Abstract
Herpes simplex virus (HSV) infections are common but there is no vaccine available. We evaluated cationic liposome-DNA complexes (CLDC) as an adjuvant for an HSV gD2 vaccine and compared it to an MPL/Alum adjuvant in a guinea pig model of genital herpes. The addition of CLDC to the gD2 vaccine significantly decreased acute and recurrent disease and most importantly the number of days with recurrent virus shedding compared to gD2 alone. Reductions in these outcomes were also detected when gD2+CLDC was compared to gD2+MPL/Alum. When the vaccine and adjuvants were evaluated as therapeutic vaccines, they were ineffective. CLDC enhanced protection compared to MPL/Alum and is the first vaccine to reduce recurrent virus shedding, a key to decreasing the spread of HSV-2.
Keywords: HSV vaccine, adjuvant, genital herpes, guinea pig, CLDC
Introduction
Herpes simplex type 2 (HSV-2) infections are common worldwide [1], [2] and are therefore an important focus for the development of vaccines. Vaccines for HSV-2 may be prophylactic and intended to prevent or reduce infection and disease or they may be therapeutic and designed to decrease subsequent sequelae of infection in those already infected [3]. Recently two HSV-2 glycoprotein sub-unit vaccines have been evaluated as prophylactic vaccines in double blinded trials. A glycoprotein D (gD2) vaccine combined with 3-deacylated monophosphoryl lipid A (MPL) and alum reduced primary disease by approximately 70% in HSV seronegative women but had no effect in men or HSV-1 seropositive women [4]. The other vaccine, a combination of gD2 and gB2 administered with MF59, a squalene oil-in-water adjuvant, was not effective [5]. Recent trials of similar vaccines administered as therapeutic vaccines revealed that the vaccines were either not effective or at most minimally effective [6], [7].
A prophylactic HSV-2 vaccine should not only decrease primary disease but also subsequent recurrences and recurrent viral shedding as this is the main means by which virus is spread. In this regard, it is interesting to note the findings of Bourne et al in which the prophylactic gD2 vaccine with MPL and alum decreased primary disease, acute vaginal viral replication and recurrent disease but did not affect the frequency of recurrent virus shedding in the guinea pig model of genital herpes [8]. If true, this vaccine adjuvant combination would have little impact on the spread of genital herpes, a primary goal of a HSV-2 vaccine.
We have previously shown that Cationic Lipid DNA complexes (CLDC) improves antibody and T cell responses to herpes simplex vaccines as well as providing increased protection in a lethal mouse model of genital herpes [9]. Cationic liposome-DNA complexes (CLDC) were originally developed as a gene delivery system for potential gene therapy [10]. During the early trials it was, however, noted that administration of CLDC activated innate immunity inducing high levels of IFN-α, suggesting potent activation of plasmacytoid dendritic cells (pDCs), and IL-12, suggesting activation of conventional DC (cDC) [11], [12], [13]. This activation was independent of the presence of a transgene expressed by the plasmid and has also been shown to occur by activation of TLR3 via poly I:C [14]. The empty vector DNA used for CLDC contains multiple unmethylated CpG motifs, which contributes to the potent induction of innate immunity through activation of TLR9. Further, addition of peptide or protein antigens to CLDC elicits marked T-cell and antibody responses [14].
In order to further study the adjuvant effects of CLDC for a HSV vaccine, we used the guinea pig model of genital herpes. Unlike the lethal mouse model, this model allows evaluation of the severity of acute disease, clinical recurrences, recurrent vaginal virus shedding and latent virus levels in the dorsal root ganglia and is considered by many the small animal of choice for evaluation of vaccines [15]. In the studies reported here, we compared a CLDC adjuvanted gD2 vaccine (gD2+CLDC) to the gD2 vaccine alone and to the gD2 vaccine adjuvanted with MPL and alum (gD2+MPL/Alum). The gD2+MPL/Alum was selected to mimic the vaccine that was recently evaluated in two large clinical trials [4] and is being further evaluated in an even larger trial [16].
Methods
Animals
Female Hartley guinea pigs (250–350 g) were obtained from Charles River Breeding Laboratories (Wilmington, MA) and housed under AAALAC approved conditions.
Vaccines
The gD2 was prepared by R. Eisenberg and G. Cohen (University of Pennsylvania) from Sf9 (Spodoptera frugiperda) cells (GIBCO BRL) infected with a recombinant baculovirus expressing gD2 as previously described [17]. All vaccine recipients received 5 μg of gD2 in 500 μl by SC inoculation. For the MPL/alum group, the gD2 was absorbed onto the Alum and then combined with MPL.
Adjuvants
The MPL/Alum combination contained 50 μg of MPL (Sigma-Aldrich Corp, St. Louis, MO) and 200 μg of aluminum potassium sulfate (Sigma-Aldrich Corp, St. Louis, MO).
The CLDC (JVRS-100, Juvaris BioTherapeutics, Inc., Burlingame, CA) was provided as a white, lyophilized powder manufactured from plasmid DNA complexed with liposomes. The plasmid (pMB75.6) was 4242 base-pairs in length and was in a Tris-HCl buffer. Liposomes were prepared from the cationic lipid DOTIM (1-[2-(oleoyloxy)ethyl]-2-oleyl-3-(2-hydroxyethyl)imidazolinium chloride) and the neutral lipid, cholesterol. The plasmid DNA and liposome intermediates were each diluted with lactose and then complexed under aseptic conditions, to form the formulated drug substance. The formulated drug substance was filled in vials and lyophilized to produce the drug product. The lyophilized CLDC drug product was reconstituted in sterile water for injection. After reconstitution, the final drug product was a colloidal dispersion of 0.3 mg/ml DNA, 1.88 mg/ml DOTIM and 1.05 mg/ml cholesterol at pH 7 containing 1.4 mM Tris-HCl and 10% w/v lactose [18].
Experimental design
For evaluation of prophylactic vaccination, sixty guinea pigs were randomized into five groups (N=12/group): Group 1, no vaccine, Group 2, CLDC alone, Group 3, gD2 vaccine alone, Group 4, gD2+CLDC, Group 5, gD2+MPL/Alum. Animals were immunized on days 49 and 21 days prior to viral inoculation. Each 0.5 ml dose was administered by subcutaneous injection at 5 separate sites on the dorsum.
One day before viral challenge animals were bled by toenail clip and the serum stored at −20°C. Animals were inoculated with virus by rupturing the vaginal closure membrane with a moistened calcium alginate tipped swab (Calgiswab #3, Spectrum Labs., Los Angeles, CA) and instilling 0.1 ml of a virus suspension containing 1×106 plaque forming units (pfu) of HSV-2 strain MS into the vaginal vault. Swab samples of cervicovaginal secretions were collected on days 1, 2, 4, 6, 8 and 10-post inoculation (PI) and stored frozen (−80°C) until assayed for virus on rabbit kidney cells grown in BME (Gibco-Invitrogen) and 10% FBS (Hyclone, Thermo Fisher Scientific) as described [19].
Guinea pigs were evaluated daily and primary genital skin disease quantified using a lesion score-scale ranging from 0 to 4 where 0 represents no disease, 1 equals 1–3 small vesicles, 2 equals >3 lesions including larger lesions, 3 equals >3 large coalescing lesions and 4 equals several large ulcers with maceration [19]. Animals with disease intermediate to these scores were assigned values in between i.e. 0.5, 1.5 etc. Following recovery from primary infection, animals were examined daily from days 22–63 PI for evidence of spontaneous recurrent herpetic lesions [19]. The number of lesion days (days on which a recurrent lesion was observed on the perineum) was recorded. Vaginal swabs were also obtained Monday, Wednesday and Fridays from days 22–60 to evaluate for recurrent virus shedding. Swabs were stored frozen (−80°C) until they were processed for PCR analysis to determine the frequency of viral shedding into the genital tract. At the end of the study, the guinea pigs were sacrificed, and the dorsal root ganglia (DRG) were harvested aseptically. DRGs were stored frozen (−80°C) until DNA was extracted for PCR evaluation of latent virus.
For evaluation as a therapeutic vaccine, 60 animals were divided into the following five groups: (N=15/group); Group 1, no vaccine, Group 2, CLDC alone, Group 3, gD2 vaccine alone, Group 4, gD2+CLDC, Group 5, gD2+MPL/Alum. Animals were immunized as described for the prophylactic vaccine on days 21 and 35 after viral inoculation. Only animals infected with the challenge HSV-2 were evaluated. Animals were observed daily for recurrent lesions (days 22–63) and vaginal swabs were collected on Monday, Wednesday and Friday (days 42–56) for evaluation of virus shedding.
gD2 Antibody assay
Serum antibody levels against gD2 were determined by ELISA using 1.0 μg/ml gD2 (vaccine preparation) diluted in coating buffer (pH 9.5) overnight at 4°C. After plates were washed with phosphate-buffered saline plus 0.05% Tween 20 (wash buffer), plates were blocked for 1 hour at 37°C using 1% Bovine Serum Albumin (Sigma, St. Louis, MO) in wash buffer. To create the standard curve, a pool of 5 different guinea pig sera known to contain antibody to gD2 was used and assigned a concentration of 500,000 units per ml. Two fold serial dilutions of the standard starting at a dilution of 1,000 and serial 10 fold dilutions of the guinea pig sera to be tested at dilutions from 1,000 to 100,000 were made. After washing the blocking solution from the plates, standards or samples were added to duplicate wells and incubated for 1 hour at room temperature. After washing, normal goat sera were added for 15 min at room temperature for blocking. Without washing, biotinylated goat anti guinea pig IgG (Vector Laboratories, Burlingame, CA) was added and incubated for 30 min at room temperature. Plates were washed and peroxidase-conjugated avidin- biotin (Vector Laboratories) was added for 30 min at room temperature. o-Phenylenediamine (Sigma) was added as substrate and incubated 15 min at room temperature in the dark. The reaction was stopped with 1 M H2SO4 and the A490 was read on an ELISA reader. The average OD values of the duplicate wells of the standard were plotted using a 4 parameter best fit method. This curve was then used to calculate the units of gD antibody using the average OD for each sample. The lower limit of the assay was 150 units. The geometric mean titer (GMT) for each group was then calculated.
HSV-2 PCR
Vaginal swabs and DRG were isolated from the vaccinated guinea pigs using sterile swabs and dissection tools pre-treated with DNA Away (Molecular BioProducts) and stored at −80°C. DRG tissue was homogenized on ice in 500 μl of 2% FBS BME. DNA was isolated from 200 μl of vaginal swab media and DRG homogenate using QIAamp DNA Mini Kit (Qiagen #51306) according to manufacturer’s protocol. The gB gene was amplified by PCR using two sets of primers [20]. The primer sequences were: gB External Forward, 5′-CCACCGGCGCTACTTCATCT -3′ and gB External Reverse, 5′-CGGATGACCGTGTCGATGTC -3′ to generate a 264 bp product, and gB Internal Forward, 5′-CCGTCAGCACCTTCATCGA -3′, and gB Internal Reverse, 5′-CGCTGGACCTCCGTGTAGTC -3′ to generate a 124 bp product, Each PCR reaction contained 50 ng purified DNA, 100 pmol each primer, Promega Master Mix (Promega) in a total volume of 25 μl. PCR was performed using a Biorad iCycler and the temperature cycling profile began with an initial 95°C for 1 min followed by annealing at 61°C for 1 min, and elongation at 72°C for 1 min 30 sec for 35 cycles. A HSV-2 viral DNA control, HSV-2 Quantitated Viral DNA (Advanced Biotechnologies Inc, Columbia MD), which harbors the entire HSV-2 genome, was used as a positive control for amplification and specificity. To determine the limit of detection of genome copy number, the HSV-2 DNA was serially diluted into uninfected tissue DNA. The amount of virus shed during recurrences was small and required nested PCR to detect on most days. Nested PCR was performed by first using the gB External primers, followed by second round PCR with 5 μl of the product formed as the template for amplification using the gB Internal primers.
Statistics
For comparison of means data were analyzed by ANOVA followed by a student’s t test comparison. The primary comparisons were gD2+CLDC to gD2 alone, and to gD2MPL/Alum. Statistics were not adjusted for the multiple comparisons. Incidence data were compared by Fisher’s exact test. All comparisons are two-tailed.
Results
Prophylactic vaccination
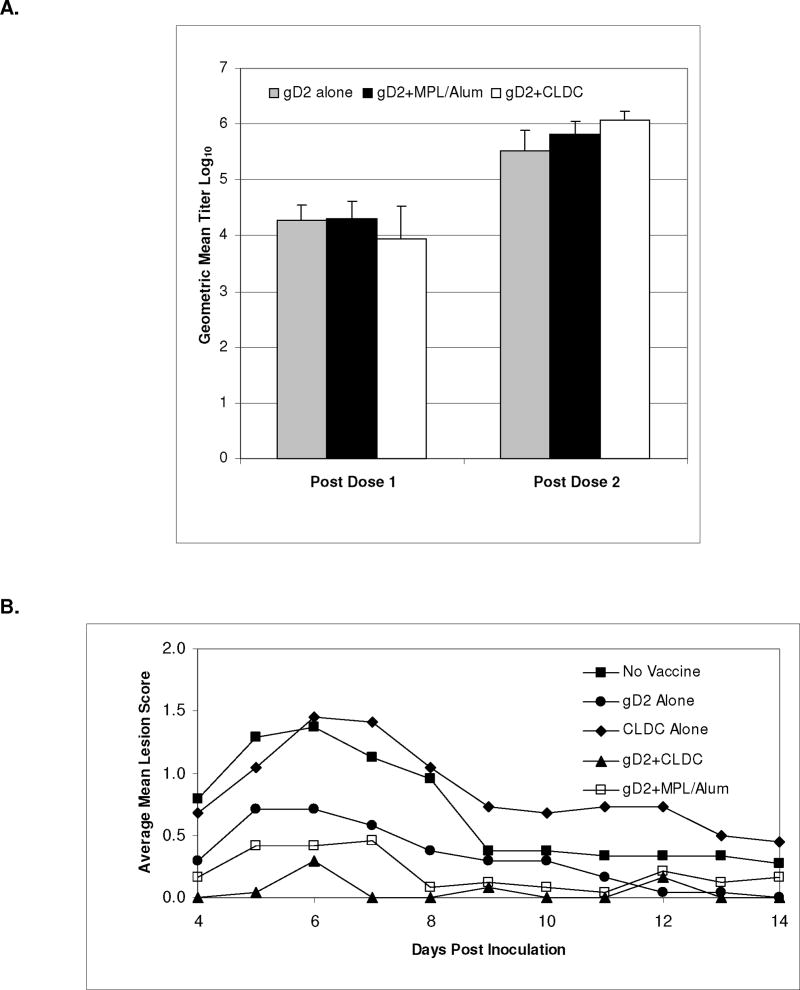
Antibody to gD2 was increased after two vaccinations by the addition of adjuvants (fig 1A) so that the GMT for the gD2+CLDC and gD2+MPL/Alum group was significantly higher than the gD alone group (P<0.001 and P=0.02, respectively) while the GMT for the gD2+CLDC group was also significantly higher than the gD2+MPL/Alum group (P=0.006).
Figure 1.
Evaluation of antibody response, clinical disease and vaginal virus shedding following vaccination with gD2 alone, or with MPL/Alum or with CLDC. Antibody responses to gD2 were evaluated by ELISA after one and two doses of vaccine (Panel A). Clinical disease was evaluated daily during the acute disease (days 1–14 following intravaginal inoculation) and the severity scored on a scale of 1–4 (Panel B). Vaginal swabs were also obtained on the days indicated and the amount of infectious virus quantitated by plaque assay (Panel C). All error bars are STD.
a P<0.001 gD2 alone vs. gD2+CLDC and P=0.02 vs. gD2+MPL/Alum
b P=0.006 gD2+CLDC vs. gD2+MPL/Alum group c P<0.05 vs. No Vaccine
d P<0.05 vs. gD2 Alone
e P<0.05 vs. gD2+MPL/Alum
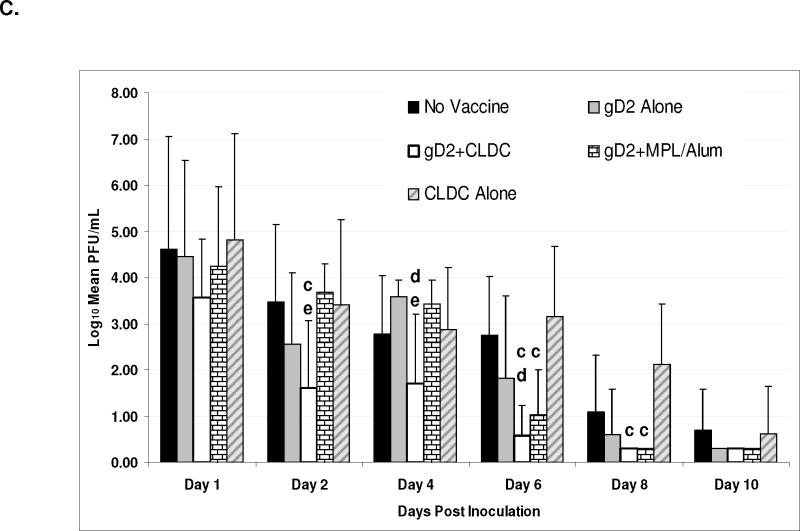
The gD2+CLDC vaccine decreased the severity of genital disease compared to vaccine alone (P<0.001) and compared to gD2+MPL/Alum (P=0.06) (Table 1 and figure 1B). The only animals that did not develop disease were in the gD2+CLDC group (5 animals) and the gD2+MPL/Alum group (2 animals). Only gD2 vaccination with an adjuvant significantly decreased the amount of virus shed during the acute disease (figure 1C). On day 4 and 6 the amount of virus shed in the gD2+CLDC group was significantly less than the group receiving gD2 alone and on days 2 and 4 it was significantly less than the gD2+MPL/Alum group (P<0.05).
Table 1. Effect of HSV gD2 vaccine and adjuvant on acute genital herpes disease.
 |
The effects of vaccination on subsequent recurrent disease are shown in figure 2. Vaccination with either gD2+CLDC or gD2+MPL/Alum significantly decreased the number of animals developing recurrences compared to the non vaccinated group (P<0.001 and P=0.008 respectively) but only the gD2+CLDC group also had significantly fewer animals than the group receiving gD2 alone (P<0.001) (figure 2A). Notably, only 1 out of 12 animals (8%) in the gD2+CLDC group developed subsequent recurrent lesions compared to 6 out of 12 (50%) for the gD2+MPL/Alum group (p=0.07). Similarly the mean number of days with recurrent lesions was decreased by immunization (figure 2B). Most importantly, the number of days with recurrences was less in the group receiving gD2+CLDC (0.4±1.4) compared to gD2 alone (2.8±3.6, P=0.04) or gD2+MPL/Alum (2.0±2.4, P=0.06).
Figure 2.
Effect of vaccination with gD2 alone or with adjuvants on the development and severity of subsequent recurrent disease. Animals were evaluated from days 22–63 after vaginal virus inoculation (when animals have recovered from the acute disease) for the development of recurrent lesions. The percent of animals developing at least one clinical recurrence is shown in panel A. The cumulative number of days with a recurrent lesion is shown in panel B.
a P<0.001 vs. gD2 alone; P=0.07 vs. gD2+MPL/Alum
b P=0.04 vs. gD2 alone; P=0.06 vs. gD2+MPL/Alum
Although the number of animals that shed virus after the acute disease had resolved was not significantly different between groups, the gD2+CLDC group had a significantly reduced number of days with recurrent virus shedding (2.2±1.3 days) compared to the gD2 alone group (3.5±1.4 days, P=0.03) and to the gD2+MPL/alum group (4.7±1.6 days, P=0<001) (figure 3).
Figure 3.
Effect of vaccination with gD2 alone or with adjuvants on recurrent vaginal virus shedding. Vaginal swabs were obtained MWF from day 22–63 after vaginal virus inoculation (when vaginal virus replication from the acute illness has resolved) for the development of recurrent lesions. The mean number of days in which virus was detected by PCR is shown in panel A. The cumulative number of days in which virus we detected by PCR is shown in panel B. All error bars are STD
a P=0.03 vs. gD2 alone; P<0.001 vs. gD2+MPL/Alum
The decreased number of recurrent shedding days and recurrent lesion days could be due to a decrease in the quantity of latent virus or to enhanced immune responses that could control recurrences. A reduction in the number of animals with detectable latent virus in the gD2+CLDC (P=0.003) or MPL/Alum (P=0.006) groups compared to gD2 alone was found (figure 4). There was no difference between the two adjuvanted groups.
Figure 4.
Effect of vaccination with gD2 alone or with adjuvants on detection of latent HSV-2 in the DRG. Animals were sacrificed at least 65 days after HSV-2 infection and latent virus assessed by PCR.
a P=0.003 vs. gD2 alone
b P=0.006 vs. gD2 alone
Therapeutic vaccination
As seen in figure 5, the number of days with recurrent lesions was reduced in the gD2+ MPL/Alum and gD2+CLDC groups but not in the gD2 alone group compared to the no vaccine group. However, none of the differences were significant, whether the data was evaluated beginning after the first vaccine, after the second vaccine or one week after the second vaccine. When the number of recurrent shedding days was evaluated no decreases in shedding days was noted (data not shown).
Figure 5.
Effect of vaccination with gD2 alone or with adjuvants as a therapeutic vaccine, administered on days 21 and 35 after virus inoculation. Animals were evaluated from day 22–63 after vaginal virus inoculation for the development of recurrent lesions. The cumulative number of days with a recurrent lesion is shown. The arrows indicate the days of vaccination. No significant differences were found.
Discussion
The ideal prophylactic HSV-2 vaccine would prevent infection by HSV-2 but this is probably an unrealistic goal for a HSV vaccine, as sterilizing immunity is difficult if not impossible to achieve. More realistically, a prophylactic HSV-2 vaccine should reduce or prevent genital herpes disease and subsequent clinical recurrences and subsequent virus shedding by either decreasing the pool of latent virus that can reactivate or by induction of immune responses that can control reactivation and shedding. HSV-2 is most frequently transmitted at times when the infected partner is asymptomatically shedding virus [21], [22], [23]. Therefore, a vaccine that decreases the time that an infected person sheds virus that can be transmitted to others should provide a major public health benefit.
In the experiments presented here, vaccination with HSV gD2 and CLDC significantly decreased the severity of primary disease, the number of animals that developed recurrent lesions, the frequency of recurrent lesions, the frequency of vaginal virus shedding and the number of animals with detectable latent HSV-2 infections compared to vaccine alone. In fact, compared to the addition of MPL/Alum, the use of CLDC with gD2 further decreased the severity of acute disease (P=0.06), the percent of animals with recurrent disease (P=0.07), the mean number of recurrent lesion days (P=0.06) and the number of days with recurrent virus shedding (P<0.001).
In previous evaluations, Bourne, et. al. also reported that vaccination with gD2 and MPL/Alum significantly decreased the severity of acute disease, the days with recurrent lesions but not the number of days with recurrent virus shedding compared to unvaccinated animals [8], [24]. Thus to our knowledge, the gD2 vaccine administered with CLDC is the first vaccine to reduce recurrent vaginal virus shedding, a critical goal for a HSV-2 vaccine. In the reports by Bourne et al the gD2 vaccine administered with MPL/Alum appeared to provide better protection than in the experiments presented here. However, it is important to recognize differences in the experimental designs including a different source for the gD2 and MPL/Alum. Most importantly, in their first study [24], animals were immunized three times (days -99, -64 and -15) before intravaginal challenge. In the second report [8] animals were immunized twice as was used in the studies reported here but on a different schedule (days 53 and 21 before challenge) and by a different route (IM) with different amounts of MPL (12.5 μg) and Alum (125 μg).
Therapeutic vaccines have had limited or no success in human trials [6], [7]. In the study reported here, vaccination with gD2 and CLDC produced the greatest reduction in recurrent disease but this did not reach significance. Thus, therapeutic vaccines for recurrent herpes disease remains an elusive goal and may require the induction of CD8+ T cell responses and thus the inclusion of other CD8+ T cell targets besides gD2.
The increased protection detected following vaccination with CLDC adjuvanted gD2 vaccine in the female guinea pig model of genital herpes warrants further investigation. The CLDC adjuvant induces a TH1 biased adaptive response to the candidate antigen which includes antibody, CD4, and CD8 responses to candidate antigens including polyfunctional CD4 and CD8 T-cell responses not seen with other adjuvants [14], [25]. It is possible that eliciting a broad based response which engages all aspects of the immune system may yield a more efficacious vaccine compared with more traditional approaches which focus on neutralizing antibody. It will be interesting to see if protection can be detected in male guinea pigs and in guinea pigs first infected with HSV-1, two populations that were not protected in the human trials of a gD2 vaccine [4].
Acknowledgments
This work was supported by the NIH/NIAID antiviral testing program (National Institute of Health Contracts No.: AI 15438) to Cincinnati Children’s Hospital Medical Center.
Footnotes
Bernstein, Farley, Bravo, Earwood, McNeal, had no conflicts. Fairman is an employee of Juvaris Bio Therapeutics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paz-Bailey G, Ramaswamy M, Hawkes SJ, Geretti AM. Herpes simplex virus type 2: epidemiology and management options in developing countries. Sexually transmitted infections. 2007 Feb;83(1):16–22. doi: 10.1136/sti.2006.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007 Dec 22;370(9605):2127–37. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein DI. Potential for immunotherapy in the treatment of herpesvirus infections. Herpes. 2001 Mar;8(1):8–11. [PubMed] [Google Scholar]
- 4.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002 Nov 21;347(21):1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 5.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. Jama. 1999 Jul 28;282(4):331–40. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 6.Straus SE, Wald A, Kost RG, McKenzie R, Langenberg AG, Hohman P, et al. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J Infect Dis. 1997 Nov;176(5):1129–34. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 7.Straus SE, Corey L, Burke RL, Savarese B, Barnum G, Krause PR, et al. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet. 1994 Jun 11;343(8911):1460–3. doi: 10.1016/s0140-6736(94)92581-x. [DOI] [PubMed] [Google Scholar]
- 8.Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis. 2005 Dec 15;192(12):2117–23. doi: 10.1086/498247. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein DI, Cardin RD, Bravo FJ, Strasser JE, Farley N, Chalk C, et al. Potent adjuvant activity of cationic liposome-DNA complexes for genital herpes vaccines. Clin Vaccine Immunol. 2009 May;16(5):699–705. doi: 10.1128/CVI.00370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N, Liggitt D, Liu Y, Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993 Jul 9;261(5118):209–11. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]
- 11.Dow SW, Fradkin LG, Liggitt DH, Willson AP, Heath TD, Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999 Aug 1;163(3):1552–61. [PubMed] [Google Scholar]
- 12.Dow SW, Schwarze J, Heath TD, Potter TA, Gelfand EW. Systemic and local interferon gamma gene delivery to the lungs for treatment of allergen-induced airway hyperresponsiveness in mice. Human gene therapy. 1999 Aug 10;10(12):1905–14. doi: 10.1089/10430349950017266. [DOI] [PubMed] [Google Scholar]
- 13.Freimark BD, Blezinger HP, Florack VJ, Nordstrom JL, Long SD, Deshpande DS, et al. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J Immunol. 1998 May 1;160(9):4580–6. [PubMed] [Google Scholar]
- 14.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006 Jun 15;176(12):7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 15.Stanberry LR. Evaluation of herpes simplex virus vaccines in animals: the guinea pig vaginal model. Rev Infect Dis. 1991 Nov-Dec;13(Suppl 11):S920–3. doi: 10.1093/clind/13.supplement_11.s920. [DOI] [PubMed] [Google Scholar]
- 16.NIAD, NIH. Herpevac Trial for Women. 2008 http://www.niaid.nih.gov/dmid/stds/herpevac/
- 17.Willis SH, Rux AH, Peng C, Whitbeck JC, Nicola AV, Lou H, et al. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J Virol. 1998 Jul;72(7):5937–47. doi: 10.1128/jvi.72.7.5937-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairman J, Moore J, Lemieux M, Van Rompay K, Geng Y, Warner J, et al. Enhanced in vivo immunogenicity of SIV vaccine candidates with cationic liposome-DNA complexes in a rhesus macaque pilot study. Human vaccines. 2008 Jul 14;5(2) doi: 10.4161/hv.5.3.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanberry LR, Bernstein DI, Burke RL, Pachl C, Myers MG. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987 May;155(5):914–20. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 20.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002 Jul;40(7):2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertz GJ. Asymptomatic shedding of herpes simplex virus 1 and 2: implications for prevention of transmission. J Infect Dis. 2008 Oct 15;198(8):1098–100. doi: 10.1086/591914. [DOI] [PubMed] [Google Scholar]
- 22.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992 Feb 1;116(3):197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 23.Mertz GJ, Schmidt O, Jourden JL, Guinan ME, Remington ML, Fahnlander A, et al. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sexually transmitted diseases. 1985 Jan–Mar;12(1):33–9. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003 Feb 15;187(4):542–9. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 25.Lay M, Callejo B, Chang S, Hong DK, Lewis DB, Carroll TD, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone((R))) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009 Jun 12;27(29):3811–20. doi: 10.1016/j.vaccine.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]