Abstract
Hepatitis B-specific memory B cell (HSMBC) frequencies were measured following hepatitis B vaccination in 15 HIV uninfected and 12 HIV infected adolescents. HSMBC were detected at significantly lower frequencies in HIV infected than in HIV uninfected individuals. The detection of HBsAb >10 mIU/ml at study week 28 was strongly associated with the detection of HSMBC and a direct correlation between HBsAb titers and HSMBC frequencies was observed. In HIV uninfected individuals, antibody titers >1000 mIU/ml were associated with higher HSMC frequencies. Lower HSMBC frequencies, reduced memory B cell (MBC) proliferation, and altered B cell phenotypes were measured in viremic HIV infected individuals compared with aviremic HIV infected or HIV uninfected individuals.
Keywords: HIV, hepatitis B vaccines, memory B cells
1. Introduction
HIV and HBV share several risk factors, and co-infection frequently occurs [1]. HIV infection prolongs HBV infectivity and significantly increases mortality risk in HBV infected patients [2, 3]. HBV can accelerate progression to AIDS; HIV infection has been associated with a rapid decline in HBV antibody titers, reactivation of latent HBV, or increased risk of re-infection [4–8]. HBV co-infection complicates clinical management of HIV co-infection and may limit antiviral options [9]. HBV vaccination is thus routinely recommended for HIV infected individuals. The HBV vaccines used in the present study (Engerix, Recombivax, and Twinrix) were licensed for general use based on immunogenicity data obtained from studies of HIV uninfected individuals [10–12]. However, suboptimal seroconversion to HBV vaccination has been documented in HIV infected individuals [4, 8, 13]. Some investigators have thus suggested that increasing the dose or number of vaccinations or including adjuvants may improve responses to HBV immunization in HIV infected individuals [4, 8, 13–18].
HBV antibody titers above 10 mIU/ml to HBV surface antigen (HBsAb) are considered protective [7, 19–21]. Even following successful HBV vaccination in healthy individuals, levels may decrease below this cut-off over time [7]. In animal models, however, some protection against subsequent re-challenge persists despite waning of antibody levels below the 10 mIU/ml threshold [19]. HSMBC have been detected in vaccinated or exposed human subjects with antibody levels less than 10 mIU/ml [22, 23], suggesting the potential importance of HBV-specific memory in protection against infection or disease.
Multiple B cell abnormalities have been described in HIV infection, including increased expression of cell-activation markers, hypergammaglobulinemia, and depletion of memory B cells (MBC) leading to ineffective recall responses [24–27]. B cell dysfunction may result in lower rates of seroconversion, accelerate the decline of antibody levels, and impair the generation or accelerate the loss of antigen-specific MBC in HIV infected patients [28, 29]. Improved B cell function was observed following the initiation of highly active antiretroviral therapy (HAART) in viremic HIV infected adults but MBC frequencies remained low over a year after viral control was attained [30].
In the present study, we evaluated the generation and maintenance of HBV-specific antibodies and MBC following HBV vaccination of HIV infected and HIV uninfected adolescents. We also explored the relationship between the detection of MBC immediately following vaccination and persistence of antibodies one year following completion of HBV vaccination. Finally, we evaluated the effects of HIV replication on circulating B cell phenotypes and proliferation. To our knowledge, this is the first longitudinal study of the generation and maintenance of HBV-specific memory B cell responses in HIV infected or uninfected HBV vaccine recipients.
2. Materials and methods
2.1. Adolescent Trials Network for HIV/AIDS Interventions (ATN) - ATN048 study design
ATN048 was a sub-study of ATN protocols 024 and 025. Briefly, ATN024 compared HBsAb responses in HIV infected adolescents 12 to 24 years of age who were randomized to one of 3 different HBV vaccine regimens: 1) standard adult dose of Engerix-B (20 mcg HBsAg); 2) increased adult dose of Engerix-B (40 mcg HBsAg); or 3) standard adult dose of Twinrix (720 EL.U Hep A/20 mcg HBsAg). Vaccines were administered at study entry, week 4, and week 24; antibody titers to HBsAg and HBV-specific memory B cell (HSMBC) frequencies were measured at study week 28. Individuals with antibody titers <10 mIU/ml at study week 28 received a booster dose of Engerix B-increased adult dose at study week 48. HBsAb titers and HSMBC frequencies were re-checked at week 72 on all ATN024 participants. CD4 T cell counts were measured using standard flow cytometric techniques. Plasma HIV-1 RNA levels were measured using commercial assays. HIV-1 viremia was defined as circulating plasma HIV-1 RNA levels of >400 copies/ml.
ATN025 compared HBsAb responses in HIV uninfected adolescents 12 to 18 years of age who were randomized to one of 2 different HBV vaccine regimens: 1) standard adult dose Recombivax (10 mcg HBsAg); or 2) Twinrix (720 EL.U Hep A/20 mcg HBsAg). Vaccines were given at entry and week 24 and HBSAb titers were measured at week 28. Study participants with HBSAb <10 mIU/ml at week 28 week received a booster dose of Recombivax between weeks 48 and 76. HBsAb titers and HSMBC frequencies were re-checked at week 76 on all ATN025 participants.
2.2. Study subjects
Individuals were eligible to participate in ATN048 study if they had not received prior HBV vaccinations and lacked antibodies to HBV core and surface antigens. Fifteen HIV uninfected and 12 HIV infected adolescents enrolled in the study, were followed through week 28, and had sufficient plasma and peripheral blood mononuclear cell (PBMC) samples for analyses (Table 1). Proliferation and B cell surface phenotyping were done on 11 HIV infected and 9 HIV uninfected study participants who had sufficient PBMC repositories to complete these assays. Seven HIV uninfected and 6 HIV infected, viremic adult donors were also evaluated as controls in the proliferation and phenotypic B cell studies. Mononuclear cells from 4 HIV and HBV uninfected cord blood specimens as well as 12 HIV uninfected, HBV uninfected and unimmunized children between 24 to 36 months of age were used to determine specificity of the ELISPOT assay. All studies were approved by the human subjects committee at each of the clinical sites and at the University of Massachusetts Medical School.
Table 1.
Baseline Patient Characteristics
| HIV-Uninfected | HIV-Infected | |
|---|---|---|
| Number | 15 | 12 |
| Male | 11 (73%) | 6 (50%) |
| Female | 4 (27%) | 6 (50%) |
| Age in years (median) | 12.2 – 18 (16.4) | 15.8 – 24.9 (21.6) |
| Plasma HIV RNA (median) | N/A | <400 – 51,600 (1038) |
| No. with plasma HIV RNA <400 copies/ml | N/A | 6 (50%) |
| Absolute CD4 count (median) | N/A | 52 – 560 (459) |
| No. on antiretroviral therapy | N/A | 5 (42%) |
N/A = not applicable
2.3. Cell preparation and flow cytometry
PBMC were separated using Ficoll Hypaque from heparin-treated whole blood and then cryopreserved for batch testing [31]. After thawing, PBMC were stained with CD14 and CD56 on Alexa 700, CD3 V450, and Live Dead Blue (LDB); subsequent gating using these parameters excluded non-B and dead cells. Appropriate single-color, matched isotype, fluorescence-minus-one (FMO), and doublet exclusion controls were also used to delineate the populations of interest. B cell surface phenotyping was evaluated by staining with fluorescent molecule-conjugated anti-human monoclonal antibodies binding to CD19 (PerCP), CD27 (APC-H7), CD21 (Fitc), BAFF-R (PE), and CD95 (APC). All fluorochromes were purchased from BD Biosciences except for LDB and CD21, which were purchased from Invitrogen (Carlsbad, CA) and Beckman Coulter (Fullerton, CA), respectively.
Samples were acquired using an LSR2 flow cytometer (BD Biosciences, San Diego, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR).
2.4. Measurement of antibody titers to HBV surface antigen (HBsAb)
HBsAb titers were measured in plasma samples taken at entry, week 28, and week 76 from HIV uninfected patients, and at entry, week 28, and week 72 from HIV infected patients using an automated immunometric technique (VITROS ECiQ) for quantitative HBsAb (Kit # 51938W, Quest Diagnostics, Madison, NJ). Individuals were considered to have made an antibody response when HBsAb titers equaled or exceeded 10 mIU/ml.
2.5. Polyclonal stimulation of PBMC
PBMC were cultured for 6 days in R-10 media (RPMI1640; GIBCO/Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum (GIBCO/Invitrogen), 0.1% Gentamicin 10 mg/ml stock reagent (GIBCO/Invitrogen), and 1% 100X L-glutamine (GIBCO/Invitrogen) [32]. This was further supplemented with 6 ug/ml CpG (Coley Pharmaceutical Group, Kanata, Canada), a 1:10,000 dilution of Staphylococus aureus Cowan A (Cat. No. P7155, Sigma Aldrich, St. Louis, MO), and a 1:10,000 dilution of pokeweed mitogen (Cat. No. L9379, Sigma Aldrich).
2.6. ELISPOT assay
Following polyclonal activation, PBMC from the 3 selected time points for each individual were tested together in a single assay to avoid inter-assay variability. ELISPOT plates (Millipore Corp. Billerica, MA) were coated with goat anti-human IgG (Invitrogen) to measure total IgG; HBsAg (Fitzgerald Industries International, Concord, MA); tetanus (positive control; Massachusetts Biologics Laboratories, Mattapan, MA); or Keyhole Limpet Hemocyanin (negative control; A.G. Scientific, San Diego, CA). High HBV responder donor PBMC were run on every plate as an additional positive control. Cells were plated at 250,000 cells per well and incubated overnight. Plates were incubated with mouse anti-human pan IgG Fc biotin conjugated antibody (Hybridoma Reagent Laboratory, Baldwin, MD) for 1 hour, followed by exposure to HRP-conjugated streptavidin for two hours. AEC substrate reagent (BD Biosciences) was used to develop the spots that were then counted with an ELISPOT reader (CTL, Cleveland, OH). HSMBC frequency was expressed as spot forming cells (SFC)/million PBMC. Responses were considered positive in wells with >12 SFC/million PBMC, and only if the antigen specific response exceeded three times the negative control value.
The specificity of the ELISPOT assay for the detection of HSMBC was demonstrated using cord blood samples from 4 infants born to HBV uninfected women and PBMC from 12 HIV uninfected, HBV seronegative children (born prior to routine infant immunization). HSMBC were not detected in the peripheral blood of these 16 HBV uninfected infants and children (data not shown).
2.7. Memory B cell proliferation assay
MBC proliferation was quantified using a membrane dye dilution assay adapted from Greenough, et al. [33]. PBMC were treated with 8uM PKH26, a fluorescent membrane dye, using the manufacturer’s protocol (Sigma Chemical Co.), and then subjected to polyclonal stimulation. After 6 days, PBMC were stained with CD19-PerCP, CD21-FITC, and CD27-APC, and were acquired using a 4-color FACSCalibur flow cytometer (BD Biosciences). CellQuest software (BD Biosciences) was used for analysis. The proliferation index was calculated using Modfit (Verity Software, Topsham, ME) as the sum of memory B cells in all generations divided by the computed number of original parent MBC present at the start of the experiment.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA) and SAS (SAS Institute, Cary, NC). The Mann-Whitney U test and mixed effects models were used to analyze the differences in antibody titers, HSMBC frequencies, and comparison of circulating B cell phenotypes among the study groups. Fisher’s exact test was used to test the hypothesis that detectable memory at week 28 was associated with antibody levels at week 28 and at weeks 72/76. Spearman’s rank correlation was used to evaluate the relationship between viral loads and B lymphocyte proliferation, and between HBsAb titers and HSMBC frequencies at week 28. Linear mixed effects models were used to compare B cell phenotypes and proliferation between study groups to accommodate for repeated measurements over time on the same individual. Linear mixed effects regression models compared proliferation with B cell surface phenotypes and similarly accounted for multiple data points per study subject over time. Statistical tests with p-values of 0.05 or less were regarded as statistically significant.
3. Results
3.1. Baseline patient characteristics
Fifteen HIV uninfected individuals were evaluated. Age at study ranged from 12.2 to 18 years (median 16.4 years; Table 1). Twelve HIV infected individuals were studied. Age at initiation of study ranged from 15.8 to 24.9 years (median 21.6 years). The median absolute CD4 count at the initiation of the study was 459 (range 52–560). Plasma HIV RNA copy number ranged from <400 – 51,600 (median 1038) copies/ml. Five (42%) HIV infected individuals were on antiretroviral therapy; 4 (80%) of these 5 individuals had plasma HIV RNA copy number <400 copies/ml (Table 1).
3.2. Seroconversion and detection of HSMBC in HIV uninfected and in HIV infected individuals
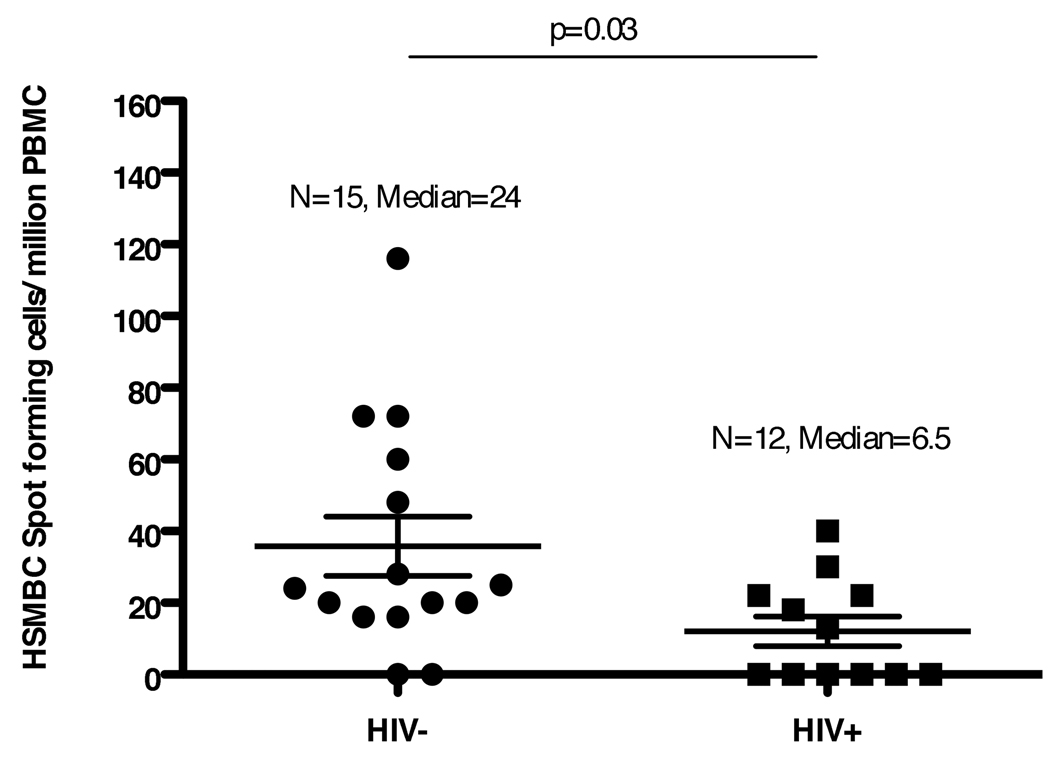
All HIV infected and HIV uninfected individuals were HBsAb negative (<10 mIU/ml) at entry. HBsAb were detected at titers >10 mIU/ml in 14 (93%) of the 15 HIV uninfected study participants and in eight (67%) of the 12 HIV infected study participants at study week 28 (Table 2). Neither the seroconversion rates nor the median antibody titers differed significantly between the two groups. However, HSMBC were detected at significantly lower frequencies in HIV infected individuals than in HIV uninfected individuals at week 28 (p = 0.03, Mann Whitney U test; Fig. 1).
Table 2.
| Infection Status |
No. of Patients |
No. with HBV Ab (%) |
p Value | No. of Patients |
No. with HSMBC (%) |
p Value | |
|---|---|---|---|---|---|---|---|
| Week 28 | |||||||
| HIV+ | 12 | 8 (67) | 0.14 | 12 | 6 (50) | 0.09 | |
| HIV− | 15 | 14 (93) | 15 | 13 (87) | |||
| Persistence of Responses at Week 72/76 (for those subjects who had positive HBV Ab responses at week 28) | |||||||
| HIV+ | 8 | 7 (88) | 1.0 | 7 | 5 (71) | 0.54 | |
| HIV− | 14 | 13 (93) | 10 | 9 (90) | |||
HBV = hepatitis B virus; Ab = Antibodies
HSMBC = hepatitis B specific memory B cells
Fig. 1. HSMBC frequencies (spot forming cells per million PBMC) in 15 HIV uninfected and 12 HIV infected study patients at study week 28.
3.3. HBsAb seroconversion at week 28 is associated with with HSMBC detection
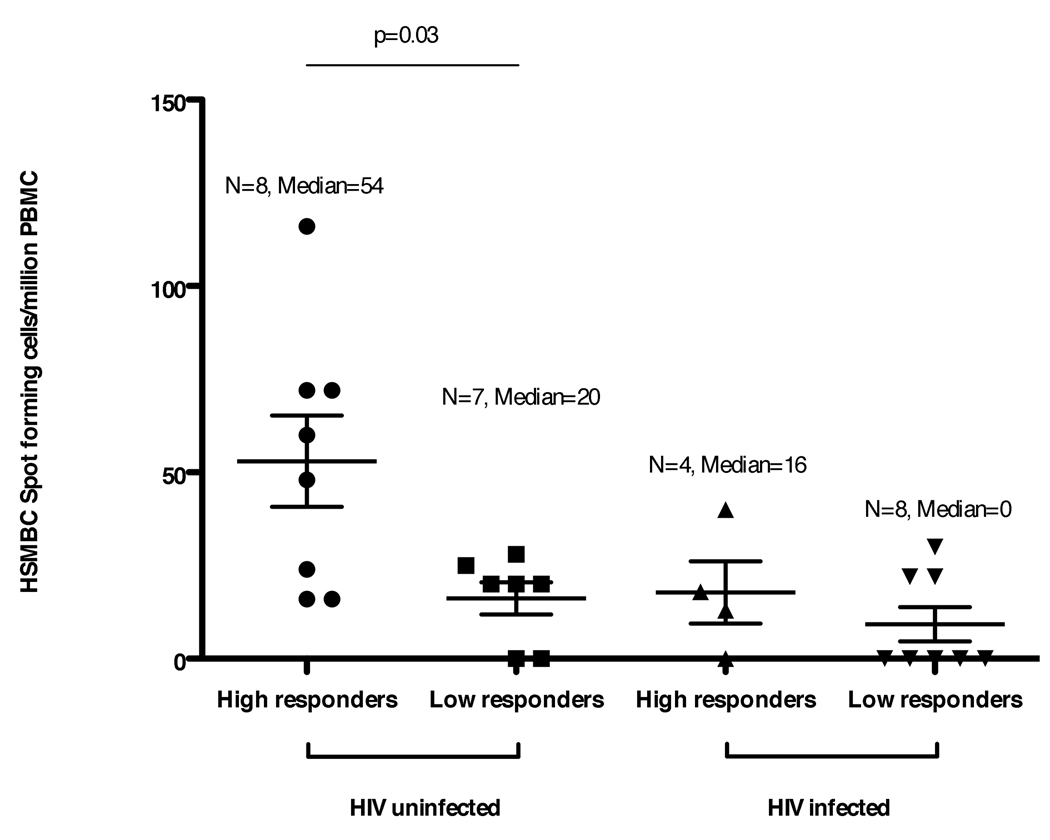
The detection of HBsAb >10 mIU/ml at study week 28 was strongly associated with the detection of HSMBC at study week 28 in both HIV uninfected and HIV infected individuals (Fisher’s exact test, p <0.0007). A similar association, between HBsAb >10 mIU/ml and HSMBC responses, was also seen at study weeks 72/76 (p = 0.02). There was a direct correlation between HBsAb titers and HSMBC frequencies (Spearman R=0.60, p = 0.02). In addition, high titer HIV uninfected responders (HBsAb >1000 mIU/ml) had higher HSMBC frequencies than low titer HIV uninfected responders (HBsAb <1000 mIU/ml; p = 0.03, Mann Whitney U test; Fig. 2).
Fig. 2. HSMBC frequencies (spot forming cells per million PBMC) in HIV uninfected and HIV infected study patients stratified as high titer responders (HBsAb >1000 mIU/ml) or low titer responders (HBsAb <1000 mIU/ml) at study week 28.
3.4. High rates of HBV antibody and HSMBC persistence in HIV infected and uninfected individuals
Overall, 13 (87%) of 15 HIV uninfected study participants had positive HBsAb responses at week 76. All 13 of these individuals had protective antibody titers at week 28 (Table 2). An additional HIV uninfected study participant with detectable HBsAb at week 28 was seronegative at week 76. The single HIV uninfected individual with undetectable HBsAb at week 28 was re-immunized at week 48 but remained seronegative at week 76. PBMC were available at week 76 from 10 HIV uninfected study participants with protective HBsAb titers and detectable HSMBC at week 28; 9 (90%) had persistently detectable HSMBC.
Overall, 7 (58%) of 12 HIV infected study participants had positive HBsAb responses at week 72 (p = 0.095 for comparison with 13/15 = 87% HIV uninfected study participants). All 7 of these individuals had detectable HBsAb at week 28 (Table 2). One additional HIV infected study participant with detectable HBsAb at week 28 was seronegative at week 72. Four HIV positive study participants who were HBsAb negative at week 28 were re-immunized at week 48 but remained seronegative at week 76. Five (71%) of 7 HIV infected study participants with protective HBsAb titers and detectable HSMBC at week 28 had persistently detectable HSMBC at week 76.
3.5. Altered circulating B cell phenotypes are observed in HIV infected individuals
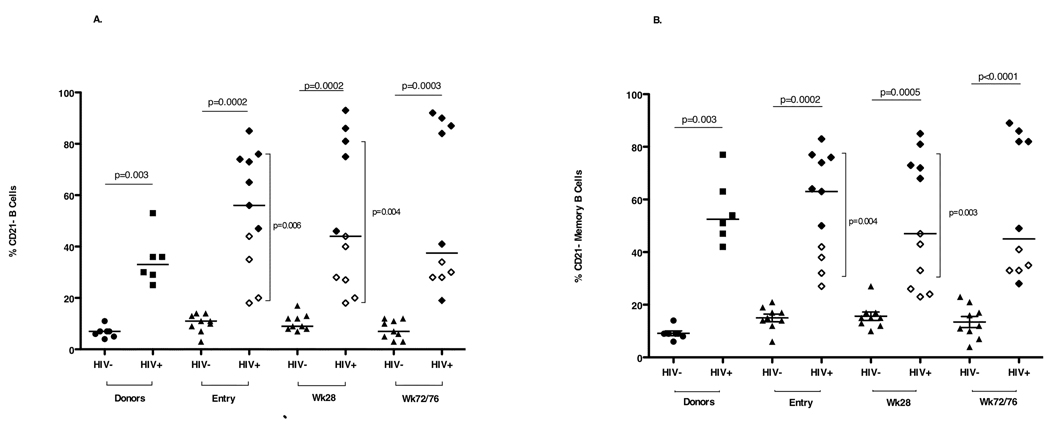
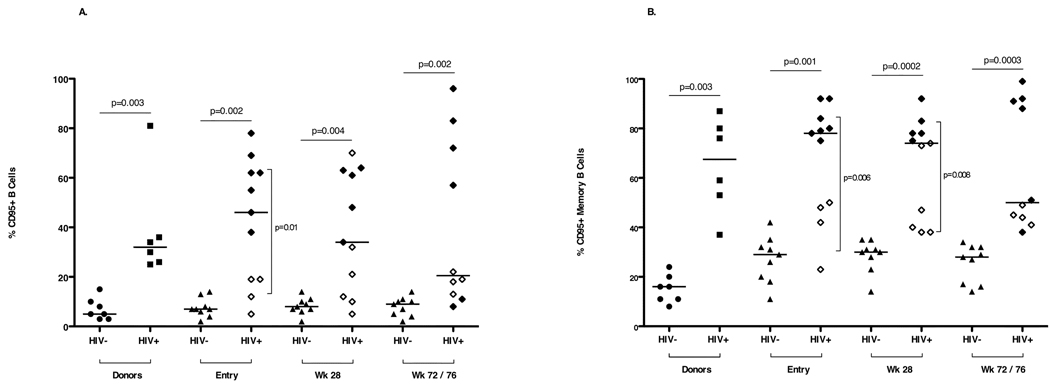
B cell (CD19+) percentages and numbers and memory B cell (CD19+CD27+) frequencies did not differ between HIV uninfected and HIV infected study subjects (data not shown). Higher frequencies of B cells and MBC with reduced CD21 expression (Fig. 3) and increased CD95 expression (Fig. 4) were measured at all three time points in HIV infected study participants compared with HIV uninfected study participants. At entry and week 28, higher frequencies of B cells and MBC with reduced CD21 expression were detected in viremic (RNA <400 copies/ml) HIV infected study participants than in aviremic (RNA >400 copies/ml) study participants. CD95 expression on MBC of aviremic HIV infected study participants was significantly lower at entry and week 28 (Fig. 4) than that measured in viremic HIV infected study participants. Cell surface Baff-R expression on B cells and MBC was similar in HIV uninfected and HIV infected study subjects.
Fig. 3. Percentages of circulating (A) CD19+ B cells and (B) CD19+CD27+ memory B cells that are CD21− in HIV uninfected and HIV infected donors and study subjects at entry, week 28 and week 72/76.
Open diamonds represent aviremic HIV infected study subjects (plasma HIV-1 RNA <400 copies/ml) and closed diamonds represent viremic HIV infected study subjects (plasma HIV-1 RNA >400 copies/ml). Horizontal bars indicate significant differences between the HIV infected and HIV uninfected study subjects. Vertical bars indicate significant differences between the HIV aviremic study subjects and the HIV viremic subjects. Seven HIV uninfected (closed circles) and 6 HIV infected, viremic (closed squares) adult donors constituted the control population.
Fig. 4. CD95 expression on CD19+ B cells (A) and CD19+CD27+ memory B cells (B) in HIV uninfected and HIV infected donors and study subjects at entry, week 28 and week 72/76.
Open diamonds represent aviremic HIV infected study subjects (plasma HIV-1 RNA <400 copies/ml) and closed diamonds represent viremic HIV infected study subjects (plasma HIV-1 RNA >400 copies/ml). Horizontal bars indicate significant differences between the HIV infected and HIV uninfected study subjects. Vertical bars indicate significant differences between the HIV aviremic study subjects and the HIV viremic study subjects. Seven HIV uninfected (closed circles) and 6 HIV infected, viremic (closed squares) adult donors constituted the control population.
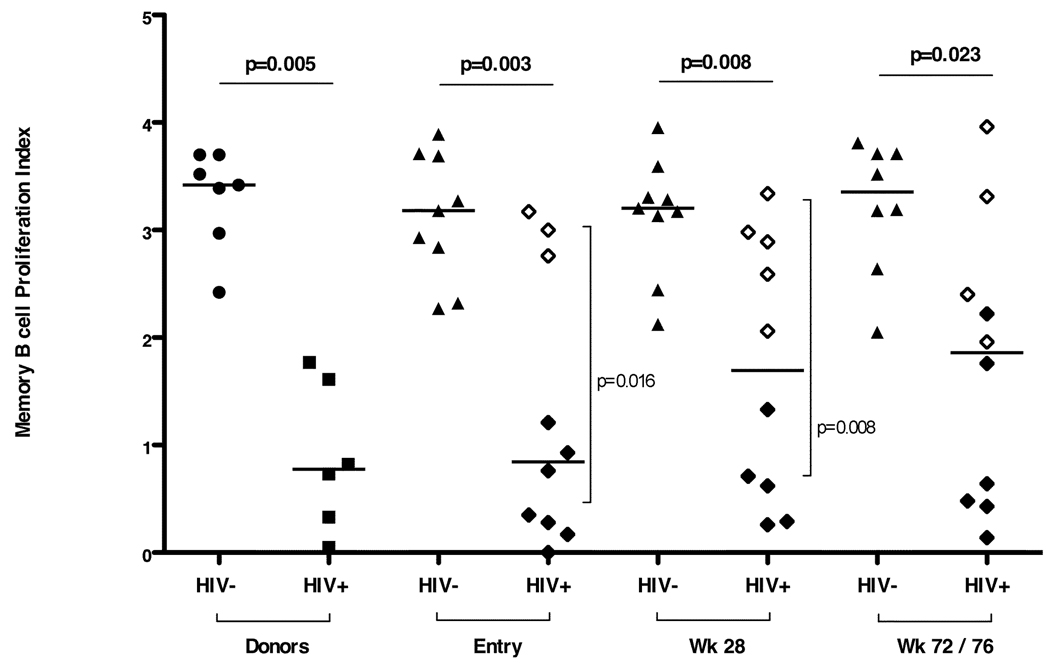
3.6. Reduced MBC proliferation in viremic HIV infected individuals
Reduced MBC proliferation was observed at all 3 time points in HIV infected study participants compared with HIV uninfected study participants (Fig. 5). At entry and week 28, reduced proliferation indices were observed in viremic HIV infected viremic individuals compared with HIV infected aviremic individuals (p = 0.016 at entry; p = 0.008 at week 28). Viral loads were negatively correlated with memory and naïve B cell proliferation (Spearman's Rank correlation, R = −0.73, p <0.02 and R = −0.7, p <0.03, respectively). Mixed effects models showed that higher frequencies of CD21+ memory B cells were associated with higher memory B cell proliferation (p <0.0001 and p <0.001, respectively).
Fig. 5. Proliferation indices for memory B cells (MBC PI) in HIV uninfected and HIV infected donors and study subjects at entry, week 28 and week 72/76.
Open diamonds represent aviremic HIV infected study subjects (plasma HIV-1 RNA <400 copies/ml) and closed diamonds represent viremic HIV infected study subjects (plasma HIV-1 RNA >400 copies/ml). Horizontal bars indicate significant differences between the HIV infected and HIV uninfected study subjects. Vertical bars indicate significant differences between the HIV aviremic study subjects and the HIV viremic subjects. Seven HIV uninfected (closed circles) and 6 HIV infected, viremic (closed squares) adult donors constituted the control population.
4. Discussion
Vaccination has played a vital role in curbing the spread of infectious diseases [13]. Ensuring successful vaccination of HIV infected patients is important for protection of individuals and the community [13]. To our knowledge, the development and maintenance of HSMBC in HIV uninfected and HIV infected individuals following HBV vaccination has not been well-characterized. This study also reports changes in B cell phenotypes and proliferation associated with lower antibody titers and HSMBC frequencies in HIV infected individuals.
Lower HSMBC frequencies were observed in HIV infected patients following vaccination. Lower rates of HBsAb seroconversion and accelerated serum antibody decline have been reported in HIV infected individuals compared with HIV uninfected individuals following primary immunization [18, 34]. Seroreversion over time is associated with lower post-vaccination antibody levels in HIV infected [16] as well as HIV uninfected [35] individuals. A recent cross sectional study reported that HIV infected patients with either natural or vaccine-elicited immunity had lower HBV-specific MBC frequencies than HIV uninfected controls [23]. Our study adds to these findings by demonstrating reduced HSMBC production in HIV infected individuals immediately following vaccination, which was associated with altered memory B cell phenotypes and reduced memory B cell proliferation. In HIV infected and HIV uninfected individuals with detectable HBV antibodies and HSMBC at week 28, rates of antibody and HSMBC persistence one year following completion of the vaccine series were not significantly different; the observed rate of protection at 72/76 weeks among all vaccinated subjects was greater for those who were HIV uninfected, though again not statistically significant. Cruciani et al, [16] have recently reported similar levels of antibody persistence at one year post-vaccination in HIV infected individuals; significant declines in anti-HBV antibody levels were observed, however, on follow-up 2 years post-vaccination. Longer follow-up of an antibody and HSMBC levels was not feasible in this study but would be of interest in future studies.
The detection of HBsAb >10 mIU/ml at study week 28 was strongly associated with the detection of HSMBC and a direct correlation between HBsAb titers and HSMBC frequencies was observed. The association between HBsAb and HSMBC response, though relatively weak (Spearman correlation = 0.60, p = 0.02), is consistent with the observed greater HBsAb and HSMBC response among HIV uninfected subjects and the corresponding lower observed response among HIV uninfected subjects. In HIV uninfected individuals, antibody titers >1000 mIU/ml were significantly associated with higher HSMC frequencies. Our results indicating a correlation between HBsAb and HSMBC are compatible with two recent studies in infants that reported an association between detectable MBC immediately following vaccination with long-term maintenance of antibodies [36, 37]. The enumeration of MBC following vaccination may improve our understanding of correlates of vaccine efficacy.
We [38] and others [37] have reported that early initiation of HAART may preserve primary responses to vaccine. Our data and others’ [30] demonstrate that in individuals with established HIV-1 infection, suboptimal B cell responses are associated with alterations in B cell and memory B cell phenotypes, including an expansion of B cells and MBC expressing low levels of CD21, higher levels of CD95, and reduced proliferative capacity at entry and week 28 (i.e. before and immediately after completing all vaccinations). Similar (but not statistically significant trends were noted at week 72/76. CD21- B cells have been shown to proliferate poorly and produce high titers of non-specific antibodies; production of antigen-specific antibodies also appears to be impaired. CD95+ B cells are more prone to apoptosis [39].
Of note, is that the majority of individuals in our study had CD4 T cell counts >350 cells/mm3. In this group of HIV infected individuals with relatively high CD4 counts, vaccine responses did not correlate with CD4 T cell counts. Lower HSMBC responses, altered B cell and memory B cell phenotypes, and reduced memory B cell proliferative capacity were observed in viremic individuals. A prior retrospective study [40] reported that the development of antibody responses following HBV vaccination of HIV infected adults was associated with undetectable viremia. In a recent prospective HBV vaccine study in HIV infected adults, receipt of 3 or more vaccines, HAART, and lower plasma HIV RNA were associated with the development of HBsAb titers >10 mIU/ml, even in individuals with high CD4 counts [41]. Lao-araya et al [42] reported impaired HBsAb responses in HIV infected, viremic children. Current recommendations regarding CD4 thresholds for initiation of ARV attempt to balance risk of progression to AIDS and death with adverse effects of ARV. Our data indicating lower HSMBC response rates in HIV infected, viremic individuals with relatively good CD4 counts support earlier initiation of ARV. Further, it may be well to postpone primary vaccinations in HIV infected individuals until viremia is controlled by ARV. In situations where vaccination has preceded effective antiretroviral therapy, revision in vaccine regimens to provide booster doses following suppression of viral replication may be required.
In conclusion, we report that HIV infection results in the impaired generation of HBV-specific memory B cell responses following vaccination in HIV infected individuals. Measurement of hepatitis B-specific memory B cell frequencies may be a valuable adjunct to antibody titers. Better understanding of how HIV disrupts the B cell compartment could have important implications for vaccine development and recommended immunization regimens.
Acknowledgments
The investigators are grateful to the members of the local youth Community Advisory Boards for their insight and counsel and are particularly indebted to the youth who participated in this study.
We acknowledge the contribution of the investigators and staff at the following ATN sites that participated and enrolled subjects into this study: Children’s Diagnostic and Treatment Center, Fort Lauderdale, FL (Puga, Leonard, Eysallanne, Inman); Children’s Hospital of Los Angeles, Los Angeles, CA (Belzer, Tucker); Children’s National Medical Center, Washington, DC (D’Angelo, Trexler, Hagler, Klamberg); John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center, Chicago, IL (Martinez, Bojan, Jackson); Montefiore Medical Center, Bronx, NY (Futterman, Enriquez-Bruce, Campos); Mount Sinai Medical Center, New York, NY (Levin, Geiger, Lee); St. Jude Children’s Research Hospital, Memphis, TN (Flynn, Gaur, Dillard, McKinley); Tulane University Health Sciences Center, New Orleans, LA (Abdalian, Baker, Kozina); University of Maryland, Baltimore, MD (Peralta, Flores, Gorle, Collinetti); University of Puerto Rico, San Juan, PR (Febo, Ayala-Flores, Fuentes); University of South Florida, Tampa, FL (Emmanuel, Lujan-Zilbermann, Callejas, Julian). Additional support was provided by the Pediatric AIDS Clinical Trials Group (PACTG) / International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) funded by grant No. 5 U01 AI068632-02. The following PACTG/IMPAACT sites also participated and enrolled subjects into this study: Hospital San Juan, Rio Piedras, Puerto Rico (Acevedo, Gonzalez, Angeli, Marrero, Perez); St. Jude Children’s Research Hospital, Memphis, TN (Flynn, Patel, Dillard, Wride); University of Alabama at Birmingham, Birmingham, AL (Pass, Craine, Beatty); University of California at San Diego, San Diego, CA (Spector, Viani, Norris, Stangl).[jl1]
We also thank Nancy Liu and Sushma Ahmad (Westat) for protocol support; Robin Brody, Erik Larson, Linda Lambrecht, James Coderre, and the UMass Pediatric Immunology staff for technical assistance; Margaret McManus for data analysis; and Wanda DePasquale for preparation of the manuscript.
Source(s) of Support: by the National Institutes of Health Grants R01-AI032391, K24-HD001489, and P30-AI042845 (to K.L.) and by The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health (U01 HD 040533 and U01 HD 040474) through the National Institute of Child Health and Human Development (B. Kapogiannis, R. Hazra, S. Lee, C. Worrell), with supplemental funding from the National Institutes on Drug Abuse (N. Borek) and Mental Health (P. Brouwers, S. Allison). The study was scientifically reviewed by the ATN’s Therapeutic Leadership Group. Network, scientific and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at The University of Alabama at Birmingham. Network operations and analytic support was provided by the ATN Data and Operations Center at Westat, Incorporated (J. Korelitz, B. Driver). Additional support for this study was provided by the P30 AI064518-04 (Duke Center for AIDS Research; CKC) and by grants from the General Clinical Research Center (GCRC) Program of the National Center for Research Resources, National Institutes of Health, Department of Health and Human Services. The following grants provided support: Children’s National Medical Center, GCRC Grant M01RR020359 and University of California at San Francisco, GCRC Grant M01RR00083-42 and Pediatric Clinical Research Grant M01RR01271. The Tulane/LSU Clinical Trial Research Center was supported in part or in full by the Louisiana Board of Regents Clinical and Translational Research, Education, and Commercialization Project. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agencies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors have contributed significantly to the work, have approved the manuscript, and concur with its submission. The manuscript material has not been previously reported, nor is it under consideration for publication elsewhere. There are no conflicting financial interests.
References
- 1.Remis RS, Dufour A, Alary M, Vincelette J, Otis J, Masse B, et al. Association of hepatitis B virus infection with other sexually transmitted infections in homosexual men. Omega Study Group. Am J Public Health. 2000;90(10):1570–1574. doi: 10.2105/ajph.90.10.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonacini M, Louie S, Bzowej N, Wohl AR. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS. 2004;18(15):2039–2045. doi: 10.1097/00002030-200410210-00008. [DOI] [PubMed] [Google Scholar]
- 3.Gilson RJ, Hawkins AE, Beecham MR, Ross E, Waite J, Briggs M, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11(5):597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29(4):1306–1310. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 5.Hadler SC, Judson FN, O'Malley PM, Altman NL, Penley K, Buchbinder S, et al. Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis. 1991;163(3):454–459. doi: 10.1093/infdis/163.3.454. [DOI] [PubMed] [Google Scholar]
- 6.Horvath J, Raffanti SP. Clinical aspects of the interactions between human immunodeficiency virus and the hepatotropic viruses. Clin Infect Dis. 1994;18(3):339–347. doi: 10.1093/clinids/18.3.339. [DOI] [PubMed] [Google Scholar]
- 7.Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep Erratum in: MMWR Morb Mortal Wkly Rep. 2007 Oct 26;56(42) 1114 2006; 55(RR-16):1-33; quiz CE1-4. [PubMed] [Google Scholar]
- 8.Wong EK, Bodsworth NJ, Slade MA, Mulhall BP, Donovan B. Response to hepatitis B vaccination in a primary care setting: influence of HIV infection, CD4+ lymphocyte count and vaccination schedule. Int J STD AIDS. 1996;7(7):490–494. doi: 10.1258/0956462961918563. [DOI] [PubMed] [Google Scholar]
- 9.Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed 6 August 2009];Department of Health and Human Services; Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008 November 3;:1–139. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 10.Diaz-Mitoma F, Law B, Subramanya A, Hoet B. Long-term antibody persistence induced by a combined hepatitis A and B vaccine in children and adolescents. Vaccine. 2008;26(14):1759–1763. doi: 10.1016/j.vaccine.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Duval B, Gilca V, Boulianne N, De Wals P, Masse R, Trudeau G, et al. Comparative long term immunogenicity of two recombinant hepatitis B vaccines and the effect of a booster dose given after five years in a low endemicity country. Pediatr Infect Dis J. 2005;24(3):213–218. doi: 10.1097/01.inf.0000154329.00361.39. [DOI] [PubMed] [Google Scholar]
- 12.Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63(10):1021–1051. doi: 10.2165/00003495-200363100-00006. [DOI] [PubMed] [Google Scholar]
- 13.Obaro SK, Pugatch D, Luzuriaga K. Immunogenicity and efficacy of childhood vaccines in HIV-1-infected children. Lancet Infect Dis. 2004;4(8):510–518. doi: 10.1016/S1473-3099(04)01106-5. [DOI] [PubMed] [Google Scholar]
- 14.Angel JB, Cooper CL, Clinch J, Young CD, Chenier A, Parato KG, et al. CpG increases vaccine antigen-specific cell-mediated immunity when administered with hepatitis B vaccine in HIV infection. J Immune Based Ther Vaccines. 2008;6:4. doi: 10.1186/1476-8518-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biggar RJ, Goedert JJ, Hoofnagle J. Accelerated loss of antibody to hepatitis B surface antigen among immunodeficient homosexual men infected with HIV. N Engl J Med. 1987;316(10):630–631. doi: 10.1056/NEJM198703053161015. [DOI] [PubMed] [Google Scholar]
- 16.Cruciani M, Mengoli C, Serpelloni G, Lanza A, Gomma M, Nardi S, et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine. 2009;27(1):17–22. doi: 10.1016/j.vaccine.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Laukamm-Josten U, Muller O, Bienzle U, Feldmeier H, Uy A, Guggenmoos-Holzmann I. Decline of naturally acquired antibodies to hepatitis B surface antigen in HIV-1 infected homosexual men. AIDS. 1988;2(5):400–401. doi: 10.1097/00002030-198810000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Zuin G, Principi N, Tornaghi R, Paccagnini S, Re M, Massironi E, et al. Impaired response to hepatitis B vaccine in HIV infected children. Vaccine. 1992;10(12):857–860. doi: 10.1016/0264-410x(92)90050-t. [DOI] [PubMed] [Google Scholar]
- 19.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16(3):197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Slifka MK, Ahmed R. Long-term antibody production is sustained by antibody-secreting cells in the bone marrow following acute viral infection. Ann N Y Acad Sci. 1996;797:166–176. doi: 10.1111/j.1749-6632.1996.tb52958.x. [DOI] [PubMed] [Google Scholar]
- 21.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 22.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24(5):572–577. doi: 10.1016/j.vaccine.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 23.Tuaillon E, Tabaa YA, Petitjean G, Huguet MF, Pajeaux G, Fondere JM, et al. Detection of memory B lymphocytes specific to hepatitis B virus (HBV) surface antigen (HBsAg) from HBsAg-vaccinated or HBV-immunized subjects by ELISPOT assay. J Immunol Methods. 2006;315(1–2):144–152. doi: 10.1016/j.jim.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med Erratum in: J Exp Med. 2004 Oct 4;200(7):587–599. following 946 2004; 200(7) [PubMed] [Google Scholar]
- 26.Titanji K, Chiodi F, Bellocco R, Schepis D, Osorio L, Tassandin C, et al. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS. 2005;19(17):1947–1955. doi: 10.1097/01.aids.0000191231.54170.89. [DOI] [PubMed] [Google Scholar]
- 27.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108(5):1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Feyen O, Jebran AF, Huck K, Jetzek-Zader M, Bas M, et al. Memory B-cell function in HIV-infected children - decreased memory B cells despite ART. Pediatr Res. 2009;66(2):185–190. doi: 10.1203/PDR.0b013e3181aa057d. [DOI] [PubMed] [Google Scholar]
- 29.Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122(1):12–19. doi: 10.1016/j.jaci.2008.04.034. quiz 20-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O'Shea MA, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197(4):572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 31.HIV/AIDS Network Coordination. [Accessed 6 August 2009];Cross-Network PBMC Processing SOP. 2009 Available at: http://www.hanc.info/labs/Pages/PBMCSOP.aspx.
- 32.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(1–2):111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Greenough TC, Cunningham CK, Muresan P, McManus M, Persaud D, Fenton T, et al. Safety and immunogenicity of recombinant poxvirus HIV-1 vaccines in young adults on highly active antiretroviral therapy. Vaccine. 2008;26(52):6883–6893. doi: 10.1016/j.vaccine.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collier AC, Corey L, Murphy VL, Handsfield HH. Antibody to human immunodeficiency virus (HIV) and suboptimal response to hepatitis B vaccination. Ann Intern Med. 1988;109(2):101–105. doi: 10.7326/0003-4819-109-2-101. [DOI] [PubMed] [Google Scholar]
- 35.Van Herck K, Van Damme P, Thoelen S, Meheus A. Long-term persistence of anti-HBs after vaccination with a recombinant DNA yeast-derived hepatitis B vaccine: 8-year results. Vaccine. 1998;16(20):1933–1935. doi: 10.1016/s0264-410x(98)00126-1. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, Yu LM, et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol. 2008;180(4):2165–2173. doi: 10.4049/jimmunol.180.4.2165. [DOI] [PubMed] [Google Scholar]
- 37.Pensieroso S, Cagigi A, Palma P, Nilsson A, Capponi C, Freda E, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci U S A. 2009;106(19):7939–7944. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta N, Pepe J, McManus M, Lin R, Somasundaran M, Yogev R, et al. Early suppression of HIV-1 replication preserves proliferative ability and measles-specific memory B cells in children [abstract C-171] Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections (Montreal, Canada) 2009 February 8–11; [Google Scholar]
- 39.Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A. 2001;98(18):10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41(7):1045–1048. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]
- 41.Landrum ML, Huppler Hullsiek K, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, et al. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27(34):4731–4738. doi: 10.1016/j.vaccine.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lao-araya M, Puthanakit T, Aurpibul L, Sirisanthana T, Sirisanthana V. Antibody response to hepatitis B re-vaccination in HIV-infected children with immune recovery on highly active antiretroviral therapy. Vaccine. 2007;25(29):5324–5329. doi: 10.1016/j.vaccine.2007.05.006. [DOI] [PubMed] [Google Scholar]