Abstract
The consolidation of memories in a variety of learning processes benefits from post-training sleep, and recent work has suggested a role for sleep slow wave activity (SWA). Previous studies using a visuomotor learning task showed a local increase in sleep SWA in right parietal cortex, which was correlated with post-sleep performance enhancement. In these as in most similar studies, learning took place in the evening, shortly before sleep. Thus, it is currently unknown whether learning a task in the morning, followed by the usual daily activities, would also result in a local increase in sleep SWA during the night, and in a correlated enhancement in performance the next day. To answer this question, a group of subjects performed a visuomotor learning task in the morning and was retested the following morning. Whole night sleep was recorded with high-density EEG. We found an increase of SWA over the right posterior parietal areas that was most evident during the second sleep cycle. Performance improved significantly the following morning, and the improvement was positively correlated with the SWA increase in the second sleep cycle. These results suggest that training-induced changes in sleep SWA and post-sleep improvements do not depend upon the time interval between original training and sleep.
Keywords: learning, memory, consolidation, motor learning, SWA
1. Introduction
Many behavioral studies have shown that performance in a variety of tasks improves following sleep [1-8]. Moreover, sleep deprivation impairs learning memory formation and inhibits plastic processes such as long-term potentiation [9, 10]. For at least some learning tasks, sleep-dependent improvement relies on sleep slow wave activity (SWA, the EEG power during NREM sleep between 0.5 and 4.5 Hz) [11-17], which reflects slow oscillations in the membrane potential of cortical neurons [18]. Sleep SWA is homeostatically regulated, generally increasing with the time spent awake, and decreasing with the time spent asleep [19, 20]. Recent evidence indicates that sleep SWA is also regulated locally, and that changes in SWA may be related to synaptic plasticity. Specifically, procedures leading to local strengthening of synapses in wakefulness induce a local increment in SWA during sleep [3, 21-23]. Conversely, behavioral or instrumental manipulations leading to synaptic depression in specific brain region produce a local decrease in SWA [21, 22, 24]. These findings suggest a direct link between the synaptic plasticity triggered by waking activities and the homeostatic regulation of sleep.
So far, studies investigating the role of sleep SWA in learning and memory in humans have placed the initial learning session shortly before sleep. This choice has the advantage of minimizing potential interference caused by subsequent waking activities and maximizing the chance of detecting learning-induced changes in brain activity during subsequent sleep. On the other hand, if sleep SWA plays an important role in learning and memory, it should be possible to document local changes in sleep SWA following learning, and such changes should be correlated with performance improvement the next day, irrespective of other daily activities. To evaluate this possibility, we took advantage of an implicit visuomotor learning task that showed sleep-dependent improvements related to a local change in sleep SWA [3]. Specifically, subjects who engaged in a rotation adaptation task in the evening before sleep showed a local increase in sleep SWA over the right posterior parietal region during the first thirty minutes of NREM sleep. Moreover, the next day subjects' performance improved in a way that was correlated with the amount of SWA during the first sleep cycle. The present work repeats the paradigm employed in the previous study. However, subjects performed the rotation adaptation task in the morning, and were left free to pursue their routine activities during the day. We then used high-density EEG to record sleep activity patterns with sufficient topographic resolution for the entire night, and not just the first hour. We asked whether morning training also triggered local changes in sleep SWA over the right posterior parietal area, and whether there would be a correlated enhancement in performance the next day.
2. Materials and Methods
Subjects and study design
Fourteen subjects (age = 20-38 years, mean: 28.4 years, eight men) were studied. Participants were right-handed, healthy, and had no history of psychiatric or neurological diseases or sleep disorders, as assessed in a preliminary screening visit. All subjects signed an informed consent form before participating in the study which was approved by the ethical committees of the participant institutions.
All subjects were tested in two sessions, separated by at least one week:
Learning session: In the morning, around 9 am, they were trained in a visuomotor adaptation task [3, 25-27] for about one hour. Thereafter, they left the laboratory to pursue their usual daily activities. Twelve hours later, around 9 pm, they returned to the sleep lab, where EEG activity was recorded during undisturbed sleep. The following morning, thirty minutes after waking up, they were retested.
Control session: After a day of usual activities, subjects arrived in the sleep lab about one hour before their habitual bedtime and EEG during sleep was recorded for the entire night.
The order of the two sessions was randomized. Subjects were instructed to maintain their usual sleep-wake schedule for at least one week before the sessions. During the two days of testing, they were required to go to sleep and to wake up at their habitual times. In addition, they were to abstain from daytime napping; wrist actigraphic recordings (Actiwatch 64, MiniMitter; Bend, Oregon) were used to ascertain that none of the subjects napped during the day.
Motor task and analysis
The motor task has been described in details elsewhere [3, 25-27]. Briefly, subjects moved a handheld cursor on a digitizing tablet. An opaque shield prevented them from seeing their arm and hand at all times. They were instructed to execute out-and-back movements from a central starting point to one of eight radially-arranged targets (distance of 4.2 cm), which appeared randomly on a computer screen together with the cursor position. Unbeknownst to the subjects, the direction of cursor movement on the screen was progressively rotated counterclockwise or clockwise relative to the hand movement by a total of 60° in four incremental steps of 15°. Each step comprised 3 blocks of 90 movements, for a total of 1080 movements. To prevent fatigue, trial blocks were separated by 1-2 minute resting periods.
For each movement, we computed several kinematic parameters as reported previously [25], including amplitude of peak velocity, reaction time, directional error at the peak velocity and movement duration. Adaptation at the end of training was measured as the decrease in percentage of the directional error at the peak velocity in a separate block [3, 26, 27]. Improvement at retest was computed as the difference in adaptation between the training and the testing sessions.
EEG recordings and analysis
EEG was recorded with a 256-channel EEG amplifier (Electrical Geodesic, Inc., Eugene, Oregon), sampled at 500 Hz and referenced to Cz. Electrode impedances were set below 50 kO. Offline, the EEG was down sampled to 128 Hz, band-pass filtered between 0.5 Hz and 50 Hz, and average-referenced after rejection of bad channels and epochs.
Sleep EEG was recorded for the entire night. For the analysis, sleep stages were visually scored in 20-s epochs according to standard criteria [28]. Matlab (The MathWorks, Natick, MA) and public license toolbox EEGLAB were used for data analysis. For a quantitative analysis of sleep EEG, we performed a spectral analysis of consecutive 20-s epochs (power spectral density estimate calculated applying a Welch's average modified periodgram with a Hanning window, based on averages of five consecutive 4-s epochs) for all included channels after visual and semiautomatic artifact removal based on the power in 0.75-4.5 Hz and 20-40 Hz bands [3]. Power spectra were calculated for NREM sleep, and the power of the two main EEG rhythms of NREM sleep, namely SWA (1-4.5 Hz) and sleep spindles (12-15 Hz) were used for analysis.
To assess significant topographic differences in sleep EEG power between the conditions, we applied statistical nonparametric mapping using a suprathreshold cluster analysis for multiple comparisons [3, 29]. For all other comparisons, t-tests, appropriate designs of the analysis of variance (ANOVA), and post hoc tests (Tukey's HSD) were applied to determine the sources of the significant effects. Kinematic measures were correlated (Pearson) with EEG power density values for individual electrodes and for the mean of all scalp electrodes for each frequency bin. Statistical analyses were computed with Matlab and SPSS for Windows 17 statistical program.
3. Results
Morning learning is followed by performance enhancement after a night of sleep
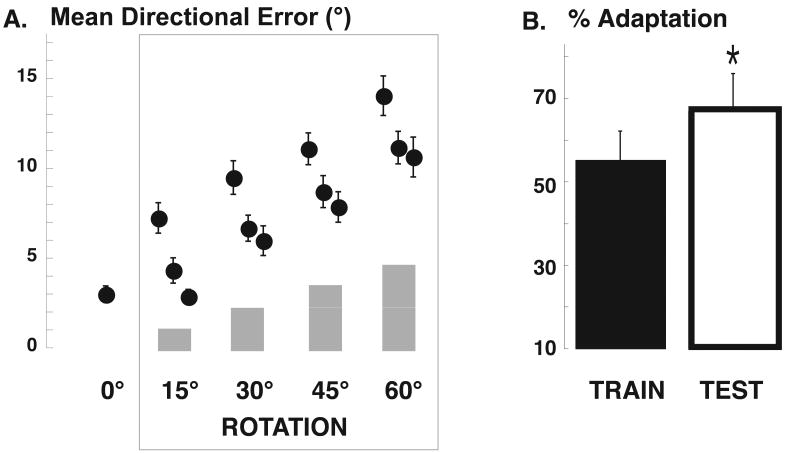
During the training in the morning, subjects adapted their movements to the imposed rotation of 60° by progressively decreasing the mean directional error and its variance across each successive incremental step of 15°. As shown in Fig. 1, the decrements of mean directional error across blocks and steps were significant (Block: F(2,167)=18.93, p<0.0001; Step: F(3,167)=35.70, p<0.0001; Block X step: F(6,167)=0.11, p=0.99). As previously reported, with the exception of the first 15° step, the directional error at the end of each step was progressively higher than the corresponding baseline values. At the last block of the 60° step, there was a residual error of approximately 10°, which was significantly greater than the baseline mean directional error (2.95°, Fig. 1, F(1,28)= 40.63 p=<0.0001). On the other hand, reaction time, movement duration and peak acceleration at the end of the training were not different from baseline (Fig. 2). After training, the degree of learning achieved was tested in a separate movement block. On average, subjects showed an adaptation of 55.31% (Fig. 1). These results are similar to those we previously reported for different groups of subjects with adaptation rates ranging from 50 and 60% [3, 27].
Figure 1.
Performance improves after sleep. (A) Learning curves for the rotation adaptation task in the morning training. The mean directional error for each block of movements is plotted. Points are means across subjects and bars represent standard errors (n=14). (B) Percentage of adaptation achieved in the morning, after training, and the next day, at test.
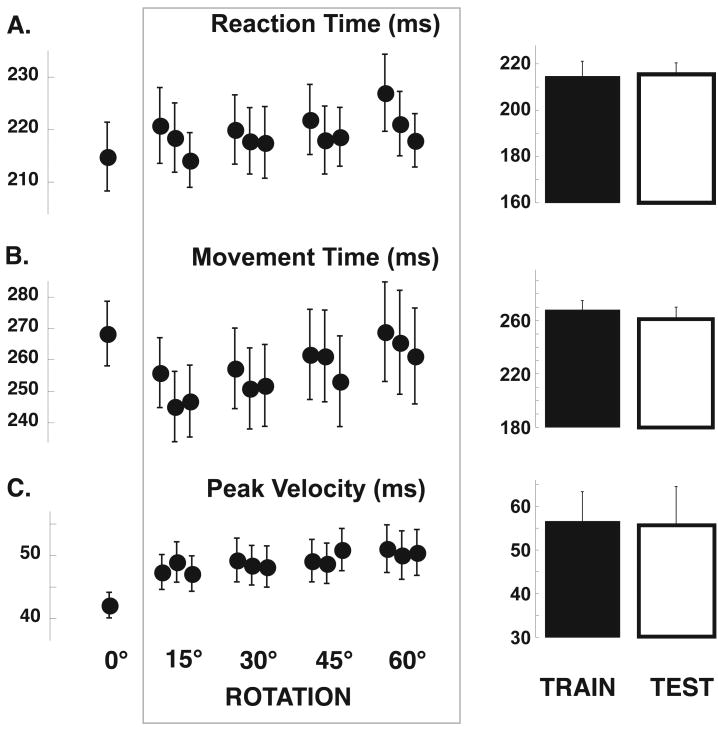
Figure 2.
Other kinematic variables do not change after sleep. Mean reaction time (A) is plotted for each movement block (left) as well as for the morning training blocks and for the following day test block. Mean movement time (B) and Peak velocity (C) are plotted as per (A).
We have previously shown that performance in this visuomotor adaptation task improves after a night of sleep [3]. As expected, upon retesting the next morning, adaptation significantly improved by 11.6 ± 0.9% (mean ± SE, p < 0.0001) an effect of similar magnitude as that observed when the training was performed just before going to sleep (11.3% [3]). In addition, as in previous works, this effect was specific for directional error, as there were no significant changes after sleep in either reaction time, movement duration or peak velocity compared to the training session.
Morning learning leads to local SWA increase during sleep
High-density EEG was recorded during sleep both 12 hours after the motor learning task and in the control session, after usual daily activities. All subjects had at least three complete sleep cycles in both sessions, and which did not differ in sleep latency, total sleep time, sleep efficiency or amount of NREM or REM sleep (Table1).
Table 1. Sleep architecture.
| Control night | Learning night | Statistics | |
|---|---|---|---|
| Sleep latency | 21 ± 19 min | 16 ± 9 min | NS* |
| Total sleep time | 419 ± 70 min | 440 ± 52 min | NS* |
| Sleep efficiency | 89 ± 5.7% | 91% ± 7.1% | NS* |
| Duration of the 1st cycle | 88 ± 20 min | 104 ± 37 min | Duration of the cycles (Condition × Cycle ANOVA) Condition: F(1,13)=.28291, p>0.05 Cycle: F(2, 26)=.45059, p>0.05 Condition × Cycle: F(2, 26)=3.2374, p>0.05 |
| % of 1st Cycle that is NREM | 78 ± 7% | 72 ± 10% | |
| Duration of the 2nd cycle | 118 ± 52 min | 91 ± 28 min | |
| % of 2nd Cycle that is NREM | 72 ± 17% | 72 ± 15% | |
| Duration of the 3rd cycle | 99 ± 28 min | 101 ± 28 min | |
| % of 3rd Cycle that is NREM | 79 ± 15% | 79 ± 13% | |
Values expressed as mean ± standard deviation.
Non-significant (p>0.05, paired t-test)
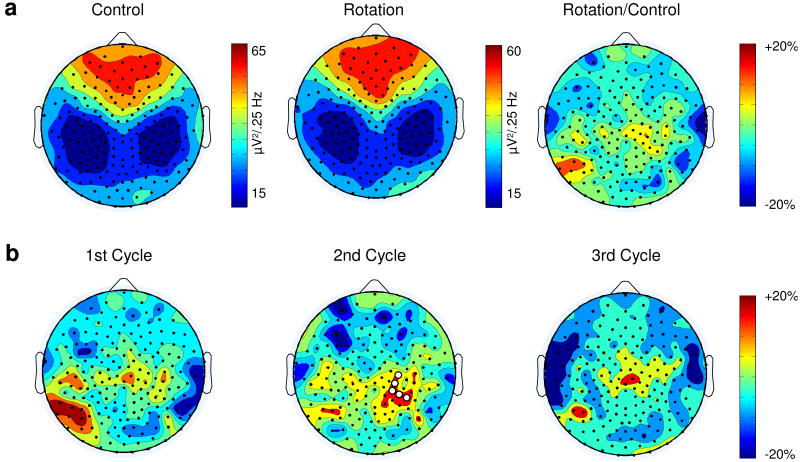
The two sessions were also similar in other respects. First, there was no difference in the mean SWA averaged across all the electrodes computed for each cycle (p>0.05). Second, the temporal evolution of SWA, computed across the three cycles, showed the same homeostatic decline in both sessions (main effect for cycle: F(1,13)= 65.42, p< 0.001). Third, the SWA topographical distribution was similar in the two conditions: the average power spectra in the SWA range computed for each electrode for both the entire night and the three sleep cycles separately showed a typical anterior predominance for SWA [30], which was similar across subjects, cycles and sessions (Fig. 3a).
Figure 3.
Morning learning leads to a SWA increase in the right parietal region. (A) Topographic distribution of average EEG NREM power density at 1-4.5 Hz (n=14 subjects) in the control (left) and rotation learning (center) sessions for the entire night. The ratio between the learning and the control session is illustrated on the right. Absolute values color-coded (μV2/0.25 Hz), plotted at the corresponding position on the planar projection of the scalp surface, and interpolated (biharmonic spline) between electrodes. (B) Topographic distribution for the average EEG NREM power density of the ratio between the learning and the control sessions. White dots indicate the five electrodes showing significantly (SnPM, p<0.01) increased SWA in the second sleep cycle 12 hours after visuomotor learning (electrodes 143, 155, 184, 154 and 163).
In previous studies we found a significant increment in SWA in the right parietal region after training with this visuomotor task [3]. To ascertain whether the two sessions differed in the local distribution of SWA, we used a region of interest (ROI) approach. We thus focused the SWA analysis on the right parietal region, which included ten electrodes (81, 130, 131, 132, 142, 143, 144, 154, 155, 184). SWA values of these electrodes were averaged, and the resulting means were used for comparison of the two sessions. Paired t-tests revealed significant differences between the conditions for the entire night of recording (p<0.05). Moreover, when we analyzed the individual sleep cycles, we found a significant increase of SWA for both the first and the second sleep cycle after the learning session compared to the control session (p<0.05 in both cases).
We then assessed the spatial specificity of the SWA increase found in the ROI using statistical nonparametric mapping (SnPM) with a suprathreshold cluster analysis for multiple comparisons. The SWA analysis was performed both for the entire night and for the first thirty minutes of each sleep cycle separately. After the visuomotor learning task, we found a significant SWA increase during the second sleep cycle over a cluster of five right parietal electrodes (p<0.01, SnPM, Fig. 3b). The peak increase in SWA, which across subjects varied in location within the cluster, was +35% (+/-17%, p<0.005, paired t-test). By means of a infrared positioning system (Nexstim, Helsinki, Finland), we co–registered electrode scalp positions overlaid onto each subject's MRI and localized the changes found in SWA to Brodmann's areas 7 and 40 (electrodes 143, 155, 184 and electrodes 154 and 163, respectively). These areas have been previously shown to be active during the task [25].
Finally, we determined if the SWA increase over the five right parietal electrodes was limited to just the SWA frequency band. We therefore computed the percentage change between the two sessions across the entire power spectrum (up to 40 Hz). This analysis confirmed that the increment was significant for the SWA and sigma ranges (Fig. 4). Changes in the spindles range, however, failed to reach significance when tested topographically using SnPm.
Figure 4.
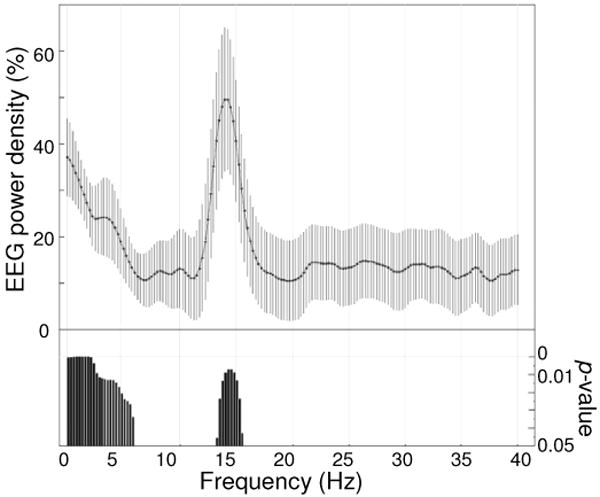
Frequency specificity of power changes in the right parietal region. EEG power density spectrum for the first thirty minutes of NREM sleep in the second sleep cycle for 5 electrodes in the right parietal region. Values represent the percentage change after the learning session with respect to the control session (mean ± s.e.m. for 0.25-Hz bins, n=14). Bottom bars indicate frequency bins for which power in the learning condition differed significantly (paired t-test, p<0.05, n=14) from that in the control condition.
In previous studies, we have found that the local increment in SWA during the first sleep cycle predicted the degree of post-sleep performance improvement [3]. In the present study, such correlation for the entire night recording or for the first 30 minutes of the first sleep cycle was not significant. However, we found a significant correlation in the first 30 min of the second sleep cycle between the degree of performance improvement and SWA increase for the electrode showing peak increment (r=0.44) as well as for the electrode cluster selected for the ROI analysis (r=0.47, p<0.05 in both cases).
4. Discussion
The present work shows that learning a visuomotor task in the morning induced a regional increase in sleep SWA the following night. In addition, the following morning performance improved, and the improvement was correlated with the local increase in sleep SWA. These results are remarkably similar to those obtained when the learning session occurred in the evening, closer to sleep time [3], suggesting that both training-induced changes in sleep SWA and post-sleep improvement in performance do not depend upon the time interval between original training and sleep.
Local changes in sleep SWA induced by a learning session twelve hours earlier
In our previous study using the rotation adaptation protocol and high-density EEG [3], we found that when the subjects went to sleep shortly after a training session in the evening, there was a local increase in sleep SWA over the right parietal cortex, a region known to be activated during this task [3, 25]. This local increase in SWA can be considered as an electrophysiological trace of previous learning, which is apparent in the spontaneous activity of cortical neurons during NREM sleep. Such increase in sleep SWA may be a reflection of an increased reactivation of cortical circuits that have undergone plastic changes. Indeed, a stronger “reactivation” of neural circuits involved in learning has been described in both the hippocampal system and in the cerebral cortex [31-33]. Recent work has shown that the level of SWA is positively correlated with the mean firing rate of neurons during the UP state of the sleep slow oscillation [34], suggesting that increased SWA may reflect increased “reactivation”. Since the local increase in SWA after learning was positively correlated with the degree of performance enhancement the next morning, the intensity of local reactivation may facilitate memory consolidation [35, 36].
In our previous study, the local increase in SWA was maximal at the beginning of sleep and had dissipated by thirty minutes into the first NREM cycle, in agreement with other reports that found the strongest changes at the beginning of sleep [3, 22-24, 37]. Unit recording studies also indicate that sleep “reactivation” is short-lasting, abating within 30-40 minutes [38, 39]. Thus, it was an open question whether a training session occurring twelve hours before would still produce a detectable electrophysiological trace during the subsequent sleep episode. Moreover, neuronal changes induced by forty-minutes of morning training might conceivably be obfuscated by the accruing of other cellular changes in the course of twelve additional hours of waking, which likely included activities calling for visuomotor plasticity.
In this context, the present results provide straightforward evidence that morning learning produces local changes in SWA during sleep the following night, thus demonstrating that a clear electrophysiological trace of learning can be detected during sleep more than twelve hours after training, and that such trace is not obfuscated by additional changes that might have occurred during subsequent wakefulness. Moreover, the local increase in sleep SWA occurs over the same cluster of right parietal electrodes that had been involved in the previous study. Finally, just as in that study, the local increase in sleep SWA correlates positively with the enhancement in performance the next morning, in line with a facilitating effect of SWA on memory consolidation [11-17].
A difference between the present results and those of our previous study should nevertheless be noted. In the current study, the local increase in sleep SWA over right parietal cortex was most significant during the second sleep cycle. We also found that only SWA in the first thirty minutes of the second sleep cycle, and not during the first cycle, was significantly correlated with the post-sleep improvement in performance. In our previous study, we found a significant increase in sleep SWA during the first cycle, lasting for only thirty minutes, which predicted the degree of the post-sleep improvement [3]. Unfortunately, in that study, because of technical limitations, we could only record slightly more than one hour of sleep using high-density EEG, so we have no information concerning EEG changes later in the night. Moreover, that study used a kinematically-equivalent control condition [3, 27] that was important in lending significance to the correlation between post-sleep and SWA increment in the first cycle [3]. Despite these caveats, the evidence from the current and the previous studies raises the intriguing possibility that plastic events occurring closer to sleep time have a larger influence on SWA at the beginning of sleep, during the first sleep cycle. By contrast, plastic events occurring at an earlier time during the waking day may be more detectable at a later time in sleep SWA, as in the second sleep cycle. Such a temporally inverted recapitulation of memory traces could occur, for instance, if the most recent synaptic changes, perhaps by being stronger, were especially effective in driving sleep reactivation, yielding high local SWA early in sleep. In turn, sleep reactivation would produce overall synaptic depression, as suggested by recent molecular and electrophysiological evidence [40-42], and presumably greater synaptic depression at those synaptic circuits that were activated the most. As a consequence, the relative weakening of the most recent memory traces would then unmask traces accrued earlier in the day, which would be reflected in higher SWA later in sleep. Further experiments addressing systematically the relationship between time of learning and the detectability of memory traces in spontaneous neural activity will be necessary to evaluate this possibility and to help understand which circuits are reactivated during sleep, at what time, and through which mechanisms.
Learning, memory consolidation and the time of initial training
Irrespective of the role of sleep SWA, the present study shows that, for rotation adaptation, post-sleep learning gains do not depend on the time interval between training and sleep. Subjects' performance after a night of sleep was significantly better than the previous day immediately after training, and the degree of improvement was comparable whether training occurred in the morning or, as in a previous study, in the evening [3]. This result is in agreement with many studies of motor learning showing that even delayed post-learning sleep can enhance performance [1, 43-45]. As in those studies, performance gains in rotation adaptation are presumably due to sleep and not to the mere passage of time, since they occur after sleep but not after a waking episode of similar duration [3, 26].
Moreover, the present results indicate that, at least with respect to rotation adaptation, the time at which training takes place - in the morning or in the evening -does not have appreciable effects on the rate of adaptation during training or on the total performance gain. The adaptation achieved by the end of the initial training in the morning was in the same range as that of subjects trained in the evening [3, 27]. The visuomotor adaptation in the rotation task is a form of implicit learning since, while reaching for targets, subjects are not aware of the distortion and unconsciously adapt to subliminal rotations imposed on the perceived trajectory. It is not known whether this lack of sensitivity to time of day may be a general feature of implicit learning. The few studies on the development of procedural skills employed motor sequence learning tasks, which often include declarative components [46], and were inconclusive about the modulation of performance and learning during the day [47]. The influence of time of day on performance and the rate of learning of new material have more frequently been investigated using declarative tasks engaging cognitive strategies and executive functions, with a heavy load on attention and working memory buffers. At variance with our finding with rotation learning, in such declarative learning tasks, performance and rate of learning are usually influenced by the time of day, and the best time for a fast acquisition is during the morning hours (for a review see [47]). Whether neural circuits involved in implicit or motor learning may be less susceptible to saturation of synaptic traces, neuronal fatigue, or other mechanisms reducing learning efficiency remains to be determined.
Acknowledgments
This work was supported by grants from the McDonnell Foundation (MFG and GT), NIH NS055185 (GT), and NS054864 (MFG). We thank Dr. Domenica Crupi and Israel Nemet for help in the analysis of kinematic data.
Footnotes
Conflict of interest: The authors declare that they have no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backhaus J, Hoeckesfeld R, Born J, Hohagen F, Junghanns K. Immediate as well as delayed post learning sleep but not wakefulness enhances declarative memory consolidation in children. Neurobiol Learn Mem. 2008;89:76–80. doi: 10.1016/j.nlm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara M, Iaria G, Tempesta D, Curcio G, Moroni F, Marzano C, De Gennaro L, Pacitti C. Sleep to find your way: the role of sleep in the consolidation of memory for navigation in humans. Hippocampus. 2008;18:844–851. doi: 10.1002/hipo.20444. [DOI] [PubMed] [Google Scholar]
- 3.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 4.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 5.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 6.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 8.Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133:911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 11.Aeschbach D, Cutler AJ, Ronda JM, Facchini S. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 13.Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 14.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 15.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- 16.Moroni F, Nobili L, Curcio G, De Carli F, Tempesta D, Marzano C, De Gennaro L, Mai R, Francione S, Lo Russo G, Ferrara M. Procedural learning and sleep hippocampal low frequencies in humans. Neuroimage. 2008;42:911–918. doi: 10.1016/j.neuroimage.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 18.Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- 19.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 20.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 21.De Gennaro L, Fratello F, Marzano C, Moroni F, Curcio G, Tempesta D, Pellicciari MC, Pirulli C, Ferrara M, Rossini PM. Cortical plasticity induced by transcranial magnetic stimulation during wakefulness affects electroencephalogram activity during sleep. PLoS ONE. 2008;3:e2483. doi: 10.1371/journal.pone.0002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-Induced Cortical Potentiation during Wakefulness Locally Increases Slow Wave Activity during Sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber R, Määttä S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of Cortical Plasticity after Transcranial Paired Associative Stimulation Predict Changes in Electroencephalogram Slow-Wave Activity during Subsequent Sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 25.Ghilardi MF, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 26.Hill S, Tononi G, Ghilardi MF. Sleep improves the variability of motor performance. Brain Res Bull. 2008;76:605. doi: 10.1016/j.brainresbull.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landsness EC, Crupi D, Hulse BK, Peterson MJ, Huber R, Ansari H, Coen M, Cirelli C, Benca RM, Ghilardi MF, Tononi G. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–1284. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rechtschaffen KA, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: 1968. [DOI] [PubMed] [Google Scholar]
- 29.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finelli LA, Achermann P, Borbely AA. Individual ‘fingerprints’ in human sleep EEG topography. Neuropsychopharmacology. 2001;25:S57–62. doi: 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 31.Euston DR, Tatsuno M, McNaughton BL. Fast-Forward Playback of Recent Memory Sequences in Prefrontal Cortex During Sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- 32.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 33.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 34.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 36.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann TO, Molle M, Marshall L, Kaya-Yildiz L, Born J, Roman Siebner H. A local signature of LTP- and LTD-like plasticity in human NREM sleep. Eur J Neurosci. 2008;27:2241–2249. doi: 10.1111/j.1460-9568.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- 38.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsuno M, Lipa P, McNaughton BL. Methodological considerations on the use of template matching to study long-lasting memory trace replay. J Neurosci. 2006;26:10727–10742. doi: 10.1523/JNEUROSCI.3317-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32:719–729. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara M, Iaria G, De Gennaro L, Guariglia C, Curcio G, Tempesta D, Bertini M. The role of sleep in the consolidation of route learning in humans: a behavioural study. Brain Res Bull. 2006;71:4–9. doi: 10.1016/j.brainresbull.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Kuriyama K, Mishima K, Suzuki H, Aritake S, Uchiyama M. Sleep accelerates the improvement in working memory performance. J Neurosci. 2008;28:10145–10150. doi: 10.1523/JNEUROSCI.2039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 46.Moisello C, Crupi D, Tunik E, Quartarone A, Bove M, Tononi G, Ghilardi MF. The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task. Exp Brain Res. 2009;194:143–155. doi: 10.1007/s00221-008-1681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24:755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]