Abstract
Electrophysiological studies have shown that adolescent ethanol (EtOH) exposure can produce long-term changes in hippocampal EEG and ERP activity. Recently, evidence has emerged suggesting that event-related oscillations (EROs) may be good indices of alcoholism risk in humans, however, have not been evaluated for their ability to index the effects of EtOH exposure. The objective of the present study was to characterize EROs generated in hippocampus in adult rats exposed to EtOH during adolescence. Adolescent male Sprague-Dawley rats were exposed to EtOH vapor for 12 h/d for 10 days. A time-frequency representation method was used to determine delta, theta, alpha and beta ERO energy and the degree of phase variation in the hippocampus of adult rats exposed to EtOH and age-matched controls. The present results suggest that the decrease in P3 amplitudes, previously observed in adult rats exposed to EtOH during adolescence, is associated with increases in evoked theta ERO energy. These studies suggest that EROs are suitable for characterizing the long-term effects of adolescent EtOH exposure. Further studies are needed to determine the relationship between the mechanisms that regulate these neurophysiological endophenotypes and the consequences of adolescent EtOH exposure.
Keywords: Adolescent, Electroencephalogram, Ethanol, Event-related oscillations, Hippocampus, Rat
Introduction
Ethanol (EtOH) is one of the most abused drugs in adolescents [34,69] and has been shown to have significant detrimental effects on brain development [12]. Early onset of drinking has also been associated with the development of EtOH-related problems during adulthood [19,26,30,56]. Moreover, adolescents with a history of EtOH abuse or dependence also display deficits in cognitive function and memory [66,67]. Studies in humans have shown that adolescent EtOH abuse is associated with decreases in hippocampal volume and reductions in the activity of frontal and parietal cortical areas during spatial working memory tasks [18,68]. Consistent with these findings, studies in animal models have shown that EtOH exposure during adolescence produces a more severe impairment of hippocampal neurogenesis in comparison to equivalent adult EtOH exposure [13]. Despite these findings, the mechanisms mediating age-related vulnerabilities to EtOH exposure remain under investigation.
The electroencephalogram (EEG) and event-related potentials (ERPs) have been used to identify neuroelectric endophenotypes associated with the risk of alcoholism and to study neurophysiologic changes in brain activity associated with aging and development [21,49,50]. Our previous studies showed that repeated exposure to EtOH during adolescence produced changes in cortical and hippocampal EEG and ERP activity of adult rats [61-63]. Findings from these studies demonstrated that EtOH exposure during adolescence decreased P2 and P3 amplitudes, while increasing N2 amplitudes in the hippocampus of adult rats [61]. In addition, these results showed that adolescent EtOH exposure increased the mean frequency of the EEG in the 16-32 Hz range in the hippocampus of adult rats. However, the mechanisms mediating these neurophysiological effects of adolescent EtOH exposure and the extent of the changes after prolonged withdrawal period are not well understood.
There is evidence indicating that some ERP components, including the P3 component, may arise from superimposed event-related oscillations (EROs) induced by sensory or cognitive processes that influence the dynamics of EEG rhythms (e.g., [20,39]). There is also ample evidence to suggest that brain oscillations represent neurophysiological correlates of information processing and cognitive function [3,39]. EROs are estimated by a decomposition of the EEG signal into phase and magnitude information for a range of frequencies and then changes in those frequencies are characterized over a millisecond time scale with respect to task events. Load dependent reductions in P3 amplitude during a working memory task have been related not only to decreased delta ERO energy but also to reductions in the delta phase locking index (PLI), suggesting higher phase variability [58].
Brain oscillations have been proposed to be endophenotypes for complex genetic disorders in humans, including drug addiction and psychiatric disorders (for reviews, see [5,50]). Findings from the Collaborative Study on the Genetics of Alcoholism (COGA) have achieved significant progress in identifying EROs associated with the human P3 component and several genes potentially involved in their regulation (for reviews see [5,50,52]). Those studies have also identified several genes that increase the susceptibility for risk of EtOH dependence (for reviews, see [5,50,52]). For instance, there is evidence to suggest that EtOH dependent individuals manifest significantly less evoked delta and theta ERO power than age-matched controls [36]. Studies from the COGA project have shown a significant linkage and association between parietal delta ERO power and the cholinergic muscarinic receptor gene (CHRM2) on chromosome 7 [35,37]. These findings have provided a better understanding of the neurophysiological mechanisms and genes contributing to the human P3 component (for reviews, see [5,50,52]).
In contrast to the considerable progress understanding the relationship among brain oscillations, the P3 component and EtOH dependence in humans, the relationship between EROs and EtOH dependence in animal models exhibiting deficits in P3 amplitudes is not well understood. We have recently focused our research efforts studying EROs associated with increased susceptibility to EtOH dependence in animal models (see [16,27]). Our recent findings showed that the decrease in P3 amplitudes in high EtOH preference C57BL/6 (B6) mice, in comparison to the low EtOH preference DBA/2J (D2) mice, are associated to reductions in evoked delta ERO energy and delta and theta phase locking [16]. Identifying changes in EROs in adult rats exposed to EtOH during adolescence could provide valuable information of specific changes in brain oscillatory activity that have been shown to be under genetic control.
We have previously shown developmental differences in hippocampal EEG and ERP responses to chronic EtOH exposure in rats [23,60,61]. Results from one of these studies demonstrated that EtOH exposure during adolescence decreased P2 and P3 amplitudes, while increasing N2 amplitudes in the hippocampus of adult rats [61]. The present study extended these initial findings by obtaining ERO energy levels and the degree of phase variability from the same datasets used to generate the hippocampal ERP data reported previously [61]. We determined whether the effects of adolescent EtOH exposure on long-latency ERP components [61] are associated with changes in hippocampal oscillatory activity in the delta, theta and beta frequency ranges. Changes in hippocampal ERO energy and PLI were estimated for time-frequency ROIs derived using standard, rare and noise tones in a passive auditory oddball paradigm. We hypothesize that differences in hippocampal P2 and P3 amplitudes previously reported in adult rats exposed to EtOH during adolescence, compared to control rats [61] are associated to differences in delta and theta ERO energy.
Materials and methods
Animals
Fifty-six (56) male Sprague-Dawley rats were used. Rats averaged 86 ± 8 g upon receipt and were maintained in a 12 h light/dark cycle (lights on at 6 am) and ad libitum access to food and water. Detailed description of the environmental conditions of rats can be found elsewhere [61]. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996) and was reviewed and approved by The Scripps Research Institute's Institutional Animal Care and Use Committee.
EtOH vapor exposure
The EtOH vapor inhalation procedure and the chambers used in this study were previously described [55,61]. In brief, rats were divided into an EtOH group (n = 16) and a control group (n = 16). EtOH-exposed rats were housed in sealed chambers, which were infused with vaporized 95% EtOH for 12 h/day during the dark cycle (from 9:30 pm to 9:30 am). For the remaining 12 h of the day, EtOH vapor was not infused into the chamber. The control group was housed in similar chambers infused with air. The EtOH group was exposed to EtOH vapors or air for 10 consecutive days (postnatal day (PND) 30-40). Age-matched controls were handled identically to EtOH-exposed rats. Food and water were available ad lib. Blood samples were collected from the tip of the tail once per week to assess blood alcohol levels (BALs). Blood samples were collected from the tip of the tail at the end of the 1st, 4th, 7th and 10th days of exposure. BALs were determined using the Analox micro-statGM7 (Analox Instr. Ltd.; Lunenberg, MA). When exposure ended, all rats were maintained in the Scripps vivarium. Recording electrodes were implanted two weeks following EtOH exposure.
Surgical and electrophysiological recording procedures
Surgical and electrophysiological recording procedures performed in this study were previously described [61]. In brief, rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally). Atropine (24 μg, subcutaneously) coadministration minimized respiratory suppression. Bipolar depth electrodes were implanted into the amygdala (AP: +1.0 mm, ML: ±5.3 mm, DV: -8.5 mm) and dorsal hippocampus (AP: -3.0 mm, ML: ± 3.0 mm, DV: -3.0 mm). The tooth bar was set at +5.0 mm. Depth electrodes were constructed from stainless steel, polyamide-coated wires (California Fine Wire: Grover Beach, CA). Stainless-steel screw electrodes were placed in the skull overlying the frontal cortex (AP: +3.0 mm, ML: ± 3.0 mm) and parietal cortex (AP: -4.0 mm, ML: ± 4.0 mm) [47]. A midline stainless-steel screw electrode was placed posterior to lambda in the skull overlying the cerebellum. Electrode connections were made to a 9-pin Amphenol connector and the assembly was anchored to the skull with dental acrylic and anchor screws. A two-week recovery period was provided before the beginning of electrophysiological studies. EEG signals were recorded with a band pass of 0.53-70 Hz with a 60-Hz notch filter in. ERP trials were digitized at a rate of 256 Hz. Potential artifacts identified by computer software were excluded only after visual analysis of raw EEG. Only neurophysiological data recorded from the hippocampus was included in the present study.
General procedures
Electrophysiological recordings were collected in one recording session in a sound-attenuated and electrically grounded BRS/LVE recording chamber (90 × 90 × 85 cm). The passive auditory ERP session consisted of 312 individual tone presentations and lasted approximately 10 mins. A three-tone auditory ‘oddball’ paradigm originally developed to directly model studies employed in humans was used [38]. Three tone types were presented: standard tones (1000 Hz square wave, 75 dB, 84% probability), rare tones (2000 Hz square wave, 85 dB, 10% probability), and noise tones (white noise, 100 dB, 6% probability). All tones were presented for 20 ms with rise and fall times of < 1 ms. Individual trials were 1000 ms in duration (100 ms pre-stimulus +900 ms post-stimulus) and were separated by variable intervals ranging from 500 to 1000 ms. Presentation of standard, rare and noise tones were randomized with at least one presentation of a standard tone between each rare tone, no more than 6 standard tones between each rare tone, and no more than 12 trials between the presentation of noise tones. Further details about the auditory ERP sessions were described previously [61].
ERO and PLI analyses
ERO and PLI analyses were accomplished from the same datasets that were used to generate the hippocampal ERP data reported in a previous publication [61]. Data from each trial generated by the stimuli were processed by a time-frequency analysis algorithm, which utilizes the S-transform [64], a generalization of the Gabor transform [29], defined as:
The equation for calculation of the S-transform of discrete time series h(kT) at time jT and frequency n/NT is where T is the sample period of the discrete time series, j is the sample index, N is the number of samples in the time series, n is the frequency index, and H[ ] is the Fourier spectrum of the discrete time series. The computer code we used is based closely on a C language S-transform subroutine available from the NIMH MEG Core Facility web site (http://kurage.nimh.nih.gov/meglab/). The defining equation of the S-transform is a convolution integral in continuous time. The method we use is equivalent to the finite discrete time version of this, but for computational efficiency, multiplications in frequency domain are used rather than convolution in time domain. The inputs to the S-transform are real, but the outputs are complex. We use the magnitudes squared of the time-frequency output values, discarding the corresponding phase angles.
The PLI was calculated to measure phase variability in relation to stimulus onset, as previously described [58]. The PLI, which ranges between zero and one, is a measure of phase. An increased PLI indicates less phase variability and stronger phase locking to the onset, whereas a reduced PLI indicates higher phase variability and weaker phase locking [58].
To reduce consequences of resulting discontinuities, we use a cosine window over the initial and final 100 msec of the input time series of each trial. The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time-frequency energy distributions. To quantify S transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI. ERO energy was determined as the measure of the energy values in the ROI. PLI was determined as the peak amplitude of the PLI in the ROI. These analyses are similar to what has been previously described [58]. Baseline corrected post-stimulus activity (900 ms) was calculated by subtracting pre-stimulus ERO energy values (100 ms) from the post-stimulus ROI values, as previously described [46]. Normalized pre-stimulus ERO energy values for each ROI were determined by calculating a normalized baseline, defined as: V1 - (Hb × A1), where V1 is the volume energy of ROI1, Hb is the average height of the baseline for ROI1, and A1 is area of ROI1. The area of ROI1 (A1) is calculated as the frequency range of ROI1 × the total duration of ROI1. Hb is defined as: Vb ÷ Ab, where Vb is the volume of the baseline for ROI1, and Ab is the area of the baseline for ROI1. The ROI frequencies were: delta (0-4 Hz), theta (4-12 Hz) and beta (12-35 Hz). The ROI time intervals correspond to the P2 component (75 - 100 ms), N2 component (150 - 200 ms) and the P3 component (250 - 400 ms). ROI frequencies and time intervals are similar to those used in our previous ERP study [61].
Statistical Analysis
Statistical analyses were performed by using SPSS for the Macintosh (SPSS, Inc., Chicago, IL). Data analyses were performed on hippocampal ERO energy and PLI for the ROIs in response to standard, rare and noise tones. The effects of treatment (EtOH exposed vs. control) on hippocampal ERO energy and PLI was assessed using a 1-way ANOVA. To correct for multiple comparisons of ROIs, P-value was set at P < 0.01 to determine the levels of statistical significance.
Results
The present study extended our initial analyses of neurophysiological endophenotypes in periadolescent rats exposed to high levels of EtOH vapor for a 10-day period by characterizing changes in ERO mean energy and the degree of phase variability in the hippocampus. Differences in body weight between the EtOH-exposed group and control group were described previously [61]. In brief, exposure of EtOH vapor (10-d intermittent exposure) produced BALs averaging 252 ± 37 mg/dl. Body weight disparity between the EtOH-exposed group and the control group were observed during the exposure phase. The results suggested that EtOH-exposed rats showed a reduction in the normal increase of body weight observed in the control group [Control group: baseline, 108 ± 3 g. After 10 days, 168 ± 9 g; EtOH-exposed group: baseline, 107 ± 1 g. End of EtOH exposure, 124 ± 3 g]. However, body weight between both groups was not significantly different prior to electrophysiological recording (i.e., approximately 1.5 months after EtOH exposure; Control group: 381 ± 28 g; EtOH-exposed group: 373 ± 28 g).
ERO differences between control and EtOH-exposed rats: Baseline activity
Adolescent EtOH exposure had no effect on baseline ERO mean energy in all frequency bands studied in the 75-100 ms (F(1,21) = 1.0; P > 0.05), 150-200 ms (F(1,21) = 0.03; P > 0.05), and 250-400 ms (F(1,21) = 1.5; P > 0.05) time windows.
ERO differences between control and EtOH-exposed rats: ERO energy and PLI
No significant effect of adolescent EtOH exposure on ERO energy in response to standard, rare and noise tones was found in the 75-100 ms window in the hippocampus (F's(1,21) < 2.5; P > 0.05). Adolescent EtOH exposure had no significant effect on hippocampal PLI (F's(1,21) < 0.8; P > 0.05).
No significant effect of adolescent EtOH exposure on ERO energy in response to standard, rare and noise tones was found in the 150-200 ms window in the hippocampus (F's(1,21) < 0.6; P > 0.05). Adolescent EtOH exposure had no significant effect on hippocampal PLI (F's(1,21) < 0.9; P > 0.05).
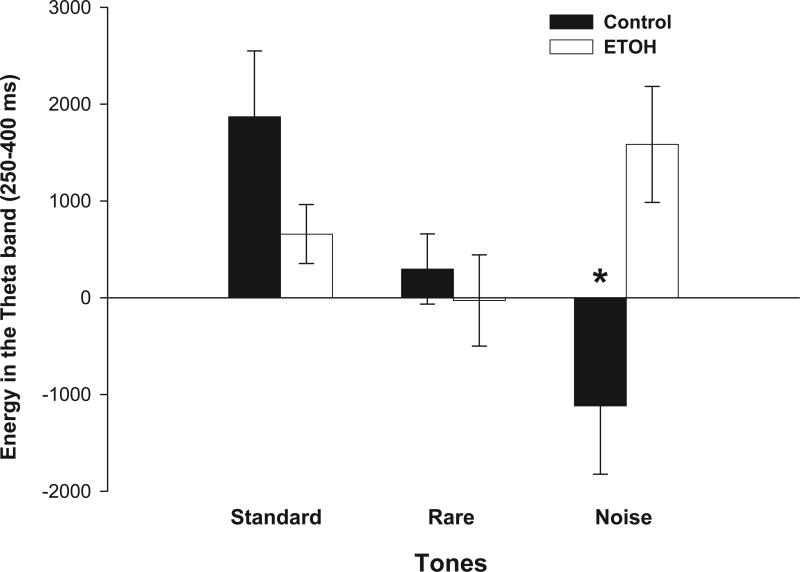
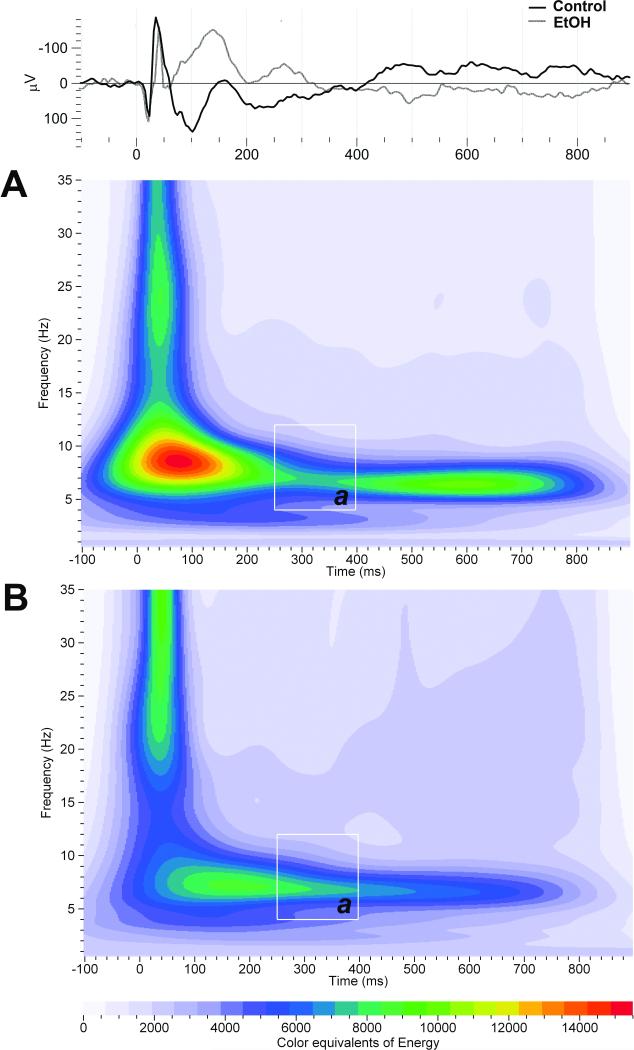
No significant effect of adolescent EtOH exposure on theta ERO energy in response to standard and rare tones was found in the hippocampus (Fig. 1; F's(1,21) < 3.0; P > 0.05). However, EtOH exposure during adolescence significantly increased hippocampal theta ERO energy in response to noise tones [F(1,21) = 8.6; P < 0.01] in the 250-400 ms time window, compared to control rats (Fig. 1). Adolescent EtOH exposure had no effect on delta and beta ERO energy in response to standard, rare and noise tones in the 250-400 ms window in the hippocampus (F's(1,21) < 2.0; P > 0.05). Adolescent EtOH exposure had no significant effect on hippocampal PLI (F's(1,21) < 1.6; P > 0.05). Figure 2 illustrates grand-averaged ERO time-frequency representations of the noise tones for the theta frequency Figure 2 (inset) also shows representative hippocampal ERP grand averages in control and EtOH groups in response to the noise tones from the data reported previously [61].
Figure 1.
Mean amplitude values of ERO energy for the theta band in response to standard, rare and noise stimuli in the hippocampus. Adult rats exposed to EtOH during adolescence showed greater hippocampal theta ERO energy than control rats in the 250-450 ms time window in response to noise tones. * Significant differences between control and EtOH-exposed rats (P < 0.05).
Figure 2.
Time-frequency representation of evoked delta, theta, alpha and beta band energy distribution of noise stimuli in the hippocampus of adult control and adult rats exposed to EtOH during adolescence. Time-frequency responses of evoked theta (a) band energy distribution to noise stimuli in control (A) and EtOH-exposed (B) groups in the hippocampus. Time-frequency ROI window used was 250-400 ms (white squares). The inset shows representative ERP grand averages in control (black line) and EtOH (gray line) groups from the hippocampus in response to the noise tones.
Discussion
The present study demonstrated that adolescent EtOH exposure increased hippocampal theta ERO energy in the 250-400 ms window, which corresponded to the time domain of the P3 component of the ERP. These data indicate that the effects of adolescent EtOH exposure responsible for reducing adult hippocampal P3 amplitudes [61] may be mediated by an increase in evoked hippocampal theta band energy. Results from the present study also showed that adolescent EtOH exposure had no effect on hippocampal ERO activity in the 75-100 ms and 150-200 ms time windows, corresponding to the P2 and N2 ERP components, respectively. These data suggest that the changes in P2 and N2 components previously shown in adult rats exposed to EtOH during adolescence [55] are not mediated by changes in hippocampal oscillatory activity.
Role of brain oscillatory activity regulating ERP responses
Most studies characterizing electrophysiological deficits in individuals with EtOH dependence and at risk of EtOH dependence have employed several ERP components to assess sensory and cognitive processing [50,52]. ERPs consist of positive and negative voltage deflections in the event-related EEG response that represents the summation of synchronous neuronal activity that takes place during information processing [28,50]. It has been proposed that the physiological basis of hippocampal and cortical ERPs lies on the field potentials generated by interacting neurons [6,44]. The neural activity integrated by field potentials recorded from hippocampal or cortical neurons can range from intracortical local field potentials (LFP) to the extracranial EEG [6]. Procedures traditionally used for ERP quantification include filtering and averaging several EEG epochs that are time-locked to a specific event (e.g., sensory, motor or cognitive) [28,50]. Those procedures are based on the evoked model that assumes that the event-related EEG response consists exclusively of the ERP, which is independent of the ongoing EEG [41].
There is also evidence to suggest that some ERP components are generated by superimposition of ongoing EEG oscillatory activity (for reviews, see [41] and [57]). For instance, it has been proposed that the P3 component and other ERP components in humans arise from changes in EEG oscillatory activity [5,20,39,50,52]. Evoked-EEG oscillatory activity generates synchronous neural activity that plays an important role regulating sensory and cognitive processes [7,8,31,40] and the timing of neural activity [41]. However, whether ERPs are generated by additive responses with a fixed latency and fixed polarity and independent of ongoing EEG (evoked model) or by a phase reorganization of ongoing oscillatory activity is a matter of current debate [41,57].
Effects of adolescent EtOH exposure on hippocampal ERO energy: The P3 ERP component
The P3 component of the ERP has been intensively used to study EtOH effects and EtOH-related risks in human subjects [4,32,33,48,50]. While EROs associated with the P3 ERP component in humans have been also linked to several relevant genes associated with EtOH dependence phenotypes [5,50,52], the relationship between EROs and EtOH dependence in animal models has not been well characterized. Findings from the present study suggest that the effects of adolescent EtOH exposure responsible for reducing adult hippocampal P3 amplitudes [61] are mediated by an increase in evoked hippocampal theta band ERO energy. The present study did not address the question of which model provides the most accurate representation of the ERP response. In fact, there is support to the notion that both models are likely responsible for the generation of the ERP [41,57].
Progress identifying neurophysiological endophenotypes associated with increased risk for EtOH dependence in humans is due in part to the success characterizing the genetics of the EROs underlying the P3 component (for review, see [50]). Findings from the present study are an initial step toward identifying a neurophysiological endophenotype associated with increased risk for EtOH dependence in animal models. Further research in rodent models is needed to characterize the EROs responsible for generating the P3 ERP component and whether those EROs are also linked to putative genes regulating EtOH dependence phenotypes. These findings are needed in order to characterize the neurotransmitter systems mediating the changes in hippocampal theta ERO energy associated with P3 amplitudes.
Analyses to determine hippocampal ERO energy were accomplished from the same datasets that were used to generate the hippocampal ERP data in response to auditory stimuli, reported in a previous publication [61]. Therefore, the neurophysiological activity integrated in the auditory hippocampal ERP is likely modulated by a larger neural network than the hippocampal LFP, which is thought to be one of the most spatially localized signals [6]. However, the mechanisms mediating the changes in hippocampal theta ERO energy and their relationship with the mechanisms regulating theta oscillatory activity in the hippocampal LFP is presently unknown.
Effects of adolescent EtOH exposure on hippocampal neurophysiology: Neurotransmitter systems
Previous studies have shown that the differential sensitivity to EtOH seen during development is most likely the result of changes in many brain systems, including GABAergic and glutamatergic systems [1,43,65] that occur over the course of adolescence. Studies characterizing the cellular and molecular mechanisms underlying the enhanced vulnerability to EtOH exposure during adolescence have frequently focused on studying glutamatergic neurotransmission (see [11]), and in particular the N-methyl-d-aspartate (NMDA) type of glutamate receptor. Findings from several electrophysiological studies have provided evidence for interactions between NMDA receptors and EtOH during adolescence. In fact, there is evidence to suggest that NMDA receptors play an important role in the short- and long-term consequences of adolescent EtOH exposure. Moreover, cortical and hippocampal NMDA receptors play an important role regulating cognitive processes that are impaired by adolescent EtOH exposure [9,12,54].
Hippocampal P3 amplitude has been demonstrated to be sensitive to altered glutamatergic function, as administration of the NMDA antagonist MK-801 has been found to produce decreases in the amplitude of the rat hippocampal P3 [24]. We recently determined the role of NMDA receptors in the development of long-term compensatory neurophysiological changes following adolescent EtOH exposure and found that MK-801 significantly reduced P3 ERP amplitude and increased P3 ERP latency in control, but not in EtOH-exposed rats [14]. These data suggest neuroadaptive changes in NMDA-mediated regulation of the P3 ERP component following adolescent EtOH exposure.
There is evidence to suggest that hippocampal theta oscillations are modulated by both GABAA and NMDA receptor systems [7]. Electrophysiological studies have demonstrated that the inhibitory effects of EtOH on NMDA-mediated cortical post-synaptic currents, hippocampal LTP, excitatory post-synaptic potentials, and on the hyperpolarization activated cation current (Ih) in hippocampal GABAergic interneurons are more pronounced in adolescent rats compared to adult rats [42,51,65,72]. However, the mechanisms mediating how the increase in theta ERO energy attenuates P3 amplitudes in the hippocampus is presently not well understood.
Effects of adolescent EtOH exposure on hippocampal P3 and theta ERO energy: Additional interpretations
The decrease in hippocampal P3 amplitude previously reported [61] and the increase in evoked theta ERO energy found in the present study were observed in response to noise tones, but not to standard and rare tones. Since hippocampal P3 amplitudes and theta ERO energy generated in response to standard and rare tones were not significantly different between adult rats exposed to EtOH during adolescence and control rats it is likely that factors other than neurotoxicity may have also play a role in these findings. An important observation is that the reduction in P3 amplitude and increase in evoked theta energy in adult rats exposed to EtOH during adolescence were revealed in response to louder noise tones (100 dB), in comparison to the standard (75 dB) and rare (85 dB) tones. Therefore, it is possible that these findings were due to startle-like response as a result to an increase in anxiety-like behaviors in adult rats exposed to EtOH during adolescence. Previous studies have used the acoustic startle response to noise as measure to assess EtOH withdrawal in rats. A study characterizing the effects of the acoustic startle response to a noise stimulus in rats with a genetic predisposition toward EtOH drinking found the 100 dB noise tone as the most effective in detecting the difference in startle magnitude between EtOH- and water-treated alcohol-preferring (P) rats [10]. Studies have shown that an increase in anxiety-like behaviors can be observed up to 28 days after EtOH withdrawal [53,70]. Moreover, recent evidence suggest that anxiety-like behaviors after EtOH withdrawal in adolescent rats last longer than in adult rats [71]. However, the present study did not assess anxiety levels in adult rats following adolescent EtOH exposure. The period of abstinence (7 weeks) in the present study was chosen based on previous studies demonstrating prolonged effects of EtOH exposure on EEG and ERPs after similar or longer abstinence periods [25,60]. The neurophysiological consequences of these long-lasting effects were also recently confirmed in adult rats exposed to EtOH during adolescence [14,15].
Another issue that must be considered is whether the effects of adolescent EtOH exposure on the processing of auditory information and hearing perception could have played a major role influencing the findings from the present study and our previous ERP study [61]. In contrast to late cognitive components of the ERP such as P2 and P3, the amplitude of the early N10 and N1 sensory components were not altered by adolescent EtOH exposure [61]. The N10 component (0-15 ms) is an early auditory evoked potential for indication of preattentional information processing [17]. Moreover, if the effects of adolescent EtOH exposure on hippocampal P2 and P3 amplitudes were due to perceptual impairments in hearing, the results would have also shown P2 and P3 attenuations in other brain regions such as the amygdala and frontal cortex. However, adolescent EtOH exposure had no effect on the amplitudes and latencies of ERP components recorded from the amygdala and frontal cortex [61]. These data suggest that the effects of adolescent EtOH exposure on hippocampal EROs were not due to perceptual impairments in hearing.
The present study did not measure whether EtOH exposed rats were EtOH dependent and exhibited signs of EtOH withdrawal. Our previous study found that adolescent EtOH exposure had no effect on locomotor activity assessed at 1 or 6-7 weeks post-EtOH exposure [61]. Those findings are consistent with results from other reports indicating that early life EtOH exposure had inconsistent effects on locomotor activity (e.g., [22]). Those data suggests that the ERO effects observed in the present study were not due to gross differences in arousal or motor activity between control and EtOH-exposed groups.
Effects of adolescent EtOH exposure on hippocampal ERO energy: The P2 and N2 ERP components
In our initial study adolescent EtOH exposure reduced P2 amplitudes in response to the noise tones [61]. Our study also suggested that adolescent EtOH exposure increased hippocampal N2 amplitude in response to the standard, rare and noise tones [61]. Results from the present study showed that adolescent EtOH exposure had no significant effect on ERO energy in the time windows corresponding to the P2 and N2 components in response to standard, rare and noise tones in the adult hippocampus. In addition, adolescent EtOH exposure had no effect on phase variability in the time windows corresponding to N2 and P2 components. Findings from the present study suggest that the effects of adolescent EtOH exposure on P2 and N2 amplitudes [61] are not due to differences in evoked ERO energy nor in PLI.
Conclusions
Considerable progress has been made to develop rat models that simulate several relevant human endophenotypic behavioral traits associated with EtOH-related disorders. Our previous studies in adult rats showed that alterations in hippocampal EEG normalize within 2 weeks following a 28-day EtOH exposure period [23]. In contrast, following just 10 days of adolescent EtOH exposure we found prolonged changes in hippocampal EEG that persisted for 6-8 weeks [61]. Consistent with our findings in hippocampal EEG, exposure to EtOH during adolescence decreased P2 and P3 amplitudes, while increasing N2 amplitudes in the hippocampus of adult rats [61]. These initial electrophysiological studies provided evidence of increased sensitivity to the effects of EtOH on EEG and ERP responses during adolescence. We extended our initial analyses of neurophysiological endophenotypes associated with adolescent EtOH exposure by characterizing changes in ERO mean energy and the degree of phase variability in the hippocampus of adult rats exposed to EtOH during adolescence [61]. Our present study showed that the inhibitory effects of adolescent EtOH exposure on adult hippocampal P3 amplitudes [61] are associated with an increase of evoked hippocampal theta band energy. In contrast, adolescent EtOH exposure had no effect on hippocampal ERO activity in the 75-100 ms and 150-200 ms time windows, corresponding to the P2 and N2 ERP components, respectively. These data suggest that the changes in P2 and N2 components previously shown in adult rats exposed to EtOH during adolescence [61] are not mediated by changes in evoked hippocampal oscillatory activity. Further studies are needed to determine whether the expression of these neurophysiological endophenotypes was triggered during adolescent EtOH exposure or during the prolonged withdrawal period following adolescent EtOH exposure. Understanding the relationship between evoked theta oscillatory activity and P3 responses may provide insight into the brain processes underlying the long-term consequences of adolescent EtOH dependence in adult rats [2,45,59].
Acknowledgements
Supported in part by National Institute on Alcoholism and Alcohol Abuse grant AA006059 and AA014339 and by the Stein Endowment fund. The computer programs were written by Dr. James Havstad. The authors thank Derek Wills, Evelyn Phillips, Phil Lau and Jennifer Roth for assistance in analyses, and Shirley Sanchez for help in editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acheson SK, Richardson R, Swartzwelder HS. Developmental changes in seizure susceptibility during ethanol withdrawal. Alcohol. 1999;18:23–26. doi: 10.1016/s0741-8329(98)00063-9. [DOI] [PubMed] [Google Scholar]
- 2.Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res. 2005;29(9):1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- 3.Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259(3):165–68. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- 4.Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- 5.Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60(2):162–71. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Bressler SL. Event-related potentials. In: Arbib MA, editor. The Handbook of Brain Theory and Neural Networks. MIT Press; Cambridge, MA: 2002. pp. 412–415. [Google Scholar]
- 7.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 8.Buzsaki G. Rhythms of the Brain. Oxford University Press; New York: 2006. p. 464. [Google Scholar]
- 9.Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86(2):200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28(5):677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- 11.Crews FT, Rudolph JG, Chandler LJ. Glutamate and alcohol-induced neurotoxicity. In: Herman BH, Frankenheim J, Litten RZ, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and addiction. Humana Press; Totowa, N.J: 2002. pp. 357–374. [Google Scholar]
- 12.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137(2):437–45. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- 14.Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008;32:1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 15.Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008;42:631–639. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Criado JR, Ehlers CL. Event-related oscillations as risk markers in genetic mouse models of high alcohol preference. Neuroscience. 2009;163(2):506–523. doi: 10.1016/j.neuroscience.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophys. doi: 10.1016/j.clinph.2009.09.027. In Press. (doi:10.1016/j.clinph.2009.09.027) [DOI] [PubMed] [Google Scholar]
- 18.De Bellis MD, Clark DB, Beers SR, Boring AM, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157(5):737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 19.De Wit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157(5):745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 20.Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topogr. 2001;13(4):251–67. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- 21.Dustman RE, Emmerson RY, Shearer DE. Life span changes in electrophysiological measures of inhibition. Brain Cogn. 1996;30(1):109–26. doi: 10.1006/brcg.1996.0007. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers CL, Chaplin RI. Chronic ethanol exposure potentiates the locomotor activating effects of corticotropin-releasing factor (CRF) in rats. Regul Pept. 1987;19:345–353. doi: 10.1016/0167-0115(87)90176-5. [DOI] [PubMed] [Google Scholar]
- 23.Ehlers CL, Chaplin RI. EEG and ERP response to chronic ethanol exposure in rats. Psychopharmacology (Berl) 1991;104(1):67–74. doi: 10.1007/BF02244556. [DOI] [PubMed] [Google Scholar]
- 24.Ehlers CL, Kaneko WM, Wall TL, Chaplin RI. Effects of dizocilpine (MK-801) and ethanol on the EEG and event- related potentials (ERPS) in rats. Neuropharmacology. 1992;31:369–378. doi: 10.1016/0028-3908(92)90069-2. [DOI] [PubMed] [Google Scholar]
- 25.Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 26.Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30(11):1856–65. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 27.Ehlers CL, Criado JR. Event-related oscillations in mice: effects of stimulus characteristics. J Neurosci Methods. 2009;181(1):52–57. doi: 10.1016/j.jneumeth.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabiani M, Gratton G, Coles MGH. Event-related brain potentials: Methods, theory and applications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd edition Cambridge University Press; Cambridge, UK: 2000. pp. 53–84. [Google Scholar]
- 29.Gabor D. Theory of Communication. J Inst Elec Eng. 1946;93(3):429–57. [Google Scholar]
- 30.Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse. 2001;13(4):493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Hill SY, Locke J, Steinhauer SR. Absence of visual and auditory P300 reduction in nondepressed male and female alcoholics. Biol Psychiatry. 1999;46:982–989. doi: 10.1016/s0006-3223(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 33.Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 34.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. [June 2, 2009];Monitoring the future national survey results on drug use, 1975-2004: Volume I, Secondary school students (NIH Publication No. 05-5727) http://www monitoringthefuture org/pubs/monographs/vol1_2004 pdf [serial online] Available from: National Institute on Drug Abuse. Bethesda, MD.
- 35.Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006;117:2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Jones KA, Porjesz B, Almasy L, Bierut L, Dick DM, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield JA, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko WM, Ehlers CL, Phillips EL, Riley EP. Auditory event-related potentials in fetal alcohol syndrome and Down's syndrome children. Alcohol Clin Exp Res. 1996;20(1):35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 39.Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neuropathol. 2000;111(10):1719–32. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- 40.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 41.Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev. 2007;31:1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Wilson WA, Swartzwelder HS. Differential effect of ethanol on NMDA EPSCs in pyramidal cells in the posterior cingulate cortex of juvenile and adult rats. J Neurophysiol. 2002;87(2):705–11. doi: 10.1152/jn.00433.2001. [DOI] [PubMed] [Google Scholar]
- 43.Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 44.Lopes da Silva F. Neural mechanisms underlying brain waves: From neural membranes to networks. Electroenceph Clin Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- 45.McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies in animal models. Recent Dev Alcohol. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- 46.Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, et al. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int J Psychophysiol. 2006;62(2):262–71. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. 2nd ed. Plenum Press; New York: 1979. p. 47. [Google Scholar]
- 48.Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- 49.Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Dev Alcohol. 1991;9:53–67. [PubMed] [Google Scholar]
- 50.Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neuropathol. 2005;116(5):993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19(2):107–11. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- 52.Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–71. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- 54.Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27(3):141–48. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27(4):466–86. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- 56.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res. 2001;25(11):1594–604. [PubMed] [Google Scholar]
- 57.Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331(2):107–10. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- 59.Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29(7):1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- 60.Slawecki CJ, Somes C, Ehlers CL. Effects of chronic ethanol exposure on neurophysiological responses to corticotropin-releasing factor and neuropeptide Y. Alcohol Alcohol. 1999;34(3):289–299. doi: 10.1093/alcalc/34.3.289. [DOI] [PubMed] [Google Scholar]
- 61.Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128(1):63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- 62.Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26(2):246–254. [PubMed] [Google Scholar]
- 63.Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170(1):41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 64.Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S Transform. IEEE Trans on Signal Processing. 1996;44(4):998–1001. [Google Scholar]
- 65.Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995(b);19(6):1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 66.Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc. 1999;5(6):481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- 67.Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- 68.Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25(2):236–245. [PubMed] [Google Scholar]
- 69.U.S. Department of Health and Human Services.Substance Abuse and Mental Health Services Administration . Results from the 2007 National Survey on Drug Use and Health: National findings. U.S. Department of Health and Human Services, Office of Applied Studies; Rockville, MD: 2008. (DHHS Publication No. SMA 08-4343, NSDUH Series H-34) [online] http://www.oas.samhsa.gov/NSDUH/2k7NSDUH/2k7results.cfm. [Google Scholar]
- 70.Valdez GR, Roberts AJ, Chan K, Davis H, Brenna M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2001;26(10):1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 71.Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2009;33(3):455–463. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan H, Li Q, Fleming R, Madison RD, Wilson WA, Swartzwelder HS. Developmental sensitivity of hippocampal interneurons to ethanol: involvement of the hyperpolarization-activated current, Ih. J Neurophysiol. 2009;101(1):67–83. doi: 10.1152/jn.90557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]