Abstract
The potent mucosal adjuvant properties of the type II heat-labile enterotoxin LT-IIa of Escherichia coli are dependent upon binding of the B pentamer of the enterotoxin (LT-IIa-B5) to ganglioside receptors on immunocompetent cells. To evaluate the immunomodulatory activities of LT-IIa-B5, in vitro experiments employing bone marrow-derived dendritic cells (BMDC) were performed. Uptake of OVA-FITC, a model antigen (Ag), was enhanced by treatment of BMDC with LT-IIa-B5, but not by treatment of cells with the B pentamer of cholera toxin (CTB). Expression of co-stimulatory molecules (CD40, CD80, CD86, and MHC-II) and cytokines (IL-12p40, TNF-α, and IFN-γ) was increased in BMDC treated with LT-IIa-B5. The capacity of LT-IIa-B5 to enhance Ag uptake and to induce expression of co-stimulatory receptors and cytokines by BMDC was dependent upon expression of TLR2 by the cell. Increased Ag uptake induced by LT-IIa-B5 was correlated with increased Ag-specific proliferation of CD4+ T cells in an in vitro syngeneic DO11.10 CD4+ T cell proliferation assay. These experiments confirm that LT-IIa-B5 exhibits potent immunomodulatory properties which may be exploitable as a non-toxic mucosal adjuvant.
Keywords: TLR2, adjuvant, antigen uptake
1. INTRODUCTION
Pathogens, including a number of viruses and various gram-negative and gram-positive bacteria, invade the body after initial contact with the mucosal surfaces of the respiratory, oral, gastrointestinal, or urogenital tracts [1]. To prevent initial colonization and/or subsequent invasion by a particular pathogen, a robust pathogen-specific immune response on the appropriate mucosal surface is required [2, 3]. Unfortunately, in most cases, endogenous cellular and molecular mechanisms which suppress B cells and T cells serve to down-regulate mucosal immune responses to foreign antigens (Ag) [4]. In other cases, tolerance to foreign Ags is induced by local production by regulatory T cells of interleukin 10 (IL-10) or transforming growth factor β (TGF-β) [5-7]. For that reason, mucosal vaccines commonly fail to evoke protective Ag-specific immune responses [8]. These immunosuppressive effects, however, can often be circumvented by the use of mucosal adjuvants. The most potent mucosal adjuvants described to date belong to the type I subfamily (e.g., cholera toxin of Vibrio cholerae, LT of E. coli) and to the type II subfamily (LT-IIa and LT-IIb of E. coli) of bacterial enterotoxins [9-11]. Within the various members of the two subfamilies, LT-IIa has been shown to exhibit unique immunomodulatory properties not observed for CT, the best described HLT adjuvant, including the ability to (i) stimulate expression of IL-4 in cervical lymph node cells from immunized mice, (ii) increase expression of IL-6 in splenic cells of immunized mice, (iii) increase expression of TNF-α by DC, and the inability to (iv) effect B cell differentiation or to (v) alter expression of CD25, CD69, and CD40L by CD4+ T cells [8, 11]. While the mechanisms by which LT-IIa, when used as a mucosal or systemic adjuvant, promotes enhanced Ag-specific immune responses have not been fully revealed, those mechanisms are undoubtedly different from those that are employed by CT and other type I HLT.
LT-IIa is a ~ 90kDa oligomeric protein composed of a single A polypeptide (~28 kDa) and a pentameric array of five B polypeptides (12 kDa) [9, 10]. The A polypeptide is functionally divided into two domains. The A1 domain harbors the toxic moiety, a strong ADP-ribosylase which confers the protein’s capacity to dramatically elevate the concentration of cAMP in intoxicated cells; the A2 domain consists of an α-helix and an extended tail which promotes assembly of the holotoxin by interacting non-covalently with amino acids located within the central pore of the B pentamer. Binding of LT-IIa to eukaryotic cells is conferred by the B pentamer which binds to cell surface gangliosides including, in order of decreasing affinity, gangliosides GD1b (GD1b), GD1a (GD1a), and GM1 (GM1) [11, 12]. The potent mucosal and systemic adjuvant properties of LT-IIa were firmly established using a mouse mucosal immunization model [11-13]. Mice intranasally co-immunized with LT-IIa and AgI/II, a surface protein from Streptococcus mutans [14], produced high levels of Ag-specific IgA in saliva and in vaginal fluids and elevated levels of Ag-specific IgG in serum [12, 13]. Treatment of dendritic cells (DC) with LT-IIa altered expression of important co-stimulatory receptors including the maturation markers CD40, CD80, CD86, and MHC-II [12]. Interaction of each of those surface proteins with their cognate partners on T cells is known to promote immunosynapsis prior to the process of Ag presentation [15-18]. Single-point substitution mutants of LT-IIa which exhibited diminished ganglioside-binding activities lacked the capacity to alter expression of these costimulatory molecules on DC [12]. Thus, the capacity of LT-IIa to modulate expression of CD40, CD80, CD86, and MHC-II on DC was highly dependent upon the ganglioside-binding activity of the enterotoxin.
While the potent mucosal adjuvant properties of LT-IIa have been established, use of LT-IIa (and other type I and type II enterotoxins) as a clinical adjuvant has been confounded by the ADP ribosylating activity of the enterotoxin. Mouse mucosal immunization experiments using single-point substitution mutants of LT-IIa (which lacked the capacity to increase intracellular cAMP) as mucosal adjuvants, however, demonstrated that the adjuvant properties of LT-IIa correlated strongly with the enterotoxin’s ganglioside binding activities [11, 12]. Since ganglioside-binding of the holotoxin is governed solely by the non-toxic pentameric array of B polypeptides (LT-IIa-B5), it was conjectured that one or more steps required to evoke enhanced Ag-specific mucosal immune responses would likely be influenced by use of the non-toxic LT-IIa-B5 as a mucosal adjuvant. To begin to investigate this hypothesis, the in vitro activities of LT-IIa-B5 on the various types of cells important in propagating an immune response need to be evaluated.
The initial step in evoking an immune response to any Ag involves acquisition and processing of that Ag by an antigen-presenting cell (APC). DC are powerful APCs that efficiently present Ag to naïve T cells. Since DC are resident in mucosal tissues [19-21], it has been surmised that these potent APC actively acquire Ags from mucosal surfaces and present those Ags to T cells. To determine whether treatment of DC with LT-IIa-B5 would influence Ag acquisition, in vitro Ag uptake experiments were performed. These experiments demonstrated that treatment of BMDC with LT-IIa-B5, in a TLR2-dependent manner, (i) enhanced intracellular uptake of FITC-OVA by BMDC, (ii) induced expression of co-stimulatory molecules on the treated BMDC, (iii) stimulated expression of important cytokines by BMDC, and (iv) promoted OVA-specific CD4+ T cell proliferation. Each of these responses provided strong support that the adjuvant properties of LT-IIa-B5 reside, in part, in the ability of the pentamer to enhance uptake of Ag by DC.
2. MATERIALS AND METHODS
2.1. Mice, antibodies, and other reagents
BALB/c mice, C57BL/6 mice, B6.129-Tlr2tm1Kir/J knock-out mice (TLR2-/-) [22] and DO11.10 [23] mice expressing the αβ-TCR specific for the OVA epitope (323-339) recognized in the context of the MHC-II molecule I-Ad were purchased from The Jackson Laboratory (Bar Harbor, ME). Females of 6-10 weeks of age were utilized for all experiments. Animal experiments were approved by the Institutional Animal Care and Use Committee at The University at Buffalo.
APC-conjugated anti-mouse CD11c (Clone HL3), FITC-conjugated anti-mouse CD11c (Clone HL3), PE-CD40 (Clone 3/23), FITC-conjugated anti-mouse CD80 (Clone 16-10A1), and FITC-conjugated anti-mouse MHC-II (Clone 2G9) were obtained from BD Biosciences (Franklin Lakes, NJ). APC-conjugated anti-mouse CD4 (Clone RM4-5) and the anti-TLR2 blocking antibody (Clone T2.5) were purchased from Biolegend (San Diego, CA). PE-conjugated anti-mouse CD86 (Clone GL1) was obtained from BD Biosciences. Pam3cys (also known as Pam3CSK) and E. coli LPS were obtained from InvivoGen (San Diego, CA). The Vyrant CFDA SE cell tracer kit (CFSE) was obtained from Molecular Probes (Eugene, OR). The viability stain 7-AAD was obtained from Calbiochem. Cholera toxin B subunit (CTB) was purchased from List Biological Laboratories, Inc. (Campbell, CA)
2.2. Cloning and purification of wt LT-IIa-B5
Cloning of the His-tagged LT-IIa-B5 was previously reported [12]. Briefly, plasmids encoding the B pentamers were introduced into E. coli DH5αF’Kan (Life Technologies, Gaithersburg, MD) and expression of LT-IIa-B5 was induced by addition of isopropyl-β-D-thiogalactoside to the culture medium. Pentamer was extracted from the periplasmic space using isotonic shock and purified by nickel affinity chromatography (Qiagen, Valencia, CA) [24, 25]. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting using polyclonal antibodies against LT-IIa holotoxin [25] were employed to demonstrate that wt LT-IIa-B5 was purified to apparent homogeneity. Purified protein was analyzed for endotoxin by use of the end-point quantitative Limulus amoebocyte lysate assay (Charles River Endosafe, Charleston, SC). The level of endotoxin in purified preparation of LT-IIa-B5 was <0.003ng/μg of protein, a level considerably below the level needed to evoke an immune response in BMDC.
2.3. Generation of BMDC
BMDC were derived from murine bone marrow cells [26]. Briefly, cells from bone marrow flushed from femurs and tibia of mice were cultured in 6-well tissue culture plates at 1×106 cells/ml in complete RPMI 1640 culture medium (Mediatech. Herndon, VA) that was supplemented with 5% fetal bovine serum (FBS) (GIBCO Invitrogen, Grand Island, NY), 50μM 2-mercaptoethanol (GIBCO Invitrogen), 1% HEPES (GIBCO Invitrogen), 1% sodium pyruvate (GIBCO Invitrogen), 1% non-essential amino acids (GIBCO Invitrogen), 10ng/ml recombinant mouse IL-4 (eBioscience, San Diego, CA) and 10% vol/vol of a supernatant obtained from the J588L cell line transfected with a recombinant GM-CSF murine gene (kindly provided by Dr. Ira Mellman, Yale University, New Haven, CT). Culture medium was replaced on day 3 and the cells were used on day 5 at which point over 90% of cells expressed CD11c+ (data not shown).
2.4. Ag uptake assay
Ag uptake was measured in BMDC using a standard method [27]. FITC labeled-chicken OVA (FITC-OVA) (Invitrogen-Molecular Probes) was used as the model Ag. Individual 100μl aliquots containing 2×105 BMDC were incubated for 10 min at 37°C with 5 μg/ml of LT-IIaB5, 5 μg/ml of CTB, 1μg/ml of LPS, 1μg/ml Pam3CSK or PBS (untreated). BMDC were then treated with FITC-OVA (0.2 mg/ml) for 10 min at 37°C. Uptake was terminated by washing the cells 3x with ice-cold PBS containing 2% FBS. Experiments were also conducted on ice to inhibit intracellular uptake as a control for surface binding of the Ag. Cells were resuspended in FACS buffer (PBS containing 2% bovine serum albumin and 0.1% sodium azide) and stained with APC-conjugated anti-CD11c (Biolegend). After staining for CD11c, cells were washed in FACS buffer and resuspended in FACS buffer containing 0.25 μg/ml of 7-AAD (Calbiochem) to distinguish living cells from dead cells. FITC fluorescence of the CD11c-positive and 7-AAD-negative cells was measured using a FACScaliber 4-color flow cytometer (Becton-Dickenson). Fluorescence values were reported as mean fluorescence intensity (MFI).
2.5. Analysis of cytokine mRNA produced by BMDC
BMDC (5×106) derived from bone marrow cells obtained from wt C57BL/6 mice (WT) or C57Bl/6 TLR2-/- mice (TLR2-/-) were purified using mouse Pan-DC MicroBeads (Miltenyi Biotec, Auburn, CA). Purified BMDC (5×106) in 10 ml of complete RPMI culture medium were incubated for 6 hrs at 37°C with PBS alone (Untreated), 5 μg/ml of LT-IIa-B5, or 100 ng/ml of LPS. Total RNA was isolated from BMDC using Trizol (Invitrogen, Carlsbad, CA) [28]. cDNA was synthesized from total RNA using iScript™ cDNA Synthesis kit (Bio-Rad, Hercules, CA) following manufacturer’s protocols. Real-time quantitative polymerase chain reaction (qRT-PCR) was performed using cDNA from BMDC and the following synthetic oligonucleotides primer sets (Integrated DNA Technologies, Coralville, IA);
forward IL-12p40 primer: 5’-GGAAGCACGGCAGCAGAATA-3’
reverse IL-12p40 primer: 5’-AACTTGAGGGAGAAGTAGGAATGG-3’
forward TNF-α primer: 5’-CATCTTCTCAAAATT CGAGTGACAA-3’
reverse-TNF-α primer: 5’-TGGGAGTAGACAAGGTACAACCC-3’
forward IL-10 primer: 5’-GGTTGCCAAGCCTTATCGGA-3’
reverse IL-10 primer: 5’-ACCTGCTCCACTGCCTTGCT-3’
forward IL-4 primer: 5’-ACAGGAGAAGGG ACGCCAT-3’
reverse IL-4 primer: 5’-GAAGCCCTACAGACGAGGTCA-3’
forward IFN-γ primer: 5’-TCAAGTG GCATAGATGTGGAAGAA-3’
reverse IFN-γ primer: 5’-TGGCTCTGCAGGATTTTCATG-3’
forward β-actin primer: 5’-AGAGGGAAATCG TGCGTGAC-3’
reverse β-actin primer: 5’-CAATAGTGATGACCTGGCCGT-3’.
qRT-PCR was performed under the following conditions; 95°C, 10min (1 cycle); 95°C, 15sec; 60°C, 1min (40 cycles) using the iQ™ SYBR Green Supermix Kit (Bio-Rad). Melting curve analysis (55°C-95°C) was performed using a MyiQ™ single color Real-time PCR detection system (Bio-Rad).
2.6. Co-stimulatory molecule expression
BMDCs (1×106) were incubated for 24 hrs in 2 ml of complete RPMI culture medium in the presence or absence of 5 μg/ml of LT-IIa-B5 or 1 μg/ml of LPS. Cells were stained with APC-conjugated anti-mouse CD11c, PE-conjugated anti-mouse CD40, FITC-conjugated anti-mouse CD80, FITC-conjugated anti-mouse MHC-II, and PE-conjugated anti-mouse CD86 (BD Biosciences). Cells were treated with 7-AAD (Calbiochem) to stain dead cells to enable gating only on living cells. Cell surface molecules on BMDC were measured using a FACScaliber 4-color flow cytometer (Becton-Dickenson).
2.7. CD4+ T cell proliferation assay
BMDC (5×104) in 100 μl of complete RPMI culture medium were incubated for 30 min at 37°C with PBS, 1.0 mg/ml OVA, 1.0 mg/ml bovine serum albumen (BSA), or 1.0 mg/ml OVA in combination with 5 μg/ml LPS, 5 μg/ml Pam3CSK, or 20 μg/ml LT-IIa-B5. After washing the cells, BMDC were incubated for 6 hrs and co-cultured with CFSE-stained naïve DO11.10 CD4+ T cells (1×106) which were isolated from spleen of 6-8 weeks female DO11.10 mice using a murine CD4+ T cell negative selection isolation kit (Miltenyi Biotec). After 4 days, the cells were harvested, washed in PBS, and stained using APC-conjugated anti-mouse CD4 (Biolegend, San Diego, CA). The levels of proliferation by the DO11.10 CD4+ T cells were determined by measuring the dilution of CFSE fluorescence. In similar experiments, BMDC (5×104) in 100 μl of complete RPMI culture medium were incubated at 37°C only with 1.0 mg/ml OVA. After 30 min of incubation, cells were washed to remove soluble OVA. BMDC were subsequently incubated for 30 min at 37°C with 5 μg/ml LPS, 5 μg/ml Pam3CSK, or 20 μg/ml LT-IIa-B5. BMDC and DO11.10 CD4+ T cells were prepared, as above, to determine CD4+ T cell proliferative responses.
2.8. Statistical analysis
Analysis of variance and the Tukey multiple-comparison test were used for multiple comparisons. Unpaired t-tests were performed to analyze differences between two groups. Statistical analyses were performed using the InStat (GraphPad, San Diego, CA) software package. Differences were considered significant at P ≤0.05.
3. RESULTS
3.1. Uptake of FITC-OVA by BMDC
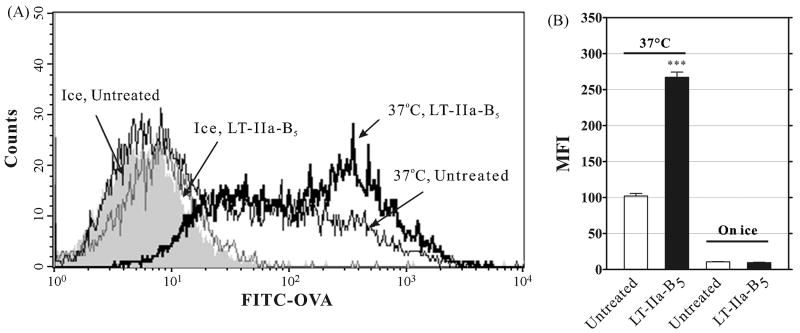
Initiation of a mucosal immune response requires participation by antigen-presenting cells (APC) such as DC which internalize, process, and present Ag to CD4+ T cells or to CD8+ T cells [29, 30]. To determine whether LT-IIa-B5 had the capacity to alter the process of Ag uptake by DC, BMDCs were incubated with FITC-OVA as a model Ag in the presence and absence of LT-IIa-B5. Fluorescence measurements demonstrated that untreated BMDC, as expected, were capable of acquiring FITC-OVA (Fig. 1). Treatment of BMDC with LT-IIa-B5, however, enhanced uptake of FITC-OVA over 2.5-fold in comparison to the amount of FITC-OVA acquired by untreated BMDC. This enhancing effect was dose-dependent (Fig. 2A) and was maximal at early time points (Fig. 2B). To confirm that the fluorescent signals which were detected were due to internalized FITC-OVA and not to FITC-OVA bound to the surface of the cell, identical experiments were performed on ice. At that temperature, acquisition of a fluorescent signal by BMDC was essentially abrogated regardless of treatment (Fig. 1).
Figure 1. Uptake of FITC-OVA by BMDC is enhanced by LT-IIa-B5.
BMDC incubated for 10 min at either 37°C or on ice with 5 μg/ml of LT-IIa-B5 or PBS (Untreated) were treated with FITC-OVA (0.2 mg/ml) for 10 min, washed with PBS, and stained with an APC-conjugated anti-CD11c mAb. The Mean Fluorescent Intensity (MFI) of CD11c-positive, 7-AAD-negative cells was obtained using flow cytometry. Triplicate samples were employed for each experiment. A. Histographical analysis of uptake of FITC-OVA by BMDC. Data from one of three independent experiments are shown. B. Graphical comparison of the uptake of FITC-OVA by BMDC. Data from one of three independent experiments is shown. Error bars denote one standard deviation of the mean obtained from triplicate samples. Key: ***, statistical difference (P<0.001) between the treated population and the untreated control.
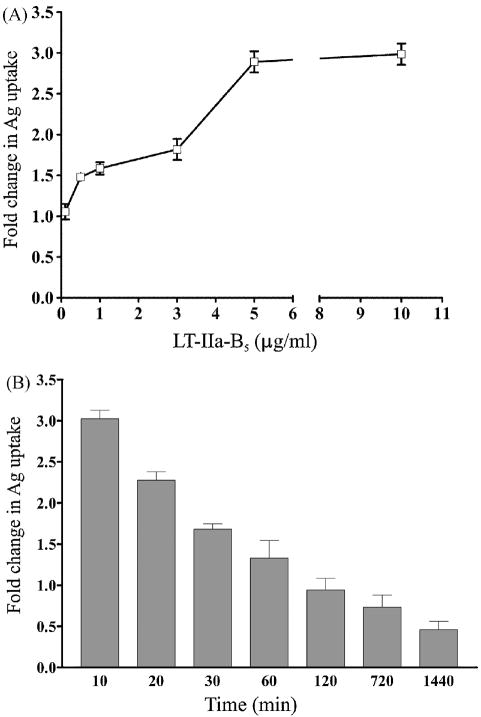
Figure 2. LT-IIa-B5-induced enhanced uptake of FITC-OVA by BMDC is time- and dose-dependent.
A. Dose-dependence. BMDC incubated for 10 min at 37°C with 0.1, 0.5, 1, 3, 5, or 10 μg/ml of LT-IIa-B5 were treated with FITC-OVA (0.2 mg/ml) for 10 min, washed with PBS, and stained with an APC-conjugated anti-CD11c mAb. Data are presented as fold change in the MFI of the FITC fluorescence of CD11c-positive, 7-AAD-negative LT-IIa-B5 treated BMDC to the MFI of the FITC fluorescence of CD11c-positive, 7-AAD-negative untreated BMDC. Error bars denote one standard deviation from the mean obtained from triplicate cultures. B. Time dependence. BMDC incubated at 37°C for 10, 20, or 30 min and 1, 2, 12, or 24 hrs with 5 μg/ml of LT-IIa-B5 were treated with FITC-OVA (0.2 mg/ml) for 10 min, washed with PBS, and stained with an APC-conjugated anti-CD11c mAb. Data are presented as fold change of MFI of the FITC fluorescence of CD11c-positive, 7-AAD-negative LT-IIa-B5 treated BMDC to MFI of the FITC fluorescence of CD11c-positive, 7-AAD-negative untreated BMDC. Error bars denote one standard deviation from the mean obtained from triplicate cultures.
Identical uptake experiments were performed using BMDC treated with LT-IIa holotoxin. No enhancement of uptake of FITC-OVA was observed in holotoxin-treated cells (data not shown). These data clearly indicated that the ability to enhance uptake of FITC-OVA by BMDC was a feature of LT-IIa-B5 which was not exhibited by LT-IIa holotoxin.
The ability of LT-IIa-B5 to enhance internalization of Ag by BMDC was not limited to FITC-OVA. Similar experiments were performed using FITC-dextran and FITC-labeled streptococcal surface antigen AgI/II [11, 12] as alternative model Ags. In both cases, uptake of FITC-dextran and FITC-AgI/II by BMDC was significantly enhanced in the presence of LT-IIa-B5 in comparison to uptake of those Ag by untreated BMDC (data not shown). These experiments indicated that LT-IIa-B5 exhibited a general property for enhancing Ag uptake by BMDC.
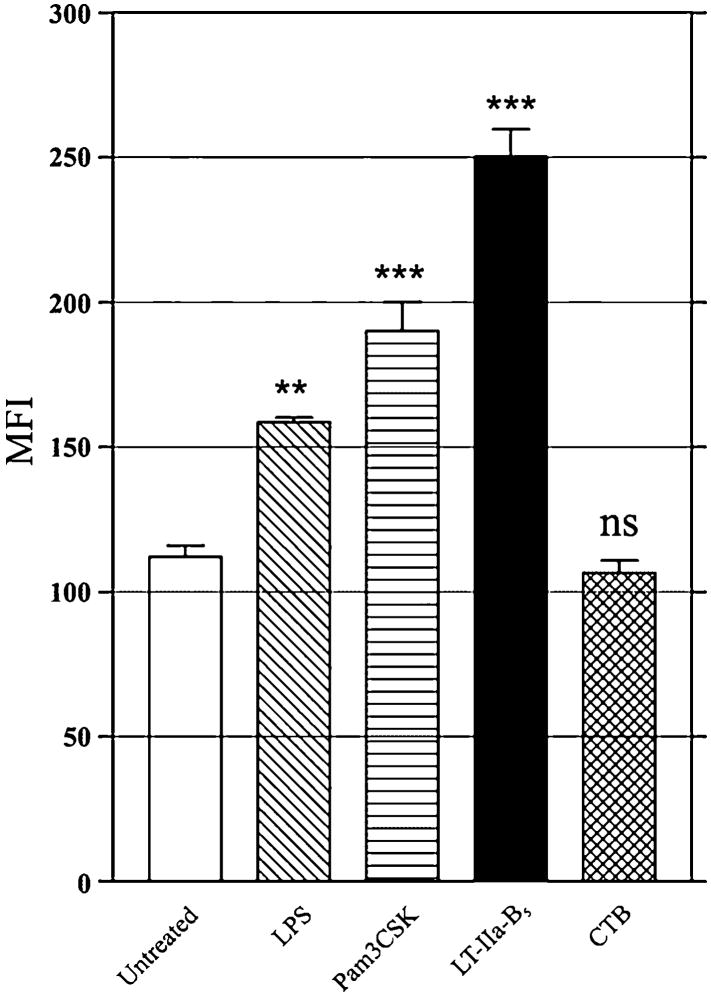
In contrast, the B pentamer of cholera toxin (CTB) which binds to ganglioside GM1 was incapable of enhancing uptake of FITC-OVA by BMDC (Fig. 3). Since prior studies demonstrated that CTB binds avidly to all splenic CD11c+ cells, the majority of which are assumed to be DC [9, 31], these data implied that the immunomodulating properties of LT-IIa-B5 and CTB are distinctive and that binding of the pentamers to ganglioside GM1 is not solely sufficient for enhancing Ag uptake by BMDC.
Figure 3. Uptake of FITC-OVA by BMDC is not enhanced by treatment with CTB.
BMDC treated for 10 min at either 37°C with PBS (untreated), 1 μg/ml of LPS, 1 μg/ml of Pam3CSK, 5 μg/ml of LT-IIa-B5, or 5 μg/ml of CTB were incubated with FITC-OVA (0.2 mg/ml) for 10 min, washed with PBS, and stained with an APC-conjugated anti-CD11c mAb. FITC fluorescence of CD11c-positive, 7-AAD-negative cells was measured by flow cytometry. Data are presented as the MFI. Error bars denote one standard deviation from the mean obtained from triplicate cultures. Key: **, statistical difference from the control at P<0.01; ***, statistical difference from the control at P<0.001; ns, no statistical difference from the control.
3.2. Induction of co-stimulatory receptors by LT-IIa-B5
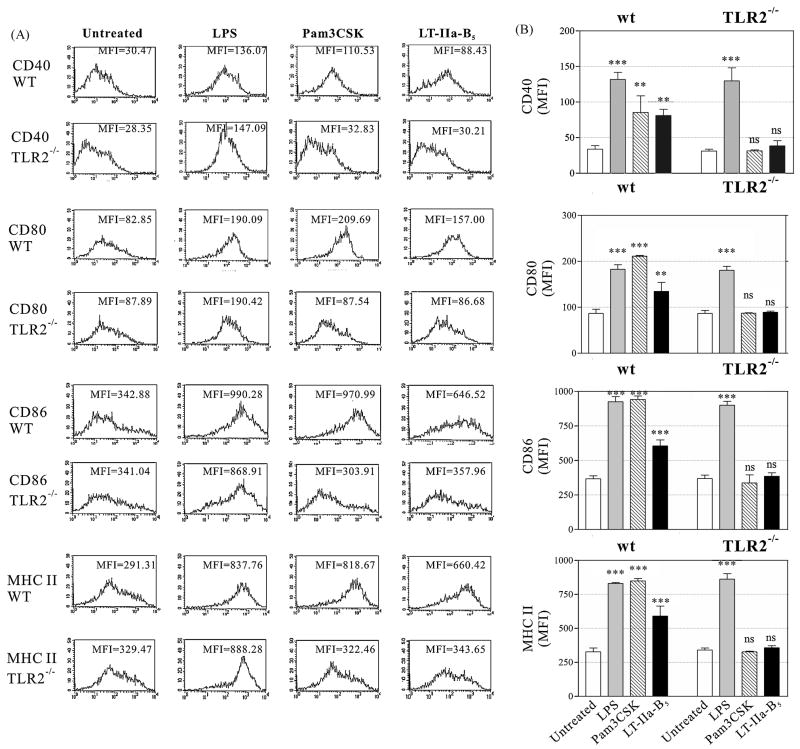
Engagement with T cells is mediated, in part, by the co-stimulatory molecules CD40, CD80, CD86, and MHC-II on DC which are upregulated upon maturation of the cell [15-18]. To determine if treatment with LT-IIa-B5 altered expression of these surface molecules, and thus induced maturation of the BMDC, unactivated BMDC incubated with LT-IIa-B5 were analyzed for expression of CD40, CD80, CD86, and MHC-II. LPS, a TLR4 agonist, and Pam3CSK, a TLR2 agonist [32, 33], were employed as controls. Untreated BMDC expressed a basal level of CDE40, CD80, CD86, and MHC-II (Fig. 4). As expected, the amounts of CD40, CD80, CD86, and MHC-II were elevated in BMDC treated either with LPS or Pam3CSK [34, 35]. Likewise, treatment of BMDC with LT-IIa-B5 significantly increased expression of all four co-stimulatory molecules above the basal levels expressed by untreated cells, although the levels of CD40 induced by treatment with LT-IIa-B5 was less than the levels induced by either LPS or Pam3CSK. Small populations of BMDC in which expression of CD40, CD86, or MHC-II was not elevated by treatment with LT-IIa-B5 was observed. That observation suggested that a small portion of the BMDC were refractory to stimulation by LT-IIa-B5. Nonetheless, treatment with LT-IIa-B5 markedly increased the maturation status of a large portion of the BMDC.
Figure 4. Expression of surface co-stimulatory receptors by wt and TLR2-deficient BMDC after treatment with LT-IIa-B5.
BMDC (1×106) derived from wt C57BL/6 mice or C57Bl/6(TLR2-/-) mice were incubated for 24 hr at 37°C with, PBS (Untreated), 1.0 μg/ml of LPS, 1.0 μg/ml Pam3CSK, or 5.0 μg/ml of LT-IIa-B5. CD11c+ cells were analyzed by flow cytometry for expression of CD40, CD80, CD86, and MHC-II. A. Histographic analysis of expression of CD40, CD80, CD86, and MHC-II. Data from one of three independent experiments are shown. The MFI values for CD40, CD80, CD86, and MHC-II are denoted within each histogram. B. Graphical comparison of the expression of CD40, CD80, CD86, and MHC-II. Error bars denote one standard deviation from the mean. Key: wt, BMDC from TLR2-proficient C57Bl/6 mice; TLR2-/-, BMDC from TLR2-deficient mice; ***, statistical difference from the untreated control (P<0.001); **, statistical difference from the untreated control (P<0.01); ns, not significant.
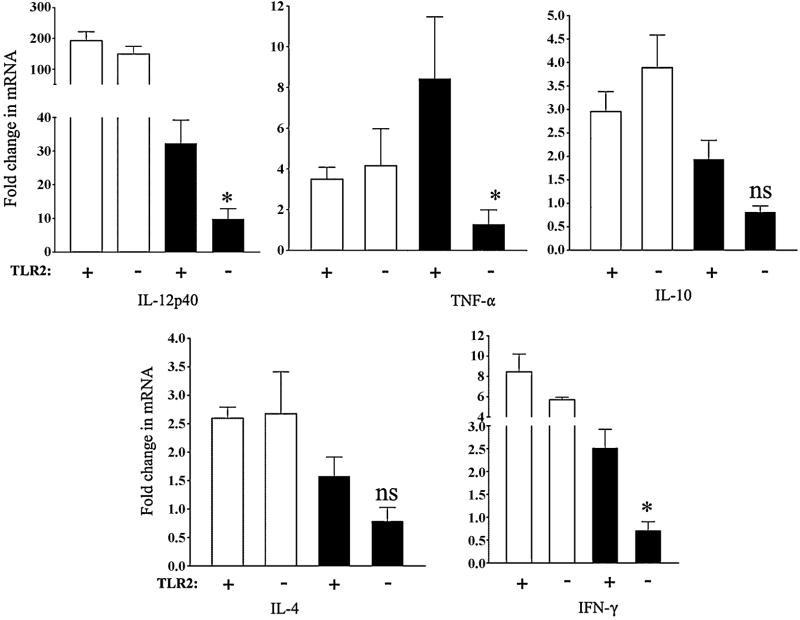
3.3. LT-IIa-B5 modulates cytokine expression by BMDC
DC produce various cytokines including IL-12, TNF-α, IL-10, IL-4, and IFN-γ. Each of these cytokines induce in various immunocompetent cells a number of cellular and molecular events needed to elicit Ag-specific immune responses [36]. To determine if the cytokine expression patterns of DC would be altered by treatment with LT-IIa-B5, transcriptional expression of IL-12p40, TNF-α, IL-10, IL-4, and IFN-γ in BMDC was evaluated.
As expected [37], BMDC stimulated with LPS responded by increasing mRNA levels of IL-12p40, TNF-α, IL-10, IL-4, and IFN-γ above those levels observed in untreated cells (increases of 192.4-fold, 3.5-fold, 2.9-fold, 2.6-fold, and a 8.4–fold over the value of the untreated control, respectively)(Fig. 5). Similarly, in comparison to levels observed in the untreated cells, treatment of BMDC with LT-IIa-B5 induced a dramatic increase in expression of mRNA encoding for IL-12p40, TNF-α, IL-10, IL-4, and IFN-γ (increases of 32.2-fold, 8.4-fold, 1.9-fold, 1.6-fold, and a 2.5-fold over the value of the untreated control, respectively). Thus, treatment with LT-IIa-B5 stimulated the expression of cytokines known for BMDC to be involved in modulating immune responses to Ags.
Figure 5. Cytokine production in BMDC induced by treatment with LT-IIa-B5.
BMDC (5×106) derived from wt C57BL/6 mice (WT) or C57Bl/6 TLR2-/- mice (TLR2-/-) were incubated for 6 hrs at 37°C with PBS (Untreated), 100 ng/ml of LPS, or with 5 μg/ml of LT-IIa-B5. Total RNAs isolated from the BMDC were analyzed by qRT-PCR for mRNA encoding IL-12p40, TNF-α, IL-10, IL-4, and IFN-γ. Data are presented as fold change in expression of the cytokine from expression of that cytokine in the untreated BMDC. Error bars denote one standard deviation from the mean obtained from triplicate cultures. Key: +, TLR2-proficient BMDC; -, TLR2-deficient BMDC; white bars, BMDC treated with LPS; black bars, BMDC treated with LT-IIa-B5; *, statistical difference from the untreated control (P<0.05).
3.4. Enhancement of CD4+ T cell proliferation
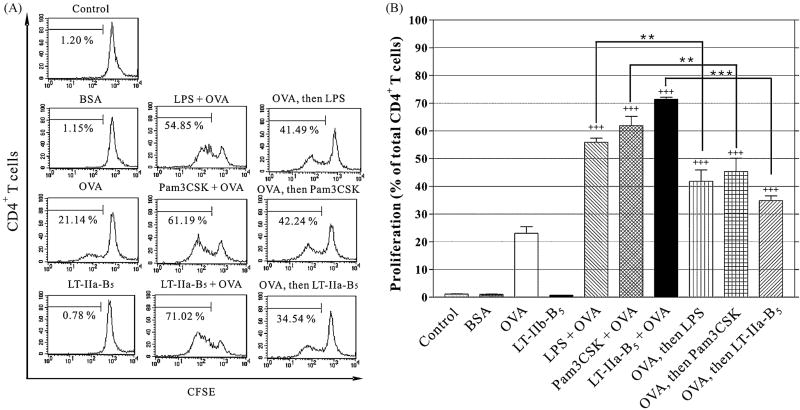
After acquisition of Ag, APC process and present Ag peptides in the context of MHC-II to CD4+ T cells which respond by clonal expansion. It was clear that LT-IIa-B5 enhanced the capacity of BMDC to acquire FITC-OVA. But, to ascribe functional significance to that effect, enhanced uptake needed to be correlated with more efficient Ag processing, presentation, and subsequent Ag-specific T cell proliferation for which a syngeneic CD4+ T cell proliferation assay was employed. BMDC were co-incubated with OVA in the presence and absence of LT-IIa-B5, washed extensively to remove unbound Ag and/or pentamer, and subsequently co-cultured with CFSE-labeled CD4+ T cells obtained from DO11.10 mice (Fig. 6). CD4+ T cells from DO11.10 mice encode a T-cell receptor that is specific for OVA presented by the MHC-II molecule I-Ad [38, 39]. Proliferation of the CD4+ T cells was evaluated by measuring dilution of the CFSE stain which is an indicator of dividing cells.
Figure 6. OVA-specific CD4+ T cells proliferation is enhanced by LT-IIa-B5.
BMDC (5×104) were incubated for 30 min with PBS (Control), 1.0 mg/ml OVA (OVA), 1.0 mg/ml BSA, 5 μg/ml LPS and 1.0 mg/ml OVA (LPS + OVA), 5 μg/ml Pam3CSK and 1.0 mg/ml OVA (Pam3CSK + OVA), or with 20 μg/ml LT-IIa-B5 and 1.0 mg/ml OVA (LT-IIa-B5 + OVA). Similar experiments were performed in which BMDC were initially incubated with 1.0 g/ml OVA, washed, and subsequent treated with 5 μg/ml LPS (OVA, then LPS), 5 μg/ml Pam3CSK (OVA, then Pam3CSK), or 20 μg/ml LT-IIa-B5 (OVA, then LT-IIa-B5). After washing, BMDC were co-cultured with CFSE-stained naïve DO11.10 CD4+ T cells (1×106). Cells were harvested after 4 days, washed in PBS, stained for CD4, and the CFSE fluorescence of the DO11.10 CD4+ T cells was determined as a measure of proliferation. A. Histographical analysis of Ag-specific CD4+ T cells proliferation. The percentage of dividing cells in each population is denoted below the bracket in each histogram. B. Graphical comparison of Ag-specific CD4+ T cell proliferation. Error bars denote one standard deviation from the mean obtained from triplicate cultures. Key: +++ and ++ statistical differences from the control at P<0.001 and P<0.01 respectively. *** and **statistical differences from the matched column at P<0.001 and P<0.01 respectively.
Essentially no proliferation of DO11.10 CD4+ T cells was observed in (i) control BMDC incubated only with PBS (1.15%), (ii) BMDC which had been incubated only with bovine serum albumin (BSA)(1.03%), or (iii) in BMDC treated only with LT-IIa-B5 (0.69%)(Fig. 6). Incubation of the BMDC solely with OVA induced Ag-specific proliferation of the DO11.10 CD4+ T cells (23.07%). In comparison to control cells, proliferation was enhanced when the CD4+ T cells were incubated with OVA in the presence of LPS (55.88%) and Pam3CSK (61.9%)(Fig. 6A). Similarly, co-incubation of BMDC with LT-IIa-B5 and OVA significantly enhanced the Ag-specific proliferative response of the DO11.10 CD4+ T cells to a level (71.42% of cells in the population proliferating) which exceeded the level of uptake induced by co-incubation of BMDC with LPS and OVA (55.88% of cells in the population proliferating). Clearly, treatment of BMDC with LT-IIa-B5 influenced Ag-specific clonal expansion of CD4+ T cells.
To determine if the increased CD4+ T cell proliferation was due to enhanced Ag-uptake or only to the enhancing effects of LT-IIa-B5 on expression of costimulatory molecules and cytokines, a sequential treatment experiment was designed. BMDC were incubated with OVA, washed extensively, and subsequently treated with LT-IIa-B5. When BMDC were incubated sequentially with OVA and LT-IIa-B5, a significantly reduced Ag-specific proliferation of DO11.10 CD4+ T cells was observed in comparison to the level of Ag-specific proliferation of DO11.10 CD4+ T cells which had been co-cultured with BMDC exposed simultaneously to OVA and LT-IIa-B5 (34.86% vs 71.42%, respectively)(Fig. 6A). OVA-treated BMDC subsequently washed and incubated with LPS or Pam3CSK also exhibited a significantly reduced Ag-specific proliferation of the DO11.10 CD4+ T cells in comparison to results elicited by use of BMDC simultaneously exposed to OVA and LPS or OVA and Pam3CSK (41.82% vs 55.88% and 45.36% vs 61. 9%, respectively)(Fig. 6A). These data suggested that the capacity of LT-IIa-B5 to enhance Ag-specific CD4+ T cell proliferation was likely an effect of enhanced uptake by Ag by LT-IIa-B5-treated BMDC rather than by the sole effects of LT-IIa-B5 on enhancing expression of either maturation markers or cytokines on BMDC.
Although this assay is an indirect assessment of the role of Ag uptake in T cell proliferation, the data support a model in which the increased Ag-specific proliferation of CD4+ T cells which was observed was a result of enhanced Ag uptake (and possibly enhanced processing and/or presentation) by BMDC that was induced by treatment with LT-IIa-B5.
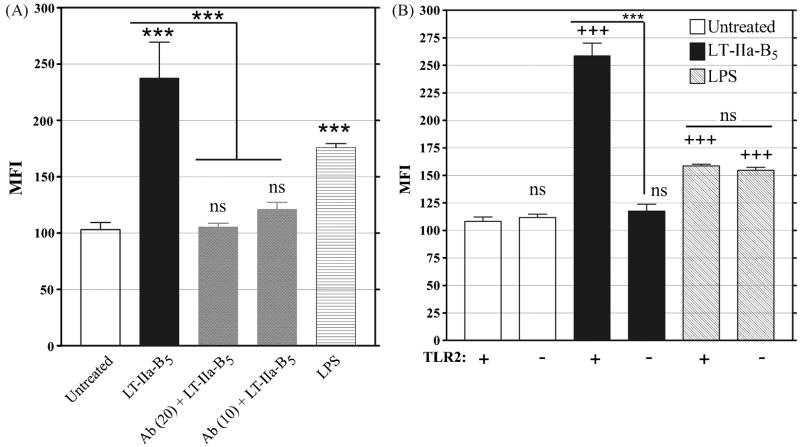
3.5. Enhanced Ag uptake and TLR2
While a basal level of uptake of FITC-OVA was observed in untreated BMDC, acquisition of the Ag was enhanced in BMDC which had been treated with Pam3CSK, a known TLR2 agonist [32, 40] (Fig. 3). In mouse and human monocytes, release of IL-1β, IL-6, IL-8, and TNF-α and activation of NF-κB [41] by LT-IIaB5 was dependent upon expression of TLR2 by the cells [24, 41]. To determine if the enhanced acquisition of FITC-OVA induced by LT-IIa-B5 also required TLR2, uptake experiments were conducted using BMDC pretreated with T2.5, an anti-TLR2 blocking antibody. LT-IIa-B5-treated BMDC which had been incubated with T2.5 exhibited significant inhibition of uptake of OVA-FITC (2.4-fold decrease) in comparison to uptake by BMDC in the absence of the blocking antibody (Fig. 7A). To confirm these results, complementary uptake experiments were performed using BMDC derived from B6.129-Tlr2tm1Kir/J knock-out mice which were deficient in expression of TLR2. No enhancement in Ag uptake above that level observed in untreated cells (MFI = 111.7) was observed for BMDC (TLR2-/-) which had been incubated with LT-IIa-B5 (MFI = 117.4)(Fig. 7B). In contrast, treatment of BMDC(TLR2-/-) with LPS, a TLR4 agonist [34], significantly increased uptake of FITC-OVA MFI = 154.6) above those levels observed in untreated cells (MFI = 111.7), thus indicating that mechanisms required for uptake of Ag were unaltered in the TLR2-deficient cells. These uptake experiments demonstrated that LT-IIa-B5-dependent enhanced acquisition of FITC-OVA by BMDC required TLR2.
Figure 7. Enhanced uptake of FITC-OVA by BMDC induced by treatment with LT-IIa-B5 is dependent on TLR2.
A. BMDC treated with PBS (untreated), 5 μg/ml of LT-IIa-B5, or 1 μg/ml of LPS were co-incubated with 10 μg/ml or 20 μg/ml of an anti-TLR2 blocking mAb (α-TLR2). After 10 min, BMDC were incubated with FITC-OVA (0.2 mg/ml), washed with PBS, and stained for CD11c. CD11c-positive cells were measured for FITC fluorescence. Data are presented as the MFI. Error bars denote one standard deviation from the mean obtained from triplicate cultures. Key: ***, statistically different from the untreated group or, for the anti-TLR2-treated cells, statistically different from the LT-IIa-B5-treated group (as denoted by the lines) (P<0.001); ns, not significant from the untreated group. B. BMDC derived from the bone marrow of wt C57Bl/6 mice or C57Bl/6(TLR2-/-) mice treated with PBS (Untreated), 5 μg/ml of LT-IIa-B5, or 1 μg/ml of LPS were incubated with FITC-OVA (0.2 mg/ml) for 10 min, washed with PBS, and stained for CD11c. Data are presented as the MFI with error bars denoting one standard deviation from the mean obtained from triplicate cultures. Key: +, TLR2-proficient BMDC; -, TLR2-deficient BMDC; +++, statistical difference from the matched (Untreated vs TLR2-deficient) control; ***, statistical difference between the wt and TLR2-deficient BMDC treated with LT-IIa-B5 (as denoted by the lines); ns, not significant to the matched untreated control or, in the case of the LPS-treated cells, no significant difference between the TLR2-proficient and the TLR2-deficient BMDC.
TLR2 was also found to be important in BMDC in modulating expression of co-stimulatory molecules in response to treatment with LT-IIa-B5. Unlike expression of CD40, CD80, CD86, and MHC-II in TLR2-proficient BMDC which was elevated upon treatment with LT-IIa-B5, expression of these four surface molecules in TLR2-deficient BMDC treated with LT-IIa-B5 was essentially equal to the basal levels of expression of these surface markers in untreated TLR2-proficient cells (MFI values: 30.21 vs 30.47; 86.68 vs 82.85; 357.96 vs 342.88; 343.65 vs 291.31; respectively) (Fig. 4). Pam3CKS, a potent TLR2 agonist, was also unable to upregulate expression of CD40, CD80, CD86, and MHC-II in TLR2-deficient cells. In comparison to expression of the four co-stimulatory receptors by untreated TLR2-deficient cells, expression of CD40, CD80, CD86, and MHC-II was enhanced in BMDC(TLR2-/-) treated with LPS, a TLR4 agonist (MFI values: 147.09 vs 28.35; 190.42 vs 87.89; 868.91 vs 341.04; 888.28 vs 329.47; respectively)(Fig. 4). These data indicated that TLR2-deficient BMDC have the ability to modulate CD40, CD80, CD86, and MHC-II using TLR4-dependent or other LPS-dependent regulatory pathways. Furthermore, the data demonstrated that LT-IIa-B5 had the capacity to modulate expression of the four co-stimulatory molecules in a TLR2-dependent manner.
Similarly, induction of cytokines by LT-IIa-B5 also required engagement of TLR2 (Fig. 5). In TLR2-deficient BMDC treated with LPS, the levels of mRNA encoding IL-12p40, TNF-α, IL-10, IL-4, and IFN-γ was essentially unchanged from the levels of mRNA of those cytokines in TLR2-proficient (wt) BMDC (149.3-fold vs 192.4-fold; 4.2-fold vs 3.5-fold; 3.9-fold vs 2.9-fold; 2.7-fold vs 2.6-fold; and 5.7-fold vs 8.4-fold change, respectively) (Fig. 5). The levels of mRNA encoding IL-12p40, TNF-α, and IFN-γ were significantly decreased in TLR2-deficient BMDC following treatment with LT-IIa-B5 in comparison to the levels of expression of the three cytokines observed in TLR2-proficient BMDC treated with LT-IIa-B5 (32.2-fold vs 9.8-fold; 8.4-fold vs 1.3-fold; and, 2.5-fold vs 0.7-fold, respectively)(Fig. 5). While the levels of expression of IL-10 and IL-4 were lower in TLR2-deficient mice in comparison to the levels of expression of those two cytokines in TLR-proficient mice, the differences were just below the level of statistical significance (>0.05). These data strongly indicated that the engagement of TLR2 was essential for LT-IIa-B5 to induce increased expression of cytokine expression in BMDC.
These data, in combination with those obtained using the anti-TLR2 blocking antibody, confirmed the role of TLR2 in the capacity of LT-IIa-B5 to enhance uptake of FITC-OVA by BMDC and in stimulating the maturation of those cells.
4.0. DISCUSSION
Immature DC are sentinels for detecting and responding to Ags produced by invading pathogens. Upon encountering a pathogen-derived Ag, DC undergo a maturation cascade which begins by acquisition of Ag and culminates in migration of the cells to proximal lymphoid tissues. During this process, DC upregulate expression of major histocompatibility complexes and other co-stimulatory surface proteins such as CD40, CD80, CD86, and MHC-II required for immunosynapse formation, processing and presenting Ag to T cells, and for production of specific cytokines required for T cell activation [15-18]. While DC preferentially evoke T helper 1 (Th1) responses, recent evidence indicates that feedback loops involving DC-elaborated IL-12 stimulate B cells to release cytokines which inhibit Th1 development [42]. Thus, DC are critical cellular components in immune cell regulatory mechanisms for polarizing the immune system toward Th1 or Th2 type of responses [43]. Boosting the efficacy in DC of one or more stages of this process would be expected to potentiate cellular and/or humoral immune responses against preferred Ags. The experiments described herein investigated the capacity of the non-toxic B pentamer of LT-IIa, a type II heat-labile enterotoxin of E. coli which binds DC, to enhance the initial step in the cascade of developmental and regulatory events which promulgate a typical Ag-specific immune response. While BMDC and mucosal resident DC are not absolutely equivalent in phenotype [44, 45], BMDC can be induced by extracellular agents to take up Ag, express maturation markers, and present Ag to T cells. Thus, BMDC were chosen as a convenient model cell type for predicting the responses of mucosal resident DC which are difficult to obtain in numbers sufficient for experimentation. In vitro experiments detailed herein firmly established that treatment with LT-IIa-B5 enhanced Ag uptake and Ag presentation by BMDC and stimulated maturation of those cells. Additional experiments were performed to reveal the initial mechanisms by which LT-IIa-B5 induced those responses.
Lacking the enzymatic activity of the holotoxin, it is likely that the immunomodulatory effects of LT-IIa-B5 were induced, in part, by binding of the pentamer to gangliosides located on the surface of one or more types of immunocompetent cells [9]. Gangliosides have often been implicated in signal transduction events that regulate a plethora of cellular responses [10]. LT-IIa-B5 binds avidly to several gangliosides including GD1b, GD1a, and GM1. It was deemed feasible, therefore, that engagement of LT-IIa-B5 with ganglioside was a sufficient trigger to induce facilitated uptake of Ag by BMDC. Ag uptake was not enhanced, however, when BMDC were treated with CTB which avidly binds to GM1. Sole engagement by LT-IIa-B5 (or CTB) to ganglioside GM1, therefore, was not a sufficient event to promote enhanced Ag uptake in BMDC. These data suggested a potential participation of another receptor for LT-IIa-B5 for stimulating BMDC. LT-IIa-B5, but not CTB, interacts physically and functionally with TLR2, one of the family of Toll-like receptors involved in innate and adaptive immunity [25, 41]. In vitro experiments demonstrated that treatment with LT-IIa-B5 induced TLR2-dependent activation of NF-κB in both murine and human monocytes [25, 41]. IL-1β, IL-6, IL-8, and TNF-α were induced in human THP-1 cells after treatment with LT-IIa-B5 [41]. Anti-TLR2, but not anti-TLR4 antibodies, inhibited expression of these four cytokines [41]. These data confirmed a functional interaction between LT-IIa-B5 and TLR2 [25, 41, 46]. The B pentamer of LT-IIb (LT-IIb-B5), a type II enterotoxin that is closely-related to LT-IIa, also was shown to functionally engage TLR2 [46]. In contrast, LT-IIb-B5(T13I), a mutant pentamer which has significantly decreased binding to its ganglioside receptors [11, 47], failed to induce TLR2-dependent production of cytokines [41]. The responses engendered by LT-IIa-B5 and LT-IIb-B5, therefore, likely require formation of a trimolecular complex consisting of the pentamer, a ganglioside, and TLR2 [47]. The formation of this complex has recently been demonstrated using FRET [47]. Genetic mapping of LT-IIb-B5 recently revealed a short domain in the pentamer that is required for binding of the pentamer to TLR2 [46, 48]. Based on crystallographic studies of LT-IIb, the related type II HLT [49], this domain is likely shielded by the A polypeptide in the LT-IIa holotoxin which explains the inability of the LT-IIa holotoxin either to interact with TLR2 [46] or to enhance uptake of antigen by BMDC. While this interaction domain is present in LT-IIa-B5, it is absent in CTB. The failure of CTB to functionally engage TLR2 is likely due to the absence of this interaction domain [24, 41, 47, 48]. A mutant LT-IIb-B5 pentamer in which a hydrophobic amino acid within the interaction domain was substituted for a charged amino acid, failed to interact with TLR2 or to elicit TLR2-dependent effects [46, 48]. TLR2 agonists are known to initiate a number of cellular events [50, 51]. Experiments conducted herein firmly demonstrated that enhanced uptake of Ag by LT-IIa-B5-treated BMDC is dependent upon expression of TLR2. These data are consistent with prior reports that implicated TLR2 agonists in the process of Ag acquisition by APC [52]. Treatment of immature DC with Pam3CSK, a TLR2 agonist, increased micropinocytosis of FITC-dextran, an event which was correlated with coordinated reorganization of actin in the cell [53]. Unlike FITC-OVA, however, uptake in that model system was likely promoted, at least in part, by binding of FITC-dextran to the mannose receptor on BMDC [54]. Fluorescent experiments using phalloidin as a reporter for polymerized actin are currently being employed to determine if LT-IIa-B5 induces similar actin reorganization in BMDC.
Immunosynapsis between naïve T cells and DC routinely requires expression of certain costimulatory receptors, such as CD40, CD80, CD86, CD40, and MHC-II, which, in the case of DC, are usually upregulated upon maturation [15-18]. Treatment of BMDC with LT-IIa-B5 induced maturation of the cells, an event evidenced by TLR2-dependent upregulated expression of CD40, CD80, CD86, and MHC-II. Induction of these critical co-stimulatory receptors by LT-IIa-B5 would be expected to promote functional interactions between T cells and DC by bolstering immunosynapse formation. This model is supported by in vitro T cell proliferation experiments. Treatment of BMDC with LT-IIa-B5 stimulated Ag-specific CD4+ T cell proliferation at levels equivalent to or exceeding those observed by treatment of the BMDC with the potent stimulant LPS [55]. The observed increase in CD4+ T cell proliferative response was likely a result of (i) pentamer-enhanced Ag uptake, processing, and presentation by the BMDC, (ii) by a more efficient process of immunosynapse formation between the DC and CD4+ T cells brought about by enhanced co-stimulatory ligand expression, (iii) effects of one or more cytokines induced by LT-IIa-B5, or (iv) some combination of the three processes. Results from proliferation experiments in which BMDC were initially exposed to OVA and, after removal of extracellular OVA, were treated with LT-IIa-B5 indicated that enhanced CD4+ T cell proliferation observed in the co-cultures when BMDC were simultaneously incubated with OVA and LT-IIa-B5 was not promoted solely by alterations on expression of the four maturation markers or by increased expression of IL-12, TNF-α, IL-10, IL-4, or IFN-γ by LT-IIa-B5. Rather, enhanced proliferation of the CD4+ T cells required enhanced uptake of Ag by the BMDC.
It will be informative to conduct experiments to determine if LT-IIa-B5 has the capacity to prolong DC in an immature state, thus enabling the cells to acquire Ag over an increased span of time. It is also feasible that LT-IIa-B5 alters the temporal expression of essential migration signals, such as CCR6 and CCR7, on DC which could enable the APC to remain for longer periods at locales in which Ag is plentiful. Notably, treatment of DC with CTB does not enhance expression of CD40, CD80, and CD86 [16, 56]. Genetic or chemical conjugation of OVA to CTB was required to promote Ag-specific proliferation of T cells [57]. The failure of CTB to elicit expression of maturation markers on DC or to induce enhanced T cell proliferation is likely due, in part, to the incapacity of CTB to engage TLR2 [46, 48]. Clearly, the immunopotentiating effects of LT-IIa-B5 on DC are very distinguishable from those of CTB.
The capacity of LT-IIa-B5 to functionally interact with TLR2, to enhance uptake of Ag by DC, to elevate expression by DC of important co-stimulatory receptors and cytokines, and to stimulate Ag-specific CD4+ T cell proliferation in vitro are distinctive immunomodulatory characteristics which may be exploitable in vivo. If, when used as a mucosal adjuvant, LT-IIa-B5 enhances uptake of a co-administered Ag by resident DC, mucosal immune responses to that Ag will likely be increased. Preliminary experiments also demonstrated that LT-IIa-B5 influences migration and/or retention of DC in draining lymph nodes; numbers of DC in the cervical lymph nodes of mice are dramatically increased after intranasal administration of LT-IIa-B5 (C.H.L, and T.D.C., data not shown).
In these experiments, LT-IIa-B5 has been shown to be a new and intriguing molecular/cellular tool for dissecting the interactions between Ag uptake mechanisms, TLR2, and signal transduction in DC. It is likely that LT-IIa-B5 will also be useful in revealing important immunological responses in other immunocompetent cell types. It will be interesting to determine whether the ability of LT-IIa-B5 to enhance antigen uptake and Ag-specific CD4+ T cell proliferation will translate to the ability to enhance Ag-specific immune responses in a mouse immunization model.
Acknowledgments
This work was supported by The National Institutes of Health research grants DE013833 and DE014357 awarded to T.D.C. and by research grant DE017138 awarded to G.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorby GL, Robinson EN, Jr, Barley LR, Clemens CM, McGee ZA. Microbial invasion: a covert activity. Can J Microbiol. 1988 Apr;34(4):507–12. doi: 10.1139/m88-087. [DOI] [PubMed] [Google Scholar]
- 2.Mestecky J, Nguyen H, Czerkinsky C, Kiyono H. Oral immunization: an update. Curr Opin Gastroenterol. 2008 Nov;24(6):713–9. doi: 10.1097/MOG.0b013e32830d58be. [DOI] [PubMed] [Google Scholar]
- 3.Baumann U. Mucosal vaccination against bacterial respiratory infections. Expert Rev Vaccines. 2008 Oct;7(8):1257–76. doi: 10.1586/14760584.7.8.1257. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 5.Anderson CC, Carroll JM, Gallucci S, Ridge JP, Cheever AW, Matzinger P. Testing time-, ignorance-, and danger-based models of tolerance. J Immunol. 2001 Mar 15;166(6):3663–71. doi: 10.4049/jimmunol.166.6.3663. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995 Jul 13;376(6536):177–80. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat Immunol. 2001 Aug;2(8):671–2. doi: 10.1038/90604. [DOI] [PubMed] [Google Scholar]
- 8.Connell TD, Metzger D, Sfintescu C, Evans RT. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol Lett. 1998 Jun;62(2):117–20. doi: 10.1016/s0165-2478(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 9.Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J Dent Res. 2005 Dec;84(12):1104–16. doi: 10.1177/154405910508401205. [DOI] [PubMed] [Google Scholar]
- 10.Connell TD. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev Vaccines. 2007 Oct;6(5):821–34. doi: 10.1586/14760584.6.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nawar HF, Arce S, Russell MW, Connell TD. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect Immun. 2005 Mar;73(3):1330–42. doi: 10.1128/IAI.73.3.1330-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nawar HF, Arce S, Russell MW, Connell TD. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect Immun. 2007 Feb;75(2):621–33. doi: 10.1128/IAI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect Immun. 2000 Jan;68(1):281–7. doi: 10.1128/iai.68.1.281-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell MW, Bergmeier LA, Zanders ED, Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–93. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathinam VA, Hoag KA, Mansfield LS. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect. 2008 Oct;10(12-13):1316–24. doi: 10.1016/j.micinf.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson K, Fredriksson M, Nordstrom I, Holmgren J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun. 2003 Apr;71(4):1740–7. doi: 10.1128/IAI.71.4.1740-1747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pascual DW, Wang X, Kochetkova I, Callis G, Riccardi C. The absence of lymphoid CD8+ dendritic cell maturation in L-selectin-/- respiratory compartment attenuates antiviral immunity. J Immunol. 2008 Jul 15;181(2):1345–56. doi: 10.4049/jimmunol.181.2.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elluru SR, van Huyen JP, Delignat S, Kazatchkine MD, Friboulet A, Kaveri SV, et al. Induction of maturation and activation of human dendritic cells: a mechanism underlying the beneficial effect of Viscum album as complimentary therapy in cancer. BMC Cancer. 2008;8:161. doi: 10.1186/1471-2407-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marland G, Bakker AB, Adema GJ, Figdor CG. Dendritic cells in immune response induction. Stem Cells. 1996 Sep;14(5):501–7. doi: 10.1002/stem.140501. [DOI] [PubMed] [Google Scholar]
- 20.Telemo E, Korotkova M, Hanson LA. Antigen presentation and processing in the intestinal mucosa and lymphocyte homing. Ann Allergy Asthma Immunol. 2003 Jun;90(6 Suppl 3):28–33. doi: 10.1016/s1081-1206(10)61657-2. [DOI] [PubMed] [Google Scholar]
- 21.Fleeton M, Contractor N, Leon F, He J, Wetzel D, Dermody T, et al. Involvement of dendritic cell subsets in the induction of oral tolerance and immunity. Ann N Y Acad Sci. 2004 Dec;1029:60–5. doi: 10.1196/annals.1309.008. [DOI] [PubMed] [Google Scholar]
- 22.Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. Journal of Immunology. 2002;168(1):348–55. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 23.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250(4988):1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 24.Hajishengallis G, Nawar H, Tapping RI, Russell MW, Connell TD. The Type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect Immun. 2004 Nov;72(11):6351–8. doi: 10.1128/IAI.72.11.6351-6358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang S, Wang M, Triantafilou K, Triantafilou M, Nawar HF, Russell MW, et al. The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J Immunol. 2007 Apr 15;178(8):4811–9. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- 26.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R. Generation of large number of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West MA, W R, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004 Aug 20;305(5687):1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 28.Haimov-Kochman R, Fisher SJ, Winn VD. Modification of the standard Trizol-based technique improves the integrity of RNA isolated from RNase-rich placental tissue. Clin Chem. 2006 Jan;52(1):159–60. doi: 10.1373/clinchem.2005.059758. [DOI] [PubMed] [Google Scholar]
- 29.Granucci F, Zanoni I, Feau S, Ricciardi-Castagnoli P. Dendritic cell regulation of immune responses: a new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J. 2003 Jun 2;22(11):2546–51. doi: 10.1093/emboj/cdg261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drakes ML, Czinn SJ, Blanchard TG. Regulation of murine dendritic cell immune responses by Helicobacter felis antigen. Infect Immun. 2006 Aug;74(8):4624–33. doi: 10.1128/IAI.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arce S, Nawar HF, Russell MW, Connell TD. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation of lymphoid cells. Infect Immun. 2005 May;73(5):2718–27. doi: 10.1128/IAI.73.5.2718-2727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardi V, Van Overtvelt L, Horiot S, Moussu H, Chabre H, Louise A, et al. Toll-like receptor 2 agonist Pam3CSK4 enhances the induction of antigen-specific tolerance via the sublingual route. Clin Exp Allergy. 2008 Jul 17; doi: 10.1111/j.1365-2222.2008.03056.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones LA, Anthony JP, Henriquez FL, Lyons RE, Nickdel MB, Carter KC, et al. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008 Sep;125(1):59–69. doi: 10.1111/j.1365-2567.2008.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Lu J, Srinivasan N, Tan BK, Chan SH. Polysaccharide-protein complex from Lycium barbarum L. is a novel stimulus of dendritic cell immunogenicity. J Immunol. 2009 Mar 15;182(6):3503–9. doi: 10.4049/jimmunol.0802567. [DOI] [PubMed] [Google Scholar]
- 35.Hope JC, Whelan AO, Hewinson RG, Vordermeier M, Howard CJ. Maturation of bovine dendritic cells by lipopeptides. Vet Immunol Immunopathol. 2003 Sep 15;95(1-2):21–31. doi: 10.1016/s0165-2427(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 36.Wan Y, Bramson J. Role of dendritic cell-derived cytokines in immune regulation. Curr Pharm Des. 2001 Jul;7(11):977–92. doi: 10.2174/1381612013397627. [DOI] [PubMed] [Google Scholar]
- 37.Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, et al. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3485–90. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamdy S, Elamanchili P, Alshamsan A, Molavi O, Satou T, Samuel J. Enhanced antigen-specific primary CD4+ and CD8+ responses by codelivery of ovalbumin and toll-like receptor ligand monophosphoryl lipid A in poly(D,L-lactic-co-glycolic acid) nanoparticles. J Biomed Mater Res A. 2007 Jun 1;81(3):652–62. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- 39.Latchman Y, Reiser H. Enhanced murine CD4+ T cell responses induced by the CD2 ligand CD48. Eur J Immunol. 1998 Dec;28(12):4325–31. doi: 10.1002/(SICI)1521-4141(199812)28:12<4325::AID-IMMU4325>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 40.Lindner SC, Kohl U, Maier TJ, Steinhilber D, Sorg BL. TLR2 ligands augment cPLA2{alpha} activity and lead to enhanced leukotriene release in human monocytes. J Leukoc Biol. 2009 Aug;86(2):389–99. doi: 10.1189/jlb.1008591. [DOI] [PubMed] [Google Scholar]
- 41.Hajishengallis G, Tapping RI, Martin MH, Nawar H, Lyle EA, Russell MW, et al. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect Immun. 2005 Mar;73(3):1343–9. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skok J, Poudrier J, Gray D. Dendritic cell-derived IL-12 promotes B cell induction of Th2 differentiation: a feedback regulation of Th1 development. J Immunol. 1999 Oct 15;163(8):4284–91. [PubMed] [Google Scholar]
- 43.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005 Jan;26(3):289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 44.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 45.Chalermsarp N, Azuma M. Identification of three distinct subsets of migrating dendritic cells from oral mucosa within the regional lymph nodes. Immunology. 2009 Aug;127(4):558–66. doi: 10.1111/j.1365-2567.2008.03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang S, Hosur KB, Lu S, Nawar HF, Weber BR, Tapping RI, et al. Mapping of a microbial protein domain involved in binding and activation of the TLR2/TLR1 heterodimer. J Immunol. 2009 Mar 1;182(5):2978–85. doi: 10.4049/jimmunol.0803737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang S, Wang M, Tapping RI, Stepensky V, Nawar HF, Triantafilou M, et al. Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. J Biol Chem. 2007 Mar 9;282(10):7532–42. doi: 10.1074/jbc.M611722200. [DOI] [PubMed] [Google Scholar]
- 48.Liang S, Hosur KB, Lu S, Nawar HF, Weber BR, Tapping RI, et al. Mapping of a microbial protein domain involved in binding and activation of the TLR2/TLR1 heterodimer. Journal of Immunology. 2009;182(5):2978–85. doi: 10.4049/jimmunol.0803737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Akker F, Sarfaty S, Twiddy EM, Connell TD, Holmes RK, Hol WG. Crystal structure of a new heat-labile enterotoxin, LT-IIb. Structure. 1996 Jun 15;4(6):665–78. doi: 10.1016/s0969-2126(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 50.Schuster JM, Nelson PS. Toll receptors: an expanding role in our understanding of human disease. J Leukoc Biol. 2000 Jun;67(6):767–73. [PubMed] [Google Scholar]
- 51.Seya T, Matsumoto M. A lipoprotein family from Mycoplasma fermentans confers host immune activation through Toll-like receptor 2. Int J Biochem Cell Biol. 2002 Aug;34(8):901–6. doi: 10.1016/s1357-2725(01)00164-9. [DOI] [PubMed] [Google Scholar]
- 52.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007 May 1;109(9):3890–4. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 53.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004 Aug 20;305(5687):1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 54.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002 Aug 1;100(3):1084–7. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 55.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol. 2003 Nov;33(11):2980–6. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 56.D’Ambrosio A, Colucci M, Pugliese O, Quintieri F, Boirivant M. Cholera toxin B subunit promotes the induction of regulatory T cells by preventing human dendritic cell maturation. J Leukoc Biol. 2008 Sep;84(3):661–8. doi: 10.1189/jlb.1207850. [DOI] [PubMed] [Google Scholar]
- 57.George-Chandy A, Eriksson K, Lebens M, Nordstrom I, Schon E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001 Sep;69(9):5716–25. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]