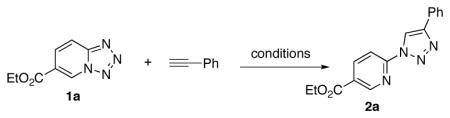
Abstract
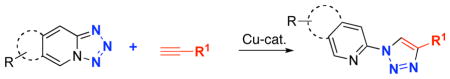
It has been shown that various pyrido-, quinolino-, pyrazino- and qunoxalinotetrazoles can efficienly be used as azide components in the Cu-catalyzed click reaction with alkynes. This method allows for efficient synthesis of a wide variety of N-heterocyclic derivatives of 1,2,3-triazoles.
1,2,3-Triazoles are biologically important units.1 Pyridotriazoles and quinolinotriazoles are particularly interesting as they exhibit a wide range of biological properties, including control of arthropod pests,2a substance-related disorders,2b ATP-competetive inhibition of vascular endothelial growth factor receptors I and II,2c antibacterial,2d and antimicrobacterial activity.2e
Unarguably, the Cu-catalyzed click chemistry3 of azide with alkyne is the most efficient way to assemble the 1,2,3-triazole ring4 (eq. 1). However, preparation of pyrido- and quinolino- triazoles is not straightforward since these azides exist in equlibrium between closed form (tetrazole A) and open form (azide B) (eq. 2).5
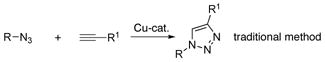 |
(1) |
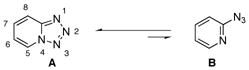 |
(2) |
Usually, the position of this equilibrium depends on several factors, such as nature of substituents,5 solvent6 and temperature.5 Thus, it has been reported7a that NO2 group at the C-6 position of tetrazole favors the open form (azide B). On the contrary, tetrazoles with NO2, COOH, and Cl groups at the C-8 position, and unsubstituted tetrazole predominantly exist7,8 in closed form A. It should be mentioned that pyridotetrazole has been employed in the preparation of organometallic complexes of late transition metals.7a Furthermore, there have been contradictory reports9,10 on the employment of tetrazoles in the click reaction. For instance, it has been shown that pyridotetrazoles, existing in closed form, are inert toward click reaction under standard conditions.9 By other hand, there have been two reports10a,b in which single examples of successful click reaction of generated in situ pyridotetrazoles with alkynes were demonstrated. Moreover, when this manuscript was under preparation, a paper describing successful click reaction of purinotetrazole, which mainly exists in open form, has appeared.10c Accordingly, motivated by the high biological importance of pyridyl- and quinolinyl-containing triazoles2 and intrigued by the contradictory results on employment of triazoles in click reaction,9,10 we undertook investigation aming at the development of efficient method for employment of differently substituted tetrazoles in synthesis of heterocyclic derivatives of 1,2,3-triazoles. Herein, we wish to report that various pyrido- quinolino-, pyrazino- and qunoxalinotetrazoles 1 can efficiently be employed in click reaction with alkynes to give the corresponding heterocyclic derivatives of 1,2,3-triazoles 2 (eq. 3).
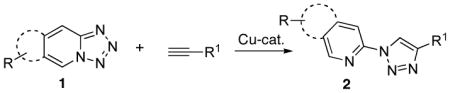 |
(3) |
We first examined the reaction of tetrazole 1a with phenyl acetylene employing the most popular[3b] click chemistry conditions (Table 1, entry 1). However, no formation of desired product 2a was observed. Employment of other copper salts was more effective. Thus, when the reaction was performed in the presence of 10 mol% CuI,4a it afforded the product 2a in 10% yield (entry 2). Use of Cu(OTf)24i gave 5% of product (entry 3). A substantial improvement of the yield (52%) has been achieved with (CuOTf)2•C6H64j (entry 4). Gratifyingly, analogous reaction at room temperature gave 81% of 2a (entry 5). THF was equally efficient as toluene in the reaction (entry 6). Switching to other solvents (entries 7 and 8) was not beneficial for this reaction.
Table 1.
Optimization of click reaction of tetrazoles.
 | |||||
|---|---|---|---|---|---|
| no. | catalyst 10 mol % | solvent 0.25 M | t [°C] | time [h] | yield[a] [%] |
| 1 | CuSO4 • 5H2O, Na-ascorbate | DCM:H2O (1:1) | 60 | 24 | 0 |
| 2 | CuI | toluene | 100 | 24 | 10 |
| 3 | Cu(OTf)2 | toluene | 100 | 24 | 5 |
| 4 | (CuOTf)2•C6H6 | toluene | 100 | 2 | 52[b] |
| 5 | (CuOTf)2•C6H6 | toluene | rt | 7 | 81 |
| 6 | (CuOTf)2•C6H6 | THF | 60 | 12 | 76 |
| 7 | (CuOTf)2•C6H6 | DCE | 100 | 24 | 0 |
| 8 | (CuOTf)2•C6H6 | 1,4-dioxane | 100 | 24 | 0 |
Isolated yields.
Some decomposition products were found.
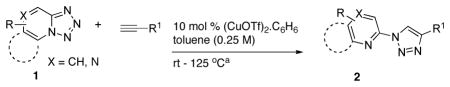
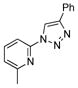
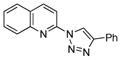
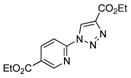
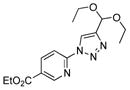
With the best-optimized conditions in hand, we tested the generality of the click reaction of tetrazoles (Table 2). To our delight, these newly developed conditions appeared to be very general for a spectrum of N-fused tetrazoles giving an easy access to 1,4-triazoles 2. Thus, reaction of ester-containing pyridotetrazole (1a) with varios alkynes proceeded smoothly at room temperature to produce differently substituted pyridyl-containing triazoles in good to excellent yields (entries 1–12). Reactions of unsubstituted (1b) and C-5 methyl-substituted (1c) tetrazoles were efficient at elevated temperatures (entries 13–21). It was also found that various N-fused heterocyclic tetrazoles, such as quinolinotetrazoles (1d, entries 22–28), pyrazinotetrazole (1e, entry 29 and 30) and qunoxalinotetrazole (1f, entries 31 and 32) successfully underwent click reaction to give the corresponding N-heterocycle-substituted 1,4-triazoles 2 in good yields. These reaction conditions appeared to be very general with respect to the alkyne component, as alkynes possessing various alkyl, aryl, alkenyl, benzyl, homobenzyl, ester, trimethylsilyl, alkyl chloride, secondary alcohol, acetal, thiophenyl, and even sugar groups provided good to high yields of triazoles 2.
Table 2.
Synthesis of functionalized 1,4-disubstituted triazoles.
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | product | yield [%]b | no. | product | yield [%]b | no. | product | yield [%]b | no. | product | yield [%]b |
| 1c |
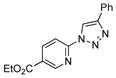 2a |
81 | 9c |
 2i |
64 | 17d |
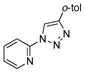 2q |
37 | 25e |
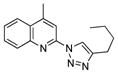 2y |
68 |
| 2c |
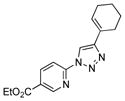 2b |
88 | 10c |
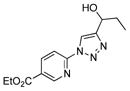 2j |
84 | 18d |
 2r |
74 | 26e |
 2z |
62 |
| 3c |
 2c |
70 | 11c |
 2k |
50 | 19d |
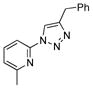 2s |
88 | 27e |
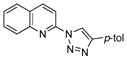 2aa |
63 |
| 4c |
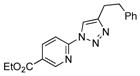 2d |
63 | 12c |
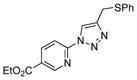 2l |
53 | 20d |
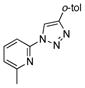 2t |
81 | 28e |
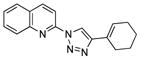 2bb |
54 |
| 5c |
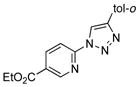 2e |
60 | 13d |
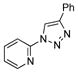 2m |
62 | 21d |
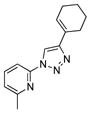 2u |
81 | 29d |
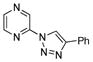 2cc |
51 |
| 6c |
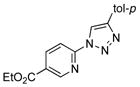 2f |
65 | 14d |
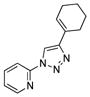 2n |
66 | 22e |
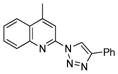 2v |
88 | 30d |
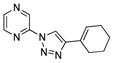 2dd |
57 |
| 7c |
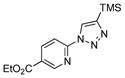 2g |
86] | 15d |
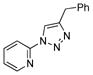 2o |
67 | 23e |
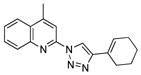 2w |
62 | 31d |
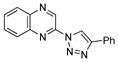 2ee |
73 |
| 8c |
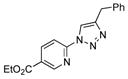 2h |
72 | 16d |
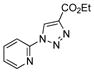 2p |
58 | 24e |
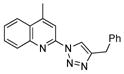 2x |
77 | 32d |
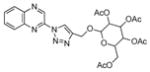 2ff |
66 |
See Supporting Information for details.
Isolated yield.
Reactions performed at room temperature.
Reactions performed at 100°C.
Reactions performed at 125°C.
After developing the “tetrazole-clicking” approach for the synthesis of 1,4-triazoles, we next examined the possibility of employment of N-fused tetrazoles in the Ru-catalyzed11 synthesis of 1,5-triazoles 5 (Table 3). However, when 1a was treated with phenyl acetylene in the presence of 5 mol% RuCpCl(PPh3)2 at 110°C for 24h in dioxane (entry 1), no desired product was formed. Employment of more active catalyst [RuCp*Cl(PPh3)2]11 gave no reaction, as well (entry 2). Probably, the azide-coordinated Ru-catalyst, in contrast to the Cu-catalyst (entry 3), is deactivated by the chelation with the nitrogen atom of the pyridine ring.12 To test this hypothesis, we performed reactions of 3-azido- and 4-azido- pyridines with this Ru(II) catalyst where no such type of chelation is possible. Indeed, it was found that 3-azidopyridine smoothly underwent cycloaddition reaction with phenyl acetylene (Table 3, entry 4) with RuCp*Cl(PPh3)2 providing inseparable mixture of 1,4-triazole and 1,5-triazole in 59% yield (1:1.5). Reaction of 4-azidopyridine gave 1,5-triazole as the major product (Table 3, entry 6). Expectedly, employment of Cu-catalysis for click reaction of 3-azido- and 4-azido- pyridines proceeded uneventfully providing 1,4-disubstituted triazoles in excellent yields (entries 5 and 7). Thus, it became evident that under the Ru-catalysis tested, pyridotetrazoles could not be used as precursors for 1,5-disubstituted triazoles.
Table 3.
Toward synthesis of 1,5-disubstituted triazoles.a
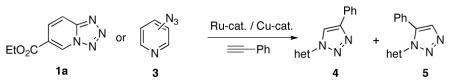 | ||||
|---|---|---|---|---|
| no. | substrate | catalyst | 4 | 5 |
| 1 | RuCpCl(PPh3)2 | - | - | |
| 2 | RuCp*Cl(PPh3)2 | - | - | |
| 3 | (CuOTf)2C6H6 | 81% | - | |
| 4 | RuCp*Cl(PPh3)2 | 23% | 36%b | |
| 5 | (CuOTf)2C6H6 | 91% | - | |
| 6 | RuCp*Cl(PPh3)2 | 3% | 56% | |
| 7 | (CuOTf)2C6H6 | 87% | - | |
Isolated yield. Reaction conditions: 5 mol % catalyst, 1,4-dioxane 0.25 M, 110°C (entries 1, 2, 4 and 6); 10 mol % catalyst, PhMe 0.25 M, 100°C (entries 3, 5 and 7).
Inseparable mixture of 4 and 5 (1:1.5).
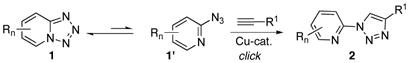 |
(4) |
In summary, it has been shown that pyrido-, quinilino-, pyrazino-, and qunoxalinotetrazoles, which exist in open/close form equilibrium (between 1 and 1′, eq. 4) can be employed as azide surrogates in the Cu-catalyzed click reaction. This reaction is efficient with a wide variety of alkynes to produce N-heterocyclic derivatives of 1,4-disubstituted triazoles 2. It has also been found that, probably due to deactivation of Ru-catalyst, pyridotetrazoles cannot be used as azide precursors in the synthesis of 1,5-disubstituted triazoles.
Supplementary Material
Acknowledgments
The support of the NIH (GM-6444) is gratefully acknowledged.
Footnotes
Supporting Information Available: Experimental procedures and characterization of new compounds.
References
- 1.(a) Alvarez R, Valaquez S, San F, Aquaro S, Penro CDCF, Karlsson A, Balzarini J, Camarasa MJ. J Med Chem. 1994;37:4185. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]; (b) Buckle DR, Rockell CJM, Smith H, Spicer BA. J Med Chem. 1986;29:2262. doi: 10.1021/jm00161a022. [DOI] [PubMed] [Google Scholar]; (d) Genin MJ, Allwine DA, Anderson DJ, Barbachyn MR, Emmert DE, Garmon SA, Graber DR, Grege KC, Hester JB, Hutchinson DK, Morris J, Reischer RJ, Ford CW, Zurenko GE, Hamel JC, Schaadt RD, Stapert D, Yagi BH. J Med Chem. 2000;43:953. doi: 10.1021/jm990373e. [DOI] [PubMed] [Google Scholar]
- 2.(a) Bretschneider T, Franken E-M, Goergens U, Fuesslein M, Hense A, Kluth J, Schwarz H-G, Koehler A, Malsam O, Voerste A. WO 2010006713 A2 20100121 PCT Int Appl. 2010; (b) Garzya V, Watson SP. WO 2009115486 A1 20090924 PCT Int Appl. 2009; (c) Kiselyov AS, Semenova M, Semenov VV. Bioorg Med Chem Lett. 2009;19:1344. doi: 10.1016/j.bmcl.2009.01.046. [DOI] [PubMed] [Google Scholar]; (d) Gordeev MF, Yuan Z, Liu J. WO 2008108988 A1 20080912 PCT Int Appl. 2008; (e) Japelj B, Recnik S, Cebasek P, Stanovnik B, Svete J. J Het Chem. 2005;42:1167. [Google Scholar]
- 3.(a) Sharpless KB, Finn MG. Angew Chem. 2001;113:11, 2056. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:2004. [Google Scholar]; (b) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem. 2002;114:2708. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:2596. [Google Scholar]; (c) Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 4.For original [3+2] cycloaddition see: Huisgen R. Angew Chem. 1963;75:604.For selected recent reviews of click chemistry see: Meldal M, Tornøe CW. Chem Rev. 2008;108:2952. doi: 10.1021/cr0783479.Bock VD, Hiemstra H, van Maarseveen JH. Eur J Org Chem. 2006:51.Nandivada, Jiang HX, Lahann J. Adv Mater. 2007;19:2197.Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med Res Rev. 2008;28:278. doi: 10.1002/med.20107.Becer CR, Hoogenboom R, Schubert US. Angew Chem Int Ed. 2009;48:4900. doi: 10.1002/anie.200900755.Spiteri C, Moses JE. Angew Chem Int Ed. 2010;49:31. doi: 10.1002/anie.200905322.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5.Lutz JF. Angew Chem Int Ed. 2007;46:1018. doi: 10.1002/anie.200604050.Fukuzawa S-i, Shimizu E, Kikuchi S. Synlett. 2007:2436.Aucagne V, Berna J, Crowley JD, Goldup SM, Hanni KD, Leigh DA, Lusby PJ, Ronaldson VE, Slawin AMZ, Viterisi A, Walker DB. J Am Chem Soc. 2007;129:11950. doi: 10.1021/ja073513f.
- 5.(a) Boyer JH, Miller EJ. J Am Chem Soc. 1959;81:4671. [Google Scholar]; (b) Lowe-Ma ChK, Nissan RA, Wilson WS. J Org Chem. 1990;55:3755. [Google Scholar]; (c) Evans RA, Wentrup C. J Chem Soc, Chem Commun. 1992:1062. [Google Scholar]; (d) Sasaki T, Kanematsu K, Murata M. J Org Chem. 1971;36:446. [Google Scholar]
- 6.Pochinok VV, Avramenko LF, Grigorenko PS, Skopenko VN. Russ Chem Rev. 1975;44:481. and references cited therein. [Google Scholar]
- 7.(a) Pizzotti M, Cenini S, Porta F. J Chem Soc, Dalton Trans. 1978:1155. [Google Scholar]; (b) Messmer A, Juhasz-Riedl HZ, Sohar P. J Org Chem. 1988;53:973. [Google Scholar]; (c) Wentrup C. Tetrahedron. 1970;26:4969. [Google Scholar]; (d) Patai S, editor. The chemistry of azido group. Interscience. London: 1971. [Google Scholar]; (b) Reimlinger H. Chem Ber. 1970;103:1900. and references cited therein. [Google Scholar]
- 8.Kanyalkar M, Coutinho EC. Tetrahedron. 2000;56:8775. [Google Scholar]
- 9.Colombano G, Travelli C, Galli U, Caldarelli A, Chini MG, Canonico PL, Sorba G, Bifulco G, Tron GC, Genazzani AA. J Med Chem. 2010;53:616. doi: 10.1021/jm9010669. [DOI] [PubMed] [Google Scholar]
- 10.(a) Klein M, Diner P, Dorin-Semblat D, Doerig C, Grotli M. Org Biomol Chem. 2009;7:3421. doi: 10.1039/b906482f. [DOI] [PubMed] [Google Scholar]; (b) Saha B, Sharma S, Sawant D, Kundu B. Synlett. 2007:1591. doi: 10.1021/cc0700445. [DOI] [PubMed] [Google Scholar]; (c) Lakshman MK, Singh M, Parrish D, Balachandran R, Day BW. J Org Chem. 2010 doi: 10.1021/jo902342z. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Chen X, Xue P, Sun HHY, Williams ID, Sharpless KB, Fokin VV, Jia G. J Am Chem Soc. 2005;127:15998. doi: 10.1021/ja054114s. [DOI] [PubMed] [Google Scholar]
- 12.For lower reactivity of 2-pyridyl diazocompounds in Rh-catalyzed transformations, see: Davies HML, Townsend RJ. J Org Chem. 2001;66:6595. doi: 10.1021/jo015617t.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


