Abstract
Introduction
The hypothalamic-pituitary-adrenal cortical (HPA) axis modulates physiological responses to stress. We previously reported sexually diergic, dose-dependent HPA responses in vivo following nicotine administration: Male rats had greater arginine vasopressin (AVP) responses than females, and female rats had greater adrenocorticotropic hormone (ACTH) and corticosterone (CORT) responses than males. The goal of the present study was to further investigate sexually diergic, dose-dependent HPA responses following nicotine addition to an in vitro model of the HPA axis, so that hormone output could be determined at each level of the axis.
Methods
Hypothalami, pituitaries, and adrenal glands were harvested from male and female rats. One-half hypothalamus, one-half pituitary, and one adrenal gland were placed individually into three jacketed tissue baths connected by tubing and perfused in series with physiological medium. Sampling ports between tissue baths were used to collect buffer before and after addition of various doses of nicotine, for measurement of AVP and corticotropin-releasing hormone (CRH) from the hypothalamus bath, ACTH from the pituitary bath, and CORT from the adrenal bath. Hormones were measured by highly specific immunoassays.
Results
Stable temperatures, flow rates, pH, and hormone baselines were achieved in the in vitro system. Consistent with our in vivo and earlier in vitro studies, nicotine added to the hypothalamus tissue bath significantly increased HPA responses in a sex- and dose-dependent manner: Males had greater AVP responses than did females, and females had greater CRH responses than did males. Sexually diergic ACTH and CORT responses were less apparent and were higher in females.
Discussion
Our in vitro system accurately models in vivo HPA responses to nicotine in both sexes and thus represents a reliable method for investigating the effects of nicotine on components of the HPA axis. These studies may be pertinent to understanding the biological differences to NIC between men and women smokers.
Keywords: ACTH, AVP, Cholinergic, CORT, CRH, Hormones, HPA Axis, Methods, Nicotine, Rat
1. Introduction
Cigarette smoking is a major worldwide health concern for both men and women (Benowitz, 2008; Rose, 2008). Nicotine (NIC) administration via various routes increases stress hormone concentrations in both humans (al'Absi, 2006; Mendelson et al., 2008; Mendelson et al., 2005; Pomerleau et al., 2004; Rohleder & Kirschbaum, 2006) and rodents (Cam & Bassett, 1983; Cam et al., 1979; Fu et al., 1997; Matta et al., 1987; Yu et al., 2008). Biological sex appears to be an important factor underlying stress hormone sensitivity following NIC (Faraday et al., 2005). Therefore, basic studies of sex differences in stress hormone responses to NIC are relevant to the understanding of biological differences between men and women smokers.
The hypothalamic-pituitary-adrenal (HPA) axis is a three-gland component of the endocrine system that modulates biological responses to acute and chronic stress (Bugajski et al., 1999). Immediately following stress, HPA axis activity increases, initiated by the release of corticotropin-releasing hormone (CRH) from neurons of the paraventricular nuclei of the hypothalamus. CRH, in turn, stimulates adrenocorticotropic hormone (ACTH) release from the anterior pituitary, and ACTH stimulates corticosterone (CORT) release from the adrenal cortex. Arginine vasopressin (AVP), released from neurons of the paraventricular and supraoptic nuclei of the hypothalamus, acts as a potent secretagogue of ACTH release, potentiating the CRH-driven stress response (Aguilera et al., 2008; Antoni, 1993; Engelmann et al., 2004; Surget & Belzung, 2008; Volpi et al., 2004).
Several neurotransmitters, including acetylcholine (ACh), modulate HPA axis activity (Rhodes et al., 2001b; Rhodes & Rubin, 1999; Whitnall, 1993) and are released in response to NIC (Albuquerque et al., 2000; Benowitz, 2008; Paterson, 2000). NIC, the main psychoactive component of cigarettes, has been shown to activate the HPA axis in a dose-dependent manner (Mendelson et al., 2005; Porcu et al., 2003; Rhodes et al., 2004; Rhodes et al., 2001a). Reports of sex differences in HPA axis responses to NIC are relatively few (e.g., (Faraday et al., 2005; Grota et al., 1997; Moidel et al., 2006; Pogun & Yararbas, 2009; Rhodes et al., 2004). Our previous in vivo studies indicate that NIC activates the HPA axis in a sexually diergic manner, males having significantly greater plasma AVP responses than females, and females having significantly greater plasma ACTH and CORT responses than males (Rhodes et al., 2001a).
We previously reported that, in a dynamic perfusion system of HPA tissues, NIC also activates the HPA axis in a sexually diergic manner (Moidel et al., 2006). However, only one NIC dose was used in the previous study and AVP was not measured. The purposes of the present study were (1) to further validate this in vitro model with improved system components and physiological parameters; (2) to determine sexually diergic CRH, AVP, ACTH, and CORT responses to cholinergic stimulation by several doses of NIC; and (3) to compare the results with previously determined in vivo and in vitro hormone responses to NIC stimulation.
2. Methods
2.1. Animals
All animal procedures were approved by the Saint Vincent College Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines for proper animal care. Eight-week-old male and female Sprague-Dawley rats weighing 200–225 g were obtained from Taconic Farms, Inc. (Germantown, NY, USA). All animals were housed doubly in a well-ventilated, temperature- and humidity-controlled environment (22–25°C, 50–75% humidity) and were maintained on a 12-h light-dark cycle (lights on at 0800 h) with food and water available ad libitum.
2.2. Tissue Isolation
Rats were euthanized by isoflurane (Baxter, Deerfield, IL, USA) inhalation prior to decapitation, methods consistent with the recommendations of the Panel of Euthanasia of the American Veterinary Medical Association. Immediately following decapitation, the hypothalamus, pituitary gland, and adrenal glands were collected. The hypothalamus was isolated by the block method (Hatton et al., 1980). The pituitary was collected from the sella turcica after severing the optic nerves and infundibulum and removing the brain from the skull. The hypothalamus and pituitary were bisected prior to weighing. Adrenal glands were removed by ventral approach and were cleared of adipose tissue. Tissue isolation time was approximately five minutes for each animal.
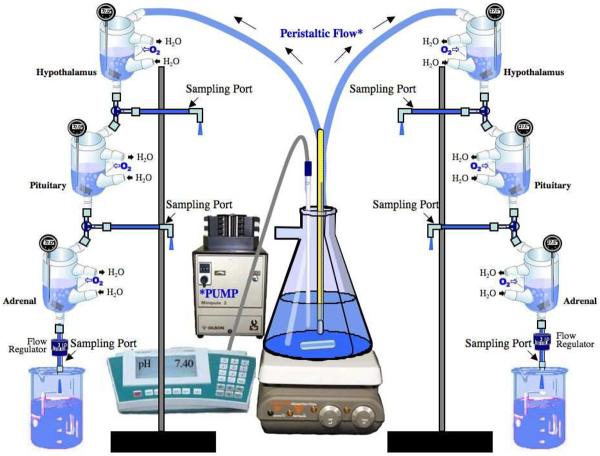
Each hypothalamic and pituitary tissue-half and each adrenal gland was weighed and placed individually in beakers containing modified Bradbury tissue culture medium (pH 7.4) at 37°C (Bradbury et al., 1974; Garrido et al., 1999). The culture medium contained (in mM) 126 NaCl, 6 KCl, 0.88 MgSO4, 1 Na2HPO4, 22 NaHCO3, 1.45 CaCl2, 11 glucose, and 0.05 ascorbic acid (all reagents from Sigma, St. Louis, MO, USA). The tissues were immediately enclosed in 100 mesh, 20 mm (3/4”) circular, stainless-steel screens (Small Parts, Inc., Miami Lakes, FL, USA) that were folded in half and held together with 9 mm stainless-steel wound clips (World Precision Instruments, Sarasota, FL, USA). These were transferred to 10-ml jacketed tissue baths (World Precision Instruments, Sarasota, FL, USA) comprising the in vitro perfusion system (Fig. 1). The hypothalamus baths were connected to the source buffer flask via Tygon flexible plastic tubing (3.2 mm [1/8”] I.D.; 6.4 mm [1/4”] O.D.; 1.6mm [1/16”] wall thickness). The jacketed tissue baths and a terminal siphon regulator were connected to each other by Tygon flexible plastic tubing (3.2 mm [1/8”] I.D.; 4.8 mm [3/16”] O.D.; 0.8 mm [1/32”] wall thickness) (Cole-Palmer, Vernon Hills, IL, USA) with Luer connectors (Cole-Palmer, Vernon Hills, IL, USA) and 3-way stopcocks (World Precision Instruments, Sarasota, FL, USA), to enable the connection of sampling ports composed of 3.2 mm Tygon tubing plus Luer connectors (Cole-Palmer, Vernon Hills, IL, USA). The interior surface of each 10-ml jacketed tissue bath was coated with silicone lubricant (Dow Corning Corp., Midland, MI, USA) to prevent ACTH from binding to the glass. Each jacketed tissue bath was oxygenated via Tygon tubing (3.2 mm [1/8”] I.D.; 6.4 mm [1/4”] O.D.; [1/16”] wall thickness) connected to a polycarbonate multi-port manifold (Cole-Palmer, Vernon Hills, IL, USA). The flow rate between baths was regulated by the speed of the perfusion pump (Minipuls 3, Gilson), by changing the height of the tissue baths in relation to one another, and by the terminal siphon regulator which was connected to the terminal end of the adrenal jacketed tissue bath via Tygon tubing. The terminal siphon regulator ensured a steady flow rate out of the adrenal jacketed tissue bath, and thus through the entire in vitro system. A Bechman Centerline II circulating water bath was used to circulate water (38 °C) through the jackets surrounding the tissue baths. The tissues were equilibrated by perfusion of 37° C culture medium for at least 30 min before experiments were initiated.
Figure 1. In Vitro Perfusion System.
The in vitro system models the hypothalamic-pituitary-adrenal (HPA) endocrine axis and consists of two sets of three jacketed tissue baths. The baths of each set contain one-half hypothalamus, one-half pituitary, and one adrenal gland connected in a series by tubing and accessories. Physiological buffer is perfused unidirectionally from a large Erlenmeyer source flask through the three baths to a waste beaker. Sampling ports are placed after each bath for sample collection before and after drug administration.
2.3. In Vitro System
Modified Bradbury culture medium warmed (49–60 °C; depending on ambient room temperature, flow rate through the system, and tissue bath temperatures) and buffered (pH 7.3–7.5) at the 1L source flask was perfused through the system by a perfusion pump (Gilson Minipuls 3, Middleton WI, USA) (Fig. 1). A 1L reserve flask containing warmed culture medium was used to replenish the medium of the source flask as necessary during the experiments. Each jacketed tissue bath was gassed with 95% O2, 5% CO2 and had a total medium volume of approximately 6ml. Oxygenation flow levels were controlled in each jacketed tissue bath via Keck Ramp Clamp Tubing Clamps (Cole-Parmer, Vernon Hills, IL, USA). Including volume loss from the system during sampling, flow rates of the medium through the system during the experiments ranged between 1.1–1.9 ml/min. Temperatures ranged between 36.1°–38.4°C in hypothalamus baths, 37.1°–38.5°C in pituitary baths, and 36.2°–38.9°C in adrenal baths. At the end of each experiment, the tissues were exposed to 60 mM KCl for 10 min to test tissue responsiveness and viability to membrane depolarization (Gao et al., 2000). Temperature and pH of buffer in the source flask, the reserve flask, and each jacketed tissue bath were continuously measured throughout each experiment with alcohol thermometers (Allegiance, McGraw Park, IL, USA), individual digital thermometers (VWR, West Chester, PA, USA) and pH electrodes and meters (Radiometer PHM92, Cedex, France). Due to heating, the pH of the Modified Bradbury buffer gradually rose throughout experimentation. The pH of the buffer was thus regulated via drop-wise additions of 1 N HCl.
2.4. Sampling for Stability of Baseline Hormone Concentrations
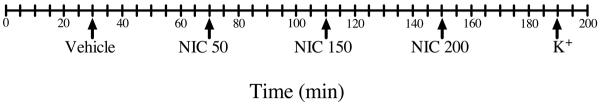
At the beginning of each experiment, baseline culture medium samples were taken from each of the sampling ports at 0, 5, 10, 15, and 20 min following the 30 min tissue equilibration for determination of CRH, AVP, ACTH, and CORT concentrations. Additional baseline culture medium samples were taken prior to each NIC administration. Following NIC stimulations at 70, 110 and 150 min, hormone concentrations returned to baseline by 100, 140, and 180 min, consistent with our earlier findings (Moidel et al., 2006). The duration of each experiment was 200 min (Fig 2).
Figure 2. Timeline indicating additions to the in vitro system.
Tissues were exposed to culture medium at 30 min, 50 ng/ml NIC at 70 min, 150 ng/ml NIC at 110 min, 200 ng/ml NIC at 150 min, and KCl at 190 min. The duration of each experiment was 200 min. Each tick mark represents 5 min.
2.5. NIC Addition and Sampling
Drug solutions were freshly prepared immediately prior to each experiment. For the 50 ng/ml dose, NIC (nicotine hydrogen tartrate salt; Sigma, St. Louis, MO, USA) was dissolved in distilled water at a concentration of 1.5 mg in 1L (3 μM). Three 0.2 ml aliquots (each 0.2 nmol free base NIC), separated by 1 min each, were added to each hypothalamus bath. The 3 NIC aliquots (0.6 nmol total free base NIC) yielded a final bath concentration of 50 ng/ml, which approximates the plasma NIC concentration after smoking one cigarette (Cam et al., 1979). Because of the high lipid solubility of NIC, plasma NIC concentrations reflect brain NIC concentrations (Chang et al., 2005).
For the 150 ng/ml dose, NIC was dissolved in distilled water at a concentration of 4.5 mg in 1 L (9 μM). Three 0.2 ml aliquots (each 0.6 nmol free base NIC), separated by 1 min each, were added to each hypothalamus bath. In this case, the 3 NIC aliquots (1.8 nmol free base NIC) yielded a final bath concentration of 150 ng/ml. For the 200 ng/ml dose, NIC was dissolved in distilled water at a concentration of 6 mg in 1 L (12 μM). Three NIC aliquots (each 0.8 nmol free base NIC), separated by 1 min each, were added to each hypothalamus bath. In this case, the 3 NIC aliquots (2.4 nmol total free base NIC) yielded a total concentration of 200 ng/ml. Culture medium samples were collected prior to and 5, 10, and 20 min after NIC addition (n = 5–6 axes per group [3 tissue sets per sex]).
2.6. Hormone Assays
Culture medium samples were analyzed in singlet for CRH, AVP, ACTH, and CORT. CRH was analyzed by enzyme immunoassay (EIA) (Phoenix Pharmaceuticals, Inc., Belmont, CA, USA). Inter- and intra-assay coefficients of variation were 5% and 14%, respectively. The minimum detectable CRH concentration was 5 pg/ml. AVP was determined by radioimmunoassay (RIA) (Alpco, Buhlmann Laboratories, Allschwil, Switzerland). Inter- and intra-assay coeffiecients of variation were 9.9% and 6.0%, respectively. The minimum detectable AVP concentration was 1.25 pg/ml. ACTH1–39 was determined by a highly specific immunoradiometric assay (IRMA) (Diasorin, Stillwater, MN) (Raff & Findling, 1989). Inter- and intra-assay coefficients of variation were 5.74% and 10.7%, respectively. The minimum detectable ACTH concentration was 1.5 pg/ml. CORT was analyzed by RIA (MP Biomedicals, Orangeburg, NY, USA). Inter- and intra-assay coefficients of variation were 9.3% and 7.3%, respectively. The minimum detectable CORT concentration was 7.7 ng/ml.
2.7. Statistical analysis
Multiple in vitro experiments were performed under the same conditions to attain n = 5–6 per group (3 tissue sets per sex) (there was insufficient sample from one axis for female CRH determinations, therefore N=6 for male CRH results and N=5 for female CRH results. N=6 for male and female AVP, ACTH, and CORT results). To account for flow rate variation, CRH concentrations are reported as fmol/mg hypothalamus-half/min, AVP concentrations as amol/mg hypothalamus-half/min, ACTH concentrations as fmol/mg pituitary-half/min, and CORT concentrations as pmol/mg single adrenal gland/min. Data are reported as mean ± standard error of the mean (SEM), and were analyzed by multifactorial analyses of variance (ANOVA), with time as a repeated measure. All data sufficiently approximated a Gaussian distribution such that no transformations were required (log-transformation did not alter the statistical results). Fisher's LSD tests were used for post-hoc comparisons. Significance was considered as P < 0.05.
3. Results
3.1. Baseline Hormone Release
The in vitro perfusion system produced minimally fluctuating CRH, AVP, ACTH, and CORT baselines prior to NIC stimulation. Basal CRH, AVP, and ACTH concentrations before and during experiments (between NIC stimulations) were not significantly different. As well, hormone concentrations were consistent between replicate experiments. Baseline values corrected for perfusion-medium flow rate and tissue weight were similar to those reported previously (Moidel et al., 2006).
3.2. Tissue Viability
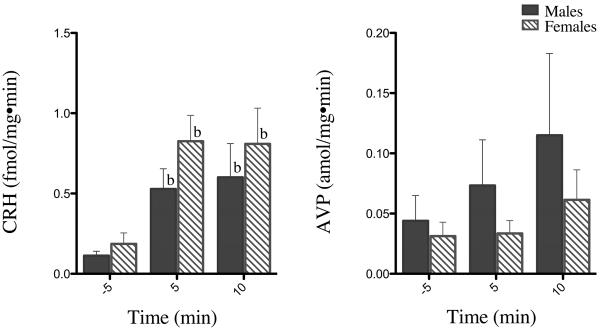
At the end of each experiment, infusion of highly concentrated potassium buffer significantly increased CRH secretion from both male and female hypothalami at 5 and 10 min (P's < 0.05) (Fig 3). Potassium buffer increased AVP secretion from male and female hypothalami approximately 3-fold and 2-fold, respectively, at 10 min (Fig 3). ACTH and CORT concentrations also increased 10 min following potassium buffer infusion (data not shown), but these increases were not statistically significant. Thus, the hypothalamic, pituitary, and adrenal tissues appeared to remain viable throughout the experiments.
Figure 3. In vitro CRH and AVP responses to high K+ buffer infusion.
Tissues were exposed to 60 mM KCl at the end of each experiment to test tissue responsiveness and viability to membrane depolarization. Data are presented as 5 min prior and 5 and 10 min following infusion. b = difference from baseline (prior to K+ buffer addition) at indicated time point (P < 0.05). Each bar represents the mean ± SEM of 5–6 axes from 3 male and 3 female rats.
3.3. Hormone Responses to NIC
Following addition of NIC to the hypothalamus bath, CRH increased promptly, followed a short time later by AVP and ACTH. ACTH responses to NIC were less pronounced in both sexes, and there was no perceptible increase in CORT, possibly because the ACTH concentration reaching the adrenal bath was too low. CRH and AVP are presented both as absolute values (Fig. 4–5) and as % changes from baseline (Fig. 6) to highlight differences in CRH and AVP hormone profiles between the sexes. ACTH and CORT are presented as absolute values only (Fig. 7–8).
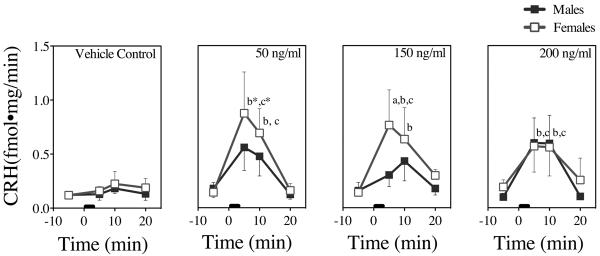
Figure 4. In Vitro CRH Dose-Responses to NIC in Male and Female Rat Tissues.
CRH concentrations 5 min prior and 5 min, 10 min, and 20 min following NIC addition from 0–3 min (black bar). Each time point represents the mean ± SEM of 5–6 axes from 3 male and 3 female rats. a = sex difference at indicated time point, b = difference from baseline (prior to stimulation) at indicated time point, c = difference from control (buffer addition) at indicated time point, and * = difference in both sexes at indicated time point (P's < 0.05).
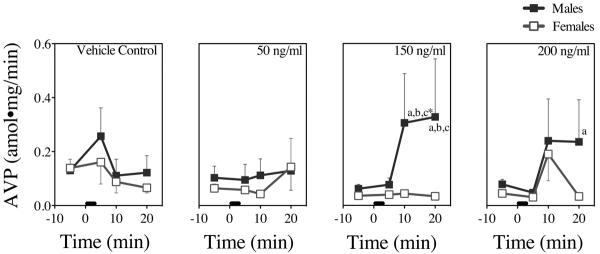
Figure 5. In Vitro AVP Dose-Responses to NIC in Male and Female Rat Tissues.
AVP concentrations 5 min prior and 5 min, 10 min, and 20 min following NIC addition from 0–3 min (black bar). Each time point represents the mean ± SEM of 6 axes from 3 male and 3 female rats. a = sex difference at indicated time point b = difference from baseline (prior to stimulation) at indicated time point, c = difference from control (buffer addition) at indicated time point, and * = difference in both sexes at indicated time point (P's < 0.05).
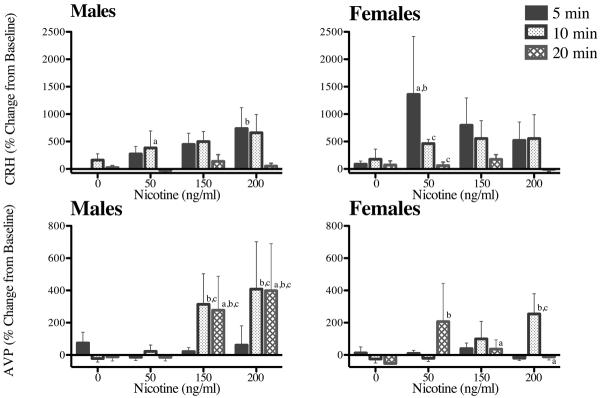
Figure 6. Percent Changes from Baseline for In Vitro CRH and AVP Dose-Responses to NIC in Male and Female Tissues.
% changes from baseline at 5, 10 and 20 min following buffer and the administration of NIC (0–3 min) in 0 ng/ml, 50 ng/ml, 150 ng/ml, and 200 ng/ml doses. Each time point represents the mean ± SEM of 5–6 axes from 3 male and 3 female rats. a = sex difference at indicated time point, b = difference from control at indicated time point, and c = difference from 5 min response at indicated time point.
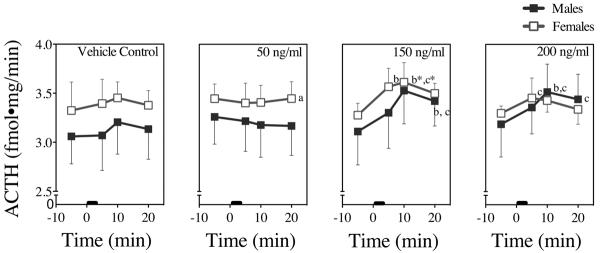
Figure 7. In Vitro ACTH Dose-Responses to NIC in Male and Female Rat Tissues.
ACTH concentrations 5 min prior and 5 min, 10 min, and 20 min following NIC addition from 0–3 min (black bar). Each time point represents the mean ± SEM of 6 axes from 3 male and 3 female rats. a = sex difference at indicated time point, b = difference from baseline (prior to stimulation) at indicated time point, c = difference from control (buffer addition) at indicated time point, and * = difference in both sexes at indicated time point (P's < 0.05).
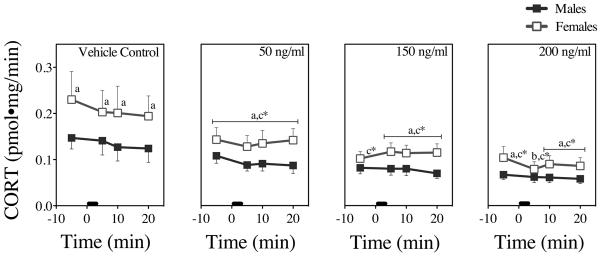
Figure 8. In Vitro CORT Dose-Responses to NIC in Male and Female Rat Tissues.
CORT concentrations 5 min prior and 5 min, 10 min, and 20 min following NIC addition from 0–3 min (black bar). Each time point represents the mean ± SEM of 6 axes from 3 male and 3 female rats. a = sex difference at indicated time point, b = difference from baseline (prior to stimulation) at indicated time point, c = difference from control (buffer addition) at indicated time point, and * = difference in both sexes at indicated time point (P's < 0.05).
Fig. 4 presents the means and SEMs of CRH responses from the hypothalamus bath 5 minutes prior to and 5, 10, and 20 min following addition of vehicle or NIC to that bath. Vehicle control slightly and non-significantly increased CRH responses in both sexes. There were no significant interactions among sex, NIC dose, or time. There was a significant main effect of time (F = [3, 132] = 15.3 P< 0.0001), reflected by both female and male CRH responses at 5 and 10 min compared to baseline values prior to NIC stimulation (P's <0.05). Female tissue showed a robust and significant response to the 50 ng/ml NIC dose (P's < 0.05) that was not enhanced by higher NIC doses; in contrast, male CRH responses, which were about half the female CRH responses following the 50 and 150 ng/ml doses, increased to the level of the female responses following the 200 ng/ml dose.
Fig. 5 presents the means and SEMs of the AVP responses. Vehicle control slightly and non-significantly increased AVP responses in both sexes. AVP showed a significant NIC dose × time interaction (F [9, 144] =2.0, P < 0.04), most likely owing to the nonlinearity of the combined male and female AVP responses at 20 min. Male tissue showed a robust and significant response to the 150 ng/ml dose that was not enhanced by the 200 ng/ml dose (P's < 0.05); in contrast, female AVP responses, which were unaffected by the 150 ng/ml dose, increased nearly to the level of the male AVP responses following the 200 ng/ml dose.
Fig. 6 presents the mean % changes from baseline and SEMs for CRH (top graphs) and AVP (bottom graphs) at 5, 10, and 20 min following NIC addition to the hypothalamus bath. As with the absolute CRH changes, CRH % changes showed a significant main effect of time (F [2, 88] = 8.34, P=0.0006). The CRH response was short-lived in both sexes, returning to baseline values by 20 min. AVP % changes from baseline also showed a significant main effect of time (F [2, 96] = 3.48, P < 0.04) and a significant dose × time interaction (F [6, 96] = 2.4, P < 0.04), reflected primarily in the male % changes at 10 and 20 min following the 150 and 200 NIC doses.
Fig. 7 presents the means and SEMs of the ACTH responses. Vehicle control slightly and non-significantly increased ACTH responses in both sexes. There were no significant interactions among sex, NIC dose, or time. ACTH showed a significant main effect of time (F [3, 144] = 4.0, P < 0.009): Following the 150 ng/ml dose, female ACTH responses were significantly higher at 5 and 10 min, and male ACTH responses were significantly higher at 10 and 20 min, compared to baseline values prior to NIC stimulation (P's < 0.05). The main effects of both sex and dose were non-significant.
Fig. 8 presents the means and SEMs of the CORT responses. Baseline CORT values from female adrenals were higher than those from male tissues. There was no discernible effect of vehicle control on CORT. There also were no significant interactions among sex, NIC dose, or time. Following NIC (and ACTH) stimulation, there were significant main effects of time (F [3, 144] = 4.3, P = 0.006) and dose (F [3, 47] =5.21, P < 0.004), reflecting varying decreases in male and female CORT responses with increasing NIC doses. There also was a significant main effect of sex (F [1, 47] =6.1, P < 0.02): Female responses were significantly higher than male responses at most time points, based largely on the higher initial baseline values in females.
4. Discussion
In the present study, we replicated the ability of our in vitro perfusion system containing hypothalamic, pituitary, and adrenal tissues to model pharmacological responses of the HPA axis we have shown in vivo. Similar to our previous in vitro study, females had greater CRH and ACTH responses, although the ACTH responses were less perceptible (Moidel et al., 2006). Also in the present study, we were able to measure AVP and found that male responses were higher than female responses, in accord with our previous NIC in vivo studies. Also similar to these in vivo studies, female rats had greater, though less pronounced, ACTH and CORT responses than did males (Rhodes et al., 2001a). The present study determined hormone responses to 50 ng/ml, 150 ng/ml and 200 ng/ml NIC, whereas our previous in vitro study only determined hormone responses to 50 ng/ml NIC.
Numerous in vitro studies have investigated hormone release following cholinergic and/or hormonal stimulation of isolated HPA axis tissues, as discussed extensively in our previous in vitro study (Moidel et al., 2006). However, no studies prior to or following our previous in vitro study have determined simultaneous, multiple hormone release from HPA tissues perfused in series. In vitro studies offer many advantages, including decreased animal use and elimination of difficult collection of portal blood for analysis of CRH and AVP (Aguilera et al., 2008; Wilhelmi et al., 2002). Our in vitro system also eliminates the influence of external factors that influence HPA axis activity, such as variable CNS input (Gray et al., 1989; Jacobson & Sapolsky, 1991; Madeira & Lieberman, 1995; Van de Kar & Blair, 1999); the stress of housing, handling, and experimentation (Belz et al., 2003; Brown & Grunberg, 1995; Brown & Grunberg, 1996; Gadek-Michalska & Bugajski, 2003); and peripheral sex hormones (Rhodes et al., 2002; Rhodes et al., 2004; Seale et al., 2004). It also eliminates negative feedback at the level of the hypothalamus and the pituitary from CORT, and can be adapted to add feedback in a controlled manner.
Several improvements to our previous in vitro model have made it possible to better emulate in vivo HPA axis physiology. The transition from Erlenmeyer flasks to jacketed tissue baths to hold the tissues allowed for greater temperature control of each bath within physiological ranges. For example, in our previous study, temperatures ranged from 32–34 °C in the adrenal flask, whereas the current system maintained temperatures from 36.2–38.9 °C for the duration of the experiments in the adrenal tissue baths. CORT concentrations in our previous study were higher in male tissues compared to female tissues, whereas in the current study CORT concentrations were higher in female tissues compared to male tissues, reflecting in vivo findings (Luine et al., 2007; Moidel et al., 2006; Rhodes et al., 2001a), perhaps due to better temperature control.
Another improvement is the reduction in perfusion flow rate to increase hormone concentrations during sampling from each bath. In our previous study, flow rate ranged from 4.5–6.0 ml/min, compared to 1.1–1.9 ml/min in the present study. This refinement was made in order to improve hormone measurements, particularly of AVP. Male AVP responses were higher than female responses, consistent with in vivo findings from our laboratory (Rhodes et al., 2004; Rhodes et al., 2001a). As well, in our previous in vitro study, culture medium was oxygenated at the source flask, which may have contributed to variable oxygen concentrations among the tissue baths. In the current study, each bath was oxygenated separately, enhancing tissue viability.
With the lower flow rates, AVP measurements were achievable, but CORT responses to NIC stimulation were less pronounced than in our earlier study. Because of the slower flow rate and the tendency of the pH of the perfusion medium to increase, the pH in the adrenal baths may not have been within the physiological range, affecting adrenal viability. Future studies will determine if a flow rate between those of the previous and present studies will allow measurable concentrations of all hormones as well as perceptible differences in CORT responses.
In the present study, bath volumes may not have approximated corresponding in vivo extracellular pools and therefore may have influenced hormone concentrations. Future studies will investigate differing bath sizes and volumes, to better approximate the expected extracellular pools of the specific tissues and therefore the drug and hormone concentrations surrounding them (e.g., a smaller pituitary bath to approximate the small volume of portal blood reaching it, and larger hypothalamic and adrenal baths to approximate the relatively larger volume of systemic blood reaching them).
NIC, as it perfused throughout the entire system, also may have differentially influenced male and female ACTH and CORT responses in the pituitary and adrenal tissues, respectively. Future studies will address the effects of NIC stimulation at various inter-bath points along the in vitro axis (NIC addition at the hypothalamic bath vs. addition at the pituitary bath vs. addition at the adrenal bath) to determine the direct effects of NIC on each tissue and perhaps better mimic delivery of NIC (and hormones) in vivo. Under non-stressful conditions, both CRH and AVP are released in a rhythmic, pulsatile fashion in vivo. The pulsations of these hormones increase in both amplitude and synchronization during stress (Chrousos, 1998; Chrousos, 2000; Chrousos & Gold, 1992). In our previous study, there were more perceptible differences in ACTH and CORT responses, perhaps because the flow of the buffer through the system was driven by gravity and the delivery of buffer to each bath was pulsatile in nature, as opposed to the continuous delivery of buffer in the present study. By dosing at inter-bath points along the in vitro axis, a concentrated, pulsatile delivery of drug can be achieved at each level of the axis, which may improve the ACTH and CORT responses measured after stimulation. Future studies using direct delivery of various doses of CRH and ACTH, to the pituitary and adrenal baths, respectively, also will address pituitary and adrenal sensitivity to these hormones.
In the current study, incremental dosing of NIC was chosen, because of the possibility of a large dose of NIC producing long-lasting effects that would mask subsequent responses to smaller doses of NIC. In order to truly explore the dose-responsiveness of NIC in our in vitro system, future studies will use a random dosing paradigm, allowing for sufficient time between doses for hormone concentrations to return to baseline. Future studies using random NIC dosing also may elucidate whether or not the tissues were primed or desensitized by incremental NIC dosing.
As stated previously, CRH responses were higher in female tissues compared to male tissues, and AVP responses were higher in male tissues compared to female tissues, similar to our in vivo studies (Rhodes et al., 2001a). As well, AVP % changes from baseline were more prolonged in males compared to females following the 150 ng/ml and 200 ng/ml NIC doses (Fig. 6). This suggests that following the higher doses of NIC, female and male hypothalami differ in both degree and timing of hormone responses to cholinergic challenge by NIC.
In an in vivo study of CRH/AVP mRNA co-expression in the parvocellular paraventricular nucleus (pcPVN) following self-administered NIC in male rats, CRH expressing neurons were markedly decreased, whereas AVP expressing neurons were increased in both the pcPVN and the magnocellular PVN (mcPVN). Further, the number of CRH neurons co-expressing AVP mRNA in the pcPVN increased fivefold (Yu et al., 2008). This shift in mRNA expression following self-administered NIC parallels the CRH vs. AVP responses observed in males in the present study.
Although Yu et al. (2008) studied only male rats, one can speculate that shifts from CRH to AVP mRNA expression and hormone release may have contributed to the sex differences in the timing and intensity of hormone responses that we observed. AVP responses appear to be sustained in male but not in female tissue, wherein responses appear to remain CRH-driven. Other studies also support the prolongation of AVP secretion in male rats (Aguilera et al., 2008; Volpi et al., 2004), and a shift to more than half of CRH neurons co-expressing AVP (Aguilera et al., 2007; Aguilera et al., 2008; Chowdrey et al., 1995; de Goeij et al., 1991; Ma et al., 1997; Volpi et al., 2004; Whitnall, 1993), but again, none of these studies specifically addressed sex differences, and they were conducted primarily in male rats.
Finally, current smokers tend to smoke more cigarettes when stressed, and following smoking cessation, women tend to relapse to smoking more quickly than men (al'Absi, 2006; Benowitz, 2008; Bjornson et al., 1995; Caldarone et al., 2008; Faraday et al., 2005; Pogun & Yararbas, 2009; Pomerleau et al., 1991). Therefore, the potentially sexually diergic influence of nicotine on anxiety pathways could be explored in our in vitro system. For example, male and female amygdala tissue could be perfused with physiological buffer and stimulated with NIC, similar to the isolated HPA axis tissues in the present study.
In summary, our improved in vitro perfusion system supports the results of our previous in vivo and in vitro studies; NIC significantly increased HPA axis responses in a sex- and dose-dependent manner. Males had greater AVP responses than females, and females had greater CRH responses than males. Sexually diergic ACTH and CORT responses were less apparent. With the application of the aforementioned additional improvements to the in vitro system, sexually diergic ACTH and CORT responses should become more apparent. The ability of our in vitro system to parallel in vivo HPA axis responses to nicotinic challenge supports its potential as a reliable laboratory method in pharmacology and toxicology.
6. Acknowledgements
The technical assistance of Anna N. Taylor, Ph.D, and Delia L. Tio, for their contributions to the AVP, ACTH and CORT assays, is gratefully acknowledged. This work was supported by NIH grant MH28380-28 to RTR and a Saint Vincent College Faculty Research Grant to MER.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Conflict of Interest Statement None of the authors have any financial or personal conflicts of interest to declare.
8. References
- Aguilera G, Kiss A, Liu Y, Kamitakahara A. Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress. 2007;10:153–161. doi: 10.1080/10253890701391192. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Subburaju S, Young S, Chen J. The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog Brain Res. 2008;170:29–39. doi: 10.1016/S0079-6123(08)00403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Mike A, Eisenberg HM, Maelicke A, Alkondon M. Neuronal nicotinic receptors in synaptic functions in humans and rats: physiological and clinical relevance. Behav Brain Res. 2000;113:131–141. doi: 10.1016/s0166-4328(00)00208-4. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, Buist AS, Hoppe-Ryan C, O'Hara P. Gender differences in smoking cessation after 3 years in the Lung Health Study. Am J Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MW, Burden J, Hillhouse EW, Jones MT. Stimulation electrically and by acetylcholine of the rat hypothalamus in vitro. J Physiol. 1974;239:269–283. doi: 10.1113/jphysiol.1974.sp010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav. 1995;58:1085–1089. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE. Effects of environmental conditions on food consumption in female and male rats. Physiol Behav. 1996;60:293–297. doi: 10.1016/0031-9384(96)00020-0. [DOI] [PubMed] [Google Scholar]
- Bugajski J, Gadek-Michalska A, Borycz J, Glod R. Social stress inhibits the nitric oxide effect on the corticotropin-releasing hormone- but not vasopressin-induced pituitary-adrenocortical responsiveness. Brain Res. 1999;817:220–225. doi: 10.1016/s0006-8993(98)01209-8. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008;439:187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam GR, Bassett JR. The plasma levels of ACTH following exposure to stress or nicotine. Arch Int Pharmacodyn Ther. 1983;264:154–167. [PubMed] [Google Scholar]
- Cam GR, Bassett JR, Cairncross KD. The action of nicotine on the pituitary-adrenal cortical axis. Arch Int Pharmacodyn Ther. 1979;237:49–66. [PubMed] [Google Scholar]
- Chang YL, Tsai PL, Chou YC, Tien JH, Tsai TH. Simultaneous determination of nicotine and its metabolite, cotinine, in rat blood and brain tissue using microdialysis coupled with liquid chromatography: pharmacokinetic application. J Chromatogr A. 2005;1088:152–157. doi: 10.1016/j.chroma.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Chowdrey HS, Larsen PJ, Harbuz MS, Jessop DS, Aguilera G, Eckland DJ, Lightman SL. Evidence for arginine vasopressin as the primary activator of the HPA axis during adjuvant-induced arthritis. Br J Pharmacol. 1995;116:2417–2424. doi: 10.1111/j.1476-5381.1995.tb15089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The HPA axis and the stress response. Endocr Res. 2000;26:513–514. doi: 10.3109/07435800009048562. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;267:1244–1252. [PubMed] [Google Scholar]
- de Goeij DC, Kvetnansky R, Whitnall MH, Jezova D, Berkenbosch F, Tilders FJ. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology. 1991;53:150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Blakeman KH, Grunberg NE. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol Biochem Behav. 2005;80:577–589. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta S, Valentine J, Sharp B. Adrenocorticotropin response and nicotine-induced norepinephrine secretion in the rat paraventricular nucleus are mediated through brainstem receptors. Endocrinology. 1997;138:1935–1943. doi: 10.1210/endo.138.5.5122. [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A, Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J Physiol Pharmacol. 2003;54:449–459. [PubMed] [Google Scholar]
- Gao L-Z, Zhang W-H, Ju G. Suppression of adrenocorticotropic hormone release by stimulation of the nerve fibres in the anterior pituitary. Journal of Neuroendocrinology. 2000;12:753–757. doi: 10.1046/j.1365-2826.2000.00518.x. [DOI] [PubMed] [Google Scholar]
- Garrido MM, Manzanares J, Fuentes JA. Hypothalamus, anterior pituitary and adrenal gland involvement in the activation of adrenocorticotropin and corticosterone secretion by gastrin-releasing peptide. Brain Research. 1999;828:20–26. doi: 10.1016/s0006-8993(99)01318-9. [DOI] [PubMed] [Google Scholar]
- Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- Grota L, Bienen T, Felten D. Corticosterone responses of adult Lewis and Fischer rats. Journal of Neuroimmunology. 1997;74:95–101. doi: 10.1016/s0165-5728(96)00209-3. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Doran AD, Salm AK, Tweedle CD. Brain slice preparation: hypothalamus. Brain Res Bull. 1980;5:405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedbackregulation of the hypothalamic-pituitary-adrenocorticol axis. Endocrine Reviews. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Matta S, Beyer S, McAllen K, Sharp B. Nicotine elevates rat plasma ACTH by a central mechanism. Journal of Pharmacology and Experimental Therapeutics. 1987;243:217–226. [PubMed] [Google Scholar]
- Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33:749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moidel MA, Belz EE, Czambel RK, Rubin RT, Rhodes ME. Novel in vitro perfusion system for the determination of hypothalamic-pituitary-adrenal axis responses. J Pharmacol Toxicol Methods. 2006;53:264–271. doi: 10.1016/j.vascn.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Paterson D, N. A. Neuronal nicotinic receptors in the human brain. Progress in neurobiology. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Pogun S, Yararbas G. Sex differences in nicotine action. Handb Exp Pharmacol. 2009:261–291. doi: 10.1007/978-3-540-69248-5_10. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Garcia AW. Biobehavioral research on nicotine use in women. Br J Addict. 1991;86:527–531. doi: 10.1111/j.1360-0443.1991.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Snedecor SM, Gaulrapp S, Brouwer RN, Cameron OG. Depression, smoking abstinence and HPA function in women smokers. Hum Psychopharmacol. 2004;19:467–476. doi: 10.1002/hup.623. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Raff H, Findling JW. A new immunoradiometric assay for corticotropin evaluated in normal subjects and patients with Cushing's syndrome. Clin Chem. 1989;35:596–600. [PubMed] [Google Scholar]
- Rhodes ME, Balestreire EM, Kenneth Czambel R, Rubin RT. Estrous cycle influences on sexual diergism of HPA axis responses to cholinergic stimulation in rats. Brain Res Bull. 2002;59:217–225. doi: 10.1016/s0361-9230(02)00868-7. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Kennell JS, Belz EE, Czambel RK, Rubin RT. Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine. Brain Res Bull. 2004;64:205–213. doi: 10.1016/j.brainresbull.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O'Toole SM, Czambel RK, Rubin RT. Male-female differences in rat hypothalamic-pituitary-adrenal axis responses to nicotine stimulation. Brain Res Bull. 2001a;54:681–688. doi: 10.1016/s0361-9230(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O'Toole SM, Wright SL, Czambel RK, Rubin RT. Sexual diergism in rat hypothalamic-pituitary-adrenal axis responses to cholinergic stimulation and antagonism. Brain Res Bull. 2001b;54:101–113. doi: 10.1016/s0361-9230(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT. Functional sex differences (`sexual diergism') of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev. 1999;30:135–152. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Rose JE. Disrupting nicotine reinforcement: from cigarette to brain. Ann N Y Acad Sci. 2008;1141:233–256. doi: 10.1196/annals.1441.019. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol. 2004;16:516–524. doi: 10.1111/j.1365-2826.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- Surget A, Belzung C. Involvement of vasopressin in affective disorders. Eur J Pharmacol. 2008;583:340–349. doi: 10.1016/j.ejphar.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Van de Kar L, Blair M. Forebrain pathways mediating stress-induced hormone secretion. Frontiers in Neuroendocrinology. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7:75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- Whitnall M. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- Wilhelmi E, Schoder UH, Benabdallah A, Sieg F, Breder J, Reymann KG. Organotypic brain-slice cultures from adult rats: approaches for a prolonged culture time. Altern Lab Anim. 2002;30:275–283. doi: 10.1177/026119290203000304. [DOI] [PubMed] [Google Scholar]
- Yu G, Chen H, Zhao W, Matta SG, Sharp BM. Nicotine self-administration differentially regulates hypothalamic corticotropin-releasing factor and arginine vasopressin mRNAs and facilitates stress-induced neuronal activation. J Neurosci. 2008;28:2773–2782. doi: 10.1523/JNEUROSCI.3837-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]