, conversion from zymogen to active enzyme, and, finally, the presence of specific inhibitors. When judging the pathophysiological relevance of increased expression of proteinases in tumor tissues, the particular context is important, that is whether endogenous inhibitors or activating (converting) enzymes in the microenvironment are present.
A key step in regulating MMP activity is the conversion of the zymogen into an active proteolytic enzyme. There are several proteinases that mediate MMP activation, such as plasmin, furin, or active MMPs (Sternlicht and Werb, 2001). Once activated, MMP-2, -3, -7, -9, and -12 may launch a negative feedback signal, for example, by degrading plasminogen and thus interfering with plasminogen conversion to active plasmin. This complex regulation of MMP activity is necessary, given that unhampered proteinase activity released from inflammatory cells may lead to tissue damage and the perpetuation of the inflammatory response in chronic inflammatory diseases and cancer (Parks et al., 2004). An example of how this process is controlled is shown by the observation that MMPs also degrade plasmin-suppressing serpin proteinase inhibitors and therefore promote the conversion of pro-MMPs. For example, MMP-3 is a potent inactivator of α2-AP (Lijnen et al., 2001), and several MMPs inactivate other serpins such as α1-PI and α1-CT and thus prolong the catalytic activity of extracellular proteinases that are normally inhibited by these molecules. A crucial interplay of MMP-9, α1-PI, and neutrophil elastase occurs in skin blister formation, in which MMP-9 efficiently degrades α1-PI, a physiological serpin inhibitor of neutrophil elastase and other serine proteinases. This promotes elastase-mediated matrix degradation that manifests in dermal-epidermal separation and blister formation in vivo (Liu et al., 2000). This study nicely illustrates the interaction between extracellular proteinases and their endogenous inhibitors as may occur in normal physiological situations and in the course of disease (Figure 2).
Figure 2. Proteolytic Cascades Regulate MMP Function.
MMPs are synthesized as inactive zymogens that need to be activated by proteolytic removal of the pro-domain (for instance, as carried out by plasmin). Several MMPs exert an autocrine feedback by degrading several physiological proteinase inhibitors that inhibit proteolytic conversion of MMPs including α1-chymotrypsin (α1-CT), α1-proteinase inhibitor (α1-PI), and α2-antiplasmin (α2-AP) (1). Some pro-MMPs can also be converted by other MMPs. Selected examples for mutual MMP conversion are given (2). Another physiological inhibitor, α2-macroglobulin (α2-MG), normally inhibits MMP activity but can also be degraded by several MMPs, which then prolongs MMP function (3). Inflammatory cells frequently infiltrate the tumor microenvironment and produce large amounts of reactive oxygen species (ROS), which may promote MMP activation via oxidation of the pro-domain cysteine. Myeloperoxidase (MPO) of infiltrating neutrophil catalyzes the transformation of ROS into hypochloric acid (HOCl), which may interfere with MMP activity by chemical modification of crucial residues of the catalytic domain (4). On the other hand, active MMPs may also launch a negative feedback, for instance, by degrading plasminogen and therefore prohibiting the conversion into MMP-activating plasmin (5). The complex interaction of proteinases and their inhibitors under physiological circumstances happens upstream of the physiological functions of MMPs, such as matrix remodeling, angiogenesis, cellular signaling, and cancer cell migration.
The function of MMPs can also be influenced by reactive oxygen species (ROS). The inflammatory response at the tumor site creates large amounts of ROS that are produced by activated neutrophils and macrophages. These oxidants initially activate MMPs via oxidation of the pro-domain cysteine (Weiss et al., 1985) but, eventually, in combination with the enzyme myeloperoxidase contributed by inflammatory cells, inactivate MMPs by modification of amino acids of the catalytic domain by hypochlorous acid (Fu et al., 2003).
The localization or compartmentalization of MMPs under physiological conditions often dictates their biological function. Several MMPs interact with surface receptors such as integrins or localize to specific areas of the ECM, which potentiates MMP activity by increasing their local concentration and also may interfere with accessibility to endogenous inhibitors (reviewed in Nagase et al., 2006). The binding of MMP-2 to integrin αvβ3 via its hemopexin domain is crucial for mesenchymal cell invasive activity (Rupp et al., 2008). Likewise, high local concentrations of active MMP-14 on the cell membrane of metastatic cancer cells play important roles in cell migration (Friedl and Wolf, 2008; Sabeh et al., 2004, 2009; Wolf et al., 2007). However, there may also be additional mechanisms to concentrate extracellular proteinases in specific sites in the microenvironment. One new example relates to neutrophilic granulocytes, which, upon cellular activation, spill out their nuclear chromatin to form so-called neutrophil extracellular traps (NETs), web-like structures with highly concentrated proteinases such as MMP-9 and leukocyte elastase localized on the extracellular chromatin scaffold (Brinkmann et al., 2004). These NETs act primarily to fight off bacterial infections, but they also contribute to the pathogenesis of autoimmune diseases (Kessenbrock et al., 2009). It remains to be determined if NETs are formed by neutrophils in the tumor microenvironment, and whether these structures play a role in malignant diseases by compartmentalizing and localizing proteinase activity to certain sites in the tumor.
Mechanical forces contribute to tumor progression (Butcher et al., 2009), potentially by modulating proteolysis of ECM components. These forces may unwind the conformation of MMP substrate proteins, thus allowing recognition and cleavage by proteinases. Von Willebrand factor (VWF), a major regulator of primary hemostasis and blood clotting, is secreted as an ultralong chain composed of hundreds of VWF monomers. The large size of this multimolecular complex renders it sensitive to high shear forces in the blood flow that are typically found at sites of injury. These increased shear forces initiate a conformational change of the complex, namely, unfolding of VWF domain 2 leading to the exposition of a cleavage site targeted by ADAMTS-13, which then cleaves VWF into smaller pieces to initiate blood clotting (Zhang et al., 2009). In a similar fashion, the ECM component fibronectin is also unfolded by mechanical forces in the ECM of living cells (Smith et al., 2007). Given that fibronectin is cleaved by several MMPs, the mechanotransduced unfolding of fibronectin might promote proteolytic degradation of this MMP substrate. Tumor progression is frequently characterized by increased tissue stiffness, elevated interstitial fluid pressure, and altered blood flow conditions (Butcher et al., 2009). Thus, it is conceivable that similar mechanisms involving mechanical force are regulatory factors for MMP function in the tumor microenvironment.
Studying MMP Function In Vivo
The physical location and the time frame of MMP enzymatic activity are fundamental to the physiological role of these enzymes in tumor progression. However, for most cancers it still remains rather elusive when and where these enzymes exhibit their proteolytic activity in the tumor microenvironment. An emerging noninvasive technological approach to address these questions utilizes the recently developed imaging probes based on MMP-specific activities. This may help to overcome a number of technical hurdles of clinical importance, such as the detection of early-stage tumors with increased sensitivity by exploiting the proteolytic activity of MMPs, the identification of tumors that might benefit from the use of metalloproteinase inhibitors (MPIs) as anticancer drugs, and the endpoint assessment of the efficacy of MPI compounds to inhibit MMP activity in vivo. To date, imaging MMP activity has mostly depended on fluorescent optical imaging modalities including fluorescent resonance energy transfer (FRET), radiolabeled imaging such as positron emission tomography (PET), single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI) (reviewed in Scherer et al., 2008a). Fluorogenic MMP substrates noninvasively show that tumors have increased MMP activity compared to non-tumor-bearing animals (Littlepage et al., 2010) and can assess the efficacy of MPIs on MMP activity in intact tumors in vivo directly (Bremer et al., 2001).
Clinical imaging technologies such as PET and SPECT that are used for cancer diagnostics and staging in the clinic also can be used to detect MMP activity in vivo. For example, PET has been used to detect specific MMP activity in cancer using an 18F-labeled MMP-2 inhibitor that accumulates in the tumor of a breast cancer model in mice (Furumoto et al., 2003). Radiolabeled 123I-MMP inhibitors designed to recognize the active site of specific MMPs can detect MMP-2 and MMP-9 activity using SPECT (Schafers et al., 2004). A radiolabeled 99 mTc-anti-MMP-14 monoclonal antibody developed as a radiolabeled SPECT probe in vivo detects malignant cells in rodents bearing breast tumors (Temma et al., 2009). Gadolinium-based paramagnetic contrast agents that carry an MMP-sensitive probe become less hydrophilic upon proteolytic cleavage resulting in a detectable contrast change that has been used with MMP-2 (Jastrzebska et al., 2009). Given that MRI facilities are widely available in clinical centers and are commonly utilized for detecting tumors, this methodology has potential as a diagnostic tool for cancer patients.
Using higher-resolution probes, it is now possible to narrow down the sites in the tumor microenvironment at which MMPs exert their activity. For example, cell-penetrating peptides, which are activated by proteolysis and carry a fluorescent cargo that accumulates in cells that are in the vicinity of active MMPs (Jiang et al., 2004), have been used successfully to visualize MMP-2 and -9 activity in cell culture systems and in mouse xenograft models and show active MMPs predominantly at the interface between tumor and stroma (Olson et al., 2009). Fluorogenic substrates based on self-quenched and near-infrared FRET pairs, so-called proteolytic beacons, created to reduce absorption and scattering and increase tissue penetration have demonstrated specific increases in MMP activity at the tumor’s leading edge in models of colon and pancreatic cancers (Scherer et al., 2008a). For example, optical imaging of MMP-7 activity in vivo using a specific near-infrared polymer-based proteolytic beacon can detect tumors as small as 0.01 cm2 in mice (Scherer et al., 2008b). Moreover, such probes can be used to image MMP activity at subcellular resolution during live-cell migration in extracellular matrices in vitro. To this end, experiments using matrices derivatized with a fluorogenic probe based on the peptide sequence present in interstitial collagen targeted by a variety of proteases including MMP-2, -9, and -14 detect MMP activity predominantly at the leading edges of migrating tumor cells (Packard et al., 2009). This new type of high-resolution probe provides site-specific reporting of protease activity and insights into mechanisms by which cells migrate through extracellular matrices.
Although in vivo imaging of MMP activity is a relatively young field of research with many technological challenges ahead, it is a promising strategy, especially regarding the future of MPIs as anticancer drugs. Given that the majority of clinical trials with MPIs have failed, presumably due to treatment of advanced-stage tumors, imaging with sensitive MMP probes will help to determine the adequate time window of MMP activity, in which MPI administration is most likely to be effective. Further advances in noninvasive imaging of MMP activity may be crucial to determine these therapy options.
New Insights into the Roles of MMPs in Cancer
MMPs have been implicated in cancer for more than 40 years, and the notion that MMP-mediated ECM degradation leads to cancer cell invasion and metastasis has been a guiding principle in MMP research (Liotta et al., 1980). The discovery that inhibition of MMPs suppresses the invasive potential of tumors in animal studies was swiftly implemented into clinical trials. Yet, these failed to increase survival rate of the patients (Coussens et al., 2002). It is now evident that MMP function is more complex than initially thought, given that these enzymes do more than degrade physical barriers. Rather, they also affect multiple signaling pathways that modulate the biology of the cell in normal physiological processes and in disease. Moreover, members of the ADAM and ADAMTS families of proteinases, which are also targeted by inhibitors with broad-spectrum anti-metzincin activity, are also associated with tumor progression (Murphy, 2008). Although the current understanding is that extracellular proteolysis is mostly implicated in cancer promotion, MMPs and other proteinases do exhibit tumor-suppressing effects in several circumstances. In addition, MMPs mediate a wide range of biological effects on their surrounding tissue. In the following sections, we discuss the recent insights gained on the physiological or pathological processes modulated by MMPs, which are likely to have significant consequences on the tumor microenvironment.
MMPs Affect Growth Signals
Unregulated proliferation is a common feature of cancer cells. There are two principal ways in which the tumor achieves this condition: by acquiring self-sufficiency in growth-promoting signals or by becoming insensitive to antigrowth signals. MMPs may be critically involved in disrupting the balance between growth and antigrowth signals in the tumor microenvironment, as they potently influence the bioavailability or functionality of multiple important factors that regulate growth.
One fundamental signaling pathway with essential roles in tissue homeostasis is the transforming growth factor-β (TGF-β) pathway. TGF-β normally exerts tumor-suppressive effects by enforcing cytostasis and differentiation. However, as the tumor advances in malignant progression, the genome often accumulates mutations in the TGF-β receptor system that render the cancer cells unresponsive to TGF-β. Moreover, its multiple effects on nonmalignant stromal cells, such as evasion of immune surveillance, can be exploited by the tumor and hence turn TGF-β into a tumor-promoting factor that leads to increased invasion and metastasis (Massague, 2008). Active TGF-β is derived from an inactive pro-form by proteolytic conversion by furin or other proteinases, such as MMP-9, which is usually expressed by inflammatory cells. MMP-9 is compartmentalized to the cell surface by docking to the surface receptor CD44 and then proteolytically activates TGF-β (Yu and Stamenkovic, 2000). Similarly, MMP-14 and MMP-2 proteolytically activate TGF-β1 (Mu et al., 2002). On the other hand, MMP-2 and MMP-9, as well as MMP-14, indirectly modulate TGF-β bioactivity by cleaving the ECM component latent TGF-β-binding protein 1 (LTBP-1), thereby solubilizing ECM-bound TGF-β (Dallas et al., 2002; Tatti et al., 2008). Given that tumor cells often acquire nonresponsiveness to TGF-β, this suggests that proteolytic activation of TGF-β by MMPs has tumor-promoting effects by selectively driving stroma-mediated invasion and metastasis of the tumor.
Ligands for the epidermal growth factor receptor (EGFR) are potent drivers of cell proliferation and important regulators of tissue homeostasis. Malfunction of this system by genetic mutations of the molecules involved is frequently observed in breast cancer and other malignant diseases (Hynes and Lane, 2005). Evidence emerging from recent studies has revealed a potential role of ADAM proteinases in the regulation of the EGFR pathway. For example, ADAM-10 triggers the release of soluble EGF, whereas ADAM-17 is a major converter of pro-forms of other EGFR ligands such as TGF-α and epiregulin. Activation of EGFR results in the upregulation of MMP-9, which in turn degrades E-cadherin, a potent control element of many cellular functions including cell-cell adhesion and differentiation. This association between EGFR, MMP-9, and E-cadherin may play an important role in ovarian cancer and metastasis, as activated EGFR and MMP-9 in these specimens colocalize with a region of reduced E-cadherin (Cowden Dahl et al., 2008). The cleavage of E-cadherin by MMPs or ADAM proteinases has an impact on cancer cell proliferation. Interestingly, ADAM-10 mediates the shedding of E-cadherin, which results in β-catenin translocation to the nucleus, driving cell proliferation (Maretzky et al., 2005). Moreover, overexpression of MMP-3 in mammary epithelium triggers a cascade of events including the cleavage of E-cadherin resulting in epithelial-mesenchymal transition (Lochter et al., 1997; Radisky et al., 2005). Combining an inhibitor of these metalloproteinases with a dual inhibitor of EGFR and HER-2/neu kinases synergistically prevents the growth of human breast cancer xenografts (Witters et al., 2008). These studies provide mechanistic insight into proteolytic acceleration of cell growth and suggest that specific inhibition of these metalloproteinases may be utilized to interfere with unregulated cell growth and proliferation in many tumors.
MMPs Regulate Apoptosis
Evading programmed cell death, or apoptosis, is another strategy that increases the cell number and size of tumors. Apoptosis is normally initiated via extracellular receptors such as the Fas receptors, which activate a proteolytic cascade of intracellular caspases once they encounter Fas ligand, ultimately leading to the selective degradation of subcellular compartments and nuclear DNA. MMP function interferes with the induction of apoptosis in malignant cells, which may involve the cleavage of ligands or receptors that transduce proapoptotic signals. MMP-7 cleaves Fas ligand from the surface of doxorubicin-treated cancer cells (Mitsiades et al., 2001), lowering the impact of chemotherapy on the tumor by abrogating apoptosis. Indeed, MMP-7 expression may serve as a predictive marker for the resistance to chemotherapy in patients with non-small cell lung cancer (Liu et al., 2008a). Similarly, ADAM-10 may suppress apoptosis induction by cytotoxic lymphocytes via the degradation of Fas ligand (Schulte et al., 2007), thus interfering with Fas receptor-triggered cell death of target cells. The interaction between MMP-7 and Fas ligand also may play a role in pancreatic ductal adenocarcinoma, as MMP-7 is expressed in human pancreatic cancer specimens and mice deficient in MMP-7 or carrying a nonfunctional Fas ligand mutation show greatly reduced metaplasia during pancreatic duct ligation (Crawford et al., 2002). Moreover, proteolytic shedding of tumor-associated major histocompatibility complex class I-related proteins MICA and MICB by ADAM-17 can potently suppress NK cell-mediated cytotoxicity toward the cancer cells (Waldhauer et al., 2008) and thereby potentially interfere with an antitumor-directed immune response. It remains unknown whether MMPs can interfere in a similar manner with NK cell-mediated tumor killing; however, the use of MMP inhibitors in combination with interleukin-15 succeeded in overcoming the resistance of small-cell lung cancer cells to NK cell killing (Le Maux Chansac et al., 2008). Hence, these examples suggest a tumor-promoting role of these metalloproteinases by blocking receptor-transmitted or lymphocyte-mediated apoptosis.
The Tumor Vasculature
The tumor vasculature is derived from sprouting of local blood vessels (angiogenesis) and circulating vasculogenic progenitor cells derived from the bone marrow (vasculogenesis). The new vessels are often irregular and leaky due to lack of the pericyte cover, with the result that tumor cells can penetrate them more easily. As compared with blood capillaries, lymphatic endothelial cells have even less developed junctions with frequently large interendothelial gaps and impaired basement membranes. The invasive margin is a critical area for stimulation of angiogenesis and lymphangiogenesis in tumors, which contributes to tumor invasion and metastasis (Padera et al., 2002). As discussed below, several lines of evidence support an important function of MMPs in angiogenic or lymphangiogenic processes. The major MMPs involved in tumor angiogenesis are MMP-2, -9, and -14, and to a lesser extent MMP-1 and -7. Given that several MMPs are expressed in all tumors, it is now evident that each MMP can contribute to distinct vascular events in the same tumor (Littlepage et al., 2010).
MMP-9 has a distinct role in tumor angiogenesis, mainly regulating the bioavailability of vascular endothelial growth factor (VEGF), the most potent inducer of tumor angiogenesis and a major therapeutic target. MMP-9, conveyed by inflammatory cells, enables an angiogenic switch by making sequestered VEGF bioavailable for its receptor VEGFR2 in pancreatic islet tumors (Bergers et al., 2000). Angiogenic switching by MMP-9 involves a complex interplay of interconnected factors. In a mouse model of glioblastoma, the hypoxia inducible factor-1α (HIF-1α) induces recruitment of CD45-positive bone marrow-derived cells, as well as endothelial and pericyte progenitor cells, to promote neovascularization. MMP-9 activity provided by these CD45-positive myeloid cells is essential and sufficient for the angiogenic switch by increasing VEGF bioavailability. This process induces angiogenesis but also regulates tumor cell invasiveness. Interestingly, VEGF prevents tumor cell migration along blood vessels, but it promotes perivascular tumor cell infiltration into the brain parenchyma (Du et al., 2008). This action of VEGF as a brake on perivascular tumor cell migration is surprising. In addition, the direct cleavage of matrix-bound VEGF by MMP-3, -7, -9, or -16 results in modified VEGF molecules with altered bioavailability, which changes the vascular patterning of tumors in vivo (Lee et al., 2005).
In addition to its role in regulating angiogenesis, MMP-9 is also implicated in vasculogenesis. Tumors transplanted into tissue irradiated to prevent angiogenesis are unable to grow in MMP-9-deficient mice. However, tumor growth is restored by transplanting CD11b-positive myeloid cells from the bone marrow of MMP-9-sufficient mice, suggesting that MMP-9 is required for tumor vasculogenesis (Ahn and Brown, 2008). MMP-9 could therefore be an important target for adjunct therapy to enhance the response of tumors to radiotherapy.
A special role is attributed to MMP-9 delivered by neutrophilic granulocytes. In contrast to other cell types, neutrophil-derived pro-MMP-9 is not complexed with TIMP-1 and therefore is more readily activated to drive tumor angiogenesis (Ardi et al., 2007). The angiogenic function of neutrophil MMP-9 requires both its active site and hemopexin domain and activates the basic fibroblast growth factor 2 (FGF-2) pathway (Ardi et al., 2009). This highlights the important effects of proteinase inhibitors on the function of MMPs and shows that MMPs produced by different cell types may function in different ways. The release of TIMP-1-free MMP-9 may be an important facet in the pro-angiogenic effects triggered by tumor-infiltrating neutrophils. Indeed, elevated numbers of neutrophils present in patients with myxofibrosarcoma correlate with microvessel density in the tumor (Mentzel et al., 2001), and depleting neutrophils in a mouse model of pancreatic cancer markedly reduces angiogenic switching in dysplasias (Nozawa et al., 2006). These findings support an important role of infiltrating neutrophils in the induction of tumor angiogenesis.
The degradation of ECM components and other extracellular molecules may generate fragments with new bioactivities that inhibit angiogenesis (Ribatti, 2009). For example, biologically active endostatin is generated via cleavage of type XVIII collagen by MMP-3, -7, -9, -13, and -20 (Heljasvaara et al., 2005). Moreover, the degradation of collagen IVα3 by MMP-9 results in the generation of the monomeric NC1 domain, called tum-statin, a potent suppressor of angiogenesis. This manifests in pathological vascularization and increased tumor growth in MMP-9-deficient mice (Hamano et al., 2003). The degradation of plasminogen by MMP-2, -9, and -12 can produce significant amounts of angiostatin, a cleavage product with antiangiogenic function (Cornelius et al., 1998; Patterson and Sang, 1997). Angiostatin production by MMP-12 may explain the suppressive effects of this MMP on outgrowth of lung metastases (Houghton et al., 2006a). Taken together, MMPs can generate both angiogenesis-inhibiting as well as -promoting signals. Depending on the time frame of MMP expression and the availability of substrates, the effects of MMPs on angiogenesis might be diverse.
MMPs also regulate vascular stability and permeability. MMPs, particularly MMP-14, appear to mediate the vascular response to tissue injury and tumor progression though activation of TGF-β (Sounni et al., 2010). Use of MPIs or TGF-β signaling inhibitors potentially could improve patient care as they may improve delivery of imaging agents or therapeutic tumor tissues.
Lymphangiogenesis plays an important role in tumor biology, given that it is directly linked with the formation of lymphatic metastases. MMPs certainly have a general impact on lymphangiogenesis as supported by the use of broad-spectrum MMP inhibitors (Nakamura et al., 2004). However, only a few reports directly link MMPs to the formation of new lymphatic vessels. The modulation of VEGF bioavailability by MMPs, especially by MMP-9, may also affect lymphangiogenesis and, in turn, promotes dissemination of metastases into the lymph. The most direct proof for MMP involvement in lymphangiogenesis has come from experiments modeling lymphangiogenesis in a three-dimensional culture system using mouse thoracic duct fragments embedded in a collagen gel in which lumen-containing lymphatic capillaries form (Bruyere et al., 2008). Increased expression of MMP-1, MMP-2 (Langenskiold et al., 2005) and MMP-3 (Islekel et al., 2007) is linked with lymphatic invasion and lymph node metastases. Inhibition of MMP-2, -9, and -14 attenuates both angiogenesis and lymphangiogenesis and reduces lymph node metastasis (Nakamura et al., 2004). The lymphatic vasculature, but not aortic vasculature, is impaired by targeted deletion of MMP-2 (Bruyere et al., 2008). Future studies are needed to clarify the specific pathways affected by MMPs in the regulation of lymphangiogenesis.
Adipocyte Regulation Affects Tumor Progression
Adipocytes are a prominent part of the tumor stroma and contribute to cancer progression. Unquestionably, there are consequences for the local paracrine crosstalk between the tumor cells and adipocytes. White adipose tissue functions in energy storage and is an endocrine organ made up of adipocytes, various stromal cells, resident and infiltrating immune cells, and an extensive endothelial network. Adipose secretory products, collectively referred to as adipokines, have been identified as contributors to the negative consequences of adipose tissue expansion in cancer, cardiovascular disease, and diabetes (Rutkowski et al., 2009). Moreover, adipokines such as leptin, regulate the expression and activation of MMPs; for example, leptin induces the MMP-13 production in glioma cells leading to increased migration and tumor invasion (Yeh et al., 2009). Human adipose tissue-derived stem cells cocultured with cancer cells produce CCL5, which, in turn, promotes breast cancer cell invasion associated with MMP-9 activity (Pinilla et al., 2009).
Recent reports link MMPs or TIMPs to the interplay between adipose tissue and the epithelium and subsequently to cancer transformation. MMPs affect the development of the adipose tissue, which, in turn, may affect the epithelium. MMPs, especially MMP-3, determine the rate of adipocyte differentiation during mammary gland remodeling during post-lactational involution, when programmed cell death of the secretory epithelium takes place concomitant with the repopulation of the mammary fat pad with adipocytes. Fibroblastic adipogenic progenitor cells express very low levels of MMPs or TIMPs. The transcription of a number of MMP and TIMP mRNAs (MMP-2, -3, -13, and -14 and TIMP-1, -2, and -3) is induced in committed preadipocytes, and differentiated adipocytes express activated MMP-2. During involution, mammary glands from transgenic mice that overexpress the tissue inhibitor of metalloproteinases, TIMP-1, or mice carrying a targeted mutation in MMP-3 show accelerated differentiation and hypertrophy of adipocytes (Alexander et al., 2001). MMP-14 also contributes to the coordination of adipocyte differentiation, as the absence of MMP-14 aborts white adipose tissue development resulting in lipodystrophic null mice (Chun et al., 2006). This defect in MMP-14 null adipocytes only becomes evident when the cells are surrounded by a three-dimensional (3D) ECM, but not in a 2D culture system. This suggests that MMP-14 acts as a 3D-specific modulator of adipogenesis by proteolytically regulating pericellular collagen rigidity.
Recent findings have identified a new member of the adipocyte “secretome” that functions to enhance MMP-2 activity. Wdnm1-like (a distant member of the whey acidic protein/four-disulfide core [WAP/4-DSC] family, which is a differentiation-dependent gene in white and brown adipogenesis) may play a role in remodeling of the extracellular milieu in adipogenesis, as well as in tumor microenvironments where adipocytes are key stromal components (Wu and Smas, 2008).
To date, the only MMP induced by adipose tissue that directly affects cancer progression is MMP-11. MMP-11 is expressed in adipose tissue as the tumor invades the surrounding environment and negatively regulates adipogenesis by reducing preadipocyte differentiation and reversing mature adipocyte differentiation. Adipocyte dedifferentiation leads to the accumulation of nonmalignant peritumoral fibroblast-like cells, which favor cancer cell survival and tumor progression. This MMP-11-mediated bidirectional crosstalk between invading cancer cells and adjacent adipocytes/preadipocytes highlights its central role during tumor desmoplasia and represents a molecular link between obesity and cancer (Motrescu and Rio, 2008).
Initiation of Neoplastic Progression
The initial process of tumor invasion shares many characteristics with the epithelial-mesenchymal transition (EMT) program during developmental processes including loss of cell-cell adhesion and increased cellular mobility (Kalluri and Weinberg, 2009). Overexpression of several MMPs, including MMP-3, -7, and -14 results in carcinoma formation (reviewed in Egeblad and Werb, 2002). A plausible mechanism is suggested by experiments showing that overexpression of MMP-3, a component of the tumor microenvironment, causes EMT and induces genomic instability in cultured mammary epithelial cells leading to all stages of neoplastic progression, malignant transformation, and mammary carcinomas in transgenic mice (Lochter et al., 1997; Sternlicht et al., 1999). These effects are linked with the expression of an alternative splice product of Rac1 that subsequently induces the generation of reactive oxygen species by mitochondria, oxidative DNA damage, and the expression of EMT-related transcription factor Snail (Radisky et al., 2005).
Tissue Invasion and Metastasis
The lethal outcome of the vast majority of all cancers is due to the dissemination of metastatic tumor cells and the outgrowth of secondary tumors at distant sites. The initiation of metastasis involves the invasion of the tumor into the peripheral tissue leading to intravasation of cancer cells into blood or lymphatic vessels from where they disseminate into secondary organs. Invasion and metastasis require the crossing of several physical barriers, such as the endothelial basement membrane. As detailed above, MMPs may account for the pathological, metastasis-prone vasculature often found in a growing tumor. Metastatic tumor cells can then enter the circulation and spread out over the body. Recent findings suggest that metastatic tumor cells specifically localize to receptive sites, called premetastatic niches, in a complex interplay with inflammatory cells and hematopoietic progenitor cells (Kaplan et al., 2005).
An interesting mechanism of MMP-mediated signal transduction linked with increased metastasis is observed in the presence of MMP-1. The proteinase-activated receptors (PARs), a set of G protein-coupled receptors with distinct functions in thrombosis and inflammation, can affect tumor invasion by inducing cancer cell migration upon proteolytic cleavage of the receptor. PAR-1 expression is increased in a number of cancers including breast, colon, and lung. A study using a xenograft model of breast carcinoma cells demonstrates a critical role for MMP-1, derived from tumor-infiltrating fibroblasts, in the cleavage of PAR-1, which appears to drive cancer cell migration and invasive behavior of the tumor (Boire et al., 2005). This exemplifies an important role of stroma-derived proteinases in the progression of tumorigenesis, carried out by specific signal transduction on cancer cells.
Bone is one of most common sites for metastasis, often leading to mortality. MMPs expressed at the interface between tumor and stromal cells play an important role in osteolysis and dissemination into bone tissue. MMP-7 expressed by osteoclasts at the tumor-bone interface triggers osteolysis and subsequent bone metastasis in a rodent model of prostate cancer (Lynch et al., 2005). The target of MMP-7 is the TNF family member RANKL (Receptor Activator for Nuclear Factor κB Ligand). Normally, RANKL is expressed on activated osteoblasts, so that the close contact between osteoclasts and osteoblasts enables binding of RANKL to its receptor RANK on osteoclast progenitors leading to osteoclast differentiation. In this context, cleavage by MMP-7 releases an active form of RANKL that promotes osteoclast activation without osteoblasts in the close proximity. A similar effect has recently been elucidated for MMP-1 and ADAMTS-1 by knocking down these proteases in a highly bone-metastatic clone of the human breast cancer cell line MDA-MB-231 (Lu et al., 2009). Further analyses reveal that ADAMTS-1 and MMP-1 proteolytically engage EGF-like ligands, resulting in activation of the RANKL pathway, which in turn promotes osteolysis and metastasis to the bone. These findings support the identification of the MMP-1 gene as part of the multigenic program that mediates bone metastasis of breast cancer cells (Kang et al., 2003). Taken together, these proteinases could serve as therapeutic targets to prevent metastasis to the bone in breast or prostate cancer.
Although there is a wide range of biological functions of MMPs in cancer, a central role is the degradation and remodeling of the ECM, paving the way through the peripheral tissue for invasion and metastasis. Recent studies using high-resolution multimodal microscopy have further corroborated the importance of ECM remodeling by MMP-14-driven pericellular proteolysis, which potently patterns the tissue to facilitate single-cell and finally collective-cell migration and invasion (Wolf et al., 2007). A number of ECM degrading proteolytic enzymes, such as MMP-1, -2, -13, and -14 and cathepsins B, K, and L have been implicated in this process; however MMP-14 may be critical and rate limiting in collagen turnover (Friedl and Wolf, 2008; Sabeh et al., 2004). A striking observation is that metastatic cancer cells can switch from a protease-dependent to a protease-independent invasion program by utilizing an amoeboid migration mode (Wolf et al., 2007). It remains a subject of ongoing debate whether the amoeboid migration mode is only relevant under in vitro conditions when the surrounding collagen network is devoid of covalent crosslinks (Sabeh et al., 2009).
Mounting evidence from in vivo analyses support the view that motility of metastatic cancer cells and their egress into the circulation occur in close cooperation with tumor-associated macrophages (Wyckoff et al., 2004; see Review by B. Qian and J.W. Pollard on page 39 of this issue). Proteolytic degradation of the endothelial basement membrane and other matrix components has long been associated with immune cell extravasation during inflammatory conditions and may be crucial for intravasation of tumor cells into the circulation. Indeed, macrophage-derived MMP-2 and -9 are important mediators of immune cell migration into the brain in a mouse model for auto-immune encephalitis, which involves degradation of the ECM component dystroglycan (Agrawal et al., 2006). This suggests that MMPs delivered by tumor-associated macrophages might contribute to intravasation of cancer cells into the blood stream. However, the significance of myeloid cell-derived MMPs in the intra- and extravasation of metastatic cancer cells needs further examination, for example by high-resolution intravital microscopy using specific MMP activity-based probes.
Metastatic Niche Formation
Certain organs such as lung, liver, or bone are the preferential sites for the formation of metastases. Metastasis not only depends on features of the cancer cells disseminating from the primary tumor but also requires the formation of a receptive environment, a metastatic niche, that is specifically suited for the engraftment of tumor cells at the distant organ. It is likely that MMPs and other proteinases are crucially involved in the formation of a metastatic niche (Figure 3). Soluble factors released from the primary tumor appear to trigger the formation of a metastatic niche that is induced initially by the expression of embryonic-type fibronectin, which is most likely produced by fibroblasts at these sites (Kaplan et al., 2005). This event takes place before disseminated tumor cells are detectable at these distant organs, hence the authors name this process the formation of a “premetastatic niche.” Increased fibronectin production at these sites allows for the infiltration of VEGFR1-positive, bone marrow-derived progenitor cells, which then establish a metastasis-supporting microenvironment.
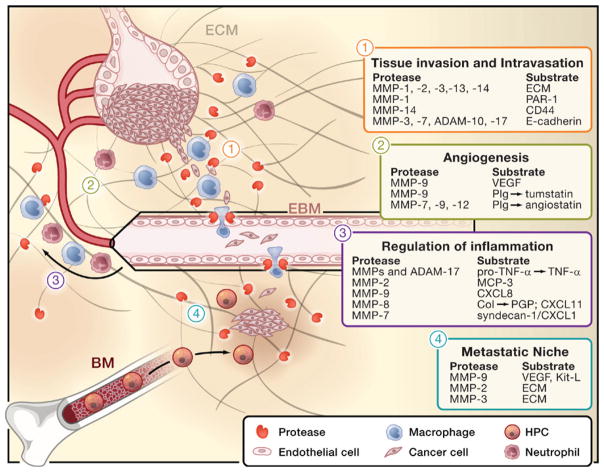
Figure 3. Multiple Functions of MMPs in the Tumor Microenvironment.
Tumor progression and metastasis involve different stages, all of which can be modulated by MMPs and other extracellular proteinases. MMPs are mainly provided by nonmalignant, infiltrating stromal cells such as neutrophils, macrophages, or endothelial cells. Selected examples of proteinases and their target substrates in each of these steps are given in numbered boxes. Tissue invasion of the tumor (1) and cancer cell intravasation into blood vessels (2) require extracellular matrix (ECM) remodeling and downregulation of cellular adhesion. MMPs, ADAMs, and other proteinases such as cathepsins (Cat)-B, -K, and -L are implicated in the turnover of ECM components, but they also regulate cancer cell migration, for example by cleaving proteinase-activated receptor (PAR)-1 or by degrading cell surface molecules that mediate cellular adhesion, such as CD44 or E-cadherin. The egress of metastatic tumor cells into the circulation is often directly accompanied by tumor-associated macrophages and may exploit proteolytic functions that mediate leukocyte migration across the endothelium and the endothelial basement membrane (EBM) under physiological conditions (2). Tumors are highly vascularized tissues and the formation of new blood vessels (angiogenesis; 3) can be triggered by the release of vascular endothelial growth factor (VEGF), which is mainly facilitated by MMP-2 and -9. Moreover, MMPs may also regulate angiogenesis by the generation of angiostatin-like peptides through the cleavage of plasminogen (Plg). MMPs are potent regulators of inflammation (4), thus they are critically involved in the recruitment of inflammatory cells to the tumor microenvironment, for example by converting TNF-α or interleukin-8 (IL-8). They also generate chemotactic peptides such as PGP through the degradation of collagen (col) and form chemotactic gradients by cleaving the ECM component syndecan to release soluble gradients of CXCL1/KC, a potent neutrophil-attracting chemokine. Some MMPs exert anti-inflammatory function, for example by degrading monocyte chemotactic protein (MCP3/CCL7). Metastasis results in the dissemination of malignant cells to secondary sites distant to the primary tumor. Recent findings indicate that these distant sites may be primed for metastasis by inflammatory cells and hematopoietic progenitor cells (HPCs) that locate to these sites to form a so-called premetastatic niche (5). MMP-9 and MMP-2 are involved in this process most likely by releasing factors such as VEGF and Kit ligand (Kit-L), which recruits HPCs from the bone marrow (BM).
Recent studies have shed more light on the factors released by the primary tumor that initiate the metastatic niche. VEGF-A, TGF-β, and tumor necrosis factor-α (TNF-α) produced by tumor cells trigger the expression of S100 chemokines by lung endothelium, which, in turn, mediate the directed migration of myeloid cells to these distant sites (Hiratsuka et al., 2006). Why these factors trigger the expression of chemokines at loci within specific tissues remains to be investigated. In a follow-up study, the authors further identified serum amyloid A3 (SAA3) as a potential upstream regulator of S100 chemokines during this process. Interestingly, the formation of a premetastatic niche depends on the inflammatory response by infiltrating myeloid cells. It appears that SAA3 triggers toll-like receptor 4 (TLR4) signaling in lung-infiltrating myeloid cells leading to activation of the nuclear factor κB (NF-κB) pathway (Hiratsuka et al., 2008). Activation of the NF-κB pathway triggers substantial production of MMPs by stromal cells (Bond et al., 1998), which then potentially contributes to the microenvironmental changes by degrading ECM and releasing growth factors.
Indeed, MMP-9 turns out to be critical for the formation of the metastatic niche (Kaplan et al., 2005), which is most likely linked with its ability to liberate VEGF and thereby support angiogenesis (Bergers et al., 2000). MMP-9 releases soluble Kit-ligand to recruit stem and progenitor cells from the bone marrow (Heissig et al., 2002), which may be of particular significance in this context, given that the niche-forming progenitor cells express c-Kit (Kaplan et al., 2005).
Lysyl oxidase (LOX) secreted by hypoxic breast cancer cells may contribute to setting up metastatic sites because it cross-links proteins including collagen IV in basement membrane structures. This results in the recruitment of myeloid cells to these sites (Erler et al., 2009), possibly owing to altered tissue stiffness (Levental et al., 2009). These invading myeloid cells release MMPs, which degrade collagen fibers and release peptides that may guide the homing of bone marrow-derived cells and metastasizing tumor cells to these sites. Interestingly, the production of MMPs, namely MMP-3 and -10, is upregulated together with the angiogenic modulator angiopoietin 2 in premetastatic lung tissue even before myeloid cells are recruited to these sites (Huang et al., 2009). Given that in vivo RNA interference of MMP-3, MMP-10, and angiopoietin 2 markedly attenuates vascular permeability and infiltration of myeloid cells into the lung, it is likely that these three factors synergize in the destabilization of the pulmonary vasculature, thus promoting metastasis. These findings imply an important role of extracellular proteolysis in premetastatic niche generation. Although future studies are needed to elucidate the critical pathways modulated by these proteinases, it appears that the action of MMPs in this instance critically involves the modulation of inflammatory processes.
MMPs Orchestrate Inflammation in Cancer
There is increasing evidence implicating MMPs as major regulators of innate and acquired immunity. The process of inflammation and production of cytokines by immune cells are in many ways linked to cancer progression (Lin and Karin, 2007). In this section, we discuss how MMPs modulate the function of cytokines and chemokines and what consequences this immunoregulatory function may have on the tumor microenvironment.
There is strong evidence from studies in knockout mice that MMPs play an important role in acute as well as chronic inflammation (Parks et al., 2004). One of the most important proinflammatory cytokines is TNF-α, which is expressed as a membrane-bound precursor (pro-TNF-α) on a variety of cells including macrophages and T cells. Conversion of pro-TNF-α into the soluble cytokinetic form requires proteolytic cleavage by ADAM-17, also known as TNF converting enzyme (TACE), or by several MMPs including MMP-1, -2, -3, -9, -12, -14, -15, and -17 (Manicone and McGuire, 2008). Although ADAM-17 is most likely the major TNF-α-generating convertase, MMPs may be important TNF-α-converting mediators in specific physiological or pathological circumstances, as described for MMP-7 in the regulation of inflammation during resorption of herniated discs (Haro et al., 2000). Many tumors produce abundant TNF-α, and it promotes cancer cell survival in an NF-κB-dependent manner (Luo et al., 2004), suggesting that the conversion of TNF-α by MMPs and ADAM-17 might be a crucial step in this tumor-promoting cascade.
A number of studies have shown the proteolytic alteration of chemokines by MMPs. Several members of the CCL/monocyte chemoattractant protein (MCP) family of chemokines are cleaved by MMPs, which specifically renders them into non-activating receptor antagonists with inflammation-dampening effects (McQuibban et al., 2002). For instance, CCL8/MCP-2 is processed by MMP-1 and MMP-3. Indeed, the proteolytic cleavage of CCL8 can counteract the antitumor capacity of this chemokine in a melanoma model (Struyf et al., 2009). This study shows that proteolytic cleavage of a chemokine can have great impact in a clinically relevant setting of tumor development.
MMP-8, -9, and -12 also modulate the bioactivity of CXCL11/I-TAC, a potent Th1 lymphocyte-attracting chemokine (Cox et al., 2008). Although MMP-mediated N-terminal truncation of CXCL11 leads to inactivation of the chemokine and creates a potent receptor antagonist, further C-terminal cleavage abolishes the antagonist function and removes heparin-binding capacity of CXCL11, thereby solubilizing the chemokine from the ECM. These findings implicate myeloid cell-derived MMPs in the regulation of T cell responses, which may have important consequences on the adaptive antitumor immune response. CXCR7, the chemokine receptor for CXCL11, is also expressed on many tumor cells and can transmit growth- and survival-promoting signals (Burns et al., 2006). Modulation of CXCL11 by MMPs might therefore reduce the antitumor immune response and thus have direct consequences on tumor growth.
The classical function of MMPs, the degradation of ECM, may have secondary effects on the immune system, as some of the proteolytic fragments of MMP-processed ECM components exert chemotactic properties. Likewise, macrophage elastase MMP-12 produces neutrophil-attracting peptides by degrading elastin (Houghton et al., 2006b). Moreover, ECM breakdown during airway inflammation generates the fragment N-acetyl Pro-Gly-Pro (PGP), a tripeptide with chemotactic activity through activation of CXC chemokine receptors on neutrophilic granulocytes (Weathington et al., 2006). MMP-8 is involved in the generation of chemotactic PGP and thus regulates neutrophil recruitment to the sites of inflammation (Rocks et al., 2008), which may cause a delay in the wound-healing response and increase inflammation over time, as observed previously in MMP-8-deficient mice (Gutierrez-Fernandez et al., 2007). MMP-8 contributed by neutrophils also has a tumor-suppressing role in a mouse model of carcinogen-induced skin cancer (Balbin et al., 2003). The defect in the resolution of inflammation and the tendency to develop chronic inflammation in the absence of MMP-8 in mice explain how loss-of-function mutations of MMP-8 contribute mechanistically to increased susceptibility of skin adenocarcinoma and melanoma formation in humans (Palavalli et al., 2009). However, expression of MMP-8 in tumor cells also tightens their adhesion to the ECM and thereby may directly suppress metastatic behavior (Gutierrez-Fernandez et al., 2008). Thus, interference with the tumor-suppressing function of MMP-8 should be regarded as one of the unwanted effects of broad-spectrum MMP inhibitors.
The infiltration of neutrophils in the tumor microenvironment often correlates with poor prognosis (de Visser et al., 2006). Neutrophils, like other inflammatory cells, sense the concentration gradient of chemokines such as CXCL1/KC, a homolog of CXCL8 in mice, which forms complexes with the heparan sulfate proteoglycan syndecan-1 on interstitial cell surfaces. MMP-7 indirectly modulates the bioactivity of CXCL1 by cleaving syndecan-1 from cell surfaces and thereby releasing chemotactic complexes of syndecan-1 and CXCL1 (Li et al., 2002). This efficiently leads to the generation of a concentration gradient of soluble chemotactic CXCL1-syndecan-1 complexes. In a comparable manner, N-terminal processing of neutrophil-attracting CXCL8/interleukin-8 (IL-8) by MMP-9 leads to 10-fold increased chemotactic activity on neutrophils compared to full-length CXCL8 (Van den Steen et al., 2000). Therefore, MMPs orchestrate the recruitment of leukocytes as an essential component of tumor-associated inflammation (Figure 3).
Nonproteolytic Functions of MMPs
An emerging area of interest is the noncatalytic function of MMPs. The discovery of such functions in MMPs is not so surprising, given that about half of all ADAMs show proteolytic capacity, whereas the other half act in a nonproteolytic manner. The hemopexin domain of MMPs plays an important role in the nonproteolytic function of MMPs. The first in vivo hint for a crucial hemopexin-mediated function of MMPs was established with the observation that TIMP-1 and -2 bind to several MMPs via their hemopexin domains. Indeed, activation of MMP-2 requires TIMP-2 that is bound to one molecule of MMP-14 via its catalytic domain and also is bound to pro-MMP-2 via its hemopexin domain. A second molecule of MMP-14 then catalytically activates MMP-2 (Strongin et al., 1995).
Several members of the MMP family trigger immune or cancer cell migration; however, recent evidence suggests that they mediate chemotaxis even without using their proteolytic domain. Precursor forms of MMP-2 and -9 enhance cell migration in a transwell chamber assay. The hemopexin domain of MMP-9, but not its proteolytic activity, is necessary for MMP-9-mediated epithelial cell migration in this assay (Dufour et al., 2008). In this context, activation of the MAP kinase and PI3 kinase pathways appear to be involved in this nonproteolytic function of MMP-9; the distinct molecular pathway as well as the in vivo role of this function yet remain elusive. Moreover, the cytoplasmic tail of MMP-14 carries out a migration-promoting function on macrophages, as genetic depletion of the cytoplasmic tail but not of the extracellular hemopexin or catalytic domain impairs the migration of macrophages during in vitro migration through Matrigel (Sakamoto and Seiki, 2009).
Clear evidence for the physiological relevance of hemopexin domains of MMPs has come more recently using genetic modification of one of the two Mmp genes of Drosophila melanogaster (Glasheen et al., 2009). These investigations reveal that, although the catalytic domain is required for all MMP functions, the hemopexin domain is specifically implicated in tissue invasion during metamorphosis but not for tracheal remodeling.
A more direct nonproteolytic function has been recently elucidated for MMP-12. The hemopexin domain, but not the catalytic site, of MMP-12 (macrophage elastase) plays an essential role in the recently described antimicrobial function of this enzyme (Houghton et al., 2009). Deleting MMP-12 genetically in mice results in impaired bacterial clearance and increased mortality when infected with gram-negative and gram-positive bacteria. The antimicrobial properties of MMP-12 map to a unique four amino acid sequence within the hemopexin-like C-terminal domain and do not require catalytic activity of the enzyme. It remains to be determined whether similar nonproteolytic motifs are present in other MMPs and whether these nonproteolytic modes of action are also implicated in cancer-related functions of these enzymes.
Several MMPs may interact with other extracellular molecules without inducing proteolytic cleavage. MMP-14 interacts with the C1q component of the complement system in a nonproteolytic, receptor-ligand manner, without inducing C1qr and C1qs proteinase activity, suggesting that this binding may inhibit activation of the complement proteinase cascade (Rozanov et al., 2004). Further studies are yet required to address the biological relevance of MMP-14-mediated inhibition of the complement system in tumorigenesis. MMPs also bind to members of the integrin family of cell surface receptors. A recent study has now linked the nonproteolytic interaction of pro-MMP-1 with α2β1 integrin on neurons with MMP-1-induced neuronal cell death in cell culture (Conant et al., 2004). Reduced dephosphorylation of AKT after MMP-1 incubation, which is inhibited by a blocking α2 integrin antibody, but not by administration of Batimastat, an inhibitor of MMP activity, suggests that integrin binding, rather than proteinase activity, is relevant for MMP-1-transmitted cytotoxicity. In chronic lymphocytic leukemia, MMP-9 promotes B cell survival in a non-proteolytic fashion via its hemopexin domain by docking to the surface receptors α4β1 and CD44v, which induce intracellular signaling involving Lyn activation and STAT3 phosphorylation that prevents B cell apoptosis (Redondo-Munoz et al., 2010).
Taken together, these data indicate that we are only beginning to understand nonproteolytic functions of MMPs and that further studies are required to clarify this principle and evaluate its role under in vivo conditions. However, it is tempting to speculate that nonproteolytic functions of MMPs could explain why previous clinical trials using inhibitors of the MMP catalytic domains failed as anticancer therapeutics.
Conclusions and Perspectives
MMPs are associated with multiple human cancers; hence they were early considered as drug targets to treat cancer. The first drug development programs based on the notion of blocking MMP-mediated angiogenesis and metastasis were started about 25 years ago and led to a number of small-molecule metalloproteinase inhibitor (MPI) drugs in phase III clinical trials. The effects of MPIs in these trials turned out to be disappointing as they failed to increase the survival rate of cancer patients. Possible reasons for the failure of MPIs have been extensively discussed previously (Coussens et al., 2002). Indeed, the clinical studies were suboptimally designed with respect to the stage of cancer, so the question remains whether MPIs might have proven more effective when used in earlier stages of the disease.
Part of the rationale to use MPIs as anticancer drugs was to block interstitial migration of metastatic cancer cells. However, recent analyses have shown that cancer cells can switch to an amoeboid-like protease-independent migration mode by forming actin-rich protrusions and “squeezing” through the ECM (Wolf et al., 2007). This would render MPIs impotent to inhibit the migratory behavior of metastatic tumor cells. Whether this alternative migration mode is actually relevant for cancer cell migration under in vivo conditions in the presence of a naturally crosslinked collagen matrix currently remains questionable. Mounting evidence supports a dominant role of MMP-14 in the migration and invasion of metastatic tumor cells; hence MMP-14 remains a promising therapeutic target (Sabeh et al., 2009). This would support the use of MPIs that specifically inhibit MMP-14 as anti-invasive drugs.
The cytostatic potential attributed to MPIs is certainly in keeping with the numerous studies describing MMP-mediated regulation of cell growth signals, such as the activation of TGF-β by MMP-2, -9, and -14 (Dallas et al., 2002; Mu et al., 2002), the proteolytic release of soluble EGFR ligands, or the degradation of E-cadherin by MMP-3 or -9 (Cowden Dahl et al., 2008; Radisky et al., 2005). Moreover, MMPs interfere with apoptosis induction, especially after chemotherapy, by cleaving Fas ligand from the surface of cancer cells as shown for MMP-7 (Mitsiades et al., 2001). In the clinical trials, MPIs were administered to patients with advanced cancer, which was most likely too late to exert any beneficial effect on survival.
Interfering with the tumor vasculature is regarded as one of the most promising strategies to inhibit tumor growth and has motivated the development of drugs like Bevacizumab (Avastin, anti-VEGF monoclonal antibody), which has been FDA approved for the treatment of metastatic cancers in combination with chemotherapy. Many studies also support a dominant role of MMP-9 in the angiogenic switch by regulating the bio-availability of VEGF tumors (e.g., Bergers et al., 2000), suggesting a beneficial effect of MPI on tumor angiogenesis. However, in other cancer models, MMP-9 generates ECM fragments like tumstatin, a potent suppressor of tumor vasculature formation, resulting in increased tumor growth in MMP-9-deficient mice (Hamano et al., 2003). This illustrates that one MMP can have opposing effects in different tumor types and highlights that the use of MPIs has to be carefully considered and evaluated for each specific kind of cancer.
Most of the initial studies utilized cancer cell lines that over-express certain members of the MMP family (reviewed in Egeblad and Werb, 2002). These studies may not recapitulate the situation in vivo, where the major source of MMPs is nonmalignant stromal cells. In fact, the cellular source of each MMP is of high significance, as the activity of the released enzyme varies substantially between cell types. This should be taken into account when assessing the expression patterns of MMPs in cancer types that should be considered for treatments with MPIs.
Certainly, the complexity of the mode of action of MMPs has expanded considerably from proteinases that simply degrade the ECM, to specific modulators of angiogenesis as well as fine-tuners of cell signaling pathways and the inflammatory response (Figure 4). One of the major, recent advances in MMP research is the discovery of specific regulatory effects of MMPs on the stromal cells in the tumor microenvironment. MMPs affect adipocyte function, which is especially likely to be implicated in adipose-rich tumor sites such as breast. They also regulate the course of the inflammatory reaction in multiple ways and facilitate the recruitment of inflammatory cells by altering the function of chemokines and the bioavailability of important proinflammatory cytokines. Regarding the link between inflammation and cancer (Lin and Karin, 2007), the interference with MMP-mediated immunoregulatory functions could prove beneficial for cancer patients. For example, given that TNF-α contributes to progression of several sorts of cancer (Balkwill, 2009), inhibiting TNF-α activation using MPIs might dampen the inflammatory milieu at the tumor microenvironment.
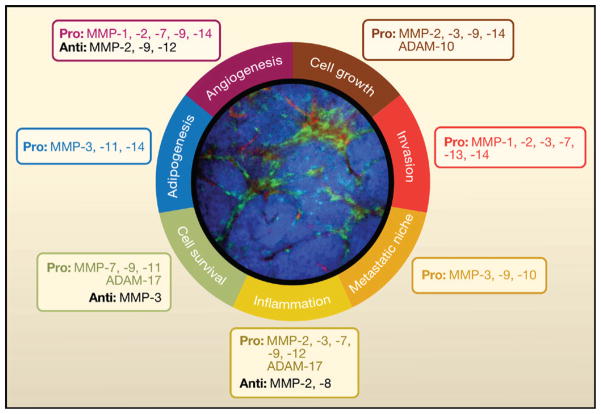
Figure 4. Modulation of the Tumor Microenvironment by MMPs.
Summary of the various processes that are modulated by MMPs in the tumor microenvironment. The selected examples of MMPs and ADAMs promote (pro) or suppress (anti) these processes. An intravital microscopy image of the mammary gland of MMTV-PyMT mice that spontaneously develop mammary carcinoma was taken using a spinning disc inverted confocal microscope (Egeblad et al., 2008). These mice also express enhanced cyan fluorescent protein (CFP) under the control of the actin promoter (ACTB-ECFP) to enable tumor cell labeling (blue) and enhanced green fluorescent protein (GFP) expression under the control of a c-fms promoter (c-fms-EGFP) to label myeloid cells (green). These mice were injected intravenously with 70 kDa rhodamine-dextran to visualize blood vessels (red). This image illustrates the complexity of the tumor microenvironment, which is largely influenced by nonmalignant cells, such as myeloid cells, all of which could be targets as well as sources for MMPs.
Effects of MMPs on myeloid cells may well be implicated in the generation of the premetastatic niche. In fact, MMP-2, -3, and -9 have already been shown to contribute to the establishment of metastasis-prone sites at tumor-distant organs (Erler et al., 2009; Huang et al., 2009; Kaplan et al., 2005). These insights argue for the use of MPIs at early stages of malignant disease, prior to the full initiation of tumor-associated inflammation and before the soil has been primed for metastasis in distant organs.
The tumor-suppressing functions of these MMPs is probably another reason for the failure of broad-spectrum MPIs as anticancer drugs (Lopez-Otin and Matrisian, 2007). The inflammation-suppressing function of MMPs accounts for increased incidence of cancer development in MMP-8 knockout mice (Balbin et al., 2003) and for the link between MMP-8 loss-of-function mutations and melanoma in humans (Palavalli et al., 2009). Also, MMP-12 delivered by macrophages can suppress the growth of lung metastases, which appears to involve regulation of the tumor vasculature (Houghton et al., 2006a). Apart from that, some MMPs carry out biological functions other than proteolytic, mediated by specific binding to certain target molecules, for instance via their hemopexin domain. Small-molecule MMP inhibitors as used in clinical trials are certainly ineffective to interfere with a nonproteolytic role of MMPs.
One of the major tasks for the future is the development of active site-directed inhibitors or antibodies that are specific for single MMPs and show little or no cross-reaction with other MMPs (Cuniasse et al., 2005). For example, a monoclonal antibody raised against the catalytic domain of MMP-14 successfully inhibits the migration and invasion of endothelial cells in collagen and fibrin gels (Galvez et al., 2001). Antibodies could also target functional noncatalytic domains of MMPs. Moreover, MMP activity can be exploited to activate cytotoxic agents such as anthrax toxin to target the tumor vasculature (Liu et al., 2008b). These agents need to be validated for specificity using MMP-deficient animals and rigorously tested in experimental cancer models. New activity-based imaging probes specific for MMPs will facilitate monitoring the effect of MPIs on the function of MMPs in vivo. The combination of these probes with minimal invasive imaging techniques will soon allow the improved endpoint assessment for the efficacy of these compounds in inhibiting the target function in vivo. Imaging activity of specific MMPs in vivo will further advance our understanding of the time frame of MMP function during the progression of certain tumors. Like the development of tailor-made therapies and medications based on individual oncogenic pathway signatures in human cancers (Bild et al., 2006), expression patterns of MMPs in cancer patients could facilitate a fully rational decision about when and in what combination MPIs and anti-cancer drugs should be used in the future.
Acknowledgments
We acknowledge support by grants (CA057621 and CA072006) from the National Cancer Institute and by fellowships from the Susan G. Komen Foundation, the Israeli Science Foundation, and the Machiah Foundation.
References
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 Proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284:25854–25866. doi: 10.1074/jbc.M109.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sheriff S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Bruyere F, Melen-Lamalle L, Blacher S, Roland G, Thiry M, Moons L, Frankenne F, Carmeliet P, Alitalo K, Libert C, et al. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods. 2008;5:431–437. doi: 10.1038/nmeth.1205. [DOI] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Nagase H, Visse R, Gary D, Haughey N, Anderson C, Turchan J, Nath A. Matrix metalloproteinase 1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J Biol Chem. 2004;279:8056–8062. doi: 10.1074/jbc.M307051200. [DOI] [PubMed] [Google Scholar]
- Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, Stack MS, Hudson LG. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–4613. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Dean RA, Roberts CR, Overall CM. Matrix metalloproteinase processing of CXCL11/I-TAC results in loss of chemoattractant activity and altered glycosaminoglycan binding. J Biol Chem. 2008;283:19389–19399. doi: 10.1074/jbc.M800266200. [DOI] [PubMed] [Google Scholar]
- Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437–1444. doi: 10.1172/JCI15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuniasse P, Dev L, Makaritis A, Beau F, Georgiadis D, Matziari M, Yiotakis A, Dive V. Future challenges facing the development of specific active-site-directed synthetic inhibitors of MMPs. Biochimie. 2005;87:393–402. doi: 10.1016/j.biochi.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J Biol Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008;217:643–651. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–167. doi: 10.1242/dmm.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J Biol Chem. 2003;278:28403–28409. doi: 10.1074/jbc.M304739200. [DOI] [PubMed] [Google Scholar]
- Furumoto S, Takashima K, Kubota K, Ido T, Iwata R, Fukuda H. Tumor detection using 18F-labeled matrix metalloproteinase-2 inhibitor. Nucl Med Biol. 2003;30:119–125. doi: 10.1016/s0969-8051(02)00393-1. [DOI] [PubMed] [Google Scholar]
- Galvez BG, Matias-Roman S, Albar JP, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem. 2001;276:37491–37500. doi: 10.1074/jbc.M104094200. [DOI] [PubMed] [Google Scholar]
- Glasheen BM, Kabra AT, Page-McCaw A. Distinct functions for the catalytic and hemopexin domains of a Drosophila matrix metalloproteinase. Proc Natl Acad Sci USA. 2009;106:2659–2664. doi: 10.1073/pnas.0804171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21:2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN, et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–2763. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heljasvaara R, Nyberg P, Luostarinen J, Parikka M, Heikkila P, Rehn M, Sorsa T, Salo T, Pihlajaniemi T. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp Cell Res. 2005;307:292–304. doi: 10.1016/j.yexcr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006a;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006b;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton AM, Hartzell WO, Robbins CS, Gomis-Ruth FX, Shapiro SD. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460:637–641. doi: 10.1038/nature08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, Shi H, Luo Y. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69:7529–7537. doi: 10.1158/0008-5472.CAN-08-4382. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Islekel H, Oktay G, Terzi C, Canda AE, Fuzun M, Kupelioglu A. Matrix metalloproteinase-9,-3 and tissue inhibitor of matrix metalloproteinase-1 in colorectal cancer: relationship to clinicopathological variables. Cell Biochem Funct. 2007;25:433–441. doi: 10.1002/cbf.1325. [DOI] [PubMed] [Google Scholar]
- Jastrzebska B, Lebel R, Therriault H, McIntyre JO, Escher E, Guerin B, Paquette B, Neugebauer WA, Lepage M. New enzyme-activated solubility-switchable contrast agent for magnetic resonance imaging: from synthesis to in vivo imaging. J Med Chem. 2009;52:1576–1581. doi: 10.1021/jm801411h. [DOI] [PubMed] [Google Scholar]
- Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenskiold M, Holmdahl L, Falk P, Ivarsson ML. Increased plasma MMP-2 protein expression in lymph node-positive patients with colorectal cancer. Int J Colorectal Dis. 2005;20:245–252. doi: 10.1007/s00384-004-0667-4. [DOI] [PubMed] [Google Scholar]
- Le Maux Chansac B, Misse D, Richon C, Vergnon I, Kubin M, Soria JC, Moretta A, Chouaib S, Mami-Chouaib F. Potentiation of NK cell-mediated cytotoxicity in human lung adenocarcinoma: role of NK-G2D-dependent pathway. Int Immunol. 2008;20:801–810. doi: 10.1093/intimm/dxn038. [DOI] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Lijnen HR, Van Hoef B, Collen D. Inactivation of the serpin alpha(2)-antiplasmin by stromelysin-1. Biochim Biophys Acta. 2001;1547:206–213. doi: 10.1016/s0167-4838(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Littlepage LE, Sternlicht MD, Rougier N, Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI, Werb Z. Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression. Cancer Res. 2010;70:2224–2234. doi: 10.1158/0008-5472.CAN-09-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang T, Li X, Huang J, Wu B, Huang X, Zhou Y, Zhu J, Hou J. Predictive value of MMP-7 expression for response to chemotherapy and survival in patients with non-small cell lung cancer. Cancer Sci. 2008a;99:2185–2192. doi: 10.1111/j.1349-7006.2008.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang H, Currie BM, Molinolo A, Leung HJ, Moayeri M, Basile JR, Alfano RW, Gutkind JS, Frankel AE, et al. Matrix metalloproteinase-activated anthrax lethal toxin demonstrates high potency in targeting tumor vasculature. J Biol Chem. 2008b;283:529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102:647–655. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, Massague J, Kang Y. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009;23:1882–1894. doi: 10.1101/gad.1824809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- Mentzel T, Brown LF, Dvorak HF, Kuhnen C, Stiller KJ, Katenkamp D, Fletcher CD. The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Arch. 2001;438:13–22. doi: 10.1007/s004280000327. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: a vicious tumor progression cycle. Biol Chem. 2008;389:1037–1041. doi: 10.1515/BC.2008.110. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:932–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nakamura ES, Koizumi K, Kobayashi M, Saiki I. Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci. 2004;95:25–31. doi: 10.1111/j.1349-7006.2004.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ES, Aguilera AT, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating pepides for targeting protease activity in cancer. Integrative Biol. 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard BZ, Artym VV, Komoriya A, Yamada KM. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol. 2009;28:3–10. doi: 10.1016/j.matbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, Davis S, Wang C, Cronin JC, Agrawal NS, et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41:518–520. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Patterson BC, Sang QA. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9) J Biol Chem. 1997;272:28823–28825. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- Pinilla S, Alt E, Abdul Khalek FJ, Jotzu C, Muehlberg F, Beckmann C, Song YH. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284:80–85. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Munoz J, Ugarte-Berzal E, Terol MJ, Van den Steen PE, Hernandez del Cerro M, Roderfeld M, Roeb E, Opdenakker G, Garcia-Marco JA, Garcia-Pardo A. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain. Cancer Cell. 2010;17:160–172. doi: 10.1016/j.ccr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res. 2009;33:638–644. doi: 10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Rocks N, Paulissen G, Quesada-Calvo F, Munaut C, Gonzalez ML, Gueders M, Hacha J, Gilles C, Foidart JM, Noel A, et al. AD-AMTS-1 metalloproteinase promotes tumor development through the induction of a stromal reaction in vivo. Cancer Res. 2008;68:9541–9550. doi: 10.1158/0008-5472.CAN-08-0548. [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Sikora S, Godzik A, Postnova TI, Golubkov V, Savinov A, Tomlinson S, Strongin AY. Non-proteolytic, receptor/ligand interactions associate cellular membrane type-1 matrix metalloproteinase with the complement component C1q. J Biol Chem. 2004;279:50321–50328. doi: 10.1074/jbc.M409174200. [DOI] [PubMed] [Google Scholar]
- Rupp PA, Visconti RP, Czirok A, Cheresh DA, Little CD. Matrix metalloproteinase 2-integrin alpha(v)beta3 binding is required for mesenchymal cell invasive activity but not epithelial locomotion: a computational time-lapse study. Mol Biol Cell. 2008;19:5529–5540. doi: 10.1091/mbc.E07-05-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Seiki M. Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity. Genes Cells. 2009;14:617–626. doi: 10.1111/j.1365-2443.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- Schafers M, Riemann B, Kopka K, Breyholz HJ, Wagner S, Schafers KP, Law MP, Schober O, Levkau B. Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation. 2004;109:2554–2559. doi: 10.1161/01.CIR.0000129088.49276.83. [DOI] [PubMed] [Google Scholar]
- Scherer RL, McIntyre JO, Matrisian LM. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008a;27:679–690. doi: 10.1007/s10555-008-9152-9. [DOI] [PubMed] [Google Scholar]
- Scherer RL, VanSaun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol Imaging. 2008b;7:118–131. [PMC free article] [PubMed] [Google Scholar]
- Schulte M, Reiss K, Lettau M, Maretzky T, Ludwig A, Hartmann D, de Strooper B, Janssen O, Saftig P. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007;14:1040–1049. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE, Dehne K, van Kempen LLCL, Egeblad M, Affara N, Cuevas I, Wiesen J, Junankar J, Korets L, Lee L, et al. Stromal regulation of vessel stability by MMP9 and TGFβ. Dis Model Mech. 2010 doi: 10.1242/dmm.00386.3. Published online March 11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Struyf S, Proost P, Vandercappellen J, Dempe S, Noyens B, Nelissen S, Gouwy M, Locati M, Opdenakker G, Dinsart C, et al. Synergistic upregulation of MCP-2/CCL8 activity is counteracted by chemokine cleavage, limiting its inflammatory and anti-tumoral effects. Eur J Immunol. 2009;39:843–857. doi: 10.1002/eji.200838660. [DOI] [PubMed] [Google Scholar]
- Tatti O, Vehvilainen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res. 2008;314:2501–2514. doi: 10.1016/j.yexcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Temma T, Sano K, Kuge Y, Kamihashi J, Takai N, Ogawa Y, Saji H. Development of a radiolabeled probe for detecting membrane type-1 matrix metalloproteinase on malignant tumors. Biol Pharm Bull. 2009;32:1272–1277. doi: 10.1248/bpb.32.1272. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by amino-terminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- Weiss SJ, Peppin G, Ortiz X, Ragsdale C, Test ST. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Witters L, Scherle P, Friedman S, Fridman J, Caulder E, Newton R, Lipton A. Synergistic inhibition with a dual epidermal growth factor receptor/HER-2/neu tyrosine kinase inhibitor and a disintegrin and metalloprotease inhibitor. Cancer Res. 2008;68:7083–7089. doi: 10.1158/0008-5472.CAN-08-0739. [DOI] [PubMed] [Google Scholar]
- Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. New York: Oxford University Press; 2000. [Google Scholar]
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- Wu Y, Smas CM. Wdnm1-like, a new adipokine with a role in MMP-2 activation. Am J Physiol Endocrinol Metab. 2008;295:E205–E215. doi: 10.1152/ajpendo.90316.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- Yeh WL, Lu DY, Lee MJ, Fu WM. Leptin induces migration and invasion of glioma cells through MMP-13 production. Glia. 2009;57:454–464. doi: 10.1002/glia.20773. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 2009;324:1330–1334. doi: 10.1126/science.1170905. [DOI] [PMC free article] [PubMed] [Google Scholar]