Abstract
Objective
Mitral annular calcification (MAC) and aortic stenosis (AS) are associated with systemic calcification and cardiovascular disease (CVD) events. Matrix Gla protein (MGP) is an inhibitor of vascular calcification and lower levels of its precursor – uncarboxylated MGP (ucMGP) – are associated with vascular calcification in pilot studies.
Methods and Results
In this cross-sectional study of 839 outpatients with stable CVD, we measured serum ucMGP, and evaluated MAC and AS by echocardiography. The association of ucMGP with MAC differed by diabetes status (interaction P<0.001). Among participants without diabetes (n=615), higher ucMGP (per standard deviation [1,178 nM] increase) was associated with lower odds of MAC (odds ratio [OR] 0.73; 95% confidence interval [CI] 0.55-0.97) in models adjusted for traditional CVD risk factors, C-reactive protein, and kidney function. Among persons with diabetes (n=221), higher ucMGP was associated with higher odds of MAC (OR 1.89; 95% CI 1.29-2.78). Results were qualitatively similar for the association of ucMGP with AS although not statistically significant.
Conclusions
Among outpatients with stable CVD, higher ucMGP is associated with lower odds of MAC in persons without diabetes, and higher odds of MAC in persons with diabetes. Future studies should determine whether ucMGP levels are associated with CVD events, and whether such associations differ by diabetes status.
Keywords: aortic stenosis, calcification, diabetes mellitus, matrix Gla protein, mitral annular calcification
Introduction
Mitral annular calcification (MAC) and aortic stenosis (AS) are associated with calcification of the aorta and other vascular beds, and may be focal manifestations of a systemic calcific condition [1]. While both are associated with cardiovascular disease (CVD) risk factors [2], the presence of either MAC or AS is associated with incident CVD events, CVD mortality, and all-cause mortality independent of traditional CVD risk factors in community-living populations [3, 4]. Previously thought to reflect a passive process, recent research has demonstrated that cardiac valve calcification is actively regulated and potentially modifiable [5]. Thus, understanding mechanisms of valvular calcification may provide novel insights into CVD.
Matrix Gla protein (MGP) inhibits vascular calcification [6]. MGP knock-out mice are characterized by severe vascular calcification and prematurely die due to spontaneous aortic rupture [7]. Recently, an assay specific for its precursor - uncarboxylated MGP (ucMGP) - has been developed [8]. In a pilot study, persons with coronary atherosclerosis, aortic stenosis, and calcific uremic arteriolopathy had lower ucMPG levels compared to healthy controls [9]. However, the number of participants was small and associations were not adjusted for age or kidney function [10], two important confounders. The goal of the present manuscript was to determine the independent association of serum ucMGP with cardiac valve calcification in a relatively large cohort of outpatients with stable CVD.
Our secondary objective was to determine whether the association of ucMGP with cardiac valvular calcification differs by diabetes status. MGP complexes with fetuin-A (another inhibitor of vascular calcification) in blood, working in concert limiting calcium crystal growth/precipitation [11]. In a prior study, we demonstrated that in persons with CVD, lower fetuin-A levels were strongly associated with cardiac valve calcification only in persons without diabetes [12]. Since MGP and fetuin-A work in concert, herein we investigate whether the association of ucMGP with valve calcification might also differ s by diabetes status. We hypothesized that lower serum ucMGP levels would be associated with both MAC and AS, with stronger relationships in persons without diabetes.
Research Design and Methods
Study Participants
The Heart and Soul Study is an observational study designed to investigate the influence of psychosocial factors on CVD progression, with methods described previously [13]. Briefly, participants were recruited from outpatient clinics around San Francisco if they had a: (i) history of myocardial infarction (MI); (ii) angiographic evidence of >50% stenosis in one or more coronary vessels; (iii) exercise-induced ischemia by treadmill or nuclear testing; (iv) history of coronary revascularization or (v) diagnosis of coronary artery disease by an internist or cardiologist. Participants were excluded if they were unable to walk one block, had a MI within the past 6 months, or were likely to move within 3 years. Participating Institutional Review Boards approved the study protocol. All participants provided written informed consent.
Between 2000 and 2002, 1024 participants underwent a daylong baseline study appointment, including an interview, physical examination, and resting echocardiogram. Fasting (12 h) serum was obtained and frozen at -70°C. The present analysis excludes subjects among whom serum was not available for ucMGP measurement (n=182, 18%) or without echocardiogram assessments of valve calcification (n=3, 0.3%) resulting in a sample of 839 subjects.
Measurements
Uncarboxylated Matrix Gla Protein
Serum ucMGP was measured by competitive enzyme linked immunosorbent assay at the VitaK BV, Maastricht, the Netherlands as previously described [9]. Anti-ucMGP (VitaK BV, Maastricht, The Netherlands) was coupled to a microtiter plate via polyclonal rabbit-anti-mouse IgG (Dako, Heeverlee, Belgium). After stringent washing, 5 μL of serum sample or standard was mixed with tracer (biotinylated peptide consisting of residues 35-54 in human MGP), transferred to the microtiter plate, and incubated overnight at 4° C. After washing, the plate was incubated with streptavidine-peroxidase (Zymed, Breda, The Netherlands) and stained with TMB (KPL, Gennep, The Netherlands). The process was stopped by adding H2SO4, and the plate was read at 450 nm. The lower limit of detection was 98 nM, and the intra-assay coefficient of variation was 6% and the inter-assay coefficient was 11%.
Mitral Annular Calcification and Aortic Stenosis
Using an Acuson Sequoia system (3.5-MHz transducer), echocardiograms were obtained at rest with all standard 2-dimentsional views and Doppler images. Echocardiograms were read by a single experienced cardiologist (NBS) who was blinded to participants' information. MAC was defined by an echo-dense structure located at the junction of the atrioventricular groove and the posterior mitral leaflet on the parasternal long-axis, apical 4-chamber, or parasternal shortaxis view [14]. Peak and mean aortic-valve pressure gradients were calculated by the Bernoulli equation; aortic valve area was calculated by the continuity equation [15]. The presence of AS was defined by a peak velocity of >2.0 m/s, a peak gradient of >15 mm Hg, and a valve area of <2.0 cm2.
Other Participant Characteristics and Laboratory Tests
Age, sex, race/ethnicity, and smoking status were determined by questionnaire. Regular alcohol use was defined by a score ≥4 measured by the AUDIT-C questionnaire [16]. Weight and height were measured at the baseline, and body mass index (BMI) was calculated (wt [kg]/height [meters]2). Medical history of hypertension, diabetes, myocardial infarction, angioplasty, coronary bypass, and heart failure were all determined by questionnaire. Systolic and diastolic blood pressures were measured after 5 minutes rest in the supine position. Serum cystatin-C concentrations [17] were used to calculate estimated glomerular filtration rate (eGFR) using the formula eGFR = 76.7 × (cystatin-C−1.19) [18]. Serum fetuin-A was measured with a BNII nephelometric assay as described in detail elsewhere (Dade Behring, Newark, Del) [12]. Remaining laboratory measures were determined using standard clinical chemistry analyzers.
Statistical Analysis
We categorized participants by tertiles of serum ucMGP levels, comparing differences in demographic and clinical variables across the tertiles using analysis of variance or the Kruskal– Wallis test for continuous variables and the Chi squared test for categorical variables, as appropriate. Pairwise correlations between ucMGP with determinants of mineral metabolism and inflammatory markers were evaluated using Pearson or Spearman correlation coefficients, as appropriate. Because of observed relations between ucMGP and many features of the metabolic syndrome, logistic regression was used to describe the relation between ucMGP and the metabolic syndrome (based on updated ATP III criteria, including a definition of impaired glucose tolerance to be ≥ 100 mg/dl as recommended by The American Diabetes Association [19]).
Next, using logistic regression we evaluated the association of serum ucMGP as a continuous variable with MAC and AS. Sequential models were developed. Model 1 was unadjusted; Model 2 adjusted for age, sex, and race; Model 3 added body mass index, hypertension, smoking, systolic blood pressure, diastolic blood pressure, albumin, total cholesterol, high density lipoprotein cholesterol, C-reactive protein, and estimated glomerular filtration rate. Subsequently, calcium, phosphorus, and fetuin-A were included as covariates, first individually, and then all three simultaneously, to determine whether these factors attenuated the observed associations. Last, we created a multiplicative interaction term (ucMGP × diabetes) to evaluate effect modification by diabetes status. Diabetes was the only effect modifier evaluated, and was chosen a priori on the basis of our previously published results [12]. All analyses were performed using Stata Statistical Software, version 9.2 (College Station, TX).
Results
Among the 839 study participants, the mean age was 68 ± 11 years, 81% were male, 40% were non-white, and 26% had diabetes mellitus. The mean ± SD ucMGP level was 3,287 ± 1,178 nM, and its distribution was approximately normal in the study sample. MAC was present in 155 participants (19%) while aortic stenosis was present in 68 (9%). Differences in baseline characteristics according to ucMGP tertile are shown in Table 1. Persons with higher ucMGP were observed to have higher prevalence of diabetes and individual components of the metabolic syndrome. Each standard deviation higher ucMGP was associated with increased odds for metabolic syndrome on crude analysis (Odds Ratio [OR] 1.43, 95% Confidence Interval [CI] 1.23-1.67, P<0.001) and with adjustment for age, sex, race, smoking, CRP, and eGFR (OR 1.55, 95% CI 1.31-1.83, P<0.001). Direct correlations were observed between ucMGP with calcium, phosphorus, and fetuin-A (Table 1), and were of modest strength, with the strongest observed with fetuin-A (r = 0.27, P<0.01). Correlations between ucMGP and markers of inflammation showed a modest correlation between lower ucMGP and lower albumin (r = 0.24, P<0.001) but no significant correlation between ucMGP and CRP or fibrinogen (Appendix Table 1).
Table 1.
Baseline demographics and clinical characteristics by uncarboxylated matrix Gla protein (ucMGP) tertiles
| Characteristics | Tertile 1 <2,757 (n = 280) |
ucMGP, nM Tertile 2 2,757-3,648 (n = 280) |
Tertile 3 >3,648 (n = 279) |
P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 71 (63-79) | 68 (60-76) | 64 (56-71) | <0.001 |
| Male Sex, No. (%) | 237 (85) | 227 (81) | 218 (78) | 0.14 |
| Race | 0.54 | |||
| Caucasian, No. (%) | 178 (64) | 168 (60) | 158 (57) | |
| African-American, No. (%) | 42 (15) | 47 (17) | 47 (17) | |
| Other, No. (%) | 60 (21) | 64 (23) | 74 (27) | |
| Regular Alcohol Use, No. (%) | 70 (25) | 76 (27) | 94 (34) | 0.07 |
| Current smoking, No. (%) | 44 (16) | 61 (22) | 66 (24) | 0.06 |
| Body mass index, kg/m2 * | 27 (24-30) | 27 (25-31) | 29 (26-33) | <0.001 |
| Medical History | ||||
| Hypertension, No. (%) | 191 (69) | 211 (75) | 201 (72) | 0.24 |
| Diabetes, No. (%) | 56 (20) | 75 (27) | 90 (32) | 0.01 |
| Myocardial Infarction, No. (%) | 157 (56) | 143 (51) | 147 (53) | 0.46 |
| Angioplasty, No. (%) | 105 (38) | 107 (38) | 116 (42) | 0.59 |
| Coronary Bypass, No. (%) | 106 (38) | 108 (39) | 91 (33) | 0.31 |
| Heart failure, No. (%) | 44 (16) | 54 (19) | 52 (19) | 0.51 |
| Measurements | ||||
| SBP, mmHg | 131 (22) | 135 (22) | 133 (20) | 0.09 |
| DBP, mmHg | 72 (11) | 75 (12) | 76 (11) | <0.001 |
| eGFR, ml/min† | 64 (22) | 71 (21) | 77 (20) | <0.001 |
| Cholesterol, mg/dL* | 164 (143-186) | 169 (145-191) | 182 (157-210) | <0.001 |
| HDL, mg/dL | 47 (14) | 45 (14) | 46 (14) | 0.30 |
| CRP, mcg/dL* | 2.27 (0.98-4.89) | 2.21 (0.87-5.43) | 2.32 (1.01-4.97) | 0.96 |
| Albumin, g/dL* | 3.8 (3.6-4.0) | 3.9 (3.7-4.1) | 4.0 (3.8-4.2) | <0.001 |
| Fibrinogen, mg/dL* | 382 (332-443) | 389 (336-453) | 379(332-443) | 0.78 |
| Calcium, mg/dL | 9.4 (0.5) | 9.5 (0.5) | 9.6 (0.5) | <0.001 |
| Phosphorus, mg/dL* | 3.6 (3.2-3.9) | 3.7 (3.3-4.0) | 3.7 (3.3-4.1) | 0.02 |
| Fetuin-A, g/L * | 0.60 (0.52-0.68) | 0.64 (0.56-0.73) | 0.68 (0.60-0.79) | <0.001 |
Abbreviations: CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate, HDL, high density lipoprotein cholesterol; SBP, systolic blood pressure
Data are presented as mean (SD) unless otherwise specified
Median (intra-quartile range)
eGFR = 76.7 × (cystatin-C−119)
The association of serum ucMGP with MAC differed by diabetes status (interaction P-value < 0.001). In participants without diabetes, each standard deviation higher ucMGP was associated with approximately 1/3 lower odds for MAC in unadjusted analysis (Table 3). This association was only modestly attenuated after adjustment for potential confounders, such that in the final multivariable model each standard deviation higher ucMGP was associated with an odds ratio of 0.73 for MAC. In contrast, in persons with diabetes each standard deviation higher ucMGP was associated with 29% higher odds of MAC in unadjusted analysis. With multivariable adjustment this association was strengthened, such that one standard deviation of higher ucMGP was associated with 89% higher odds of MAC in the final adjusted model (Table 3). The association of serum ucMGP with AS also differed qualitatively by diabetes status, although the p-value for interaction did not reach statistical significance (P = 0.21). When analyzed according to tertiles of ucMGP to facilitate graphical representation (Figure 1) results were similar. In participants without diabetes the highest tertile of ucMGP was associated with lower odds for MAC (OR 0.61; 95% CI 0.32-1.13) while in diabetics the lowest tertile was of ucMGP was associated with lower odds for MAC (OR 0.14; 95% CI 0.04-0.53).
Table 3.
Table 3A. Association of ucMGP (per standard deviation increase) with mitral annular calcification, stratified by diabetes status*
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| No diabetes (n = 615) | |||
| Unadjusted | 0.64 | 0.50 to 0.81 | <0.001 |
| Adjusted for age, sex, & race | 0.77 | 0.60 to 1.00 | 0.05 |
| Multivariable adjusted† | 0.73 | 0.55 to 0.97 | 0.03 |
| Diabetes (n = 221) | |||
| Unadjusted | 1.29 | 0.97 to 1.71 | 0.08 |
| Adjusted for age, sex, & race | 1.49 | 1.10 to 2.04 | 0.01 |
| Multivariable adjusted† | 1.89 | 1.29 to 2.78 | 0.001 |
| Table 3B. Association of ucMGP (per standard deviation increase) with aortic stenosis, stratified by diabetes status‡ | |||
| Odds Ratio | 95% CI | P Value | |
| No diabetes (n = 587) | |||
| Unadjusted | 0.80 | 0.56 to 1.14 | 0.22 |
| Adjusted for age, sex, & race | 0.86 | 0.60 to 1.24 | 0.42 |
| Multivariable adjusted† | 0.84 | 0.56 to 1.27 | 0.42 |
| Diabetes (n = 211) | |||
| Unadjusted | 1.08 | 0.75 to 1.57 | 0.68 |
| Adjusted for age, sex, & race | 1.23 | 0.84 to 1.81 | 0.29 |
| Multivariable adjusted† | 1.51 | 0.91 to 2.52 | 0.11 |
P-value for interaction < 0.001 in the multivariable model
Adjusted for age, sex, race, body mass index, hypertension, smoking, systolic blood pressure, diastolic blood pressure, albumin, total cholesterol, high density lipoprotein cholesterol, C-reactive protein, estimated glomerular filtration rate
P-value for interaction = 0.21 in the multivariable model
Figure 1.
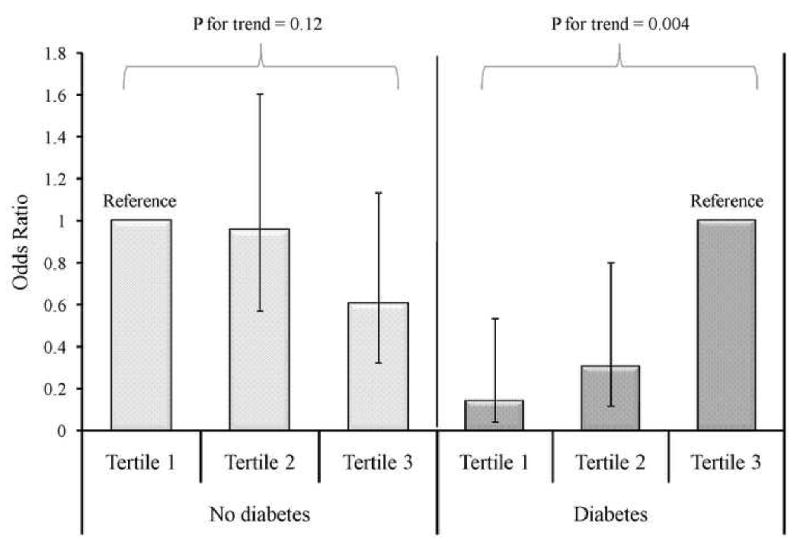
Odds of Mitral Annular Calcification According to Diabetic Status and Tertile of Uncarboxylated Matrix Gla Protein*
*Adjusted for age, sex, race, body mass index, hypertension, smoking, systolic blood pressure, diastolic blood pressure, albumin, total cholesterol, high density lipoprotein cholesterol, C-reactive protein, estimated glomerular filtration rate; Tertile 1 <2757 nM; Tertile 2757-3648 nM; Tertile 3 >3648 nM; error bars represent 95% confidence intervals
We evaluated whether these associations were attenuated when models were adjusted further for serum calcium, phosphorus, and fetuin-A levels, as we hypothesized that these factors might mediate the observed associations of ucMGP with cardiac valve calcification. Inclusion of each of these variables, either individually or collectively, had little effect on the observed associations (Table 4).
Table 4.
Table 4A. Evaluation of candidate mediators on the association of ucMGP (per standard deviation increase) with mitral annular calcification, stratified by diabetes status
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| No diabetes (n = 615) | |||
| Calcium (Ca) adjusted* | 0.73 | 0.55 to 0.97 | 0.03 |
| Phosphorus (Phos) adjusted* | 0.73 | 0.55 to 0.96 | 0.03 |
| Fetuin-A adjusted* | 0.76 | 0.57 to 1.00 | 0.05 |
| Ca + Phos + Fetuin-A adjusted* | 0.75 | 0.56 to 1.00 | 0.05 |
| Diabetes (n = 221) | |||
| Calcium (Ca) adjusted* | 1.85 | 1.25 to 2.73 | 0.002 |
| Phosphorus (Phos) adjusted* | 1.89 | 1.28 to 2.79 | 0.001 |
| Fetuin-A adjusted* | 2.12 | 1.40 to 3.23 | <0.001 |
| Ca + Phos + Fetuin-A adjusted* | 2.08 | 1.36 to 3.17 | 0.001 |
| Table 4B. Evaluation of candidate mediators on the association of ucMGP (per standard deviation increase) with aortic stenosis, stratified by diabetes status | |||
| Odds Ratio | 95% CI | P Value | |
| No diabetes (n = 587) | |||
| Calcium (Ca) adjusted* | 0.86 | 0.58 to 1.30 | 0.48 |
| Phosphorus (Phos) adjusted* | 0.82 | 0.55 to 1.24 | 0.35 |
| Fetuin-A adjusted* | 0.90 | 0.60 to 1.36 | 0.62 |
| Ca + Phos + Fetuin-A adjusted* | 0.88 | 0.59 to 1.33 | 0.55 |
| Diabetes (n = 211) | |||
| Calcium (Ca) adjusted* | 1.50 | 0.90 to 2.50 | 0.12 |
| Phosphorus (Phos) adjusted* | 1.51 | 0.91 to 2.51 | 0.12 |
| Fetuin-A adjusted* | 1.44 | 0.86 to 2.44 | 0.17 |
| Ca + Phos + Fetuin-A adjusted* | 1.43 | 0.84 to 2.42 | 0.19 |
Also adjusted for age, sex, race, body mass index, hypertension, smoking, systolic blood pressure, diastolic blood pressure, albumin, total cholesterol, high density lipoprotein cholesterol, C-reactive protein, estimated glomerular filtration rate
Discussion
Serum ucMGP levels are strongly associated with MAC in out-patients with stable CVD, but that the direction of association differs by diabetes status. Higher serum ucMGP levels are associated with lower odds for MAC in persons without diabetes, and higher odds for MAC in those with diabetes. These associations were independent of age, kidney function, traditional CVD risk factors, albumin, and CRP and were not materially altered with adjusting for calcium, phosphorus or fetuin-A levels. While results were qualitatively similar by diabetes status for the association of ucMGP with aortic stenosis, these results did not reach statistical significance.
A prior study using the same ucMGP assay demonstrated an association between lower ucMGP in persons with vascular disease. This study included 25 patients with AS (7 with diabetes) and 30 patients referred for angiography (5 with diabetes). On average, both groups had lower serum ucMGP levels than healthy controls, but these results were not evaluated separately by diabetes status, nor subjected to multivariable modeling, perhaps because of the small sample size [9]. The data presented herein confirm an association between lower ucMGP and higher prevalence of mitral annular calcification, independent of traditional CVD risk factors, or kidney function; an association limited to subjects without diabetes.
The mechanism(s) responsible for the effect modification by diabetes is uncertain but there are several possibilities. We have shown previously that lower ucMGP is associated with decreased eGFR [10] and present results show that higher ucMGP is associated with diabetes and the metabolic syndrome (independent of age and eGFR). While a low eGFR and diabetes/metabolic syndrome are both risk factors for vascular calcification, the biology that each condition engenders may have important differences that may, in part, be reflected by circulating levels of ucMGP. One possibility is that insulin resistance/diabetes induces greater vascular ucMGP generation or decreased clearance, and the association of higher ucMGP with valve calcification in the diabetic strata may reflect longer duration of exposure to diabetes, or greater severity of disease. However, ucMGP is produced in places other than the vasculature [20], and a second possibility is that ucMGP is produced in greater quantities in non-vascular tissues in diabetes, thereby rendering greater quantities in the serum. In support of this, a small study that evaluated the peripheral arteries in amputated limbs from 9 persons with diabetes and 12 without diabetes showed that tissue MGP mRNA levels were globally lower in vascular tissue from diabetics [21].
MGP works in concert with fetuin-A to inhibit vascular calcification [11], and the association of fetuin-A with cardiac valve calcification also differs by diabetes status [12]. The observed differences in the association of ucMGP with cardiac valve calcification may reflect this overlapping biology. While the association of ucMGP with valve calcification was unaltered when adjusted for serum fetuin-A levels in our analysis, it remains possible that either the association is stronger and driven by ucMGP, or that blood level measurement alone does not fully inform the complex interplay of these two proteins. For example, fetuin-A is a circulating inhibitor of vascular calcification produced by the liver, while the anti-calcific effects of MGP in the vasculature are mediated locally where it is produced [22]. In this cohort, fetuin-A is associated with the inflammatory markers fibrinogen and CRP (although weakly), but ucMGP is not.
These findings add to a growing body of literature demonstrating that many novel inhibitors of calcification function differently among persons with or without diabetes mellitus. For example, fetuin-A knock-out mice develop soft-tissue calcification and are simultaneously insulin sensitive [23, 24]. Similarly, mice with gene knock-outs for fibroblast growth factor-23 [25] and klotho [26, 27] demonstrate extensive vascular calcification and are insulin sensitive or hypoglycemic. Future studies that elucidate the mechanisms responsible for these findings should receive high priority, as they may provide insights into mechanisms of vascular calcification, glucose regulation, and cardiovascular disease.
The data provided herein should serve principally to provide novel insights to mechanisms of valvular calcification, and we do not yet recommend ucMGP measurement for clinical purposes. However, if the results observed here are confirmed, measurement of ucMGP may ultimately prove useful to identify individuals at higher risk for cardiac valve disease progression, and by extension, may identify persons at greater risk for heart failure and CVD events. Intriguingly, in this context, serum ucMGP may yield unique predictive information in subjects with or without diabetes. The pattern of vascular disease in persons with diabetes differs. For example, subjects with diabetes are protected from abdominal aortic aneurysms [28], and more frequently have diffuse coronary artery disease [29] and medial arterial calcification [30] than their non-diabetic counterparts. Study of ucMGP may provide insights to these distinct patterns of disease.
Strengths of this study include the relatively large sample size, availability of echocardiography and potential confounding variables including fetuin-A in all subjects. The study also has important limitations. Only 68 participants (9%) had aortic stenosis, and 23 of these had diabetes. This provided limited statistical power to evaluate the association of serum ucMGP with aortic stenosis, particularly within diabetic strata. All participants had prevalent CVD and most were older men. Results may differ in younger persons, women, and persons without CVD.
In conclusion, higher serum ucMGP levels are associated with lower odds of mitral annular calcification in participants without diabetes whereas they are associated with higher odds of mitral annular calcification in persons with diabetes. Similar, albeit statistically nonsignificant directions of association were observed for the association of serum ucMGP level with aortic stenosis. This study adds to a growing body of literature demonstrating that regulation of cardiac valvular calcification differs by diabetes status. Future studies with are required to determine whether serum ucMGP levels are associated with CVD events and heart failure, and to determine if such associations may also differ by diabetes status.
Table 2.
Correlation of serum uncarboxylated matrix Gla protein (ucMGP), calcium, phosphorus, and fetuin-A
| ucMGP | Calcium | Phosphorus | Fetuin-A | |
|---|---|---|---|---|
| ucMGP | 1 | |||
| Calcium | 0.19 | 1 | ||
| Phosphorus | 0.10 | 0.16 | 1 | |
| Fetuin-A | 0.27 | 0.16 | 0.09 | 1 |
| All P-values < 0.01 | ||||
Acknowledgments
Sources of Funding: This study was supported by a Hypertension Training Grant (T32 HL007261) through the National Heart Lung and Blood Institute (BDP) and an American Heart Association Fellow-to-Faculty transition grant (JHI). The Heart and Soul Study was supported by the Department of Veterans Epidemiology Merit Review Program; the Department of Veterans Affairs Health Services Research and Development service; the National Heart Lung and Blood Institute (R01 HL079235); the American Federation for Aging Research (Paul Beeson Scholars Program); the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program); and the Ischemia Research and Education Foundation. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Appendix
Table 1.
Correlation of serum uncarboxylated matrix Gla protein (ucMGP) with fetuin-A, C-reactive protein, albumin, and fibrinogen
| ucMGP | Fetuin-A | C-reactive protein | Albumin | Fibrinogen | |
|---|---|---|---|---|---|
| ucMGP | 1 | ||||
| Fetuin-A | 0.31 P < 0.001 |
1 | |||
| C-reactive protein | 0.01 P = 0.70 |
0.10 P = 0.01 |
1 | ||
| Albumin | 0.24 P < 0.001 |
0.19 P < 0.001 |
-0.25 P < 0.001 |
1 | |
| Fibrinogen | 0.003 P = 0.91 |
0.09 P = 0.01 |
0.55 P < 0.001 |
-0.14 P < 0.001 |
1 |
Data are Spearman correlation coefficients, P-values
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–866. doi: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 2.Boon A, Cheriex E, Lodder J, Kessels F. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78:472–474. doi: 10.1136/hrt.78.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barasch E, Gottdiener JS, Marino Larsen EK, Chaves PH, Newman AB. Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study) Am J Cardiol. 2006;97:1281–1286. doi: 10.1016/j.amjcard.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Vasan RS, Parise H, Levy D, O'Donnell CJ, D'Agostino RB, Benjamin EJ. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- 5.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zebboudj AF, Shin V, Bostrom K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J Cell Biochem. 2003;90:756–765. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 7.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 8.Hermans MM, Vermeer C, Kooman JP, Brandenburg V, Ketteler M, Gladziwa U, Rensma PL, Leunissen KM, Schurgers LJ. Undercarboxylated matrix GLA protein levels are decreased in dialysis patients and related to parameters of calcium-phosphate metabolism and aortic augmentation index. Blood Purif. 2007;25:395–401. doi: 10.1159/000108629. [DOI] [PubMed] [Google Scholar]
- 9.Cranenburg EC, Vermeer C, Koos R, Boumans ML, Hackeng TM, Bouwman FG, Kwaijtaal M, Brandenburg VM, Ketteler M, Schurgers LJ. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res. 2008;45:427–436. doi: 10.1159/000124863. [DOI] [PubMed] [Google Scholar]
- 10.Parker BD, Ix JH, Cranenburg EC, Vermeer C, Whooley MA, Schurgers LJ. Association of kidney function and uncarboxylated matrix Gla protein: data from the Heart and Soul Study. Nephrol Dial Transplant. 2009;24:2095–2101. doi: 10.1093/ndt/gfp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price PA, Nguyen TM, Williamson MK. Biochemical characterization of the serum fetuin-mineral complex. J Biol Chem. 2003;278:22153–22160. doi: 10.1074/jbc.M300739200. [DOI] [PubMed] [Google Scholar]
- 12.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:2533–2539. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. Jama. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meltzer RS, Martin RP, Robbins BS, Popp RL. Mitral annular calcification: clinical and echocardiographic features. Acta Cardiol. 1980;35:189–202. [PubMed] [Google Scholar]
- 15.Skjaerpe T, Hegrenaes L, Hatle L. Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two-dimensional echocardiography. Circulation. 1985;72:810–818. doi: 10.1161/01.cir.72.4.810. [DOI] [PubMed] [Google Scholar]
- 16.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 17.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 20.Fraser JD, Price PA. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J Biol Chem. 1988;263:11033–11036. [PubMed] [Google Scholar]
- 21.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 22.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews ST, Rakhade S, Zhou X, Parker GC, Coscina DV, Grunberger G. Fetuin-null mice are protected against obesity and insulin resistance associated with aging. Biochem Biophys Res Commun. 2006;350:437–443. doi: 10.1016/j.bbrc.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 24.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 27.Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, Aizawa H, Itoh H, Kurabayashi M, Kawazu S, Tomono S, Oka Y, Suga T, Kuro-o M, Nabeshima Y, Nagai R. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000;49:1118–1123. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 28.Iribarren C, Darbinian JA, Go AS, Fireman BH, Lee CD, Grey DP. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann Epidemiol. 2007;17:669–678. doi: 10.1016/j.annepidem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part I: recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631–642. doi: 10.1016/j.jacc.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 30.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]


